1. Introduction
Citrus (
Citrus sinensis L. Osb.) represents one of the most important woody crops in Spain, with 306,000 ha, and the third most important crop in Andalusia in terms of surface, occupying 94.400 ha, being the sweet orange the most representative specie with 64.300 ha [
1].
Regarding to the natural resources availability, citrus sector faces an increasingly uncertain scenario due to the limitations water resources and their management [
2], and the main challenge is related to the development of an adequate irrigation water management as adaptation to water scarcity scenarios and the adverse climatic conditions. In this agreement, several works have been developed with the aim of describing the potential effects of Climate Change on ecosystems, and how these scenarios could affect to the crops, especially as response to water scarcity and the air temperature increase [
3]. For the case of citrus, few works aimed at modeling their response to different water stress scenarios and heat stress have been developed, especially in relation to the physiological changes that occurs when drought conditions are imposed; and how their responses affect plant growth and crop productivity [
4].
Citrus trees are classified as C
3 evergreen plants, with hypo-stomatal leaves covered with a fine cuticle and a relative low CO
2 assimilation (A
n) and stomatal conductance (g
s) rates under well-irrigated conditions. Its leaves have an average lifespan of one year, although under optimal conditions they can live up to three. This crop has a high capacity for acclimatization to different seasonal changes, such as incident radiation or air temperature. When citrus trees are grown under optimum management conditions, their leaf area index can be high, even higher than 9 [
5]. This circumstance is largely explained by a marked g
s reduction when the air vapor pressure deficit (VPD) increases, as well as by a high diffusive resistance to CO
2 in the stoma and mesophyll [
6]. Although these characteristics could represent a productive disadvantage for the crop, they contribute to avoiding dehydration situations and the occurrence of embolism in conditions of severe drought.
Under the current scenarios of water scarcity, deficit irrigation (DI) strategies on perennial crops in semi-arid areas of South Spain is not an option but mandatory; and for the case of citrus crop is not a different fact. In this agreement, when these strategies are implemented, it is crucial to define those threshold values of drought stress that could cause several damages on crop (
i.e., affecting their potential growth, survival capability and significant reductions on final yield and fruit quality [
7,
8]. All these major responses are the result of the physiological traits affected by water deficit, with holistic changes that encompasses physiological, biochemical and metabolic routes [
9].
In this agreement, water stress promotes negative impacts on the crop physiological status, being affected different routes responses (depending on the level of water stress and duration); and hence yield losses can be produced [
10]. In this line, yield response is not only determined by the phenological stage in which the water stress is imposed [
11] but also the drought intensity and the crop responses induced during these stages [
9].
In physiological terms, drought conditions promote direct effects on the crop potential to keep water into their tissues, being affected the relative water content (RWC) [
12]. This response is directly related to the crop capability to regulate the gas-exchange rates under water stress conditions. According to [
13], ABA plays an important role in citrus subjected to drought, with significant increases detected under water stress in both leaves and roots. Additionally, have highlighted the role played not only by the presence of ABA in the physiological response to the combination of water stress and heat stress, but also the role played by salicylic acid (SA) and jasmonic acid (JA) [
12,
14]. These authors verified that SA levels increased significantly in response to situations of water and thermal stress, and that the combination of both stresses exerted an additive effect. If RWC is affected, it is traduced on the chlorophyll degradation. The degradation of chlorophylls results, on the one hand, in a reduction of the crop’s capacity for fluorescence emission, and therefore, as light radiation cannot be used in the electron transport chain and cannot be reflected in the form of fluorescence, the excess radiation ends up causing an increase in reactive oxygen species (ROS), causing significant oxidative damage to plant tissues and proline (Pro) accumulation as an osmoprotectant specie [
15]. If this happens, ROS are accumulated and it can promote the cell destruction, causing lipid peroxidation and damages into the membrane, proteins, chlorophyll, nucleic acids and cell death [
16,
17]. Thus, if the crop is able to increase their capability of ROS reduction, it is considered as a signal of water stress tolerance [
18]. Moreover, these damages are highly dependent on the water stress intensity and its duration over time. When an increase of ROS is produced, crops have different mechanisms to avoid oxidative damages (
i.e., the synthesis of antioxidative enzymes and other antioxidants to protect the cell). There are different chemical markers that show the oxidative damage degree, among them the malondialdehyde (MDA), a subproduct derived from the lipidic peroxidation of biologic membranes [
19]. If MDA significantly increases, this is a clear signal of the oxidative damage existence [
20,
21].
To avoid transpiration losses during drought stress, the first response of plants is to close the stomata [
22]. As a consequence of the closure of the stomata, the concentration of available CO
2 is reduced, which leads to an overproduction of the components of the electron transport chain [
23]. In a situation where water stress is very intense, the loss of water leads to tissue damage and wilting. To avoid this process, plants reduce their osmotic potential by accumulating solutes in the cytoplasm, helping the movement of water into the cell and preserving tissue turgor. Proline is one of the amino acids that accumulates most in more or less prolonged situations of drought stress [
24]; and it acts as an osmolyte whose synthesis is related to abiotic stress situations [
25].
Taking into consideration the different routes as response to drought stress on citrus trees, the aim of this work was to describe the main metabolic routes induced to avoid damages under drought conditions, determining the threshold values that would suggest moderate-to-severe damages that ultimately will affect the final yield.
2. Materials and Methods
2.1. Experimental Site and Irrigation Treatments
The trial was conducted during two consecutive months (July and August, these coinciding with the fruit-growth period) in the experimental farm “Las Torres” belonging to the Andalusian Institute of Teaching and Agricultural Research (37º 30’ 38,55” N; 05º 57’ 44,98” W) in two-years old sweet orange trees (Citrus sinensis L. Osb. cv. Navelina) grafted onto Citrange Carrizo (Citrus sinensis Osb. x Poncirus trifoliata Raf.). Trees were planted at outdoor conditions in 40-L pots, with 46 cm of diameter and 35.4 cm of height. These were filled with the same soil from the experimental farm, this being a typical clayey-loam texture Fluvisol (USDA, 2010), with a field capacity (-0.033 MPa) and permanent wilt point (-1,5 MPa) of 0,42 and 0,17 m3/m3, respectively.
Trees had an average height of 50 cm and a canopy diameter 1 m, approximately. These were irrigated with two emitters per pot, with a flow rate of 2,3 L/h, following the irrigation scheduling procedure based on the estimation of the reference evapotranspiration values (ET
0) through the modified Penman equation [
26], and using the information from a weather station located in the same experimental orchard. Crop evapotranspiration (ET
C) values were daily estimated, using a crop coefficient (K
C) of 0,7 and assuming a crop surface per tree ~ 1 m
2.
Three irrigation treatments were arranged:
-A full irrigation treatment; (FI), which received 100% of the irrigation requirements (IR) throughout the experience.
-A low-frequency deficit-irrigation treatment (LFDI) which was subjected to irrigation-restriction cycles. This treatment is based on keeping the crop within a range of physiological threshold values previously defined, and hence, its application has required proper crop water monitoring. In this case, during restriction periods, irrigation was suppressed until reaching stem water potential values (ΨStem) between −1.8 and 2.0 MPa. Then, irrigation withholding was finished, and trees were irrigated with the same frequency as FI until ΨStem reached similar values to those registered in FI. The length of the irrigation-restriction cycles varied depending on the weather conditions, although, on average, these ranged between 8–10 days, with 4–5 days of water restriction and something similar for the recovery periods.
-A sustained-deficit irrigation (SDI) treatment, which were irrigated at 75% of FI.
2.2. Physiological Measurements
2.2.1. Stem Water Potential and Stomatal Conductance
Stem-water potential (Ψ
Stem) readings were recorded using a Scholander pressure chamber with an external source of nitrogen (3000 Plant Water Status Console, Soil-moisture Equipment Corp., CA, USA) [
27]. Measurements were done in two leaves per tree and four trees per treatment. Readings were carried out at noon (solar time), with a periodicity of 48-72 h, depending on the observed evolution in the previous measurements. Before the measurements, the selected leaves (mature, healthy and well-developed) were previously covered with aluminium bags at least 30 minutes before readings in order to achieve an equilibrium between leaf and stem water potential.
In these same trees, the stomatal conductance to water vapor (gs), was measured in these same trees, by using a porometer SC-1 (Decagon Devices, INC, WA, USA), on two sunny leaves per tested tree.
2.2.2. Canopy Temperature
Tc was measured at the same time that Ψ
Stem readings, by using a ThermaCam (Flir SC660, Flir Systems, USA, 7–13 μm, 640 × 480 pixels), using an emissivity (ε) set of 0.96. Images were taken in the sunlit side of four trees per irrigation treatment, with the imager placed at 0,5 m of the canopy. Background temperature was determined by measuring the temperature of a crumpled sheet of aluminium foil placed close to the leaves of interest using ε =1 [
28]. To ease the further analysis of these images, a cooled white screen was used as background, this being placed behind of each monitored tree to simplify the isolation of the canopy surface through image processing. Finally, thermal images at tree level were analysed with the software developed by [
29].
2.4. Fluorescence Measurements
Additionally, chlorophyll fluorescence (ChF) measurements were developed, during both cycles of restriction-recovery. Two measurements per monitored tree and four trees per treatment (n=4) were done during each sample period, using a modulated fluorimeter (FMS-2, Hansatech Instrument Ltd., England). This instrument ensured the light saturation of PS reaction centers with an actinic light pulse of 15000 µE m
-2 s
-1 for 0.7 s allowing to calculate fluorescence quenching’s combining light and dark-adapted fluorescence measurements. The same leaf section of each plant used for dark-adapted records was then exposed to ambient light for 30 min and the steady state fluorescence yield (
Fs) was recorded. Then, leaves were again exposed to a saturating actinic light pulse of 15000 µmol m
-2 s
-1 for 0.7 s to produce the maximum fluorescence yield (
Fm’) by temporarily inhibiting PSII photochemistry. Using the fluorescence parameters determined in both light- and dark-adapted conditions, the following parameters were calculated: quantum efficiency of PSII (Φ
PSII = (
Fm’ –
Fs) /
Fm’), photochemical quenching (qP = (
Fm’ –
Fs) / (
Fm’ –
F0)), and non-photochemical quenching (NPQ = (
Fm –
Fm’) /
Fm’) [
30].
2.5. Determination of Proline and MDA
During the second cycle of restriction-recovery, and coinciding with the measurements of chlorophyll fluorescence, samples leaves were collected in each monitored tree (n=4). These simples were kept at –20 ºC until processing in the Lab. Proline (Pro) quantification was done according to the methodology proposed by [
31], developing an extraction with sulfosalicylic acid. Additionally, malondialdehyde (MDA) was determined with a previous extraction in acid medium with thiobarbituric acid + tricloracetic acid [
32]. Both, as Proline as MDA were analyzed by using a spectrophotometer Hitachi U-1900 (Gemini, BV, Güeldres, Netherlands).
2.6. Statistical Analysis
Data were checked for normality and homoscedasticity, and analysis of variance (ANOVA) was applied using Sigma Plot Software 15.0 (Sigma Plot, 2479 E. Bayshore Rd, Suite 195 Palo Alto, CA 94303) and treatment means were separated by the Tukey Multiple Range Test at 5% level of significance for the different physiological variables monitored.
3. Results and Discussion
3.1. Water Potential, Stomatal Conductance and Canopy Temperature Readings
Table 2 and
Table 3 show the average values for Ψ
Stem, g
s and T
C in each treatment and measuring day. During the first cycle of water-stress and recovery, the stem water potential was shown to be the most sensitive physiological variable for detecting moderate to severe water stress. Thus, differences in terms of static conductance were only evident for potential values below -1.7 MPa. In the case of T
C, differences were only appreciable between treatments when a very significant drop in conductance values was detected, corresponding to very severe stress situations. Finally, after a recovery period of 96 hours, the two stress treatments showed a good recovery capacity in terms of tissue rehydration, evidencing that at least the hydraulics of the crop had not been affected.
During the second cycle of water stress and recovery the pattern of the physiological variables was very similar to that observed in the previous one. Thus, in accordance to this, once again the first differences in gs were observed when ΨStem had reached values below to -1.7 MPa. Additionally, these differences were accompanied with higher values on TC, because of the reduction of evaporative cooling process. Finally, and as it was previously observed after 96 h of re-watering the tissue of rehydration had been possible.
3.2. Fluorescence Chlorophyll
During the first irrigation-restriction cycle, no significant differences were observed in the maximum quantum efficiency of PSII photochemistry (F
v/F
m), nor quantum efficiency of PSII electron transport in the light (ϕ
PSII) and non Photochemical Quenching (NPQ) at midday (
Table 4). Thus, F
v/F
m values were very similar in the studied treatments without relevant differences along the cycle, and values that were around 0.72. Something similar was observed for the case of ϕ
PSII, without significant reductions on the electron transport efficiency for PSII. By the contrast, although non significant differences between treatments were observed in the NPQ, these values were higher in RDI and LFDI in comparison to FI, with a sensible reduction after the recovery period.
Something similar was observed during the second water stress cycle. Thus, no significant differences were observed on the chlorophyll fluorescence parameters, with values very similar to those detected on the previous cycle (
Table 5).
The chlorophyll fluorescence allows to analyze the possible damages occurred in the electron transport chain that do not allow to use the light radiation to ATP and NADPH synthesis, this excess of radiation being dissipated as fluorescence. Among the possible limitations caused by drought stress, biochemical limitations are closely related to moderate to severe stress, including changes or loss of efficiency in the electron transport chain. In relation to the results observed, we cannot affirm that in any of the deficit irrigation treatments there was a reduction in the electronic transport capacity, nor a decrease in the maximum photochemical efficiency, nor a significant increase in the non-photochemical quantum. However, as it was stated, in both cycles, a greater dissipation of radiation was observed during the moment of maximum stress in RDI and LFDI, although this situation was reversed after the rehydration process. This fact suggests the absence of cumulative damage throughout the stress period, and that the crop was therefore capable of adequately managing these situations, at least for the water potential values measured. As it was discussed by [
8]Ningombam et al. (2022) a reduction on stomatal conductance usually occurs just before or in line with a reduction on mesophyll conductance, an inhibition of CO
2 metabolism, inactivation of RuBisCO, and hence a downregulation of PSII and decreasing in Y(II).
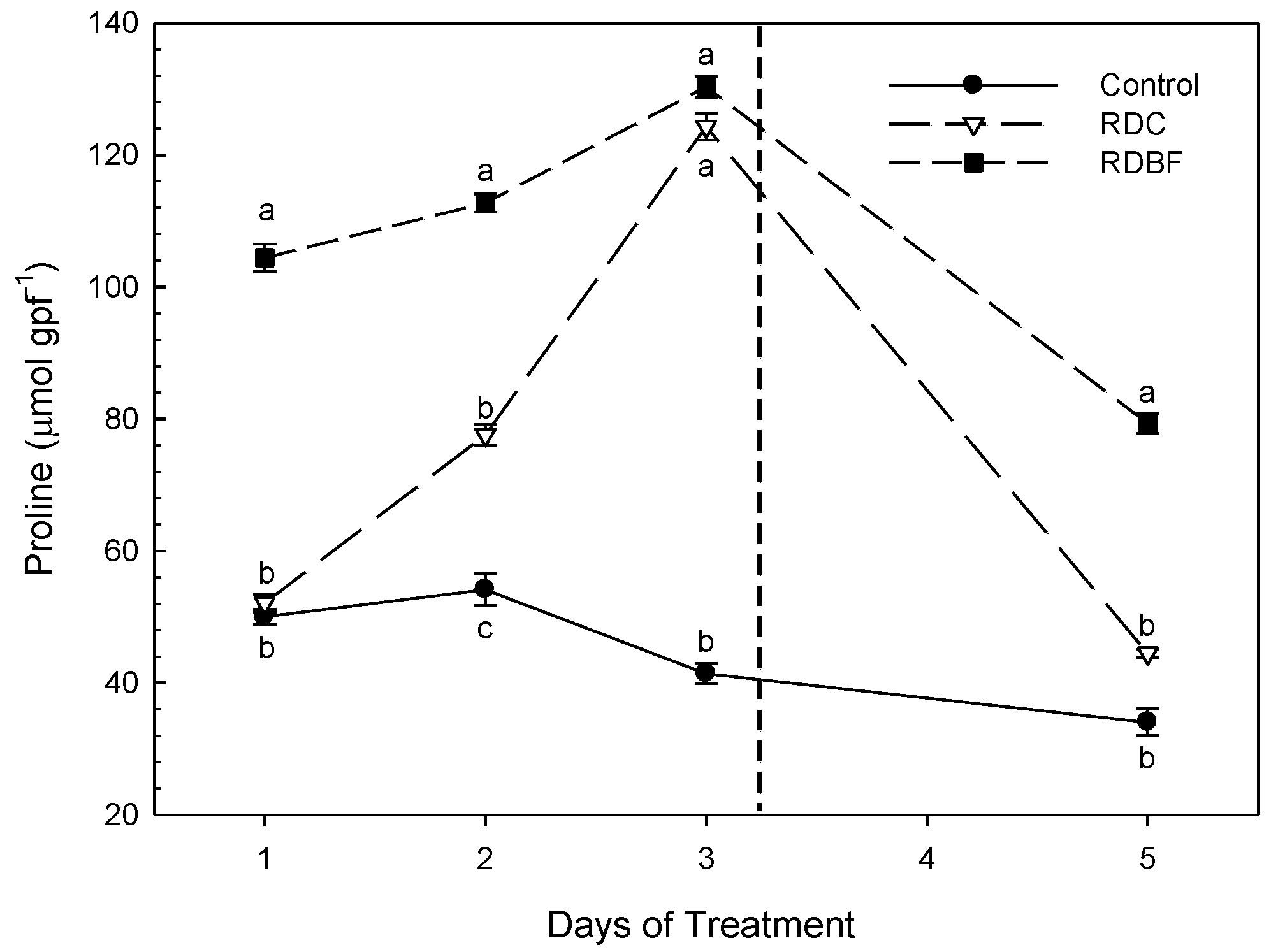
3.3. Proline and MDA Content
As described above, during the second stress cycle, leaf samples were analyzed for proline and malondialdehyde content in order to determine possible oxidative damage in response to severe drought stress (
Figure 1). As we observed, the proline content in the control treatment remained fairly stable throughout the second cycle. On the contrary, the RDI treatment showed an increasing evaluation throughout the stress cycle, with a total recovery in proline levels after the rehydration period, matching these values with those observed in the control treatment.
Particularly striking were the proline values observed during the second stress cycle in the LFDI treatment. Thus, at the beginning of the stress cycle this treatment already showed significantly higher propine values than those detected in the control and RDI, increasing even more during the restriction period. Moreover, at the end of the cycle, and after rehydration, the tip values achieved did not differ substantially from those recorded at the beginning of the second stress cycle, being much higher than those detected in the other irrigation treatments. This behavior would be compatible with the existence of mechanism of osmotic adjustment throughout the stress cycles, in order to avoid additional damages related to the hydraulic of the studied trees.
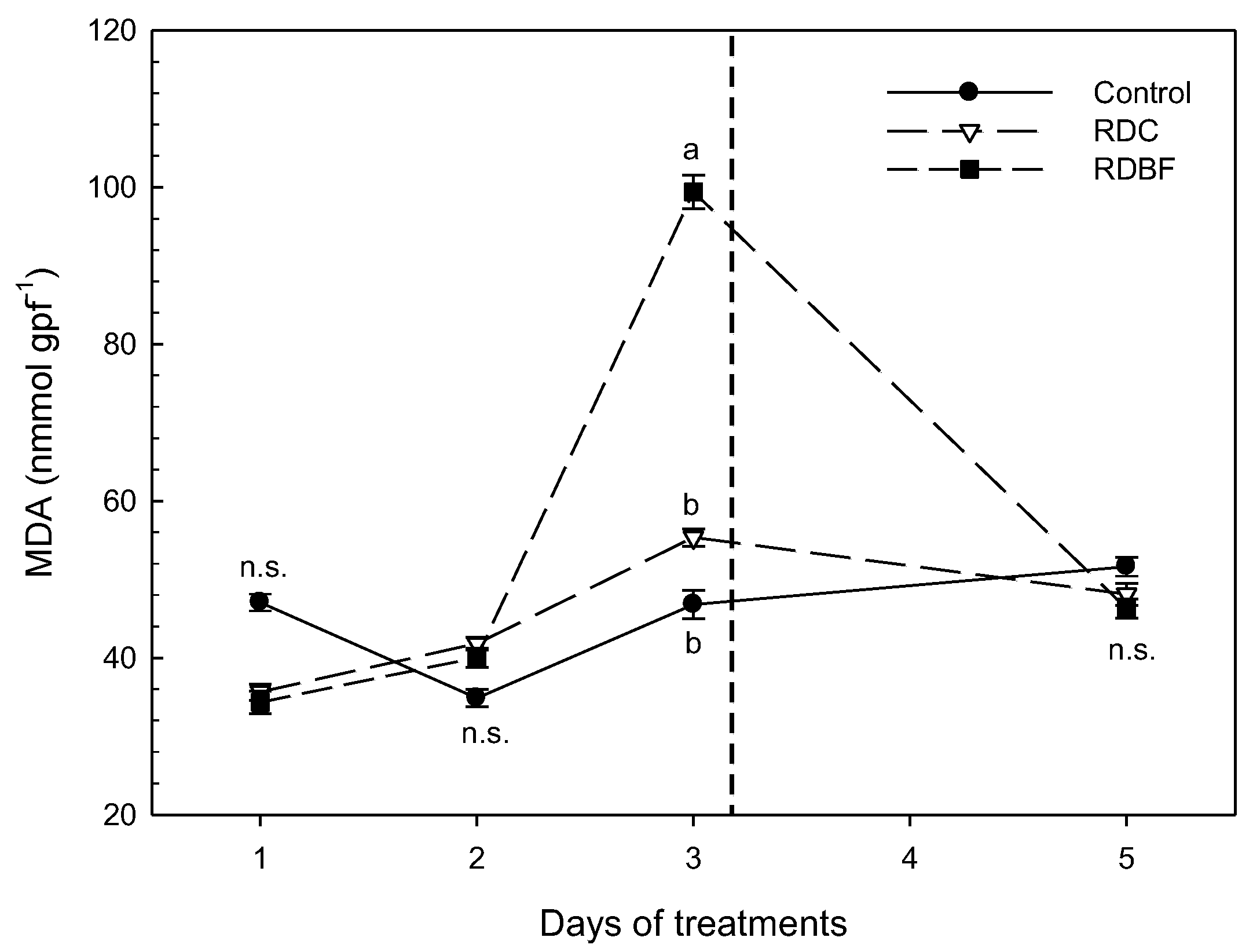
Regarding malondialdehyde content, the contents of the RDI and LFDI treatments were similar to those detected in the control treatment (
Figure 2). As the stress cycle progressed, these contents increased in the LFDI treatment, but not in the RDI, which was able to maintain constant values similar to those recorded in the control treatment. Finally, and after the rehydration period, the LFDI treatment was able to equal the MDA contents with those recorded in Control and RDI.
This behavior would be consistent with signs compatible with increased lipid peroxidation at the highest stress levels in the LFDI treatment, but with good recovery after the rehydration period.
Solutes accumulation is within the main responses of citrus to moderate-to-severe situations of water stress [
33]. This response is typical of those species with a good response to drought conditions [
34], this being considered as a good water-tress indicator in biochemical terms [
35]. According to a recently published work by [
24], proline contents increase significantly in citrus crops even after short periods of stress, which is directly related to the ability of citrus crops to respond adequately to stress cycles, even before decreases in gas exchange levels are detected, thus allowing them to maintain photosynthesis rates similar to those recorded in the absence of water stress. This response in terms of proline synthesis would explain the good capacity of the crop to recover water potential values after the recovery period and the few differences in terms of stomatal conductance detected in the first moments of the restriction cycles. Not surprisingly, good correlations between leaf proline content and stem potential values in citrus were found, both in sunny and shaded leaves, this being suggested as a good indicator of water stress level in citrus trees [
36].
Relating to the MDA content, the obtained the results suggest that possible oxidative damage was only reached at the peak of stress, with full recovery after the rehydration period. These results would be in line with the adequate response of LFDI treatments in sweet orange trees as it was stated by [
10].
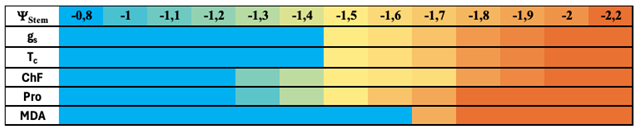
Taking into consideration the whole of data,
Table 6 shows a non-exhaustive classification of the different responses of sweet young orange trees subjected to drought conditions. According to this, the first stomatal limitations would be detected for Ψ
Stem values below to -1.4 MPa, this being identified as the point in which the CO
2 intake would be affected, as well as a reduction in water losses through the stomata, as evidenced by an increase in canopy temperature due to a decrease in the evaporative cooling process.
Moreover, as it can be seen in the results obtained, even before a gs reduction could be observed, an increase in proline levels would be detected, thus related to an osmotic adjustment prior to the reduction in gs values. In addition to these increases in Pro, an increase in chlorophyll fluorescence levels could be produced. This shows a coupling between the first diffusive limitations with the preparation of the plant to a future situation derived from biochemical limitations that would finally become evident with the presence of oxidative damage from potential values below -1.8 MPa, as shown by the MDA values observed during the second stress cycle.
The set of results observed show a first stress threshold, around -1.3 MPa for the stem water potential at midday, when crop would start to prepare for a moderate stress situation with the increase of osmolytes to ensure the osmotic adjustment and still allow gas exchange rates to remain stable, as well as a start in the dissipation of excess light radiation in the form of fluorescence to avoid oxidative damage. The second threshold would be established around potential values below -1.6 MPa, corresponding to the appearance of diffusive limitations at the stomata and an increase in leaf temperature. Finally, a third stress threshold would start around potential values below -1.8 MPa, when oxidative damage becomes evident and even osmolyte synthesis levels are maintained after recovery, a sign that exceeding these thresholds could be causing damage to the crop hydraulics itself.