Submitted:
17 April 2024
Posted:
18 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strains and Cultural Conditions
2.2. Bacteriophage Isolation, Purification, and Propagation
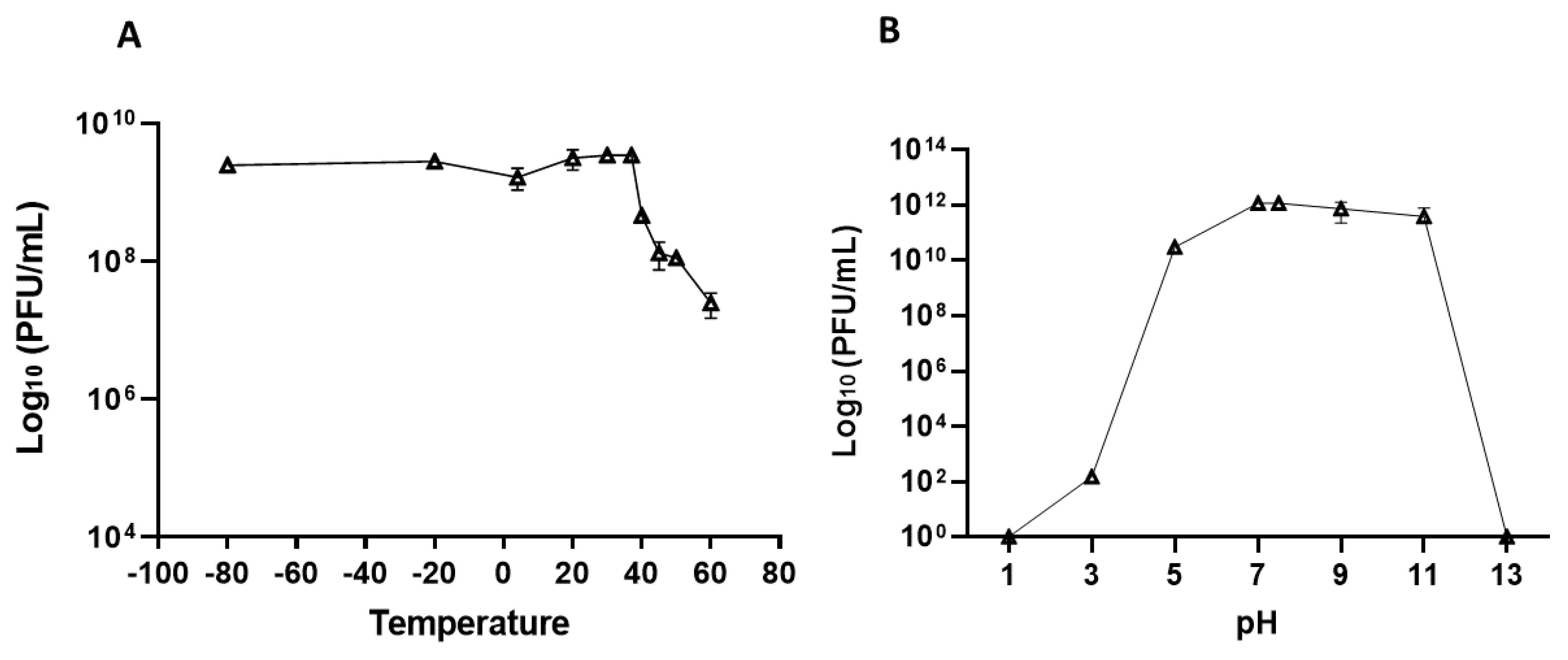
2.3. Phage Stability
2.3.1. Thermal Stability
2.4. Host Range Analysis
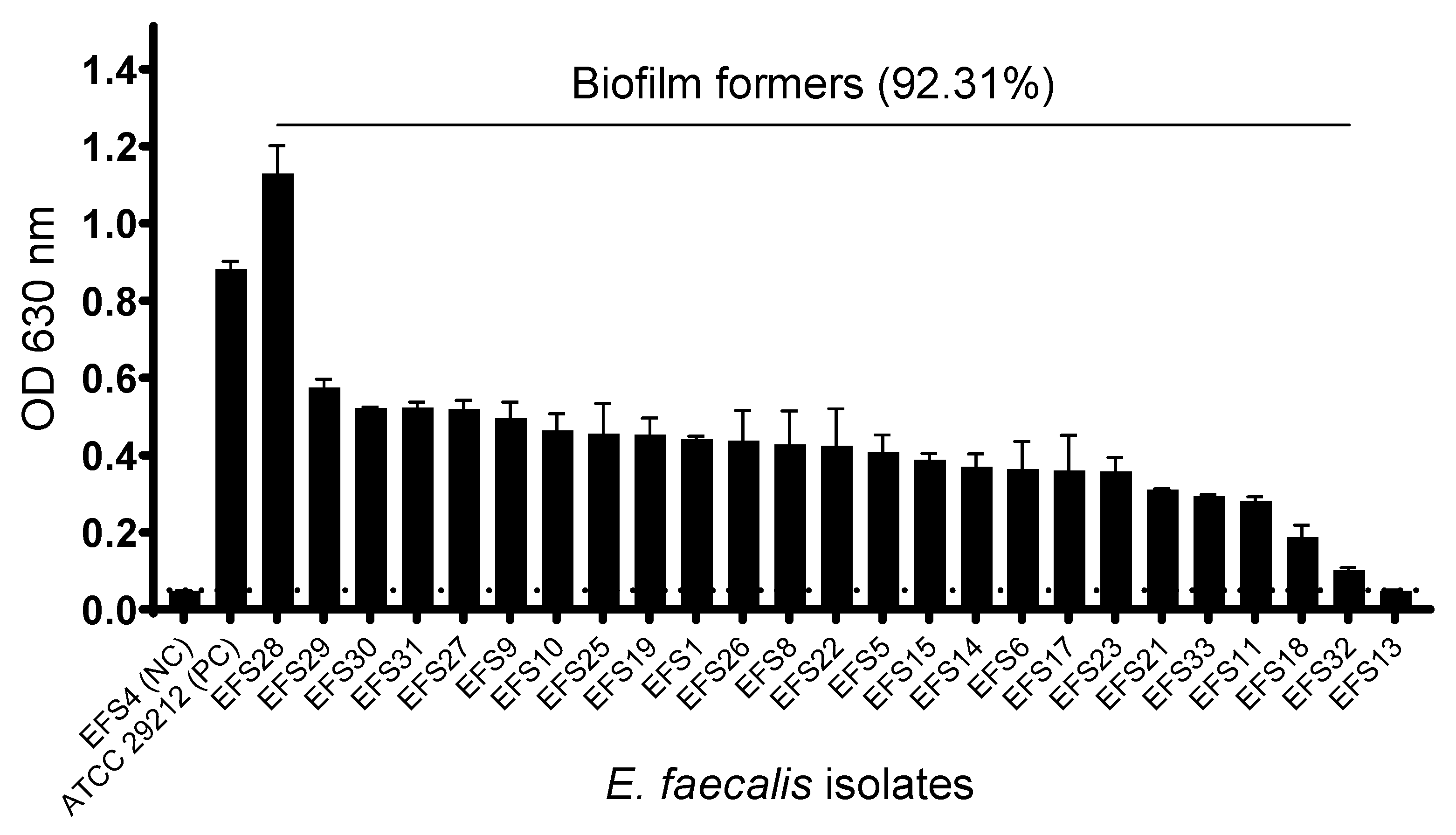
2.5. Biofilm Formation Assay
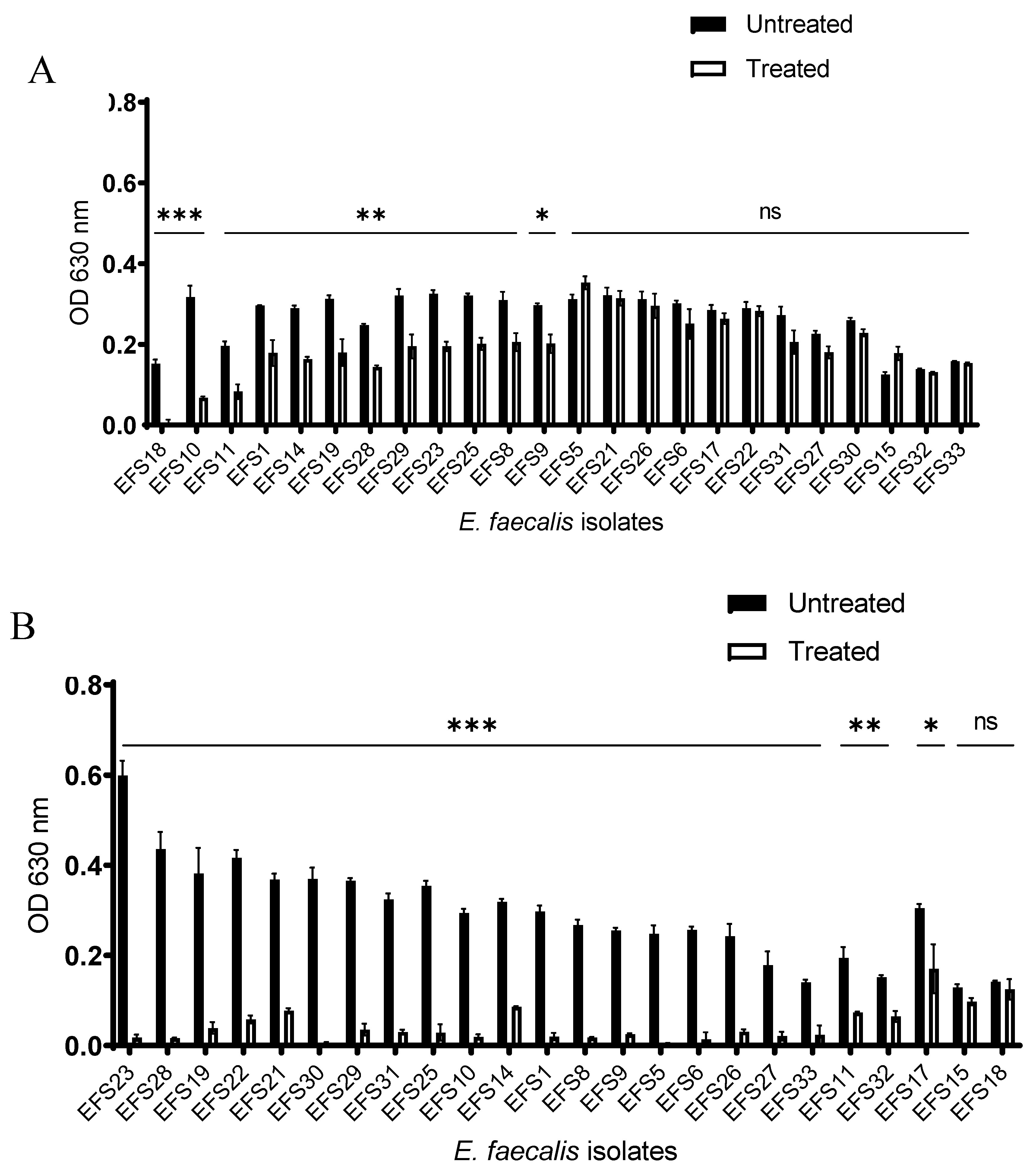
2.6. Biofilm Inhibition Assay by Phage
2.7. Biofilm Disruption Assay by Phage
2.8. Genomic DNA Extraction
2.9. Genome Sequencing and Bioinformatic Analysis of Sequencing Data
2.10. Phylogenetic Tree and Comparative Genomics of Phage Genomes
2.11. Statistical Analysis
3. Results
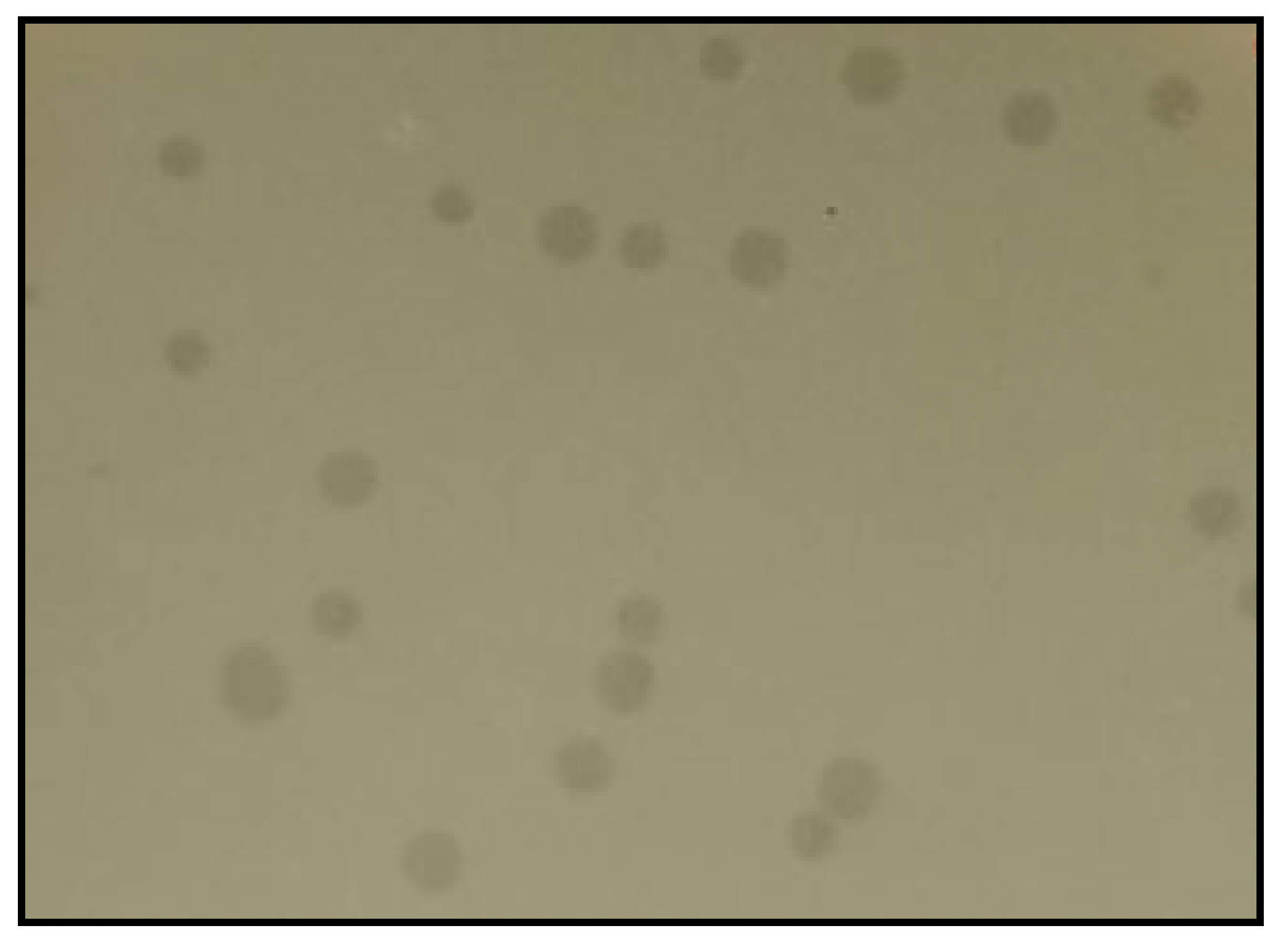
3.1. Bacteriophage Isolation and Purification
3.2. Host Range Analysis
3.3. Phage Stability
3.4. Biofilm Formation of Enterococcus faecalis
3.5. Biofilm Inhibition and Disruption by Phage vB_Efs8_KEN04.
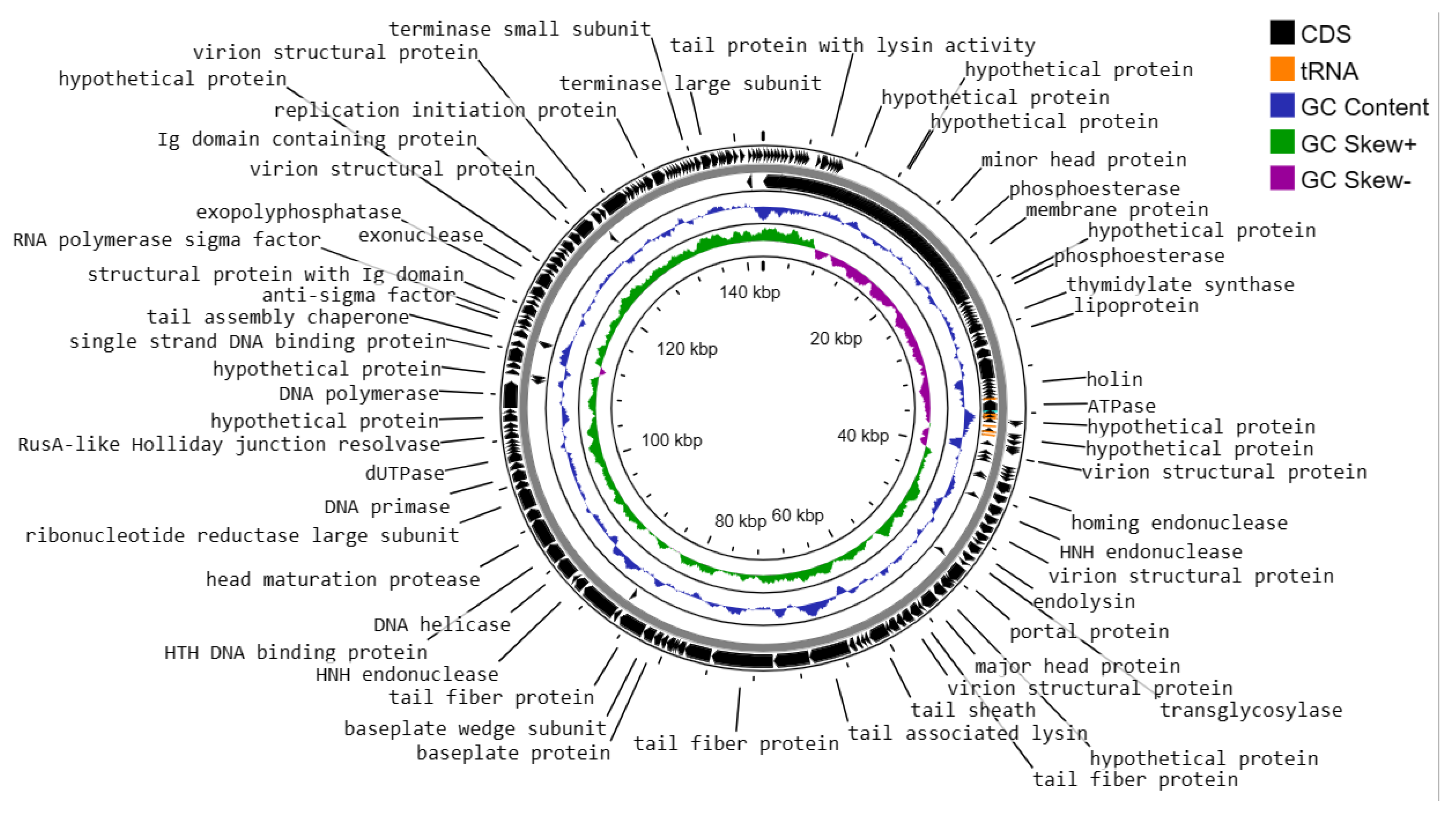
3.6. Genome Characteristics of Enterococcus faecalis Phage vB_Efs8_KEN04
- i)
- DNA replication, transcription, translation, and nucleotide metabolism: A total of 25 CDSs were predicted to encode for DNA replication, transcription regulation, translation, and metabolism-related proteins, such as HNH homing endonuclease, DNA helicase, exonucleases, transcriptional repressor, DNA helicase, DNA primase, and a transcriptional regulator, RNA polymerase beta subunit, and thymidylate synthase.
- ii)
- Structural and packaging proteins: 27 CDS were predicted to encode for tail, head, and packaging proteins such as portal proteins, head proteins, tail fiber proteins, head maturation proteases, virion structural proteins, tail proteins, tail assembly chaperones, minor and major head proteins, and terminase large and small subunits.
- iii)
- Host lysis and adhesion proteins: Two CDS were predicted to encode holin and endolysin proteins. BLASTP analysis of the Enterococcus phage vB_Efs8_KEN04 genome revealed no similarities to the genes encoding integrase or excisionase. The genome of phage vB_Efs8_KEN04 lacks genes encoding toxins, virulence factors, antibiotic resistance genes, and CRISPR. These data indicate that phage vB_Efs8_KEN04 is a strictly lytic phage that can be used to treat E. faecalis infection.
- iv)
- Sixteen CDS encode for moron, auxiliary metabolic genes, and host takeover.
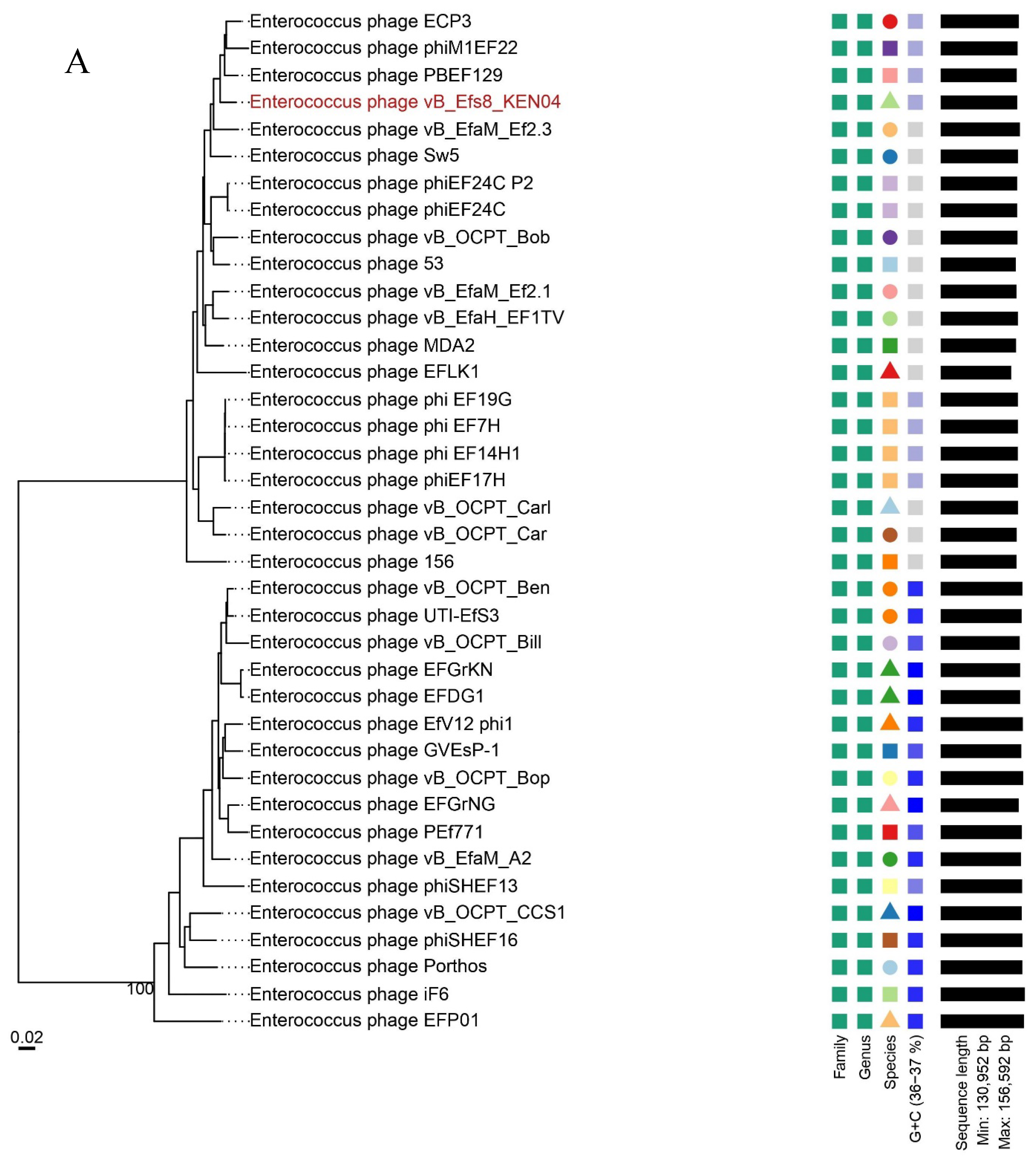
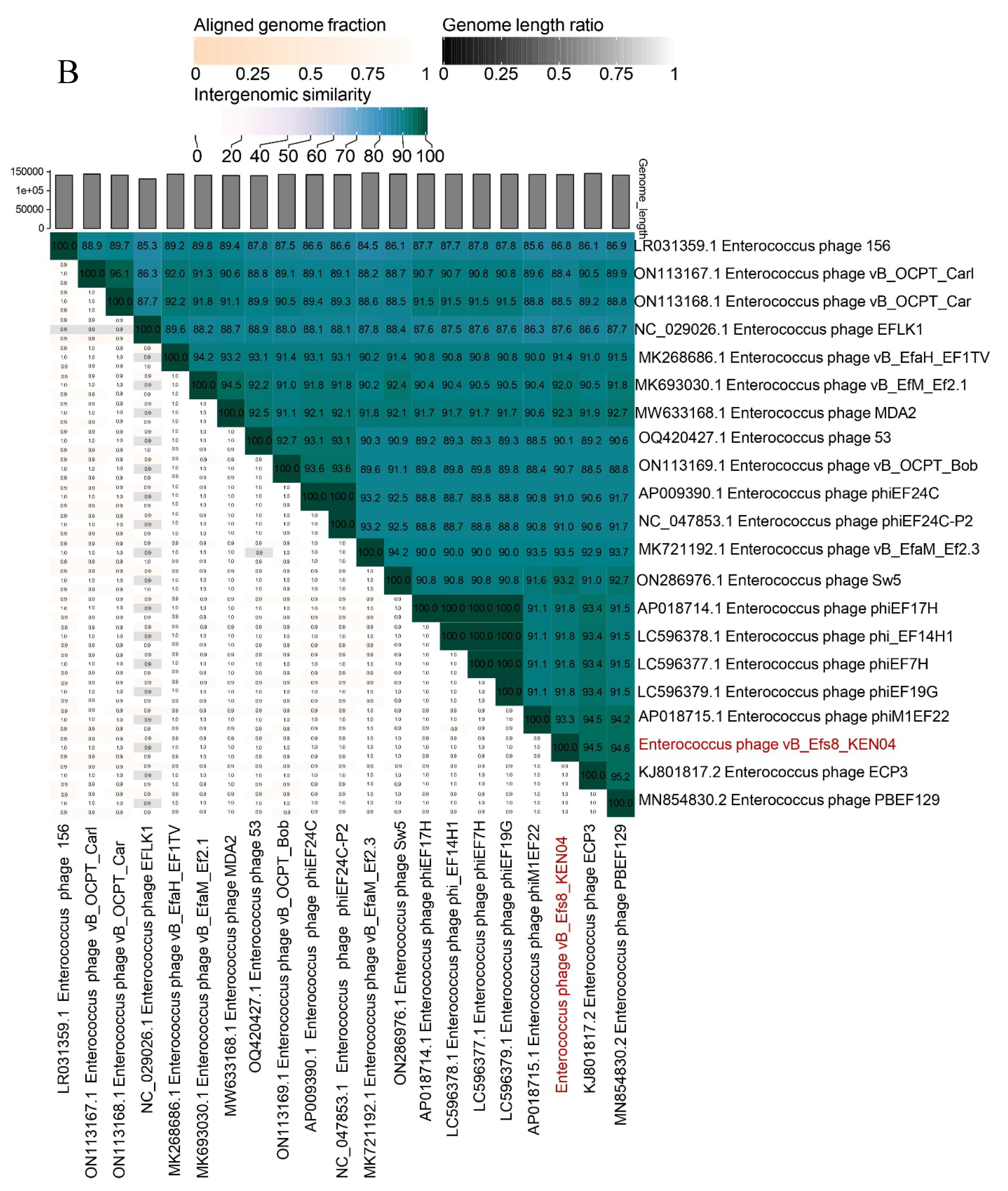
3.7. Phylogenetic Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus Infections. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Raza, T.; Ullah, S.R.; Mehmood, K.; Andleeb, S. Vancomycin Resistant Enterococci: A Brief Review. JPMA J. Pak. Med. Assoc. 2018, 68, 768–772. [Google Scholar]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as Probiotics and Their Implications in Food Safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of Combination Antimicrobial Therapy for Enterococcus Faecalis Bloodstream Infections and Infective Endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari, N.; Mathur, P.; Misra, M.C. Soft Tissue and Wound Infections Due to Enterococcus Spp. Among Hospitalized Trauma Patients in a Developing Country. J. Glob. Infect. Dis. 2014, 6, 189–193. [Google Scholar] [CrossRef]
- Shiadeh, S.M.J.; Pormohammad, A.; Hashemi, A.; Lak, P. Global Prevalence of Antibiotic Resistance in Blood-Isolated Enterococcus Faecalis and Enterococcus Faecium: A Systematic Review and Meta-Analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Dale, J.L.; Nilson, J.L.; Barnes, A.M.T.; Dunny, G.M. Restructuring of Enterococcus Faecalis Biofilm Architecture in Response to Antibiotic-Induced Stress. Npj Biofilms Microbiomes 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Blackledge, M.S.; Worthington, R.J.; Melander, C. Biologically Inspired Strategies for Combating Bacterial Biofilms. Curr. Opin. Pharmacol. 2013, 13, 699–706. [Google Scholar] [CrossRef]
- Chaudhary, N.; Maurya, R.; Singh, D.; Mohan, B.; Taneja, N. Genome Analysis and Antibiofilm Activity of Phage 590B against Multidrug-Resistant and Extensively Drug-Resistant Uropathogenic Escherichia Coli Isolates, India. Pathogens 2022, 11, 1448. [Google Scholar] [CrossRef]
- Shrestha, L.; Fan, H.-M.; Tao, H.-R.; Huang, J.-D. Recent Strategies to Combat Biofilms Using Antimicrobial Agents and Therapeutic Approaches. Pathogens 2022, 11, 292. [Google Scholar] [CrossRef]
- Brives, C.; Pourraz, J. Phage Therapy as a Potential Solution in the Fight against AMR: Obstacles and Possible Futures. Palgrave Commun. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T. Bacteriophages and Their Enzymes in Biofilm Control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Song, M.; Wu, D.; Hu, Y.; Luo, H.; Li, G. Characterization of an Enterococcus Faecalis Bacteriophage vB_EfaM_LG1 and Its Synergistic Effect With Antibiotic. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Di Lallo, G.; Falconi, M.; Iacovelli, F.; Frezza, D.; D’Addabbo, P. Analysis of Four New Enterococcus Faecalis Phages and Modeling of a Hyaluronidase Catalytic Domain from Saphexavirus. PHAGE New Rochelle N 2021, 2, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Lin, Y.; Yuan, L.; El-Telbany, M.; Maung, A.; Abdelaziz, M.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Isolation, Characterization of Enterococcus Phages and Their Application in Control of E. Faecalis in Milk. J. Appl. Microbiol. 2023, 134. [Google Scholar] [CrossRef]
- Zhang, W.; Mi, Z.; Yin, X.; Fan, H.; An, X.; Zhang, Z.; Chen, J.; Tong, Y. Characterization of Enterococcus Faecalis Phage IME-EF1 and Its Endolysin. PLoS ONE 2013, 8, e80435. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, F.; Hallajzadeh, M.; Sholeh, M.; Talebi, M.; Pirhajati Mahabadi, V.; Amirmozafari, N. Anti-Biofilm Activity of a Lytic Phage Against Vancomycin-Resistant Enterococcus Faecalis. Iran. J. Pathol. 2022, 17, 285–293. [Google Scholar] [CrossRef]
- El-Telbany, M.; Lin, C.-Y.; Abdelaziz, M.; Maung, A.; El-Shibiny, A.; Noor Mohammadi, T.; Zayda, M.; Wang, C.; Lwin, S.; Zhao, J.; et al. Potential Application of Phage vB_EfKS5 to Control Enterococcus Faecalis and Its Biofilm in Food. AMB Express 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- El-Atrees, D.M.; El-Kased, R.F.; Abbas, A.M.; Yassien, M.A. Characterization and Anti-Biofilm Activity of Bacteriophages against Urinary Tract Enterococcus Faecalis Isolates. Sci. Rep. 2022, 12, 13048. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, L.; Brosh, Y.; Gelman, D.; Coppenhagen-Glazer, S.; Beyth, S.; Poradosu-Cohen, R.; Que, Y.-A.; Beyth, N.; Hazan, R. Targeting Enterococcus Faecalis Biofilms with Phage Therapy. Appl. Environ. Microbiol. 2015, 81, 2696–2705. [Google Scholar] [CrossRef]
- Zhang, H.; Fouts, D.E.; DePew, J.; Stevens, R.H. Genetic Modifications to Temperate Enterococcus Faecalis Phage Ef11 That Abolish the Establishment of Lysogeny and Sensitivity to Repressor, and Increase Host Range and Productivity of Lytic Infection. Microbiol. Read. Engl. 2013, 159, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, J.M.; Buttaro, B.; Zhang, H.; Liss, N.; Sassone, L.; Stevens, R. Effect of a Genetically Engineered Bacteriophage on Enterococcus Faecalis Biofilms. Arch. Oral Biol. 2016, 71, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; López-Hernández, I.; Rodríguez-Fernández, M.; Pérez-Florido, J.; Casimiro-Soriguer, C.S.; Djebara, S.; Merabishvili, M.; Pirnay, J.-P.; Rodríguez-Baño, J.; Tomás, M.; et al. Case Report: Analysis of Phage Therapy Failure in a Patient with a Pseudomonas Aeruginosa Prosthetic Vascular Graft Infection. Front. Med. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Song’oro, E.; Nyerere, A.; Magoma, G.; Gunturu, R. Occurrence of Highly Resistant Microorganisms in Ruai Wastewater Treatment Plant and Dandora Dumpsite in Nairobi County, Kenya. Adv. Microbiol. 2019, 9, 479–494. [Google Scholar] [CrossRef]
- Clokie, M.R.J.; Kropinski, A.M. Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions. Available online: https://link.springer.com/book/10.1007/978-1-60327-164-6 (accessed on 3 March 2024).
- Chang, Y.; Shin, H.; Lee, J.-H.; Park, C.J.; Paik, S.-Y.; Ryu, S. Isolation and Genome Characterization of the Virulent Staphylococcus Aureus Bacteriophage SA97. Viruses 2015, 7, 5225–5242. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Nilsson, A.S. Correction: Isolation of Phages for Phage Therapy: A Comparison of Spot Tests and Efficiency of Plating Analyses for Determination of Host Range and Efficacy. PloS One 2015, 10, e0127606. [Google Scholar] [CrossRef]
- Mutai, I.J.; Juma, A.A.; Inyimili, M.I.; Nyachieo, A.; Nyamache, A.K. Efficacy of Diversely Isolated Lytic Phages against Multi-Drug Resistant Enterobacter Cloacae Isolates in Kenya. Afr. J. Lab. Med. 2022, 11. [Google Scholar] [CrossRef]
- Chaudhary, N.; Mohan, B.; Kaur, H.; Modgil, V.; Kant, V.; Bhatia, A.; Taneja, N. Vibrio Phage VMJ710 Can Prevent and Treat Disease Caused by Pathogenic MDR V. Cholerae O1 in an Infant Mouse Model. Antibiotics 2023, 12, 1046. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Brabban, A.D.; Diez-Gonzalez, F. Isolation and Characterization of Lytic Bacteriophages against Enterohaemorrhagic Escherichia Coli. J. Appl. Microbiol. 2011, 110, 1323–1331. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus Spp. Isolated from Urinary Tract Infections. Pathogens 2022, 12, 34. [Google Scholar] [CrossRef]
- Tiria, F.; Odoyo, E.; Georges, M.; Nyerere, A.; Musila, L. Molecular Detection of Key Virulence-Associated Genes and Phenotypic Analysis of Virulence Traits of Klebsiella Pneumoniae Clinical Isolates from Kenya. J. Pure Appl. Microbiol. 2023, 17. [Google Scholar] [CrossRef]
- Donelli, G.; Vuotto, C.; Cardines, R.; Mastrantonio, P. Biofilm-Growing Intestinal Anaerobic Bacteria. FEMS Immunol. Med. Microbiol. 2012, 65, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, A.; Ranjbar, R. Antibiotic Resistance, Virulence-Associated Genes Analysis and Molecular Typing of Klebsiella Pneumoniae Strains Recovered from Clinical Samples. AMB Express 2021, 11, 122. [Google Scholar] [CrossRef]
- Goodarzi, F.; Hallajzadeh, M.; Sholeh, M.; Talebi, M.; Pirhajati Mahabadi, V.; Amirmozafari, N. Anti-Biofilm Activity of a Lytic Phage Against Vancomycin-Resistant Enterococcus Faecalis. Iran. J. Pathol. 2022, 17, 285–293. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.; Filippov, A.A.; Sergueev, K.V.; He, Y.; Ward, A.M.; Goglin, K.; Vashee, S.; Nikolich, M.P.; Fouts, D.E. Complete Genome Sequence of Broad-Host-Range Staphylococcus Aureus Myophage ESa1. Microbiol. Resour. Announc. 2020, 9, e00730–20. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 21 February 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinforma. Oxf. Engl. 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bouras, G.; Nepal, R.; Houtak, G.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Pharokka: A Fast Scalable Bacteriophage Annotation Tool. Bioinforma. Oxf. Engl. 2023, 39, btac776. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Rajandas, H.; Parimannan, S.; Manickam, R.; Marimuthu, K.; Petersen, B.; Clokie, M.R.J.; Millard, A.; Sicheritz-Pontén, T. PhageLeads: Rapid Assessment of Phage Therapeutic Suitability Using an Ensemble Machine Learning Approach. Viruses 2022, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.R.; Depardieu, F.; Fortier, L.-C.; Bikard, D.; Monot, M. PhageTerm: A Tool for Fast and Accurate Determination of Phage Termini and Packaging Mechanism Using next-Generation Sequencing Data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARAGORN, a Program to Detect tRNA Genes and tmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol. Biol. Clifton NJ 2019, 1962, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. ABRicate: Mass Screening of Contigs for Antimicrobial and Virulence Genes. Github 2017.
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.-H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, 10.1128/aac.00483-19. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A Comparative Pathogenomic Platform with an Interactive Web Interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-Based Phylogeny and Classification of Prokaryotic Viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Henz, S.R.; Huson, D.H.; Auch, A.F.; Nieselt-Struwe, K.; Schuster, S.C. Whole-Genome Prokaryotic Phylogeny. Bioinforma. Oxf. Engl. 2005, 21, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinformatics 2013, 14, 60. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Vincent, M.; Sun, Y.; Yu, H.; Wang, J.; Bao, Q.; Kong, H.; Hu, S. Complete Genome Sequence of Bacteriophage T5. Virology 2005, 332, 45–65. [Google Scholar] [CrossRef]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. A Systematic and Critical Review of Bacteriophage Therapy Against Multidrug-Resistant ESKAPE Organisms in Humans. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 167–178. [Google Scholar] [CrossRef]
- Goodarzi, F.; Hallajzadeh, M.; Sholeh, M.; Talebi, M.; Mahabadi, V.P.; Amirmozafari, N. Biological Characteristics and Anti-Biofilm Activity of a Lytic Phage against Vancomycin-Resistant Enterococcus Faecium. Iran. J. Microbiol. 2021, 13, 691–702. [Google Scholar] [CrossRef]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the Intriguing Presence of tRNAs in Phages. Genome Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Y.; Wang, Y.; Yang, H.; Shen, W.; Chen, X. Transcription Regulation Mechanisms of Bacteriophages: Recent Advances and Future Prospects. Bioengineered 2014, 5, 300–304. [Google Scholar] [CrossRef]
- van den Berg, D.F.; van der Steen, B.A.; Costa, A.R.; Brouns, S.J.J. Phage tRNAs Evade tRNA-Targeting Host Defenses through Anticodon Loop Mutations. eLife 2023, 12, e85183. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host Receptors for Bacteriophage Adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Riede, I.; Montag, D.; Eschbach, M.-L.; Henning, U. Receptor Specificity of the Escherichia Coli T-Even Type Phage Ox2: Mutational Alterations in Host Range Mutants. J. Mol. Biol. 1989, 207, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W.; Tremblay, D.; Moineau, S. Long-Term Bacteriophage Preservation. WFCC Newsl. 2004, 38, 35–40. [Google Scholar]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases. Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Disease. J. Clin. Periodontol. 2017, 44, S5–S11. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Bamford, N.C.; MacPhee, C.E.; Stanley-Wall, N.R. Microbial Primer: An Introduction to Biofilms – What They Are, Why They Form and Their Impact on Built and Natural Environments. Microbiology 2023, 169, 001338. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Biofilms and Antimicrobial Resistance. Clin. Orthop. 2005, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Burgess, J.G. Extracellular DNA in Oral Microbial Biofilms. Microbes Infect. 2015, 17, 531–537. [Google Scholar] [CrossRef]
- Goodarzi, F.; Hallajzadeh, M.; Sholeh, M.; Talebi, M.; Mahabadi, V.P.; Amirmozafari, N. Biological Characteristics and Anti-Biofilm Activity of a Lytic Phage against Vancomycin-Resistant Enterococcus Faecium. Iran. J. Microbiol. 2021, 13, 691–702. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Li, Y.; Lu, Y.; Xiong, K.; Zhong, Q.; Wang, J. Bacteriophage-Resistant Mutant of Enterococcus Faecalis Is Impaired in Biofilm Formation. Front. Microbiol. 2022, 13, 913023. [Google Scholar] [CrossRef] [PubMed]
- Winans, J.B.; Wucher, B.R.; Nadell, C.D. Multispecies Biofilm Architecture Determines Bacterial Exposure to Phages. PLoS Biol. 2022, 20, e3001913. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Mlynek, K.D.; Hettiarachchi, H.; Alamneh, Y.A.; Biggemann, L.; Zurawski, D.V.; Black, C.C.; Bane, C.E.; Kim, R.K.; Granick, M.S. Extracellular Polymeric Substance (EPS)-Degrading Enzymes Reduce Staphylococcal Surface Attachment and Biocide Resistance on Pig Skin in Vivo. PloS One 2018, 13, e0205526. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Bacteriophage Endolysins: Applications for Food Safety. Curr. Opin. Biotechnol. 2016, 37, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm Formation and Control Strategies of Foodborne Pathogens: Food Safety Perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
| No. | Bacterial isolates | Sequence Types (ST) | Origin | Spot assay | Efficiency of Plating (EOP) |
|---|---|---|---|---|---|
| 1 | E. faecalis EFS8 * | 1904 | Urinary tract infection | ++ | 1 |
| 2 | E. faecalis EFS1 | 6 | Skin and soft tissue infection | ++ | 0.15 |
| 3 | E. faecalis EFS4 | 947 | Skin and soft tissue infection | + | <0.001 |
| 4 | E. faecalis EFS5 | 6 | Skin and soft tissue infection | ++ | 0.076 |
| 5 | E. faecalis EFS6 | 6 | Skin and soft tissue infection | ++ | 0.05 |
| 6 | E. faecalis EFS9 | 6 | Urinary tract infection | ++ | 0.01 |
| 7 | E. faecalis EFS10 | 6 | Urinary tract infection | ++ | 1.1 |
| 8 | E. faecalis EFS11 | 368 | Urinary tract infection | ++ | 0.0004 |
| 9 | E. faecalis EFS13 | 59 | Skin and soft tissue infection | ++ | 0.5 |
| 10 | E. faecalis EFS14 | 6 | Skin and soft tissue infection | ++ | 1.7 |
| 11 | E. faecalis EFS15 | Urinary tract infection | + | <0.001 | |
| 12 | E. faecalis EFS17 | 6 | Skin and soft tissue infection | + | <0.001 |
| 13 | E. faecalis EFS18 | 368 | Urinary tract infection | ++ | 0.0011 |
| 14 | E. faecalis EFS19 | Urinary tract infection | ++ | 0.12 | |
| 15 | E. faecalis EFS21 | 44 | Skin and soft tissue infection | + | <0.001 |
| 16 | E. faecalis EFS22 | Skin and soft tissue infection | + | <0.001 | |
| 17 | E. faecalis EFS23 | 6 | Urinary tract infection | ++ | 3 |
| 18 | E. faecalis EFS25 | 6 | Surgical site infection | ++ | 1.5 |
| 19 | E. faecalis EFS26 | 6 | Skin and soft tissue infection | ++ | 1.2 |
| 20 | E. faecalis EFS27 | 1903 | Urinary tract infection | ++ | 0.14 |
| 21 | E. faecalis EFS28 | 28 | Skin and soft tissue infection | ++ | 0.6 |
| 22 | E. faecalis EFS29 | 6 | Blood infection | ++ | 0.8 |
| 23 | E. faecalis EFS30 | 28 | Skin and soft tissue infection | ++ | 0.8 |
| 24 | E. faecalis EFS31 | 6 | Urinary tract infection | ++ | 1.2 |
| 25 | E. faecalis EFS32 | 1903 | Urinary tract infection | ++ | 0.9 |
| 26 | E. faecalis EFS33 | 1903 | Skin and soft tissue infection | ++ | 6 |
| 27 | E. faecium EFM5 | 80 | Urinary tract infection | + | <0.001 |
| 28 | E. faecium EFM1 | Skin and soft tissue infection | - | N/A | |
| 29 | E. faecium EFM2 | 80 | Skin and soft tissue infection | - | N/A |
| 30 | E. faecium EFM3 | Skin and soft tissue infection | - | N/A | |
| 31 | E. faecium EFM4 | Skin and soft tissue infection | - | N/A | |
| 32 | E. faecium EFM6 | 612 | Skin and soft tissue infection | - | N/A |
| 33 | E. faecium EFM7 | Skin and soft tissue infection | - | N/A | |
| 34 | E. faecium EFM8 | 80 | Urinary tract infection | - | N/A |
| 35 | E. faecium EFM9 | 80 | Skin and soft tissue infection | - | N/A |
| 36 | E. faecium EFM10 | Urinary tract infection | - | N/A | |
| 37 | E. faecium EFM11 | 761 | Surgical site infection | - | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





