1. Introduction
Yeast, a single-celled microorganism belonging to the fungal kingdom, has long been recognized for its pivotal role in various industrial processes, most notably in the production of alcoholic beverages such as beer (Bastos et al., 2022; Ferreira, Pinho, Vieira, & Tavarela, 2010; Horn et al., 2023). Beyond its fermentation application, yeast has garnered scientific interest due to its rich composition, encompassing not only proteins extracted in the form of yeast extract but also valuable polysaccharides residing within its cell wall (Bastos et al., 2022; Pacheco, Caballero-Córdoba, & Sgarbieri, 1997; Tao et al., 2023).
Yeast cell walls are a source of useful polysaccharides with a range of biological activity and uses in medicine (Bastos et al., 2022; Horn et al., 2023; Yang Liu, Huang, & Lv, 2018; Tang et al., 2017; Utama, Oktaviani, Balia, & Rialita, 2023; Zhang et al., 2023). Past research has clarified the complex structure and composition of Saccharomyces cerevisiae cell walls, demonstrating that they make up 15–35% of the dry weight of the cell and are composed of up to 90% polysaccharides. These layers are typically arranged in a cell wall system consisting of three layers: an inner rigid layer that is composed of chitin and alkali insoluble glucans, and an outer amorphous layer that is composed of phosphorylated mannoproteins. Through (β1→3)- and (β1→6)-D-Glc connections (Glc — glucose), the β-glucans are interconnected. These polysaccharides impart a range of functional qualities to the cell, including immunomodulatory, antioxidative, and prebiotic benefits, while also maintaining its structural integrity (Tang et al., 2017; Zhou et al., 2023).
The biomedical applications of yeast cell wall polysaccharides have been extensively explored, with promising outcomes in various fields. β-glucans, renowned for their immunomodulatory properties, have shown potential in enhancing host defense mechanisms and combating infectious diseases (Castro et al., 2021). Mannans, on the other hand, have garnered attention for their prebiotic effects and ability to promote gut health (Al-Manhel & Niamah, 2017; Kath & Kulicke, 1999a; Kumar Suryawanshi & Kango, 2021). Additionally, chitin-derived compounds have exhibited wound healing properties and have been utilized in biomedical applications such as tissue engineering and drug delivery systems.
Despite the biomedical potential of yeast cell wall polysaccharides, their utilization has primarily been restricted to niche applications, with yeast extract serving as the predominant source for industrial purposes. Brewer’s spent yeast (BSY), a by-product of beer production, represents a vast untapped reservoir of yeast cell wall polysaccharides. It is the second largest beer production by-products and annually, significant quantities of BSY are disposed of, with current utilization limited to animal feed applications (Ferreira et al., 2010; Reis et al., 2023).
Given the rich polysaccharide content of yeast cell walls, BSY presents a promising, cost-effective alternative source of functional polysaccharides. This study investigates the composition and soluble cell wall polysaccharides extracted from BSY, comparing them with those from pure yeast cell cultures. By elucidating the polysaccharide profiles, we aim to assess the feasibility of utilizing BSY-derived polysaccharides for various applications, including functional foods and nutraceuticals. Furthermore, we highlight the economic advantage of using BSY over preparing pure yeast cell cultures for polysaccharide extraction, emphasizing its viability, sustainability, and associated economic and environmental benefits.
2. Materials and Methods
2.1. Materials
The Brewer’s spent yeast (BSY) utilized in this experiment was produced through drum drying by Korea Yeast Co., Ltd., (Mungyeong, Gyeongsangbuk-do, Korea. The strain used was Saccharomyces cerevisiae. Pure strain cultures were prepared following the method described by Lee et al. (2023) using Saccharomyces cerevisiae D452-2 (SC) and Saccharomyces var. boulardii ATCC MYA-796 (SB). Pre-cultures of the strains were grown at 30°C and 250 rpm overnight in YP medium containing 20 g L−1 glucose. The pre-cultured cells were then harvested and inoculated into main cultures with an initial optical density at 600 nm of 1.0.
Fed-batch fermentation was conducted in a 2.5 L bioreactor (Kobiotech Co., Incheon, Republic of Korea) containing 1 L of YP medium with 20 g L−1 glucose and an initial OD 600 of 1.0. Upon depletion of the initially added glucose, a feeding solution comprising 600 g L−1 glucose, 200 g L−1 yeast extract, and 100 g L−1 peptone was introduced at a rate of 15 mL h−1. The medium’s pH, temperature, agitation speed, and air supply were maintained at pH 5.5, 30°C, 500 rpm, and 1.0vvm, respectively. Following incubation, 60 to 70 g of cell dry matter was recovered and used for analysis. The yeast cells were washed twice with distilled water and subjected to centrifugation (8,000 rpm, 10 min). Subsequently, the yeast cells were freeze-dried for further experimentation, and their weight was measured to calculate the yield.
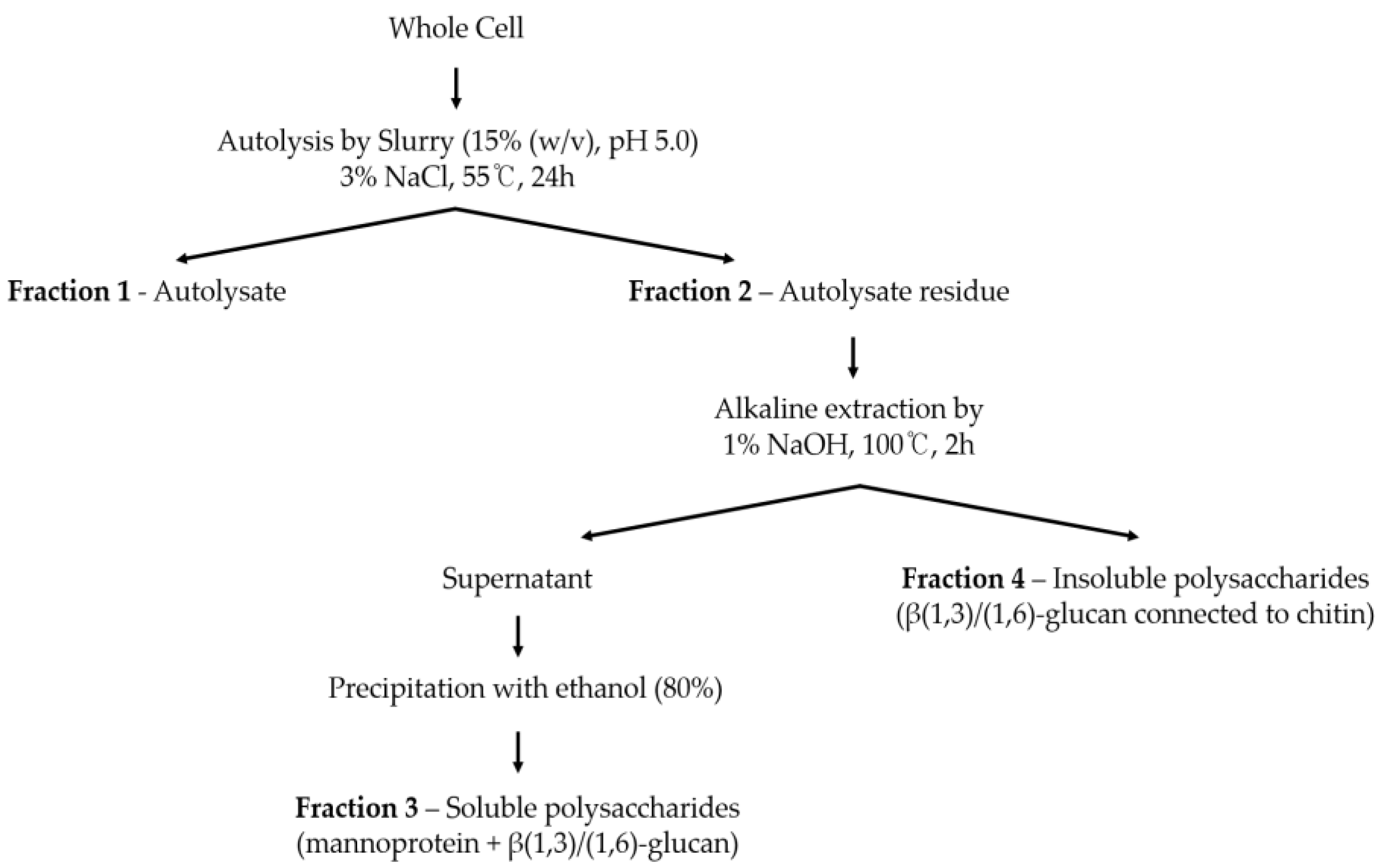
2.2. Autolysis of Yeast Cell
Autolysis was performed following the method outlined by Tanguler et al. (2008) to separate yeast into cell walls and extracts (
Figure 1). Initially, 3% sodium chloride was added to yeast cell slurry (15% (w/v), pH 5.0) as a self-decomposition promoter, and the mixture was incubated for 24 hours. Subsequently, the mixture was centrifuged at 5000 rpm, and the resulting residue was freeze-dried to obtain the cell wall fraction.
2.3. Water-Soluble Polysaccharide Extraction from Autolysate Residue
The isolation of water-soluble polysaccharides from autolysate residue (cell wall fraction) following autolysis was conducted based on the method described by Kath & Kulicke, (1999a) (
Figure 1). Initially, 5g of yeast cell wall was treated with 1% NaOH and extracted in a water bath at 100°C for 2 hours. The alkali-treated suspension was then centrifuged at 4,500 rpm for 10 minutes, resulting in the separation of an insoluble polysaccharide pellet and a soluble polysaccharide supernatant. The supernatant solution pH was neutralized with 2 mol/L HCl and treated with cold ethanol at a ratio of 1:4. The mixture was subsequently precipitated at -80°C for more than 2 hours and then centrifuged at 8,000 rpm for 10 minutes at -10°C to recover the precipitate. This ethanol precipitation process was repeated two times to ensure thorough purification. Finally, the obtained precipitate was freeze-dried to yield the water-soluble polysaccharide fraction of yeast cells.
2.4. Characterization of Yeast Cell Components
2.4.1. Proximate Composition
The general composition of BSY, SC and SB cells and their respective autolysate residue were determined using (Official Analytical Chemistry International, 2005). Briefly, the moisture content was determined by heating drying method at 105°C, the crude fat by the Soxhlet extraction method, and the nitrogen by the micro-kjeldahl method and converted to crude protein by multiplying with the coefficient factor 6.25, and the crude ash was measured by the heating the sample at 550°C in furnace. From 100, the value obtained by adding the moisture content, crude fat, crude protein, and reference content was subtracted and the remaining value was used as carbohydrate.
2.4.2. Sugar Composition
To quantify sugars constituents, hydrolysis with sulfuric acid was performed based on the method by (Dallies, François, & Paquet, 1998). This acid hydrolysis released glucose from β-glucan, mannose from mannans and glucosamine from chitin (since the N-acetyl residue in N-acetyl-glucosamine is acid-labile). in 10mg of cell wall was soaked with 75㎕ of 72% (w/v) H2SO4 at room temperature for 3 hours. Then the slurry was diluted to 2 N H2SO4 by addition of 0.95 ml MilliQ water containing 1mg/ml Sorbitol (used as an internal standard to control the recovery) and heated at 100℃ for 4 hours. Sulfate ions were neutralized by drop-wise addition of NaOH until neutral pH was reached. The volume was adjusted to 20ml. 1 mL was collected and centrifuged at 13,000 rpm for 5 minutes, and then the supernatant was filtered with 0.22 μm syringe filter and used for analysis. High performance anion exchange chromatography (HPAEC) was used to separate polysaccharides (as glucose, mannose, and N-acetyl-glucosamine). ICS 5000 Dionex chromatography system (ICS-5000+, Thermo Fisher Scientific Co, USA) CarboPac PA100 column (250x4 mm, Dionex), PEEK tube (0.24 mm i.d.), a gradient mixer (2 mm), ED amperometry cell with 0.25 µL channel volume, a pH-Ag/AgCl reference electrode, 0.002 in gasket, and gold electrodes were included. 100 mM sodium hydroxide and 1 M sodium acetate were used as analysis solvents, and samples were separated and analyzed under gradient conditions set to increase to 0-20 mM for 10-15 minutes and 20-500 mM for 15-20 minutes. The column temperature was maintained at 40°C, and the solvent flow rate was 1 mL/min.
2.4.3. Gel Permeation Chromatography (GPC)
GPC was conducted to analyze the molecular weight distribution of water-soluble polysaccharides in each sample. First, the freeze-dried sample (10 mg/ml) was dissolved in distilled water and filtered using a 0.22µm syringe filter (13mm, 0.22 µl, Thermo Fisher Scientific Co.). The resulting filtered solution was utilized as the analysis sample. TSKgel G3000PW columns (7.8mm × 30cm, Tosoh, Japan) were employed in an HPLC system (Agilent Technologies, Inc, USA) for the analysis. Distilled water served as the mobile phase, with the column temperature maintained at 40°C. The sample injection volume was 10 μL, and the solvent flow rate was set to 0.5 mL/min.
2.4.4. Glycosidic Linkage Analysis Using 1H-NMR
To analyze of ⍺-1, 6 and ⍺-1, 2 glycosidic bonds between sugar molecules of water-soluble polymer samples, 1H-NMR spectroscopy (500 MHz FT-NMR, JEOL, Tokyo, Japan) was performed recovered by alkali extraction of autolysate residue and ethanol precipitation. The freeze-dried sample namely soluble polysaccharides (20 mg/ml) was dissolved in deuterium oxide (D2O), reacted at 45°C for 20 minutes, and then 1H-NMR analysis was performed.
2.4.5. FT-IR
FT-IR spectral analysis of soluble polysaccharides was carried out using the potassium bromide (KBr) pellet method with a Spectrum 3 FT-IR spectrophotometer (PerkinElmer Inc., Billerica, MA, USA) in the range of 400–4000 cm− 1.
2.5. Antioxidant Enzyme Activity of Water-Soluble Polysaccharide
2.5.1. Superoxide Dismutase-Like Activity (SOD)
The SOD assay kit-WST (Biomax, Seoul, Korea) was utilized for analysis. The sample was dissolved in double distilled water (DDW) at a concentration of 100 μl/ml. Next, 20 μL of the sample was added to the sample well and Blank 2 well on a 96-well plate. Additionally, 20 μL of DDW was added to Blank 1 and Blank 3 wells. Following this, 200 μL of WST working solution was added to each well. To Blank 2 and Blank 3 wells, 20 μL of dilution buffer was added, while to Blank 1 and the sample well, 20 μL of Enzyme working solution was added. The reaction was then carried out at 37°C for 20 minutes.
Subsequently, the absorbance was measured at 450 nm using a microplate reader (Multiskan Sky, Thermo Scientific Co.).
Where:
B1: Blank 1/Maximum absorbance
B2: refers to blank 2/Sample’s Background Absorbance
B3: Blank 3/Background absorbance of the rest of the solution except sample
Sample: sample absorbance
2.5.2. ABTS Radical Scavenging
Following the method outlined by (K. J. Lee, Oh, Cho, & Ma, 2015), ABTS and potassium persulfate were dissolved in distilled water to achieve final concentrations of 7.4 mM and 2.6 mM, respectively. This mixture was then stored in a dark place at room temperature for 18 hours. Prior to use, it was diluted with phosphate-buffered saline (pH 7.4) to attain an absorbance of approximately 0.70 at 732 nm. Subsequently, 50 μL of the sample was combined with 1.0 mL of the diluted ABTS mixture and allowed to stand in darkness for 30 minutes. Following incubation, the absorbance was measured at 732 nm. Trolox, a standard antioxidant, was employed for calibration purposes, and the results were expressed as milligrams of Trolox equivalent per gram (mg Trolox equivalent/g) of the sample. Furthermore, the sample concentration (IC
50), corresponding to a 50% scavenging rate, was calculated by establishing the relationship between concentration and scavenging rate.
where: C refers to OD of control/black and S: refers to OD of sample
2.5.3. DPPH Radical Inhibition
The DPPH radical scavenging ability, often employed as a representative indicator of antioxidant activity, was assessed following the method outlined by (Senba, Nishishita, Saito, Yoshioka, & Yoshioka, 1999). Initially, the sample was diluted to various concentrations, with each aliquot measuring 200 μL. Subsequently, 800 μL of a 0.4 mM DPPH solution was added to each sample, and the mixture was then incubated at 37°C for 30 minutes. Following the incubation period, the absorbance was measured at 525 nm. The DPPH radical scavenging rate was determined by calculating the ratio of the decrease in absorbance observed in the sample treatment group in comparison to the absorbance of the control group (without sample).
where: C refers to OD of control/black and S: refers to OD of sample
2.5.4. Total Phenolic Content
According to the method outlined by Horn et al. (2023), with minor modifications, the total phenol content was measured at 760 nm. This was achieved by adding 0.08 N Folin-Ciocalteu reagent to the sample and allowing it to stand at room temperature for 6 minutes. Subsequently, 3% Na2CO3 solution was added and left for 90 minutes. A standard curve was prepared using gallic acid as a standard reagent, and the total phenol content was expressed as mM gallic acid equivalent.
2.6. Statistical Analysis
The experimental results were presented as the average value and standard deviation (means ± SD) based on triplicate analyses. Significance testing between experimental groups was conducted using the SPSS statistical program (Ver. 23, Statistical Package for Social Sciences, SPSS Inc, Chicago, IL, USA) with a significance level set at p < 0.05.
3. Results and Discussion
3.1. Proximate Composition
The protein, carbohydrate, and ash contents per dry weight ranged from 52.0 to 57.4%, 28.8 to 38.9%, and 6.4 to 8.5%, respectively. Our findings align with previous studies by Chae et al. (2001), Teresa et al. (1997), Heringer et al. (2023), and Wan et al. (2021), which reported similar parameters for yeast cells. Despite being rich in nutrients compared to other sources, BSY exhibited the lowest protein, carbohydrate, and mineral contents compared to pure cultured SC and SB. This disparity may be attributed to SC and SB being cultured in YPD medium under constant conditions and recovered at the end of the stationary phase, where organelles are well-developed, resulting in higher nutrient content (Bzducha-Wróbel, Kieliszek, & Błażejak, 2013).
Unlike pure cultured yeast, BSY is obtained after approximately 1 week of fermentation, during which composed of active and inactive yeast cells (Horn et al., 2023). Additionally, BSY is mixed with wort and hop juice during fermentation. These factors likely contribute to the differences in composition observed between BSY and SC or SB. These comparative results provide valuable insights for the future utilization of cell debris residue as a food ingredient, particularly considering the lack of reports demonstrating the general composition of each strain of SC and SB cultured at high concentrations.
The proximate composition of autolysate residue from BSY and pure cultured cells (SC and SB) was determined to assess the effect of autolysis. Upon autolysis, the protein content of all samples decreased due to the removal of yeast cell internal components, which are protein-rich, such as yeast extract. Conversely, carbohydrate and mineral content increased, as the autolysate residue was primarily composed of cell wall. These results for BSY were consistent with the reported composition and shredded residue content of BSY. In contrast to the whole cell results, the protein and ash contents of BSY were higher than those of cultured cells, while carbohydrate content was lower.
Table 1.
Proximate composition of whole and autolyzed cell residue from BSY, S. cerevisiae and S. boulardii.
Table 1.
Proximate composition of whole and autolyzed cell residue from BSY, S. cerevisiae and S. boulardii.
| Components (% |
BSY |
S. cerevisiae |
S. boulardii |
| WC |
AR |
WC |
AR |
WC |
AR |
| Crude protein |
52.04±1.98c
|
30.50 ± 0.21a
|
53.46±2.43b
|
18.64 ± 0.06c
|
57.43±2.71a
|
19.57 ± 0.38b
|
| Carbohydrate |
28.80±0.54c
|
53.13 ± 0.23c
|
38.87±1.25a
|
67.17 ± 0.09a
|
33.65±0.95b
|
64.65 ± 0.41b
|
| Ash |
6.43±0.04c
|
10.39 ± 0.06a
|
7.35±0.04b
|
7.77 ± 0.01c
|
8.47±0.07a
|
± 0.04b
|
3.2. Yield Components of Yeast Cell Fractionations
Yeast cell walls account for 15 – 30% of the yeast cell dry weight, of which up to 90% is composed of polysaccharides (Bastos
et al., 2022; Fu
et al., 2022, Wang et al., 2018).
Table 2 shows the yield of 4 fractions of autolysis and extraction including cell walls for BSY, SC and SB and its polysaccharides content. Upon autolysis, BSY yielded lower autolysate which refers to yeast extract/cytoplasmic components and higher residue (constitute the cell wall) than SC and SB cultures cell. This mainly due to nutrient depleted beer manufacturing environment for BSY as compared to SC and SB grown in fermenter. The findings of these autolysate fractions are consistent with a study by Tanguler & Erten (2008) and suggest that the autolysate residue contains more than just cell wall components, as evidenced by a total polysaccharide content of approximately 600 µg/mg.
To estimate the proportion of yeast cell wall, the composition of insoluble residue polysaccharides resulting from autolysis was determined. BSY exhibited the highest cell wall component content (34.09%), followed by SB (26.61%) and SC (24.25%), respectively. This disparity can be attributed to the higher concentration of β-glucan in the cell wall, likely due to modifications introduced by the brewing process (Bastos, Coelho, & Coimbra, 2015).
The total carbohydrate, β-glucan, and mannan composition of the autolysate residue results align with previous studies on probiotic and brewer’s yeast cell wall composition under different growth conditions (Aguilar-Uscanga & François, 2003; Bzducha-Wróbel, Kieliszek, & Błażejak, 2013). And the values in this study were in the range reported as which β-glucans constitute 50–60% whereas are mannoproteins 35–40% (Klis, Boorsma, & De Groot, 2006). Among the cell wall composition results, SB exhibited the highest mannan content (297.40 µg/mg), consistent with reports that SB, a lactic acid yeast, possesses a distinctive cell wall architecture with a thick mannan layer and higher mannan content (Bzducha-Wróbel et al., 2013).
These results suggest that BSY has comparable cell wall polysaccharide content to cultured SC and SB and serves as a rich source of functional cell wall polysaccharides such as β-glucan and mannan. However, through alkali extraction, BSY yielded lower soluble polysaccharide sugar and insoluble residue compared to SC and SB, as described in detail in section 3.3.
3.3. Characterization of Soluble Cell Wall Polysaccharides
3.3.1. Soluble Cell Wall Polysaccharides Composition
Water-soluble polysaccharides (SP) were extracted from the autolysate residue using an alkaline solution, and their constituent saccharides, including β-glucan, mannan (mannoprotein), and chitin, were analyzed (see
Table 3). SP contents were found to be higher in BSY (700.90 µg/mg), followed by SC (688.84 µg/mg) and SB (634.70 µg/mg), respectively. Upon detailed examination, a notably high mannan: glucan ratio was observed, consistent with previous studies on probiotic and brewer’s yeast cell wall composition under glycerol cultivation (Bzducha-Wróbel et al., 2013).
Research by Bastos et al. (2015) demonstrated an increase in soluble β-glucan with β-1,3 and β-1,6-D-Glc bonds derived from wort during brewing. This finding explains the higher proportion of water-soluble β-glucan in BSY (70:30) compared to SC (84:14) and SB (90:10). Additionally, alkaline-extracted fractions exhibited notably high levels of mannan, known for its water solubility compared to other yeast cell wall components such as β-glucan and chitin (Huang, Yang, & Wang, 2010; Yang Liu et al., 2018; Silva Araújo et al., 2014). Given mannan’s immune-enhancing properties, this mannan-rich soluble polysaccharide (mannan: β-glucan 70:30) holds significant potential in nutraceutical industries. Furthermore, the alkali-insoluble fraction predominantly comprises β-glucan, as reported by Lee et al. (2008) (see supplementary
Table S1).
3.3.2. Molecular Weight Determination
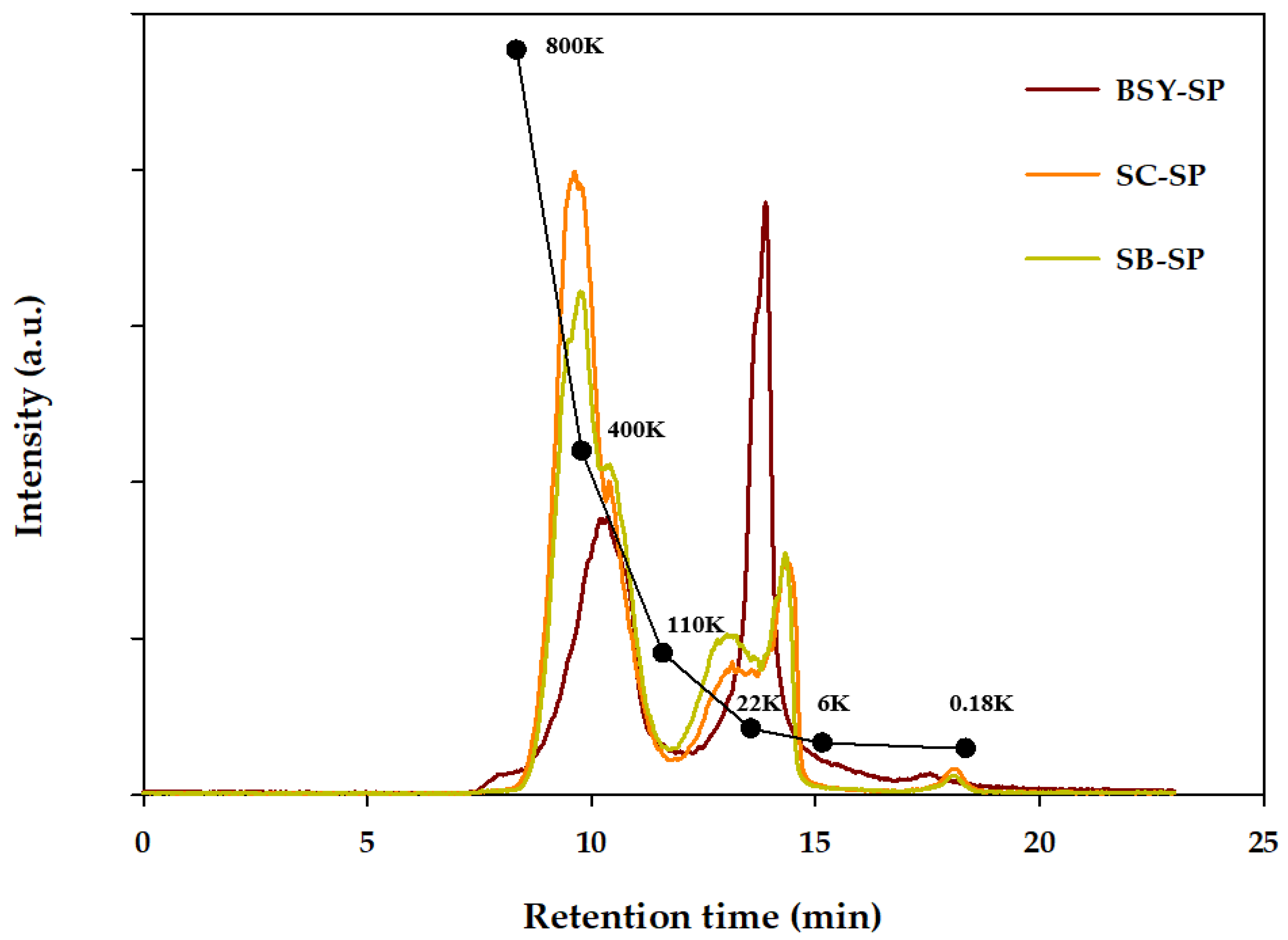
The molecular weight size distribution of soluble polysaccharides (SP) obtained from each yeast autolysate residue was assessed via gel permeation chromatography (GPC), as illustrated in
Figure 2. Previous research indicates that SP extracted from yeast cell walls typically exhibit molecular weights ranging from 166 to 700 kDa. (Kath & Kulicke, 1999b) Notably, the total weight average molecular weight (Mw) of SP derived from spent yeast (185.8 kDa) was significantly lower than that of the cultured SC and SB strains, measuring 302.9 kDa and 261.7 kDa, respectively. Further analysis revealed the molecular weight of the height peak (Mp) for SC-SP and SB-SP to be 452.5 kDa and 405.3 kDa, respectively, while BSY-SP exhibited a notably lower Mp of 19.3 kDa. Subsequent confirmation of degree of polymerization (DP) at Mp unveiled values of 2793.2 for SC-SP, 2502.1 for SB-SP, and 118.9 for BSY-SP, indicating a higher degree of polymerization in SC-SP.
The presence of a large quantity of water-soluble glucose in BSY-SP likely influences its molecular weight, contributing to its lower size compared to other polysaccharides. Numerous studies have highlighted that polysaccharides with smaller molecular weights tend to possess simpler structures and enhanced water solubility, thus exhibiting heightened biological activity (Du et al., 2016; Wang et al., 2010).
Comparison between strains (SC and SB) revealed distinct characteristics in their polysaccharide fractions. SC exhibited a greater proportion of polymeric fractions with a size of around 400 kDa, whereas SB displayed a slightly higher content of polysaccharides with sizes approximately 22 kDa and above. This aligns with previous findings indicating that SB strains typically feature a thicker mannan layer, a characteristic component of the outer membrane.
Table 4.
Molecular weight distributions of Water-Soluble polysaccharide.
Table 4.
Molecular weight distributions of Water-Soluble polysaccharide.
| Sample |
Mw1)
|
Mp2)
|
DP3) |
| BSY |
185.8 |
19.3 |
118.9 |
| S. cerevisiae |
302.9 |
452.5 |
2793.2 |
| S. boulardii
|
261.7 |
405.3 |
2502.1 |
3.3.3. 1H-NMR Spectroscopic Identification
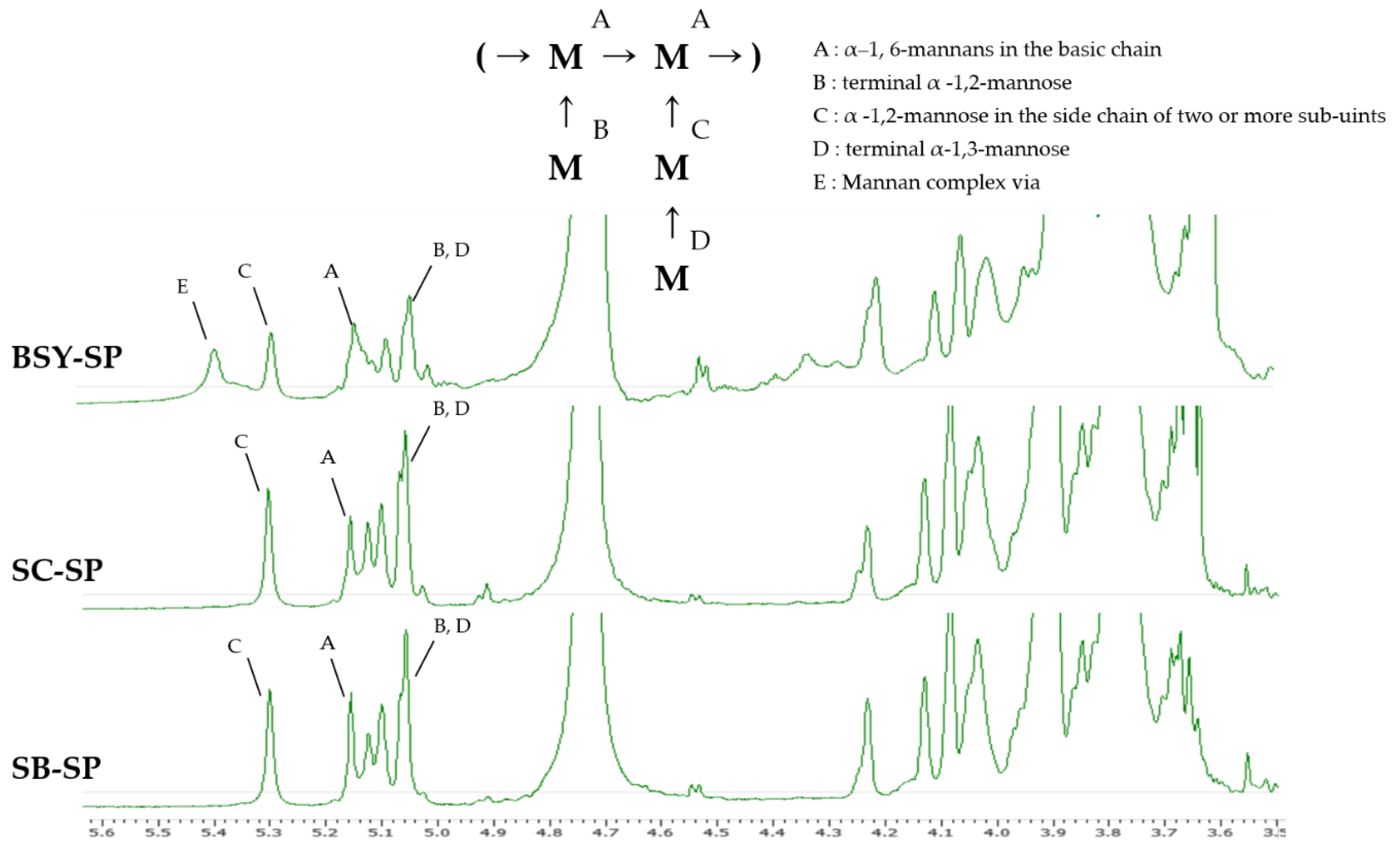
The results illustrating the confirmation of the intermolecular binding form of water-soluble polysaccharide (SP) extracted from the cell wall are depicted in
Figure 3. Anomeric H atoms, crucial for identification, resonate within the range of 4.9–5.5 ppm, while the remaining H atoms fall within 3.5–4.5 ppm, as per a previous study (Kath & Kulicke, 1999b). However, due to numerous overlapping signals in this range, precise assignment becomes unfeasible. The assignment of anomeric H atoms relied upon published data for higher mannan oligosaccharides, which were further corroborated by methylation analyses and other findings (H. Z. Liu, Liu, Hui, & Wang, 2015). Notably, the signal observed at 5.1-5.2 ppm (positions A) signifies the presence of α-1,6-mannans in the basic chain, upon which the mannose side groups are attached. Additionally, a signal corresponding to an anomeric H atom of a terminal α-1,3-linked mannose is discernible in this region (position D). Another distinctive signal at 5.3 ppm is indicative of mannose in α-1,2-linked side chains composed of two or more sub-units (position C). Furthermore, the signal at 5.04 ppm denotes terminal α-1,2-linked mannose and α-1,2-linked mannose with a mannose substituent in the 3-position (position B, D). Occasionally, a band appears at 5.41 ppm (positions E), attributed to a mannose bonded to the mannan complex via a phosphodiester bridge (Kath & Kulicke, 1999b).
BSY-SP exhibited a characteristic peak at a chemical shift of 5.4 ppm, likely arising from complex formation due to interactions with residual substances present in the wort during beer fermentation. Furthermore, the intensity of this peak tends to be relatively lower compared to that of SC-SP and SB-SP. This difference is believed to stem from the mixture of various components during the brewing process and the lower purity inherent in yeast cells cultured in a non-controlled environment.
Tamano et al. (2019) reported that the soluble form of beta-glucan typically manifests a peak at a chemical shift of 4.5 ppm, attributable to beta-1,6-linked glucose bonds. Additionally, Chen et al. (1995) noted in a structure determination study involving hydrolysis of barley that the peak corresponding to 5.3 ppm in the spectral results of 1-3 or 1-4-linked beta-glucan via 1H-NMR could be attributed to residual anomeric protons of the 1,4-glucoside bond within beta-glucan.
3.3.4. FT-IR Spectroscopic Identification
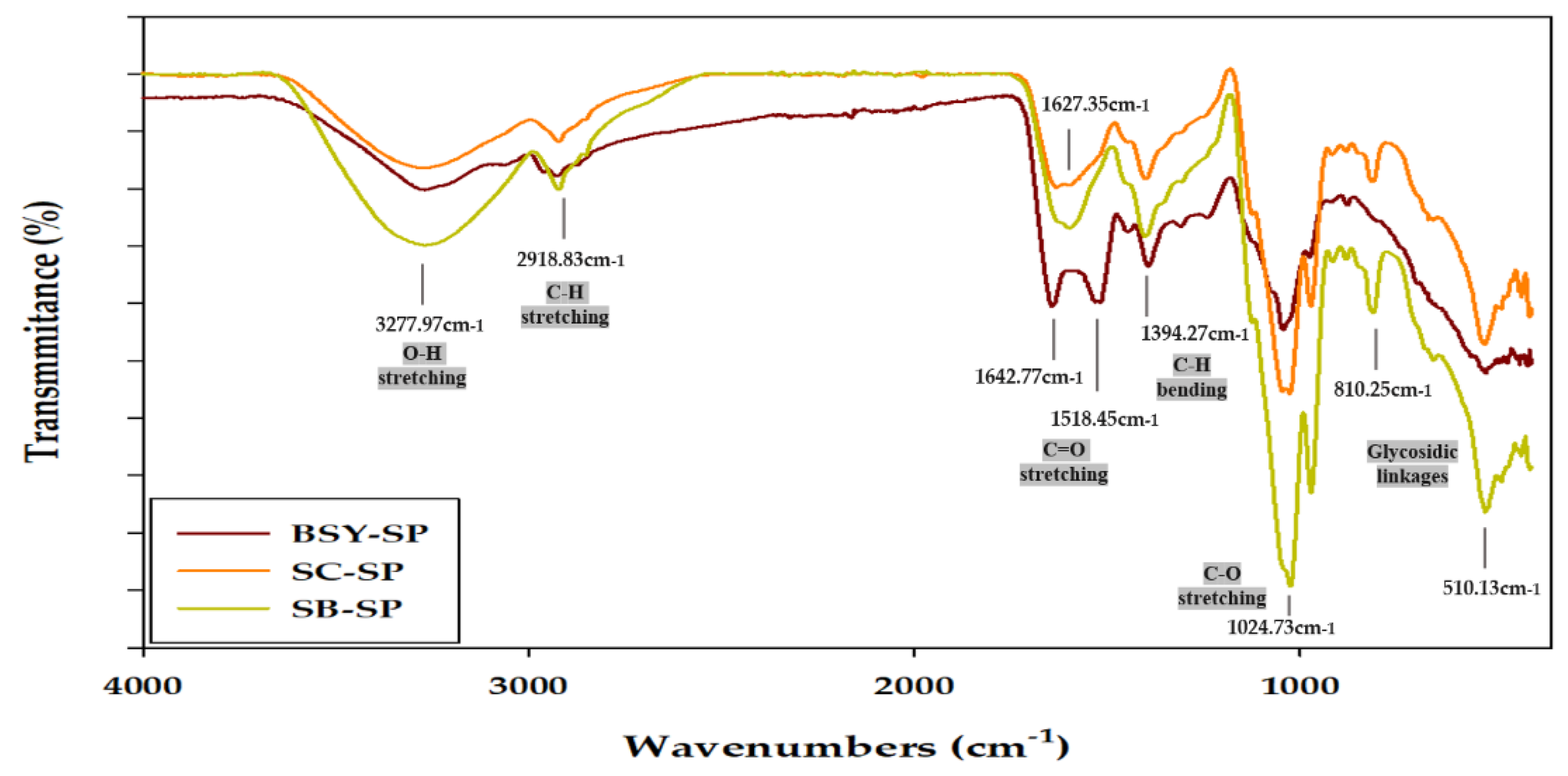
The FT-IR spectrum results of cell wall-soluble polysaccharides (
Figure 4) exhibit typical polysaccharide patterns, consistent with findings from various studies (Cerqueira et al., 2011; S. H. Lee et al., 2015; Y. Liu et al., 2018; Zeng, Zhang, Gao, Jia, & Chen, 2012). A broad peak spanning 3650-3200 cm
-1 corresponds to O-H stretching bonds, indicative of changes in bond length with hydroxyl groups, while a weaker bend observed between 3000-2800 cm
-1 is associated with C-H stretching bonds in carbonyl groups (Ballesteros, Cerqueira, Teixeira, & Mussatto, 2015; Huang, 2008). Takalloo et al., (2020) suggested that the wavelength range of 3650-3200 cm
-1, representing hydroxyl groups, may influence the formation or alteration of intramolecular and intermolecular hydrogen bonds, or variations in the polymerization of cell wall polysaccharides or yeast cell wall polysaccharides, affecting their helical structure.
Moreover, a peak observed between 2985-3015 cm-1 is attributed to lipid residues within the cell wall (Bzducha-Wróbel et al., 2014). The regions spanning 1700-1500 cm-1 are associated with C=O asymmetric and symmetric stretching bonds, while a peak at 1374 cm-1 corresponds to C-H bending bonds, where changes in bond angles occur (Ren et al., 2014). Additionally, a peak between 1155-1080 cm-1 signifies a C-O bending bond, while the presence of a C-O stretching bond is indicated by a peak at 1024 cm-1 (Heringer et al., 2023). Oscillations between 520 and 1100 cm-1 are linked to various polysaccharides (α-glucan, β-glucan, α-mannan, etc.) and can be subdivided into the sugar region (950–1200 cm-1) and the anomeric region (750–950 cm-1) (Hromádková et al., 2003).
Notably, in SB-SP, the hydroxyl groups at the 3650-3200 cm-1 peak appear prominent, suggesting a predominance of O-H stretching bonds. The C-O stretching binding, indicated by the 1024 cm-1 peak, is more pronounced in SC-SP and SB-SP compared to BSY-SP. Within the range of 700-1500 cm-1, large peaks corresponding to C=O asymmetric and symmetric stretching bonds, as well as C-H bending bonds at 1374 cm-1, are observed in BSY-SP, indicative of β-glucan presence. This observation suggests the presence of chitin and proteolysis by-products from yeast cell wall components (Piotrowska & Masek, 2015). Moreover, the strong absorption peak at 810 cm-1 suggests the presence of α-anomeric configurations in the polysaccharides (Huang, 2008).
Comparison of the 810 cm-1 peak areas reveals that SC-SP and SB-SP exhibit more α-anomeric configurations and a higher percentage of mannose compared to BSY-SP. SB-SP notably exhibits a pronounced absorption of O-H and C-O stretching peaks, likely due to the thick mannoprotein layer characteristic of SB strains (Hudson et al., 2016). Conversely, BSY-SP demonstrates intense absorption of the C=O stretching peak, attributed to the presence of double-bonded substances such as Hop Acids, derived from the wort and hop juice mixture used in the brewing process (Bryant & Cohen, 2015).
3.3.5. Antioxidant Enzyme Activity of Water-Soluble Polysaccharide
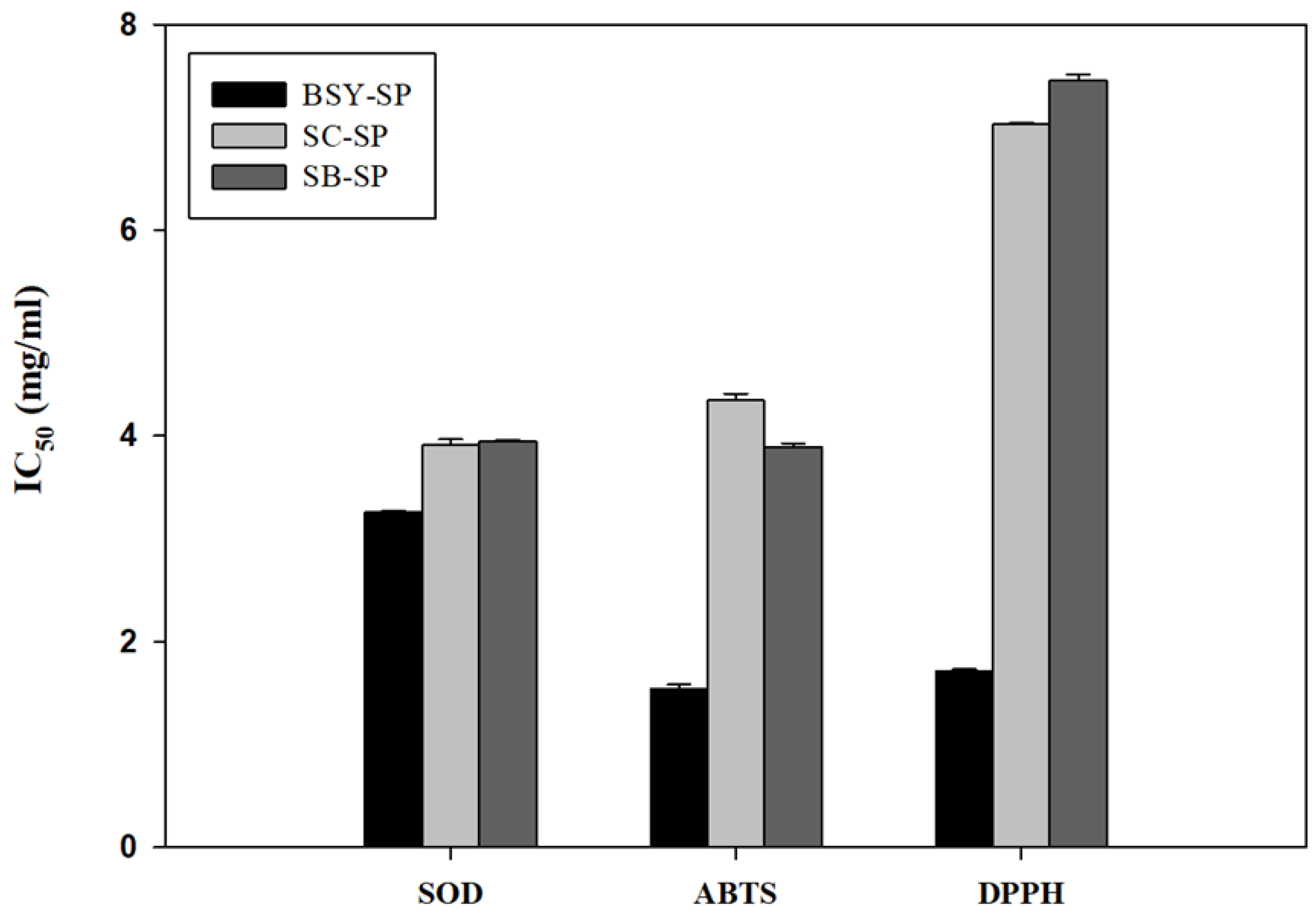
The water-soluble polysaccharide component of yeast cells exhibited antioxidant activity against superoxide, ABTS, and DPPH radicals (
Table 5). The results indicate that brewers’ spent yeast polysaccharides displayed significantly higher antioxidant activity compared to the soluble polysaccharides from cultured yeast cells. This disparity may be attributed to phenolic compounds, such as hop acids, derived from the beer manufacturing process. Previous studies have shown that hop acids from beer bind to yeast cells and become soluble only at higher pH levels (Tanguler & Erten, 2008). There is substantial evidence suggesting that hop acids possess antioxidant properties (Bryant & Cohen, 2015). Further analysis of Total Phenolic Acid (TPA) content supports this finding, revealing that the soluble polysaccharide fraction of brewers’ spent yeast contains three times more TPA content.
Additionally, Figure 5 also indicates that BSY polysaccharides exhibited significantly lower IC50 values than pure cultured polysaccharides.
Previous research on the antioxidant activity of yeast polysaccharides has indicated that the insoluble β-glucan exhibits lower antioxidative activity compared to the alkali-soluble mannan and β-glucan (Jaehrig et al., 2007). The authors of this study concluded that the protein residue on the mannan contributes significantly to its antioxidative properties. However, it’s noteworthy that β-glucan derived from malt also possesses considerable radical scavenging potential (Kofuji et al., 2012). This finding might contribute to explaining the higher antioxidant activities observed in brewers’ spent yeast compared to pure cultured yeast strains SC and SB, which also contain higher β-glucan content.
4. Conclusion
Based on the findings, it is evident that BSY represents a promising alternative to cultured yeast cells due to its enriched nutrient profile and functional polysaccharide composition. Its autolysis residue, predominantly composed of the cell wall, demonstrates higher polysaccharide content, particularly β-glucan and mannan, than that of pure cultured SC and SB. Furthermore, although mannan-rich in all yeasts, soluble polysaccharide composition in BSY reveals elevated levels of β-glucan and reduced mannan concentration compared to SC and SB. Moreover, these soluble extracts exhibit superior antioxidant activity, as evidenced by their higher scavenging potential against SOD, ABTS, and DPPH radicals compared to cultured SC and SB yeast cells, attributed in part to the presence of hop acid residue, which offers an additional advantage. Overall, BSY emerges as a cost-effective and sustainable source of functional soluble polysaccharides, offering a viable alternative to cultured yeast cells as a food ingredient. This contributes also to waste reduction in beer production processes. These findings underscore the potential of BSY in enhancing the nutritional and functional aspects of food products, warranting further exploration and utilization in various food formulations and applications.
Supplementary materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1. Insoluble polysaccharide of alkali extraction derived from autolysate residue.
Author Contributions
Hyun Ji Lee: Conceptualization, methodology, validation, and formal analysis; investigation, data curation; writing—original draft. Bo Ram Park: Conceptualization, writing—original draft and editing, resources, supervision, project administration, funding acquisition. Legesse Shiferaw. Chewaka: Writing, methodology, review and editing, visualization, supervision.
Funding
“This research was funded by Research Program for Agricultural Science & Technology Development (Project No. PJ01577002)”, Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Al-Manhel, A. J., & Niamah, A. K. (2017). Mannan extract from Saccharomyces cerevisiae used as prebiotic in bioyogurt production from buffalo milk. International Food Research Journal, 24(5), 2259–2264.
- Ballesteros, L. F., Cerqueira, M. A., Teixeira, J. A., & Mussatto, S. I. (2015). Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydrate Polymers, 127, 347–354. [CrossRef]
- Bastos, R., Coelho, E., & Coimbra, M. A. (2015). Modifications of Saccharomyces pastorianus cell wall polysaccharides with brewing process. Carbohydrate Polymers, 124, 322–330. [CrossRef]
- Bastos, R., Oliveira, P. G., Gaspar, V. M., Mano, J. F., Coimbra, M. A., & Coelho, E. (2022). Brewer’s yeast polysaccharides — A review of their exquisite structural features and biomedical applications. Carbohydrate Polymers, 277(August 2021). [CrossRef]
- Bzducha-Wróbel, A., Blłazejak, S., Kawarska, A., Stasiak-Rózańska, L., Gientka, I., & Majewska, E. (2014). Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules, 19(12), 20941–20961. [CrossRef]
- Bzducha-Wróbel, A., Kieliszek, M., & Błażejak, S. (2013). Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. European Food Research and Technology, 237(4), 489–499. [CrossRef]
- Cerqueira, M. A., Bourbon, A. I., Pinheiro, A. C., Martins, J. T., Souza, B. W. S., Teixeira, J. A., & Vicente, A. A. (2011). Galactomannans use in the development of edible films/coatings for food applications. Trends in Food Science and Technology, 22(12), 662–671. [CrossRef]
- Dallies, N., François, J., & Paquet, V. (1998). A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast, 14(14), 1297–1306. [CrossRef]
- Ferreira, I. M. P. L. V. O., Pinho, O., Vieira, E., & Tavarela, J. G. (2010). Brewer’s Saccharomyces yeast biomass: characteristics and potential applications. Trends in Food Science and Technology, 21(2), 77–84. [CrossRef]
- Heringer, H. C. E., Kuhn Marchioro, M. L., Meneguzzi, D., Barbosa-Dekker, A. M., Dekker, R. F. H., & Alves da Cunha, M. A. (2023). Valorization of spent Brewers yeast in the integrated production of the fungal exopolysaccharide (1 → 6)-β-D-glucan (lasiodiplodan) and single-cell protein. Biocatalysis and Agricultural Biotechnology, 54(November), 102971. [CrossRef]
- Horn, P. A., Zeni, A. L. B., Cavichioli, N., Winter, E., Batista, K. Z. S., Vitali, L., & de Almeida, E. A. (2023). Chemical profile of craft brewer’s spent yeast and its antioxidant and antiproliferative activities. European Food Research and Technology, 249(8), 2001–2015. [CrossRef]
- Hromádková, Z., Ebringerová, A., Sasinková, V., Šandula, J., Hříbalová, V., & Omelková, J. (2003). Influence of the drying method on the physical properties and immunomodulatory activity of the particulate (1 → 3)-β-D-glucan from Saccharomyces cerevisiae. Carbohydrate Polymers, 51(1), 9–15. [CrossRef]
- Huang, G. L. (2008). Extraction of two active polysaccharides from the yeast cell wall. Zeitschrift Fur Naturforschung - Section C Journal of Biosciences, 63(11–12), 919–921. [CrossRef]
- Kath, F., & Kulicke, W. (1999a). Mild enzymatic isolation of mannan and glucan from yeast Saccharomyces cerevisiae. Die Angewandte Makromolekulare Chemie. 268(4667), 59–68.
- Kath, F., & Kulicke, W. M. (1999b). Polymer analytical characterization of glucan and mannan from yeast Saccharomyces cerevisiae. Angewandte Makromolekulare Chemie, 268(4668), 69–80. [CrossRef]
- Klis, F. M., Boorsma, A., & De Groot, P. W. J. (2006). Cell wall construction in Saccharomyces cerevisiae. Yeast, 23(3), 185–202. [CrossRef]
- Kofuji, K., Aoki, A., Tsubaki, K., Konishi, M., Isobe, T., & Murata, Y. (2012). Antioxidant Activity of β -Glucan . ISRN Pharmaceutics, 2012, 1–5. [CrossRef]
- Kumar Suryawanshi, R., & Kango, N. (2021). Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chemistry, 334(November 2019), 127428. [CrossRef]
- Lee, K. J., Oh, Y. C., Cho, W. K., & Ma, J. Y. (2015). Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evidence-Based Complementary and Alternative Medicine, 2015. [CrossRef]
- Lee, S. H., Baek, S. Y., Kang, J. E., Jeon, C. O., Kim, D. H., Kim, M. D., & Yeo, S. H. (2015). Effects of temperature on the changes of enzymatic activities and metabolite during wheat nuruk fermentation. Korean Journal of Microbiology and Biotechnology, 43(4), 378–384. [CrossRef]
- Liu, H. Z., Liu, L., Hui, H., & Wang, Q. (2015). Structural characterization and antineoplastic activity of saccharomyces cerevisiae mannoprotein. International Journal of Food Properties, 18(2), 359–371. [CrossRef]
- Liu, Y., Huang, G., & Lv, M. (2018). Extraction, characterization and antioxidant activities of mannan from yeast cell wall. International Journal of Biological Macromolecules, 118, 952–956. [CrossRef]
- Association of Official Analytical Chemistry, [AOAC].. (2005). Official Methods of Analysis of AOAC INTERNATIONAL. Aoac.
- Pacheco, M. T. B., Caballero-Córdoba, G. M., & Sgarbieri, V. C. (1997). Composition and nutritive value of yeast biomass and yeast protein concentrates. Journal of Nutritional Science and Vitaminology, 43(6), 601–612. [CrossRef]
- Piotrowska, M., & Masek, A. (2015). Saccharomyces cerevisiae cell wall components as tools for ochratoxin A decontamination. Toxins, 7(4), 1151–1162. [CrossRef]
- Reis, S. F., Fernandes, P. A. R., Martins, V. J., Gonçalves, S., Ferreira, L. P., Gaspar, V. M., … Coelho, E. (2023). Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules, 28(8). [CrossRef]
- Ren, L., Hemar, Y., Perera, C. O., Lewis, G., Krissansen, G. W., & Buchanan, P. K. (2014). Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioactive Carbohydrates and Dietary Fibre, 3(2), 41–51. [CrossRef]
- Senba, Y., Nishishita, T., Saito, K., Yoshioka, H., & Yoshioka, H. (1999). Stopped-flow and spectrophotometric study on radical scavenging by tea catechins and the model compounds. Chemical and Pharmaceutical Bulletin, 47(10), 1369–1374. [CrossRef]
- Takalloo, Z., Nikkhah, M., Nemati, R., Jalilian, N., & Sajedi, R. H. (2020). Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): a comparative study. World Journal of Microbiology and Biotechnology, 36(5), 1–14. [CrossRef]
- Tang, Q., Huang, G., Zhao, F., Zhou, L., Huang, S., & Li, H. (2017). The antioxidant activities of six (1 → 3)-β-D-glucan derivatives prepared from yeast cell wall. International Journal of Biological Macromolecules, 98, 216–221. [CrossRef]
- Tao, Z., Yuan, H., Liu, M., Liu, Q., Zhang, S., Liu, H., … Wang, T. (2023). Yeast Extract: Characteristics, Production, Applications and Future Perspectives. Journal of Microbiology and Biotechnology, 33(1), 151–166. [CrossRef]
- Utama, G. L., Oktaviani, L., Balia, R. L., & Rialita, T. (2023). Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers, 15(16). [CrossRef]
- Wang, J., Li, M., Zheng, F., Niu, C., Liu, C., Li, Q., & Sun, J. (2018). Cell wall polysaccharides: before and after autolysis of brewer’s yeast. World Journal of Microbiology and Biotechnology, 34(9), 1–8. [CrossRef]
- Zeng, W. C., Zhang, Z., Gao, H., Jia, L. R., & Chen, W. Y. (2012). Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydrate Polymers, 89(2), 694–700. [CrossRef]
- Zhang, J., Fang, Y., Fu, Y., Jalukar, S., Ma, J., Liu, Y., … Zhao, L. (2023). Yeast polysaccharide mitigated oxidative injury in broilers induced by mixed mycotoxins via regulating intestinal mucosal oxidative stress and hepatic metabolic enzymes. Poultry Science, 102(9), 102862. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).