1. Introduction
In the Mediterranean region, pomegranate (Punica granatum L.) has been cultivated since 3000 BC (Stover and Mercure 2007) and has its name in the Holy Bible (Blumenfeld et al., 2000). Being originated in Iran, pomegranate is cultivated all over the world due to its medicinal properties, health benefits, and high market value (Parvizi et al., 2016). Edible parts of pomegranate are aril (Selahvarzi et al., 2017), which contains high bioactive compounds and antioxidants (Holland et al., 2009). Important health benefits are that it helps in boosting immunity, reduces the risk of anemia, boosts heart health, soothes stomach infections, alleviates arthritis, and prevents cancer & premature aging (Kandylis and Kokkinomagoulos 2020). It is a kind of crop that is grown across arid, semi-arid, and even under desert conditions. Under irrigated conditions, it grows well in hot, dry summer and cold winter (Aseri et al., 2008). It is a stress-tolerant crop (Holland et al., 2009) and has the potential to grow in different agro-climatic conditions.
Deficit irrigation (DI) is an alternative technology for improving the water productivity (WP) of the crop under water scarcity regions without having an adverse effect on yield and quality of crops (Nangare et al., 2016; Noitsakis et al., 2016) whereas PRD technique involves irrigating part of the root zone on an alternate basis according to the scheduled soil water depletion and crop water consumption (Jovanovic and Stikic 2018). Alternate drying and wetting of the root zone lead to partial closing of stomata which reduces water loss and increases water use efficiency (Stoll et al., 2000; Beis and Patakas 2015). Effect of PRD in improving water use efficiency (WUE) has also been reported in grapes (Romero et al., 2016), apples (Du et al., 2017), and oranges (Hutton and Loveys 2011). However, stomatal conductance, net photosynthesis, and transpiration were higher in full irrigation and mild water stress treatments in comparison with the plants under severe water stress treatments (Li et al., 2018; Parvizi et al., 2016; Rodriguez et al., 2012).
Further, PGRs play an important role in the qualitative and quantitative improvement in the growth and development of pomegranates by regulating responses to environmental and cultural conditions. Spraying of NAA @25 ppm three times in 21 days intervals increased fruit set, fruit retention and quality of pomegranate compared to the control (Ghosh et al., 2009; Kishor et al., 2016; Anawal et al., 2016). Further, Nuncio-Jauregui et al., (2014) reported higher concentrations of glucose, fructose, and major organic acids in the plants treated with SA. Ghassemi-Golezani et al., (2020) reported that variable fluorescence, the maximal quantum yield of photosystem, effectiveness of water-splitting complex, physiological parameters, and seed production under stress conditions were all increased by SA. In this approach, SA applied topically to the leaves of plants may be crucial to their capacity to withstand stress.
Most of the research in this area has been focused on the effect of DI and PGRs individually, however their combined effect on pomegranate grown under semi-arid conditions having shallow soils with less water holding capacity is yet to be assessed in detail. Therefore the objective of the study was to study physiological and biochemical changes under deficit irrigation and PGRs in pomegranate. The findings of this experiment will aid in standardizing the recommendations for growing pomegranates in these agro-ecological regions without compromising their yield and quality while saving the water resource.
2. Material & Methods
2.1. Experimental Site
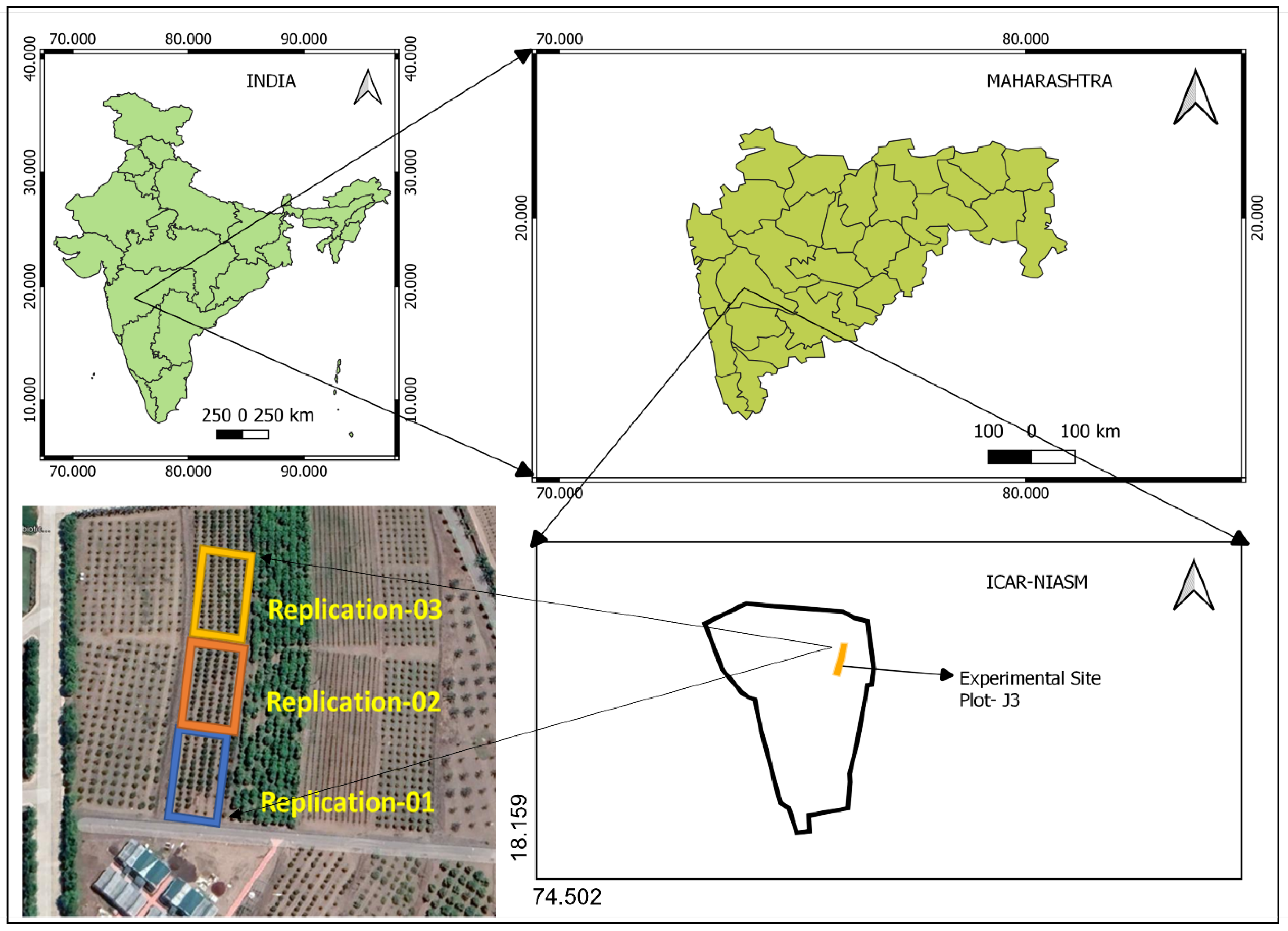
The experiment was conducted at Plot No.-J3 of the North block orchard of ICAR- National Institute of Abiotic Stress Management, Malegaon, Baramati during the year 2021-2022 (18.159
0 N, 74.502
0 E, 570 m from MSL) as shown in
Figure 1. This area belongs to the Agro-climatic zone (AZ-95) & Agro-ecological region (AER-6) of the Deccan plateau with a hot and semi-arid climate. The physical & chemical properties of the soil at the experimental site are given in
Table 1.
2.2. Meteorological Conditions
All the meteorological data used in this experiment were collected from the meteorological observatory situated at ICAR-NIASM, Malegaon, Baramati (
Table 2). Average daily maximum temperature, average daily minimum temperature, relative humidity, wind speed, and pan evaporation in the study period were 32.24
0C, 19.20
0C, 76.56%, 7.56 kmhr
-1, and 5.59 mm respectively. The total pan evaporation observed during the experiment was 1479.50 mm while the total rainfall received during the experiment was 194.60 mm.
2.3. Experimental Details
Pomegranate trees (cv. Bhagwa) of 8 years old and planted at 3 x 4.5 m spacing were selected for the study. Initially, Hasta bahar (September-October ) was practised and stress was imposed on plants by withholding irrigation for the month of September-October followed by other intercultural operations like pruning. The experiment was designed in a randomized block design (RBD) with 11 treatments with 3 replications (
Table 3). PGRs were applied at 4 different phenological stages
viz, flower bud initiation, flowering, fruit setting, and fruit enlargement. SA & NAA were applied at @300 ppm and 45 ppm, respectively. Drip irrigation system was installed in the experimental plot for providing irrigation. Single lateral was installed in all the plants near the trunk except for PRD-treated plants, where two laterals were installed at a 50 cm distance from the trunk to supply irrigation in alternation. Further, each plant was provided with two drippers, which had a discharge rate of 4Lh
-1 @1 kg-cm
-2. In PRD, shifting of laterals was done every 15 days interval.
2.4. Orchard Management & Cultural Practices
Weeds & suckers were removed each 15 days interval. Spraying of insecticides, pesticides & fertilizer application was done as per the package of practices given by ICAR-NRCP, Solapur, Maharashtra, India.
2.5. Water Requirement
Irrigation was applied as per different treatment levels to plants based on the USDA Class A Pan evaporimeter data (Doorenbos and Pruitt 1977). The quantity of water to be applied through drip irrigation in litres per day per plant was calculated by the formula:
where,
WRp = Water requirement by plant, (litre day-1 tree-1)
Epan = Pan Evaporation, (mm)
Kp = Pan Coefficient
Kc = Crop coefficient
A = Area occupied by each tree, (m2)
WA = Wetted area in fraction
ŋ = Irrigation Efficiency in fraction
2.6. Crop Coefficient
For different phenophases (i.e., initiation of new leaves, development, maturity, harvesting) crop coefficient values were calculated by the shaded area approach by using the equation developed by (Gorantiwar et al., 2011).
where,
Kc = Crop Coefficient
X = Percentage of the shaded area, (%)
2.7. Plant Yield/Fruit Yield
After harvesting all the fruits were weighed in a weighing balance. Total yield per plant and total yield per treatment were recorded and expressed in kilograms (kg).
2.8. Water productivity
Water productivity (WP) shows the efficiency of plants in utilizing water. WP was calculated by the formula:
2.9. Soil Moisture Content (SMC)
Soil moisture content was measured at fortnightly interval to know the available soil moisture in the field.
2.10. Physiological Parameters
Relative Water Content (RWC)
RWC is the ratio of the amount of water present in a leaf at the time when sampling was done to the maximum amount of water that can be beheld by the leaf. Recently matured leaf was collected from each tree. The fresh weight of all the leaves was taken and recorded. After that, leaves were kept inside water in a falcon tube for 4-6 hrs. Then turgid weight was measured. These samples were then kept in a hot air oven at 70
0C for a period of 24 hrs and finally, the dry weight of all the leaves was measured. RWC was then calculated by using the formula:
where
RWC = Relative water content of leaf (%)
FW = Fresh Weight of the leaf (g.)
TW = Turgid weight of the leaf (g.)
DW = Dry weight of the leaf (g.)
Chlorophyll Content
Measurement of chlorophyll content in the leaf was done with the help of a chlorophyll meter (SPAD 502 plus, Konica-Minolta, Japan). Average measurement was taken from five leaves of each plant and then the average of all the readings was done to record the chlorophyll content.
Membrane stability Index (MSI)
Methods suggested by Sairam (1994) were used to calculate the MSI of tissue. It shows the leakage in the tissue. First, the leaf sample was collected from each treatment and 25g of each leaf was kept inside a test tube filled with 10 ml of distilled water. Then all the samples were heated inside a hot water bath at 45
0C for 30 minutes. Then it was cooled to room temperature and the electrical conductivity (C
1) was measured by using a conductivity meter (Systronics India Ltd., Mumbai, India). And again, all the samples were incubated at 100
0C for 15 minutes inside a water bath and the electrical conductivity (C
2) of each sample was measured after cooling down to room temperature. Then MSI was calculated by using the following formula
Normalized Difference Vegetative Index (NDVI)
NDVI was measured with the help of a green seeker (Trimble handled crop sensor, Ukraine). It is a handy tool that shows the vigour of crops instantly and helps in making decisions for effective crop management.
Canopy Temperature (CT)
CT was measured with the help of an Infrared (IR) thermometer. All the readings of canopy temperature were taken between 10.30-11.00 AM under a clear sky to get uniformity.
Chlorophyll Fluorescence (CF)
CF was measured with the help of Fluorcam instruments (Photon Systems Instruments, Czech Republic). Before the start of the experiment, leaf samples were collected and kept in dark conditions at least for 30 minutes. Leaf samples were kept under the LED light panel and analysis was done by using the software FluorCam7 to calculate the maximum PS-II quantum yield in the dark-adapted state (QYmax) which is the ratio of variable fluorescence in the dark-adapted state (Fv) to maximum fluorescence in dark-adapted state (Fm).
Gas Exchange Parameters
Leaf gas exchange parameters like photosynthesis rate (An) and transpiration rate (Tr) were measured with the help of a portable photosynthesis system (GFS-3000, Waiz, Germany). All the data were taken at 11 AM morning under a clear sky.
Proline Content of Leaves
The proline content in matured leaves of each treatment was estimated by the method suggested by Bates et al. (1973). A fresh leaf (0.5 g) was homogenized in 10 ml of 3 percent sulpho-salicylic acid in a pre-chilled mortar and pestle. Then, it was centrifuged at 10,000 rpm for 10 minutes at 4oC (Eppendorf, Centrifuge 5810 R, Germany). 2 ml of supernatant was taken in the test tube. Then 2 ml of acid ninhydrin reagent and 2 ml of glacial acetic acid were added to the test tube. After that, all the test tubes were placed in a hot water bath for one hour at 100oC. Thereafter, the reaction was terminated by keeping the solution in an ice bath. Then, 4 ml toluene was added and mixed vigorously with the help of a vortex stirrer (Vortex Mixer, Benchtop Lab Systems) for 20-30 seconds. The chromophore containing toluene layer (light pink) was aspirated from the aqueous phase, warmed to room temperature, and then absorbance was read at 520 nm on UV-VIS double-beam PC 8 scanning Auto-cell spectrophotometer (UVD-3200, Labomed, Inc., Culver City, CA, USA), using pure toluene as a blank. The proline concentration in the samples was determined from a standard curve.
2.11. Antioxidant Activity
Total Phenols
The folin-Ciocalteu method suggested by (Bharathi et al., 2014) was used for the estimation of total phenols in the sample. Samples of 20 g were crushed in the 10-time volume of 80% aqueous methanol at room temperature using a pestle and mortar. After that, the homogenate was centrifuged at 7500 RPM for 15 min at 4 0C Eppendorf, Centrifuge 5810 R, Germany. From the supernatant 0.5 ml of the extract was taken and then 0.2 ml of Folin–Ciocaltau reagent was added to it. Followed by 3.3 ml of distilled water was added to the sample. Then 1 ml of 20% Sodium carbonate (Na2CO3) was added to each tube. Then all the samples were kept in dark for 30 minutes after that a UV–vis spectrophotometer (Lab India., Hyderabad, India) was used to measure the absorbance at 765 nm. Then total phenol content in the sample was expressed as gallic acid equivalent (mg/100 g GAE fwb).
Total Flavonoids
For estimating flavonoids samples were first crushed in 80% methanol. Methods were followed as per (Bharathi et al., 2014). The homogenate was then centrifuged at 7500 RPM for 15 min. at 4 0C Eppendorf, Centrifuge 5810 R, Germany. 0.5 ml of extract was taken in a test tube 0.4 ml of distilled water was added to it. After that 0.3 ml, 5% sodium nitrite was added to it and the mixture was allowed to react for 5 min. Later, 0.3 ml 10% aluminium chloride was added, and the mixture was allowed to stand for another 5 min then 3.4 ml of 4N Sodium Hydroxide was added to it. Then the samples were incubated at room temperature for 30 min and the absorbance was taken at 415 nm, UV–vis spectrophotometer (Lab India., Hyderabad, India). The total flavonoid content in the sample was expressed at milligrams of catechin equivalent (mg/100 g CE fwb).
FRAP Enzymatic Assay
By following the methods of Benzie and Strain (1996) Ferric ion reducing antioxidant power (FRAP) was estimated. Before the experiment FRAP reagent, was prepared fresh by adding 1 mM 2,4,6-tripyridyl-2-triazine (TPTZ) and 20 mM ferric chloride in 0.25 M sodium acetate. After crushing the sample with 80% methanol and then it was centrifuged. 0.2 ml of aliquot extract was taken in the test tube and 1.8 ml of FRAP reagent was added. Samples are then incubated at room temperature for 40 min. After incubation, the absorbance was read at 593 nm using a spectrophotometer (Lab India., Hyderabad, India). The antioxidant capacity of each sample (leaf & fruit) was calculated based on its ability to reduce ferric ions. Readings were expressed as the ascorbic acid equivalent antioxidant capacity (AEAC; in mg ascorbic acid 100 g−1fwb).
DPPH Radical Scavenging Activity
The electron-donating capacity of extracts of leaf and fruit samples was determined using the 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) method. Samples were crushed in 80% methanol and then centrifuged. Then 0.5 ml of sample extract was added to 3 ml of 0.001M DPPH methanol solution. The reaction mixture was left in the dark at room temperature for 45 min. The absorbance was measured at 517 nm using a UV–vis spectrophotometer (Lab India., Hyderabad, India) every 10 min up to 120 min. The absorbance was also taken from a blank sample containing 1 ml of DPPH and 5 ml of 80% methanol. All determinations were performed in triplicate. The percentage of scavenging activity against DPPH radical was calculated by the following equation:
Statistical Analysis
The experiment was conducted in a randomized complete block design (RBD) with 11 treatments replicated three times. Statistically, analysis was performed using analysis of variance (ANOVA) at a 5% level of significance (Gomez and Gomez 1984) using SPSS (ver 16.0). Treatment means were separated as per Duncan’s Multiple range test (DMRT).
3. Results & Discussion
Soil Moisture Content
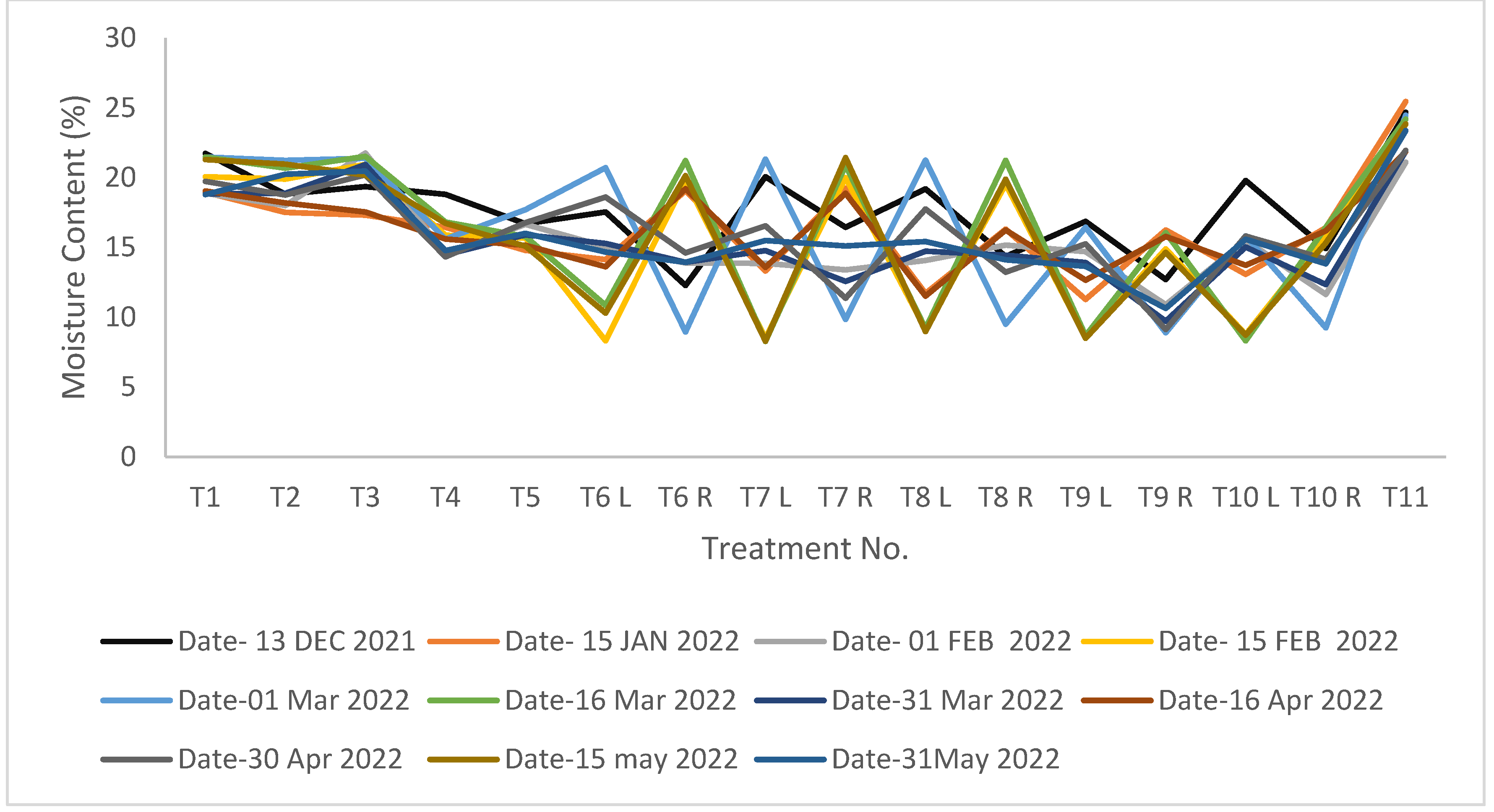
The average value of variation in SMC in different treatments was shown in
Figure 2. A significant difference among different irrigation levels was found. In the case of PRD-treated plants, SMC of both the right and left sides were measured. Initially, SMC was uniform for the whole experimental plot. Later, it showed variation as per the irrigation water applied according to the treatments. In plants treated with DI-80 and PRD-80, the SMC was close to that of control plants. So optimum SMC can be maintained by adopting DI-80 and PRD-80. Similar results were concluded by Kang et al., (2003); Shahnazari et al., (2007); Hutton and Loveys (2011).
Crop Coefficient
Crop coefficient was found to be higher during the crop development stage and at the maturity stage due to an increase in leaves, and good canopy cover. The amount of water applied through irrigation was also high during these two phases due to high climatic demand. The Kc values used are 0.35 at initial stage, 0.6 at development stage, 0.8 at the maturity stage and 0.55 at the harvesting stage due to the falling of leaves and less amount of irrigation required during this period (Bhantana and Lazarovitch 2010; Gorantiwar et al.,2011; Meshram et al.,2019).
Irrigation Water Applied
The total irrigation water applied in control plants was 2331.3 m3ha-1. Similarly, 1865.0 and 1398.7 m3ha-1 water was applied to the plants treated with 80% of Epan (DI-80, PRD-80) and 60% of Epan (DI-60, PRD-60), respectively. Water applied per tree in control plants was 3147.20 litres while water applied per tree under 80% of Epan (DI-80, PRD-80) & 60% Epan (DI-60, PRD-60) were 2517.7 and 1888.3 litres, respectively.
Physiological & Biochemical Parameters
Relative Water Content
RWC is a measure of water deficit in plants, and it changes according to environmental conditions (Rostami and Zahra 2016). However, it was reported that deficit irrigation strategies coupled with PGRs were found to be effective in maintaining water in plant leaves as a significant increase in the RWC value was observed in treated plants (75.18% in DI-80+SA+NAA & 74.06% in DI-80+SA) in comparison to the same irrigation level (70.90% in DI-80). It may be associated with the closing of stomata which prevented water loss through transpiration (Lee 1998). A decreasing trend was observed in the RWC value from flower bud initiation to the fruit enlargement stage due to an increase in environmental temperature and more transpiration (
Figure 3). The results obtained in RWC (%) values are in accordance with Abid et al., 2018; Sakya et al., 2018; Ju et al.,2018.
Chlorophyll Content
Chlorophyll content in the leaf is directly linked with the photosynthesis rate. Deficit irrigation with 80% ET & 60% ET decreased the chlorophyll content as compared to control plants (
Figure 3). Water stress causes hindrance in the phosphorylation process and decreases the chlorophyll content (Hura et al., 2007). However, a significant increase in chlorophyll content was observed by the application of PGRs like SA & NAA which decreased the destruction of chlorophyll pigments in response to stress (Tohma and Estiken 2011).
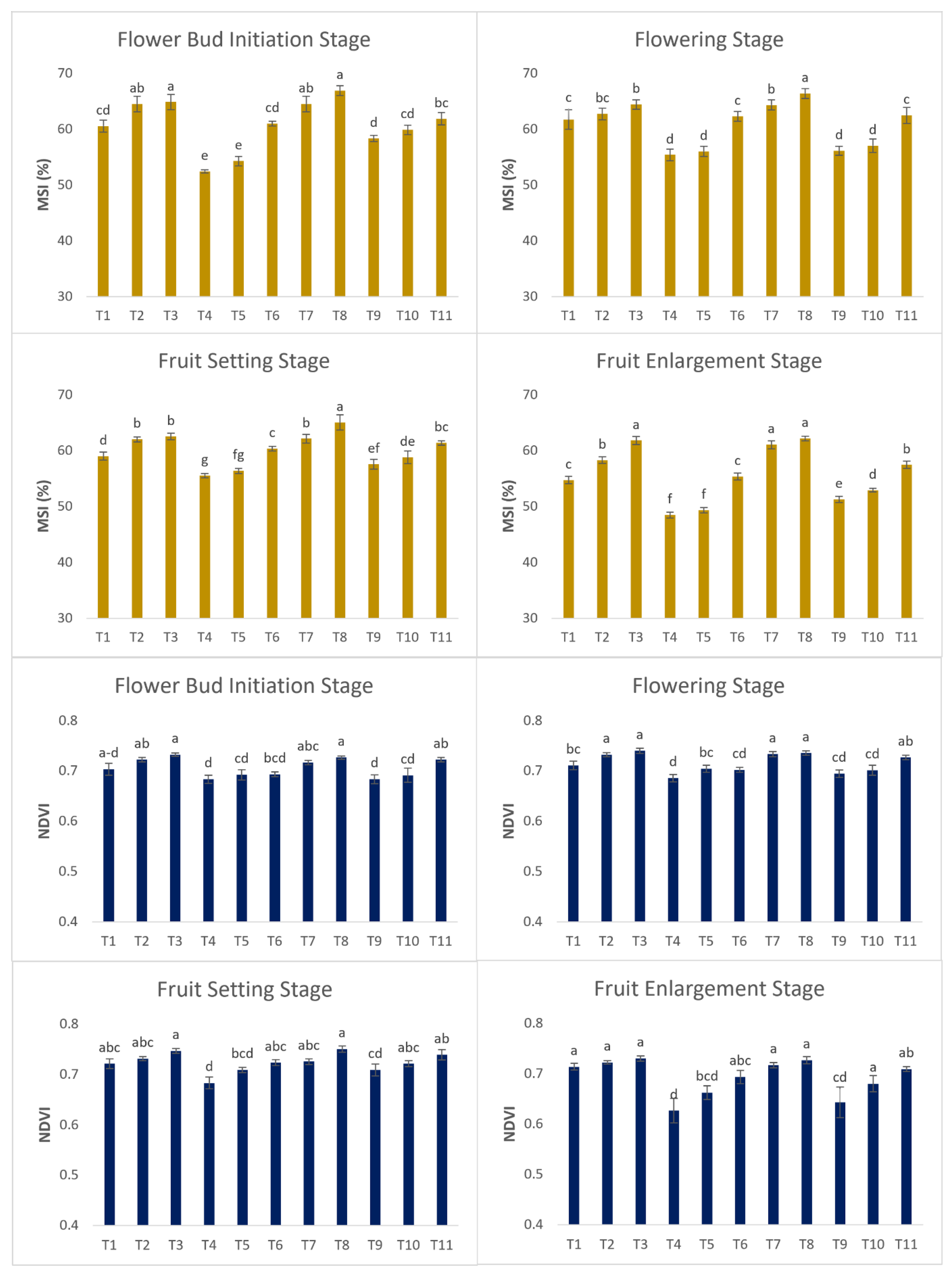
Membrane Stability Index
The ability of the cell membrane to survive in response to stress can be studied by measuring MSI. Decreasing irrigation level decreased the MSI values in plants supplied with 60% irrigation as compared to control plants in the flower bud initiation stage. In all the treatments, MSI values decreased after the flowering stage due to an increase in atmospheric temperature (
Figure 4). Growing the plants in water-deficit conditions induces oxidative stress which produces ROS. An increase in ROS oxidizes the lipid membrane and increases the permeability of the membrane which leads to leakage in tissue, which is the reason for lower MSI in plants treated with DI. SA increased the MSI value in different treatments and protected the tissue from leakage by increasing the production of antioxidants which in turn protect the cell membrane from ROS (Samea-Andabjadid et al., 2018; Hayat et al., 2010; Youssef et al., 2017).
Normalized Difference Vegetation Index
Plants treated with DI-80 & PRD-80 in combination with SA+NAA showed significantly higher NDVI values as compared to other treatments in different phenological stages (
Figure 4). An increase in chlorophyll content, number of leaves, and better nitrogen utilization due to the application of PGRs helped the plants to achieve greenness in various growth stages. A lower NDVI value was observed in plants treated with 60% irrigation because of the destruction of chlorophyll and the decrease in the number of leaves.
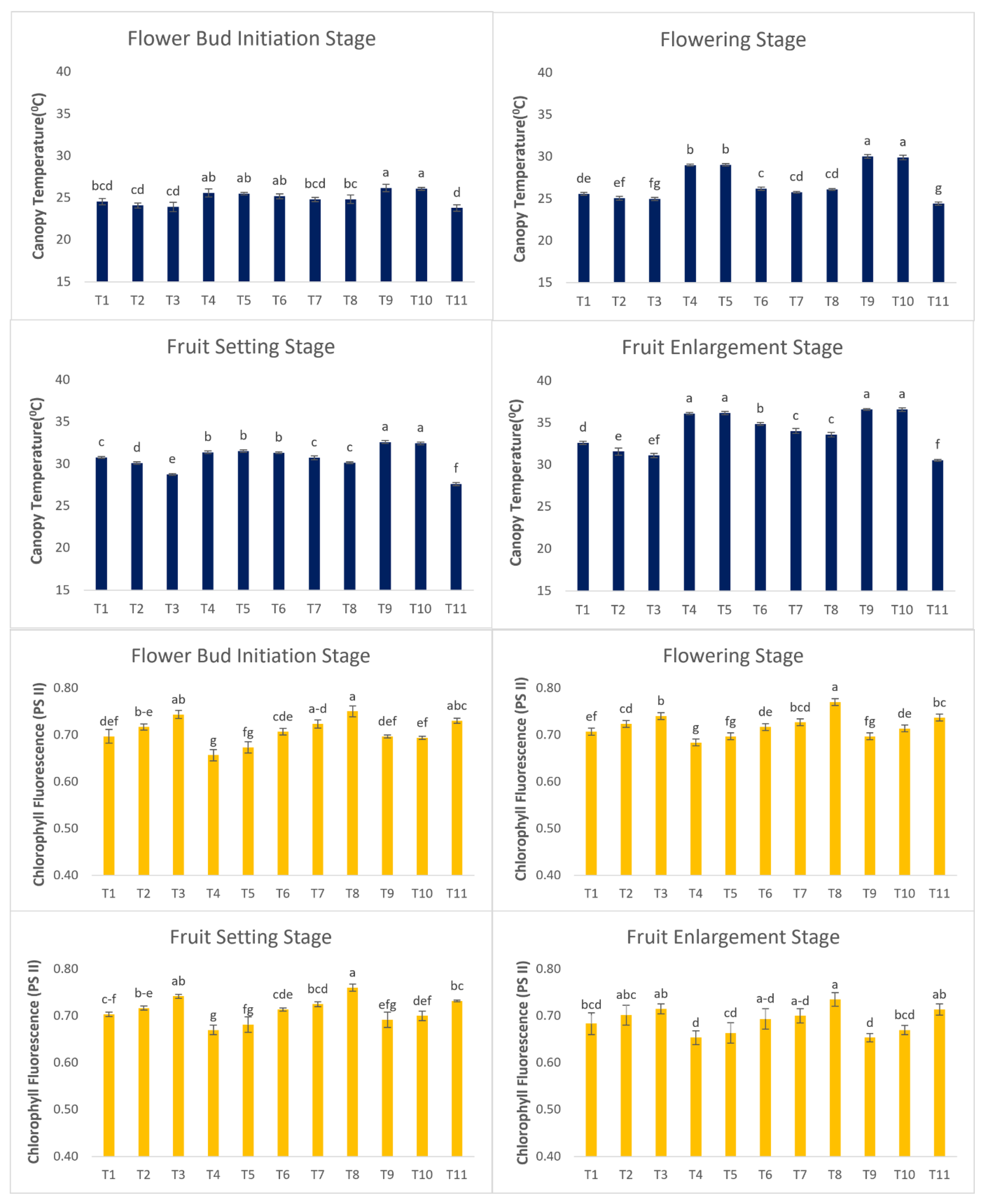
Canopy Temperature
It was observed that CT increased from the flower bud initiation stage to the fruit enlargement stage in all the treatments (
Figure 5). Among all the treatments CT was found lower in control plants at 23.78
0C, 24.42
0C, 27.59
0C, and 30.52
0C in flower bud initiation, flowering, fruit setting & fruit enlargement stage due to the opening of the stomatal apparatus which cools the leaf surface area. Higher CT was observed in plants treated with a 60% irrigation level through PRD techniques in different growth stages. Reduction in water supply and application of SA caused the plants to close the stomata in response to water stress (Wakchaure et al., 2020). Closing of the stomatal apparatus reduced the transpiration rate which leads to an increase in leaf CT. DI-80+SA+NAA was found to be keeping the CT on par with control plants.
Chlorophyll Fluorescence
The highest CF value was observed in plants treated with PRD-80+SA+NAA and the values were observed as 0.75, 0.77, 0.76, and 0.74 in flower bud initiation, flowering, fruit setting & fruit enlargement stage, respectively. The positive effect of SA & NAA was observed in improving fluorescence values in different treatments (
Figure 5). In general water deficit, increase in temperature, and nutrient deficiency decrease QY
max (F
v/F
m) value in plants. Plants with 100% ET showed higher chlorophyll fluorescence values. The reduction in QY
max in water-stressed plants is due to the destruction of the photosystem and reduction in the electron transport system (Stirbet et al., 2018). However, in plants, treated with the same irrigation level say 80% ET, (QY
max) was found to be higher in those treated with SA. As SA increased the efficiency of the water-splitting complex at the doner side of photosystem-II which is attributed to the improvement of the photosynthetic electron transport system (Ghassemi-Golezani et al., 2020). No significant difference was observed in PRD-treated plants and DI-treated plants.
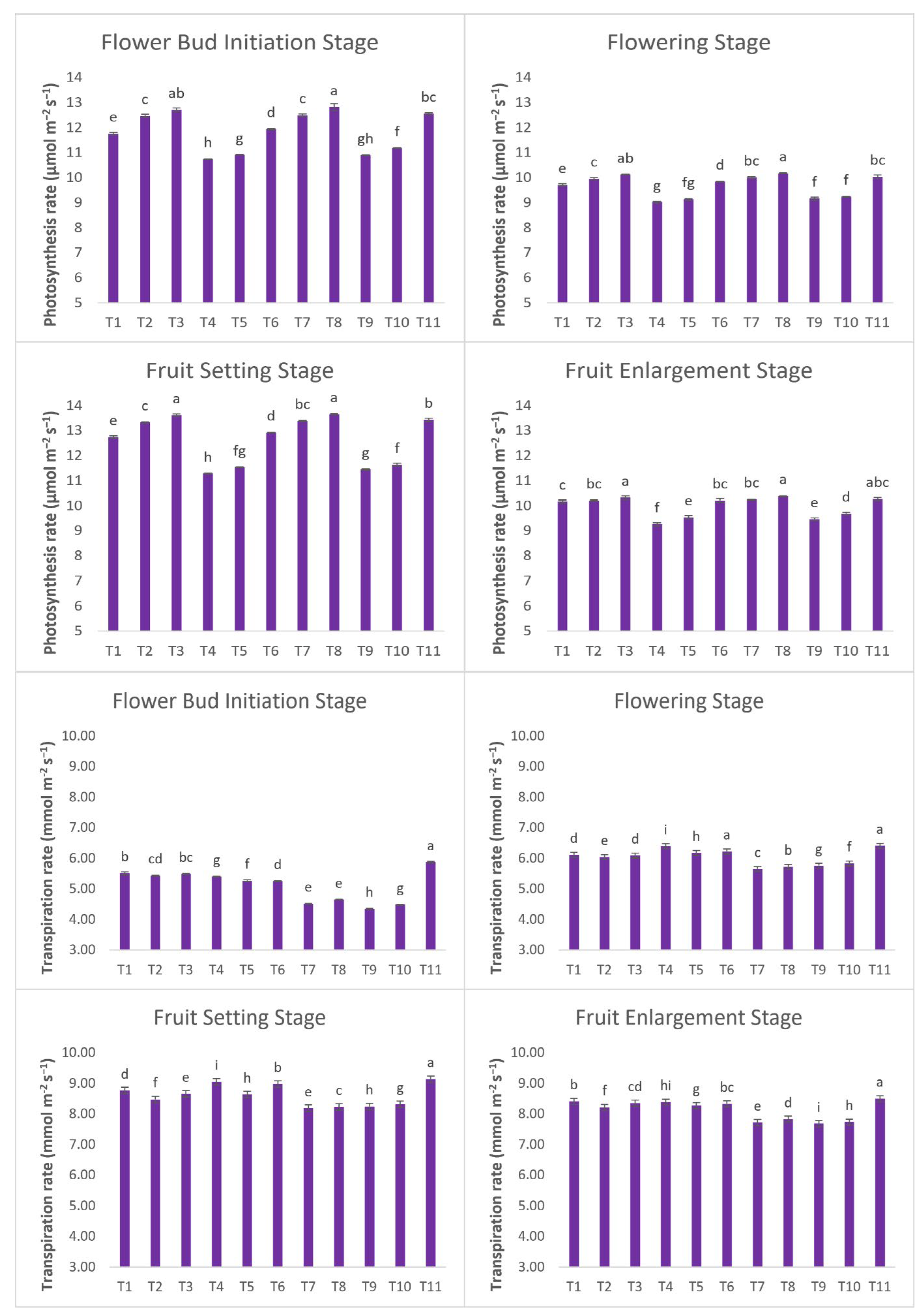
Gas Exchange Parameters
During the flower bud initiation stage, A
n was higher in plants treated with PRD-80+SA+NAA (12.82 μmol m
−2 s
−1), and the value of A
n decreased from the flower bud initiation stage to the flowering stage (
Figure 6). At the flowering stage, higher A
n was found in PRD-80 +SA+NAA (10.18 μmol m
−2 s
−1) which was significantly higher as compared to the control plants (10.03 μmol m
−2 s
−1). However, no significant difference was found between DI-80+SA & PRD-80 + SA and they were found to be at par with the control plants in the flowering stage. DI decreased the A
n due to a reduction in CO
2 assimilation as stomata closed in response to water stress (Parvizi et al., 2016). However, SA & NAA were found to increase the A
n in flowering and fruit setting stage in DI and PRD treated plants. SA increases the fresh weight of leaves along with it also increases chlorophyll, carotenoid pigments, and sugar concentration in leaves which enhances the photosynthesis rate (Zafar et al., 2021). A significantly higher transpiration rate was found in the control plants in all the phenological stages 5.873, 6.413, 9.14, and 8.497 mmol m
-2 s
−1 respectively. At the flowering stage, PRD-80 (6.39 mmol m
-2 s
−1) was found to be on par with the control plants (6.413 mmol m
-2 s
−1). The transpiration rate increased from the flower bud initiation stage to the fruit setting stage and thereafter it decreased in the fruit enlargement stage (
Figure 6). Water stress decreased the transpiration rate due to the closing of stomata. A lower transpiration rate was found in PRD-treated plants due to the supply of irrigation on one side of the plant while the other part remained dry. Furthermore, in plants treated with SA transpiration rate decreased than untreated ones due to the further closing of stomata in response to water deficit conditions. Root drying signal from root to shoot in PRD-treated plants caused a reduction in stomatal conductance (Stoll et al., 2000; Kang et al., 2003). The effect of SA was observed in reducing water loss through stomata. Gas exchange parameters decreased from the flower bud initiation stage to the harvesting stage. Similar results were also reported by Hussain et al., (2021) in pomegranate (cv. Bhagwa).
Proline Content of Leaves
A significant difference was found in the variation of proline content in leaves in different treatments
Table 4. Proline content in the leaf increased with increasing stress. The highest proline content (2.05 µg g
-1 f.w.) was recorded in plants treated with PRD-60+SA+NAA followed by plants treated with PRD-60+SA (2.01 µg g
-1 f.w.). However, the lowest proline was found in control plants with an average value of 1.01 µg g
-1 fw. Decreasing irrigation levels increased the proline concentration of levels. When a plant undergoes stress; the concentration of one of the ꭤ-amino acids, proline increases which acts as an osmolyte and acts as a defense system against stress and this is the reason for the high proline content in PRD-60+SA (2.01 µg g
-1 fw) & PRD-60+SA+NAA (2.05 µg g
-1 fw). In the stress condition ROS & H
2O
2 content increase. Proline helps in capturing those ROS & H
2O
2 by producing NADP
+ which in turn accepts those ROS. NADP
+ is produced from NADPH in the proline biosynthesis process. Thus, apart from the scavenging of ROS, proline also helps in maintaining cellular redox balance. Further exogenous application of SA triggered the proline biosynthesis pathway and thus NADPH/ NADP
+ ratio in the plant is maintained which is attributed to an increase in proline content in plants treated with SA (Sorkheh et al., 2012; Abdelaal et al.,2020; La et al.,2019).
FRAP Enzymatic Assay
Frap enzymatic assay compares the antioxidants based on the ability of the oxidants to reduce Fe
+3 present in the ferric-tripyridyl-triazine to two electron-deficient compounds (Fe
+2) ferrous-tripyridyl-triazine. The former is a colorless compound and its color changes to blue color once it interacts with antioxidants in the sample. Higher FRAP enzymatic activity in PRD-60+SA & PRD-60+SA+NAA shows higher antioxidant activity in those plants. It was observed that deficit irrigation increased FRAP enzymatic activity (Nangare et al., 2016; Kumar et al., 2015). However, the positive effect of SA on increasing antioxidant activity was also reported in treated plants (Wakchaure et al., 2020).
Table 4 shows the variation of FRAP in different treatments. A significantly higher & lower value of FRAP was observed in the fruit juice of plants treated with DI-60+SA+NAA (39.15 mg AAE/100 ml) & control plants (15.44 mg AAE/ 100 ml), respectively. However, equal significance was observed in plants treated with DI-60 and all the PRD-treated plants.
DPPH Radical Scavenging Activity
The variation of DPPH radical scavenging activity in different treatments is depicted in
Table 4. Among all the treatments, DPPH radical scavenging activity was found to be more in plants treated with PRD-60+SA (75.23%). A significant difference was observed in DPPH activities among plants treated with DI-80 (41.98%), PRD-80 (58.72%), & control plants (28.79%). Significant high non-enzymatic antioxidants like FRAP & DPPH are found in plants treated with high water deficit conditions. DPPH is a free radical & is used as a determinant of the scavenging capacity of antioxidants towards it. By receiving a hydrogen atom from the antioxidants, the odd electron present in the nitrogen atom of DPPH is neutralized quickly. Which is related to more antioxidant activity in plants treated with 60% ETc. However, SA also increased enzymatic activity as it enhances the biosynthesis of phenolic compounds by triggering the 1
st enzyme of the biosynthetic pathway, this, in turn, increased the scavenging activity in SA-treated plants (Junmatong et al., 2015; Shamili et al., 2021; Yang et al.,2022).
Total Phenols
Total phenol content in different treatments is shown in
Table 4. The total phenols were found to be significantly more in plants treated with 60% irrigation level irrespective of DI & PRD and the total phenol was found ranging between 85.34-205.50 mg GAE/100 ml. However, significantly lower phenolic content among all the treatments was observed in control plants (85.34 mg GAE/100ml).
Total Flavonoids
The total flavonoid content in different treatments is shown in
Table 4. Flavonoids followed a similar trend to that of phenols and the highest flavonoid content was found in plants treated with PRD-60+SA+NAA (46.3 mg CE/100 ml). The flavonoid content in different treatments ranged between 18.3-46.3 mg CE/100 ml. statistically, no significant difference was observed between plants treated with DI-80, PRD-80 & control plants.Total phenol and flavonoid content increased with the reduction of water supply. To protect the plant cells from oxidative stress, the synthesis of these non-enzymatic activities increased in plants. However, the effect of SA in the accumulation of those antioxidants was also observed in treated plants, which helps the plants to resist different abiotic and biotic stress.
Total Fruit Yield
The total fruit yield as affected by different treatments is shown in
Table 6. The total fruit yield per plant was found to be more in plants treated with PRD-80+SA+NAA. Both the treatments PRD-80+SA+NAA (16.86 kg plant
-1) & DI+80+SA+NAA (15.85 plant
-1) were found to be at par. The treatments like DI-80+SA (13.93 kg plant
-1) & PRD-80+SA (14.23 kg plant
-1) were found to be at par with control plants (12.01 kg plant
-1). However, as only irrigation level was concerned, plants with a 60% irrigation level produced a lower yield per plant (8.61 kg plant
-1 in PRD-60 +SA, 8.07 kg plant
-1 in DI-60+SA+NAA & 6.7 kg plant
-1 in DI-60+SA) as compared to control plants. No significant difference was observed between plants treated with DI-80 (11.33 kg plant
-1) & PRD-60+SA+NAA (9.42 kg plant
-1). Treating the plants with PRD changes modifies morphological characteristics like smaller guard cells and a decrease in stomatal density. Under a lower transpiration rate, better utilization of nutrients and water occurs, which has a positive impact on the net photosynthesis rate. Remobilization of photosynthates from vegetative tissues to the fruits consequently increased yield and quality (Jovanovic & Stikic 2018; Garcia-Pastor et al., 2020).
Correlation Analysis
The correlation matrix developed for various physical and quality parameters was was expressed in
Table 5. A positive correlation was obtained between yield and different physiological parameters like RWC, MSI, NDVI, PS-II, chlorophyll content, and photosynthesis rate with correlation coefficients of 0.97, 0.90, 0.95, 0.95, 0.91 and 0.96, respectively. However, all these parameters were found to be negatively correlated with canopy temperature. A positive correlation coefficient of 0.84 was obtained between canopy temperature and proline content.
Water Productivity
Water productivity (WP) among treatments was found to be significant (
Table 6). Both the DI & PRD treated plants with 80% irrigation levels coupled with SA+NAA significantly increased the WUE (6.30 & 6.69 kg m
-3, respectively). WP was higher in plants under DI conditions, which increased further with applications of PGRs. PRD-treated plants with a 60% irrigation level coupled with PGRs (4.56-4.99 kg m
-3) were found to have higher WP than control plants (3.82 kg m
-3). However, plants treated with DI-60+SA (3.55 kg m
-3) and DI-60+SA+NAA (4.27 kg m
-3) were at par with control plants. The reason for high WP in DI-80+SA+NAA & PRD-80+SA+NAA was that the application of NAA increased the number of fruits and fruit setting percentage (Kishor
et al., 2016; Anawal
et al., 2016) and further application of SA helped the plants to minimize water loss (Ju
et al., 2018). It was also observed that DI-80 & PRD-80 coupled with SA can be adapted in the water-scarce zone for achieving high water productivity than control plants. As, no such significant difference was observed in plants treated with DI-60+SA, DI-60+SA+NAA, PRD-60+SA, & PRD-60+SA+NAA in comparison to control plants; so, plants can be grown with 60% ETc with the application of PGRs in water scarcity areas.
Table 6.
Effect of deficit irrigation & PGRs on yield attributes and water productivity.
Table 6.
Effect of deficit irrigation & PGRs on yield attributes and water productivity.
| Treatment details |
Water applied
(Lplant-1) |
Yield
(kg ha-1) |
Water applied
(m3ha-1) |
WP
(kgm-3) |
| DI-80 |
2517.8 |
8.39 |
1865.0 |
4.50 d-g
|
| DI-80 + SA |
2517.8 |
10.31 |
1865.0 |
5.53 bcd
|
| DI-80+SA+NAA |
2517.8 |
11.74 |
1865.0 |
6.30 ab
|
| DI-60 + SA |
1888.3 |
4.96 |
1398.8 |
3.55 g
|
| DI-60+SA+NAA |
1888.3 |
5.97 |
1398.8 |
4.27 efg
|
| PRD-80 |
2517.8 |
9.11 |
1865.0 |
4.89 c-f
|
| PRD-80 + SA |
2517.8 |
10.54 |
1865.0 |
5.65 abc
|
| PRD-80 +SA+NAA |
2517.8 |
12.48 |
1865.0 |
6.70 a
|
| PRD-60 + SA |
1888.3 |
6.37 |
1398.8 |
4.56 c-g
|
| PRD-60 +SA+NAA |
1888.3 |
6.97 |
1398.8 |
4.99 cde
|
| CONTROL |
3147.2 |
8.89 |
2331.3 |
3.82 fg
|
| CD (P=0.05) |
|
|
|
1.09 |
Conclusions
Due to low organic matter content and poor water holding capacity, shallow basaltic soil of the Deccan plateau is not suitable for crop production. However, in such water-scarce environment, DI along with potential PGRs can aid in growing perennial fruit crops like pomegranate. Physiological parameters like RWC in leaves, MSI, NDVI, and CF were affected in deficit irrigation. CT increased in plants in response to DI conditions. The role of SA was observed in lowering the CT and can be used as a potential PGR in alleviating water stress by increasing photosynthesis rate, MSI, and lowering CT by 5.95 %, 8.18 %, and 2.17%, respectively. Further, antioxidant activity was higher in SA treated plants. Irrigating the plants with 80% of ETc in conjunction with foliar spray of SA+NAA increased fruit yield by 31.9% and 40.3%, and water productivity (WP) by 64.9% and 75.3% in DI and PRD irrigation strategies, respectively for pomegranate grown in shallow basaltic soils, particularly in water-scarce environments or limited water conditions.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request
References
- Abdelaal KAA, Attia KA, Alamery SF, El-Afry MM, Ghazy AI, Tantawy DS, Al-Doss AA, El-Shawy E-SE, M. Abu-Elsaoud A, Hafez YM., (2020). Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability. 12(5),1736. [CrossRef]
- Aseri, G.K., Jain, N., Panwar, J., Rao, A.V., Meghwal, P.R., (2008). Biofertilizers improve plant growth, fruit yield, nutrition, metabolism, and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Scientia Horticulturae.117 (2), 130–135. [CrossRef]
- Bates, L. S., Waldren, R. D. and Teare, I. D., (1973). Rapid determination of free proline for water stress studies. Plant and Soil. 39: 205-207. [CrossRef]
- Beis, A. and A. Patakas., (2015). Differential physiological and biochemical responses to drought in grapevines subjected to partial root drying and deficit irrigation. European Journal of Agronomy. 62: 90-97. [CrossRef]
- Benzie, I.F.F., Strain, J.J., (1996). Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Analytical Biochemistry. 239, 70–76. [CrossRef]
- Bhantana, P., Lazarovitch, N., (2010). Evapotranspiration, crop coefficient and growth of two young pomegranate varieties under salt stress. Agricultural Water Management. 97, 715–722. [CrossRef]
- Bharathi, L.K., Singh, H.S., Shivashankar, S., Ganeshamurthy, A.N., Suresh Kumar, P., (2014). Assay of nutritional composition and antioxidant activity of three dioecious Momordica species of Southeast Asia. Proceedings of the National Academy of Sciences India. Section B, Biological Sciences. 84 (1), 31–36. [CrossRef]
- Blumenfeld A, Shaya F, Hillel R., (2000). Cultivation of pomegranate. Options Mediterraneennes Ser A 42:143–147.
- García-Pastor ME, Zapata PJ,Castillo S, Martínez-Romero D,Guillén F, Valero D and Serrano M (2020).The Effects of Salicylic Acid and Its Derivatives on Increasing Pomegranate Fruit Quality and Bioactive Compounds at Harvest and During Storage. Frontiers in Plant Sciences 11:668. [CrossRef]
- Ghassemi-Golezani, K., Hosseinzadeh-Mahootchi, A., Farhangi-Abriz, S., (2020). Chlorophyll a fluorescence of safflower affected by salt stress and hormonal treatments. SN Applied Sciences. 2, 1306. [CrossRef]
- Ghosh S.N., Bera B., Roy S., Kundu A., (2009). Effect of plant growth regulators in yield and fruit quality in pomegranate cv. Ruby. Journal of Horticultural Science. Vol. 4 (2): 158-160.
- Gomez, K.A., Gomez, A.A., (1984). Statistical Procedures for Agricultural Research. John Wiley and Sons, New York. https://pdf.usaid.gov/pdf_docs/PNAAR208.pdf.
- Gorantiwar, S.D., Meshram, D.T., Mittal, H.K., (2011). Water requirement of pomegranate (Punica granatum L.) for Ahmednagar district of Maharashtra State, India. Journal of Agrometeorology. 13 (2), 123–127. [CrossRef]
- Hayat, S., Hasan, S.A., Hayat, Q., Irfan, M., Ahmad, A., (2010). Effect of salicylic acid on net photosynthetic rate, chlorophyll fluorescence, and antioxidant enzymes in Vigna radiata plants exposed to temperature and salinity stresses. Plant Stress. 4,62-71.
- Hussain, S.F., Murthy, B.N.S., Reddy, M.L.N., Upreti, K.K., Satisha, J., Laxman, R.H., Srinivasulu, B., Reddy, P.S.S. (2021). Effect of different chemical and seasons on gas exchange parameters, phytohormones, and chlorophyll content in tissue culture plants of Pomegranate (Punica granatum L.) cv. Bhagwa. Environment Conservation Journal. 22 (1&2): 127-135. [CrossRef]
- Hutton, R.J., Loveys, B.R., (2011). A partial root zone drying irrigation strategy for citrus effects on water use efficiency and fruit characteristics. Agricultural Water Management. 98, 1485–1496. [CrossRef]
- Joon-Sang, Lee. (1998). The mechanism of stomatal closing by salicylic acid in Commelina communis L. Journal of Plant Biology. 41, 97–102. [CrossRef]
- Jovanovic Z and Stikic R, (2018). Partial Root-Zone Drying Technique: From Water Saving to the Improvement of a Fruit Quality. Frontiers in Sustainable Food Systems. 1:3. [CrossRef]
- Ju, Yan-lun; Yue, Xiao-feng; Zhao, Xian-fang; Zhao, Hui; Fang, Yu-lin, (2018). Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiology and Biochemistry. 130, 501–510. [CrossRef]
- Junmatong, C., Faiyue, B., Rotarayanont, S., Uthaibutra, J., Boonyakiat, D., & Saengnil, K., (2015). Cold storage in salicylic acid increases enzymatic and non-enzymatic antioxidants of Nam Dok Mai No. 4 mango fruit. Science Asia. 41, 12-21. [CrossRef]
- Kandylis, P., & Kokkinomagoulos, E., (2020). Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods. 9(2), 122. [CrossRef]
- Kang SZ, Zhang JH., (2004). Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. Journal of Experimental Botany. 55:2437–2446. [CrossRef]
- Kang, S.Z., Hu, X., Jerie, P., Zhang, J.H., (2003). The effects of partial root zone dryingon root, trunk sap flow and water balance in an irrigated pear (Pyrus communisL.) orchard. Journal of Hydrology. 280, 192–206. [CrossRef]
- Kishor, S., Maji, S., Govind, Y.R., Meena, K.R., & Kumar, A., (2016). Influence of plant bio- regulators and chemicals on yield and fruit quality of young pomegranate (Punica granatum L.) cv. Bhagwa. Environment and Ecology, 34, 2566–2570.
- Kumar PS, Singh Y, Nangare DD, Bhagat K, Kumar M, Taware PB., (2015). Influence of growth stage specific water stress on the yield, physiochemical quality and functional characteristics of tomato grown in shallow basaltic soils. Scientia Horticulturae. 197C;261-71. [CrossRef]
- La, V.H., Lee, BR., Zhang, Q. et al., (2019). Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Horticulture Environment and Biotechnology. 60, 31–40. [CrossRef]
- Li, X., Kang, S., Zhang, X., Li, F., Lu, H., (2018). Deficit irrigation provokes more pronounced responses of maize photosynthesis and water productivity to elevated CO2. Agricultural Water Management. 195, 71–83. [CrossRef]
- Meshram D T., Gorantiwar S D., Singh NV., Babu K D., (2019a). Response of micro-irrigation systems on growth, yield and WUE of Pomegranate (Punica granatum L.) in semi-arid regions of India. Scientia Horticulturae. 246: 686–692. [CrossRef]
- Muhammad Abid, Ali Shafaqat, Qi Lei Kang, Zahoor Rizwan, Tian Zhongwei, Jiang Dong, Snider John L., Dai Tingbo., (2018). Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Scientific Reports. 8:4615. [CrossRef]
- Nangare D.D., Suresh Kumar P., Singh Y., Minhas P.S., (2016). Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agricultural Water Management. 171:73-79. [CrossRef]
- Noitsakis, B., Chouzouri, A., Papa, L., & Patakas, A., (2016). Pomegranate Physiological responses to Partial Root Drying Under Field Conditions. Emirates Journal of Food and Agriculture. 28, 410-414. [CrossRef]
- P. Rodríguez, C.D. Mellisho, W. Conejero, Z.N. Cruz, M.F. Ortuño, A. Galindo, A. Torrecillas, (2012). Plant water relations of leaves of pomegranate trees under different irrigation conditions. Environmental and Experimental Botany. 77, 19-24. [CrossRef]
- Parvizi Hossein, Sepaskhah Ali Reza, Ahmadi Seyed Hamid., (2016). Physiological and growth responses of pomegranate tree (Punica granatum (L.) cv. Rabab) under partial root zone drying and deficit irrigation regimes. Agricultural Water Management. 163, 146–158. [CrossRef]
- Rostami, Majid & Movahedi, Zahra., (2016). Evaluating the effects of Naphthalene acetic acid (NAA) on morpho-physiological traits of valerian (Valeriana officinalis L.) in aeroponic system. Iranian Journal of Plant Physiology. 6. 1751-1759.
- Sairam, R.K., (1994). Effect of moisture stress on physiological activities two contrasting wheat genotypes. Indian Journal of Experimental Biology. 32:593-594.
- Sakya, A.T.; Sulistyaningsih, E.; Indradewa, D.; Purwanto, B.H. Physiological characters, and tomato yield under drought stress. In Proceedings of the International Conference on Climate Change (ICCC 2018), Solo City, Indonesia, 27–28 November 2018; IOP Publishing: Bristol, UK, 2018; Volume 200, p. 012043. [CrossRef]
- Samea-Andabjadid S, Ghassemi-Golezani K, Nasrollahzadeh S, Najafi N., (2018). Exogenous salicylic acid and cytokinin alter sugar accumulation, antioxidants, and membrane stability of faba bean. Acta Biologica Hungarica. 69(1):86-96. [CrossRef]
- Shahnazari, A., Liu, F., Andersen, M. N., Jacobsen, S-E., & Jensen, C. R. (2007). Effects of partial root-zone drying on yield, tuber size and water use efficiency in potato under field conditions. Field Crops Research. 100(1), 117-124. [CrossRef]
- Shamili M, Ghalati RE, Samari F., (2021). The impact of foliar salicylic acid in salt-exposed guava (Psidium Guajava L.) seedlings. International Journal of Fruit Science. 21:323–333. [CrossRef]
- Sorkheh, K., Shiran, B., Khodambashi, M., (2012). Exogenous proline alleviates the effects of H2O2-induced oxidative stress in wild almond species. Russian Journal of Plant Physiology. 59, 788–798. [CrossRef]
- Stirbet, A., Lazár, D., Kromdijk, J., (2018). Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses?. Photosynthetica. 56, 86-104. [CrossRef]
- Stoll, M., Loveys, B., Dry, P., (2000). Hormonal changes induced by partial root zone drying of irrigated grapevine. Journal of Experimental Botany. 51, 1627–1634. [CrossRef]
- Stover, E., Mercure, E.W., 2007. The Pomegranate: A new look at the fruit of paradise. Hort Science. 1088–1092. [CrossRef]
- Tohma, O. and Esitken, A., (2011). Response of salt stressed strawberry plants to foliar salicylic acid pre-treatments. Journal of Plant Nutrition. 34 (4), 590-599. [CrossRef]
- Vidya V. Anawal, Narayanaswamy, P. and Suresh. D. Ekabote., (2016). Effects of plant growth regulators on fruit set and yield of pomegranate cv. Bhagwa. International Journal of Current Research. 8, (04), 29008-29010. [CrossRef]
- Wakchaure GC, Minhas PS, Meena KK, Kumar S, Rane J., (2020). Effect of plant growth regulators and deficit irrigation on canopy traits, yield, water productivity and fruit quality of eggplant (Solanum melongena L.) grown in the water scarce environment. Journal of Environmental Management. 15; 262:110320. [CrossRef]
- Yang, Z.; Guan, Y.; Ji, H., (2022). Enhanced Antioxidant Activity of Fresh Fruits through Salicylic Acid/b-CD Hydroalcoholic Gels. Gels. 8, 61. [CrossRef]
- Youssef, S., Abu El-Azm, N., Abd Elhady, S., (2017). Frequent Foliar Sprayings of Salicylic Acid with Elevated Concentrations Enhance Growth, Yield and Fruit Quality of Strawberry (Fragaria x ananassa Duch. cv. Festival) Plants. Egyptian Journal of Horticulture. 44(1), 61-74. [CrossRef]
- Zafar, Z.; Rasheed, F.; Atif,R.M.; Javed, M.A.; Maqsood, M.; Gailing, O., (2021). Foliar Application of Salicylic Acid Improves Water Stress Tolerance in Conocarpus erectus L. and Populus deltoides L. Saplings: Evidence from Morphological, Physiological, and Biochemical Changes. Plants. 10, 1242. [CrossRef]
- Romero, P., García García, J., Fernández-Fernández, J. I., Muñoz, R. G., del Amor Saavedra, F., and Martínez-Cutillas, A. (2016). Improving berry and wine quality attributes and vineyard economic efficiency by long-term deficit irrigation practices under semiarid conditions. Sci. Hortic. 203, 69–85. [CrossRef]
- Selahvarzi, Y., Zamani, Z., Fatahi, R., & Talaei, A. R. (2017). Effect of deficit irrigation on flowering and fruit properties of pomegranate (Punica granatum cv. Shahvar). Agricultural Water Management, 192, 189-197. [CrossRef]
- Du, S., Kang, S., Li, F., and Du, T. (2017). Water use efficiency is improved by alternate partial root-zone irrigation of apple in arid northwest China. Agric. Water Manage. 179, 184–192. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).