Submitted:
18 April 2024
Posted:
18 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Fungal Cultivation and Polysaccharide Extraction Process
2.3. Plant Material and Seed Priming
2.4. Examination of seed quality and initial growth of pea plants under optimal and drought conditions
2.4.1. Seed Germination Assessment
2.4.2. Determination of the Shoot and Root Length and the Root/Shoot Ratio
2.4.3. Determination of the Fresh and Dry Shoot and Root Biomass Accumulation
2.4.4. Determination of Shoot Elongation Rate (SER) and Root Elongation Rate (RER)
2.4.5. Determination of Seed Vigor Index
2.4.6. Electrolyte Permeability Assay
2.4.7. Determination of Membrane Stability Index
2.4.8. Determination of Relative Water Content
2.4.9. Determination of Pea Stress Tolerance Indices
2.5. Statistical Analysis
3. Results
3.1. Effect of Seed Bio-Priming Treatments on Seed Germination and Initial Seedling Growth of Pea
3.2. Correlation analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesk, C.; Anderson, W.B.; Rigden, A.J.; Coast, O.; Jägermeyr, J.; McDermid, S.P.; Davis, K.F.; Konar, M. Compound heat and moisture extreme impacts on global crop yields under climate change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Verma, P.; Hiremani, N.S.; Gawande, S.P.; Sain, S.K.; Nagrale, D.T. , Narkhedkar, N.G., Prasad, Y.G. Modulation of plant growth and antioxidative defense system through endophyte biopriming in cotton (Gossypium spp.) and non-host crops. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Tamindžić, G.; Đorđević, V.; Tintor, B.; Milošević, D.; Ignjatov, M.; Nikolić, Z. Bio-priming of soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to improve seed germination and the initial seedling growth. Plants 2022, 11, 1927. [Google Scholar] [CrossRef]
- Metwally, R.A.; Abdelhameed, R.E.; Soliman, S.A.; Al-Badwy, A.H. Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol. 2022, 22, 108. [Google Scholar] [CrossRef]
- Deshmukh, A.J.; Jaiman, R.S.; Bambharolia, R.P.; Patil, V.A. Seed biopriming-a review. Int. J. Econ. Plants 2020, 7, 038–043. [Google Scholar] [CrossRef]
- Rasskazova, I.; Kirse-Ozolina, A. Field pea Pisum sativum L. as a perspective ingredient for vegan foods: A review. Food Sci. 2020, 35, 125–131. [Google Scholar]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Tulbek, M.C.; Wang, Y.L.; Hounjet, M. Pea—A sustainable vegetable protein crop. In: Sustainable Protein Sources, Academic Press, Chapter 9, pp. 143-–162. 2024. [CrossRef]
- Wu, D.T.; Li, W.X.; Wan, J.J.; Hu, Y.C.; Gan, R.Y.; Zou, L. A comprehensive review of pea (Pisum sativum L.): chemical composition, processing, health benefits, and food applications. Food 2023, 12, 2527. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Ţura, D.; Wasser, S.P.; Zmitrovich, I.V. Wood-inhabiting fungi: Applied aspects. In: Fungi: and Management Strategies, CRC Press, 2018, Chapter 12, pp. 245–292.
- Mišković, J.; Rašeta, M.; Krsmanović, N.; Karaman, M. Update on mycochemical profile and selected biological activities of genus Schizophyllum Fr. 1815. Microbiol. Res. 2023, 14, 409–429. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Ghani, A.; Lazim, M.I.M.; Khulidin, K.A.; Shahidi, F.; Ismail, A. Schizophyllum commune (Fries) mushroom: A review on its nutritional components, antioxidative and anti-inflammatory properties. Curr. Opin. Food Sci. 2024, 56, 101129. [Google Scholar] [CrossRef]
- Krause, K.; Jung, E.M.; Lindner, J.; Hardiman, I.; Poetschner, J.; Madhavan, S.; Matthäus, C.; Kai, M.; Menezes, R.C.; Popp, J.; Svatoš, A.; Koth, E. Response of the wood-decay fungus Schizophyllum commune to co-occurring microorganisms. PLoS One 2020, 15, e0232145. [Google Scholar] [CrossRef]
- Rangkhawong, P.; Issaranuwat, P.; Gaensakoo, R. Mycelial fermentation in submerged culture of Schizophyllum commune and its properties. SCJMSU 2014, 33, 420–428. [Google Scholar]
- Mišković, J.; Karaman, M.; Rašeta, M.; Krsmanović, N.; Berežni, S.; Jakovljević, D.; Piattoni, F.; Zambonelli, A.; Gargano, M.L.; Venturella, G. Comparison of two Schizophyllum commune strains in production of acetylcholinesterase inhibitors and antioxidants from submerged cultivation. J. Fungi 2021, 7, 115. [Google Scholar] [CrossRef]
- Kumar, A.; Bharti, A.K.; Bezie, Y. Schizophyllum commune: A fungal cell-factory for production of valuable metabolites and enzymes. BioResources 2022, 17, 5420–5436. [Google Scholar] [CrossRef]
- Chen, C.-C.; Nargotra, P.; Kuo, C.-H.; Liu, Y.-C. High-molecular-weight exopolysaccharides production from Tuber borchii cultivated by submerged fermentation. Int. J. Mol.Sci. 2023, 24, 4875. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Cheema, M.A.; Rehman, H. Chilling tolerance in maize hybrid induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008, 194, 161–168. [Google Scholar] [CrossRef]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Attallah, S.Y. Seed priming boost adaptation in pea plants under drought stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef]
- Tamindžić, G.; Červenski, J.; Milošević, D.; Vlajić, S.; Nikolić, Z.; Ignjatov, M. The response of garden pea cultivars to simulated drought. Acta Agric. Serb. 2021, 26, 167–173. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; Seed Science and Technology: Zurich, Switzerland, 2022. [Google Scholar]
- Tamindžić, G.; Ignjatov, M.; Miljaković, D.; Červenski, J.; Milošević, D.; Nikolić, Z.; Vasiljević, S. Seed priming treatments to improve heat stress tolerance of garden pea (Pisum sativum L.). Agriculture 2023, 13, 439. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Murtazova, K.M.S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating seed germination and seedling growth of nano-primed wheat and flax seeds using seven biogenic metal-based nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of some rapeseed (Brassica napus L.) genotypes to different drought stress levels during germination and seedling growth stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 594–594. [Google Scholar]
- Maiti, R.K.; de la Rosa-Ibarra, M.; Sandoval, N.D. Genotypic variability in glossy sorghum lines for resistance to drought, salinity and temperature stress at the seedling stage. J. Plant Physiol. 1994, 143, 241–244. [Google Scholar] [CrossRef]
- Aswathi, K.R.; Kalaji, H.M.; Puthur, J.T. Seed priming of plants aiding in drought stress tolerance and faster recovery: A review. Plant Growth Regul. 2022, 97, 235–253. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants' physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Braccini, A.L.; Ruiz, H.A.; Braccini, M.C.L.; Reis, M.S. Germinaçáo e vigor de sementes de soja sob estresse hidrico induzido por soluções de cloreto de sódio, mannitol e polietilenglicol. Rev. Bras. Sementes 1996, 18, 10–16. [Google Scholar] [CrossRef]
- Pereira, I.C.; Catão, H.; Caixteta, F. Seed physiological quality and seedling growth of pea under water and salt stress. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 95–100. [Google Scholar] [CrossRef]
- Ghezal, N.; Rinez, I.; Sbai, H.; Saad, I.; Farooq, M.; Rinez, A.; Haouala, R. Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. S. Afr. J. Bot. 2016, 105, 240–250. [Google Scholar] [CrossRef]
- Dalal, S.R.; Hussein, M.H.; El-Naggar, N.E.A.; Mostafa, S.I.; Shaaban-Dessuuki, S.A. Characterization of alginate extracted from Sargassum latifolium and its use in Chlorella vulgaris growth promotion and riboflavin drug delivery. Sci. Rep. 2021, 11, 16741. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Dey, R.; Raghuwanshi, R. Exopolysaccharide-producing rhizospheric bacteria enhance yield via promoting wheat (Triticum aestivum L.) growth at early stages. Microbiology 2022, 91, 757–769. [Google Scholar] [CrossRef]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Imran, M.; Kang, S.M.; Pande, A.; Lee, I.J. Biopriming of maize seeds with a novel bacterial strain SH-6 to enhance drought tolerance in South Korea. Plants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Dwivedi, P. Trichoderma-induced promotion of nitrogen use efficiency is mediated by nitric oxide generation leading to improved growth and yield in pea (Pisum sativum L.) plants. J. Plant Growth Regul. 2022, 42, 6397–6412. [Google Scholar] [CrossRef]
- Chandra Nayaka, S.; Niranjana, S.R.; Uday Shankar, A.C.; Niranjan Raj, S.; Reddy, M.S.; Prakash, H.S.; Mortensen, C.N. Seed biopriming with novel strain of Trichoderma harzianum for the control of toxigenic Fusarium verticillioides and fumonisins in maize. Arch. Phytopathol. Pflanzenschutz. 2010, 43, 264–282. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Prathibha, M.D.; Singhal, R.K. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Mašková, T.; Herben, T. Root: shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef]
- Sakya, A.T.; Sulistyaningsih, E.; Indradewa, D.; Purwanto, B.H. Physiological characters and tomato yield under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 200, 012043. [Google Scholar] [CrossRef]
- Almeselmani, M.; Abdullah, F.; Hareri, F.; Naaesan, M.; Ammar, M.A.; ZuherKanbar, O. Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. J. Agric. Sci. 2011, 3, 127. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Moreno-Casasola, P.; Barrera, O. Interaction between algae and seed germination in tropical dune slack species: a facilitation process. Aquat. Bot. 1998, 60, 409–416. [Google Scholar] [CrossRef]
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species.In: Water Stress in Plants, Rahman, I.M.M.; Begum, M.Z.A.; Hasegawa, H. (Eds.), InTechOpen, 2016, 43, 62. [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Van Staden, J. Identification of the cytokinin isopentenyladenine in a strain of Arthronema africanum (Cyanobacteria). J. Phycol. 1999, 35, 89–92. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2015, 166, 171–182. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Plant growth-promoting rhizobacteria (PGPR): A rampart against the adverse effects of drought stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]

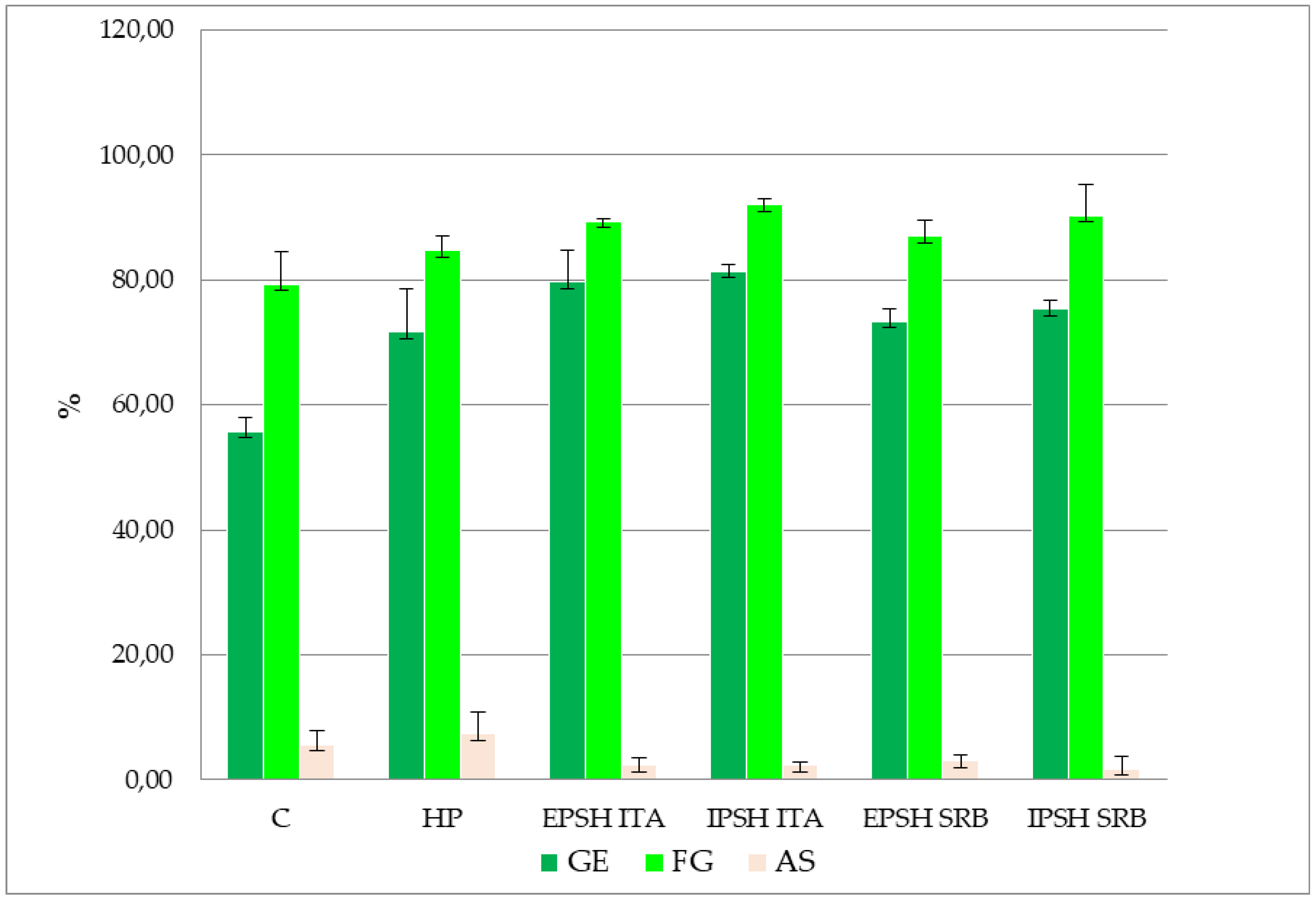
| Trait | S | Т | S × Т |
| Germination energy (GE) | *** | *** | *** |
| Seed Germination (SG) | *** | *** | *** |
| Abnormal seedlings (AS) | ** | ** | NS |
| Shoot length (SL) | *** | *** | *** |
| Root length (RL) | *** | *** | *** |
| Shoot fresh weight (SFW) | *** | ** | *** |
| Root fresh weight (RFW) | *** | *** | *** |
| Shoot dry weight (SDW) | *** | *** | *** |
| Root dry weight (RDW) | *** | *** | *** |
| Shoot elongation rate (SER) | *** | *** | *** |
| Root elongation rate (RER) | *** | *** | *** |
| Seedling vigor index (SVI) | *** | *** | *** |
| Membrane stability index (MSI) | *** | *** | *** |
| Electrolyte leakage (EL) | *** | *** | *** |
| Relative water content (RWC) | *** | *** | ** |
| Drought tolerance index (DTI) | *** | *** | *** |
| Shoot length tolerance index (SLSI) | *** | *** | *** |
| Root length tolerance index (RLSI) | *** | *** | *** |
| Тreatment* | SL** (mm) | RL (mm) | SFW (g) | RFW (g) | SDW (g) | RDW (g) | SVI |
|---|---|---|---|---|---|---|---|
| Optimal conditions | |||||||
| C | 55.00 ± 1.50d | 94.70 ± 2.90d | 2.37 ± 0.17 ab | 2.08 ± 0.04 c | 0.2064 ± 0.009 bc | 0.1820 ± 0.001 c | 1356.80 ± 7.30e |
| HP | 59.0 ± 0.90bc | 97.0 ± 2.0 d | 2.48 ± 0.11 b | 2.16 ± 0.02 b | 0.2421 ± 0.015 ab | 0.1785 ± 0.002 c | 1450.80 ± 21.50 d |
| EPSH ITA | 64.50 ± 0.09 a | 114.5 ± 3.30b | 2.76 ± 0.11 b | 2.45 ± 0.02 a | 0.2569 ± 0.023 a | 0.1831 ± 0.001 c | 168.70 ± 39.70ab |
| IPSH ITA | 60.80 ± 0.70b | 105.7 ± 3.3 c | 2.48 ± 0.06 b | 2.37 ± 0.05 a | 0.2092 ± 0.005bc | 0.1847 ± 0.001 c | 1576.1 ± 30.6 c |
| EPSH SRB | 57.20 ± 0.70cd | 115.90 ± 1.10b | 1.98 ± 0.16 a | 2.20 ± 0.01 b | 0.1844 ± 0.008 c | 0.2020 ± 0.005 a | 1615.50 ± 24.00bc |
| IPSH SRB | 57.60 ± 2.20bcd | 123.90 ± 1.50a | 2.03 ± 0.25 a | 2.13 ± 0.02 bc | 0.1957 ± 0.021 c | 0.1950 ± 0.002 b | 1694.60 ± 29.80a |
| Drought conditions | |||||||
| C | 26.00 ± 0.50d | 53.50 ± 4.30e | 1.18 ± 0.12 c | 1.42 ± 0.03 d | 0.1139 ± 0.003 c | 0.1091 ± 0.005 c | 632.00 ± 73.40d |
| HP | 27.30 ± 1.30d | 72.50 ± 3.50d | 1.37 ± 0.05 bc | 1.61 ± 0.06 bc | 0.1288 ± 0.006 b | 0.1291 ± 0.002 b | 844.70 ± 9.50c |
| EPSH ITA | 33.90 ± 0.90b | 88.80 ± 4.20c | 1.35 ± 0.07 bc | 1.64 ± 0.01 b | 0.1315 ± 0.002 b | 0.1420 ± 0.006 a | 1096.5 ± 9.50c |
| IPSH ITA | 30.80 ± 1.10c | 87.30 ± 2.90c | 1.31 ± 0.04 bc | 1.81 ± 0.04 a | 0.1324 ± 0.003 b | 0.1360 ± 0.002 ab | 1087.40 ± 44.10b |
| EPSH SRB | 37.70 ± 0.90a | 119.80 ± 1.90a | 1.62 ± 0.10 a | 1.56 ± 0.02 bc | 0.1434 ± 0.002 bc | 0.1325 ± 0.002 ab | 1369.80 ± 42.60a |
| IPSH SRB | 37.30 ± 0.50a | 108.9 ± 2.20b | 1.42 ± 0.09 ab | 1.54 ± 0.03 c | 0.1329 ± 0.003 b | 0.1332 ± 0.006 ab | 1320.90 ± 88.90a |
| Treatment* | SER** | RER | R/S ratio | MSI (%) | EL (%) |
|---|---|---|---|---|---|
| Optimal conditions | |||||
| C | 20.21 ± 0.41c | 9.19 ± 1.10b | 1.72 ± 0.10b | 81.00 ± 0.18b | 19.00 ± 0.18b |
| HP | 10.21 ± 0.22c | 9.14 ± 0.91b | 1.64 ± 0.06b | 81.88 ± 0.52b | 18.12 ± 0.52b |
| EPSH ITA | 11.37 ± 0.21ab | 10.94 ± 1.22b | 1.78 ± 0.06b | 81.78 ± 0.38b | 18.22 ± 0.38b |
| IPSH ITA | 10.47 ± 0.37bc | 10.80 ± 1.36b | 1.74 ± 0.05b | 83.99 ± 0.22a | 16.01 ± 0.22c |
| EPSH SRB | 12.01 ± 0.39a | 14.78 ± 0.47a | 2.03 ± 0.04a | 71.07 ± 0.51c | 28.93 ± 0.51a |
| IPSH SRB | 10.07 ± 0.62c | 13.92 ± 0.65a | 1.00 ± 0.07c | 71.67 ± 1.06c | 28.33 ± 1.06a |
| Drought conditions | |||||
| C | 5.91 ± 0.30c | 5.73 ± 1.50 d | 2.05 ± 0.38 b | 72.66 ± 1.04 b | 27.34 ± 1.04a |
| HP | 4.40 ± 0.41d | 6.83 ± 1.25 d | 2.65 ± 0.21 a | 72.48 ± 0.79 b | 27.52 ± 0.79a |
| EPSH ITA | 7.03 ± 0.36b | 13.31 ± 1.73 c | 2.63 ± 0.48 a | 81.99 ± 0.93 a | 18.01 ± 0.93b |
| IPSH ITA | 6.09 ± 0.35c | 11.50 ± 1.17c | 2.83 ± 0.09a | 82.37 ± 0.81a | 17.63 ± 0.81b |
| EPSH SRB | 9.14 ± 0.28a | 22.23 ± 1.69a | 0.92 ± 0.02c | 71.93 ± 0.67b | 28.07 ± 0.67a |
| IPSH SRB | 8.91 ± 0.25a | 18.23 ± 0.58b | 1.00 ± 0.11c | 72.15 ± 1.04b | 27.85 ± 1.04a |
| Treatment* | RWC** | DTI | SLSI | RLSI |
|---|---|---|---|---|
| Optimal condition | ||||
| C | 81.80 ± 0.70bc | 1.00 ± 0.00 b | 100.00 ± 0.00c | 100.00 ± 0.00d |
| HP | 81.90 ± 0.30bc | 1.08 ± 0.03 ab | 107.30 ± 1.80b | 102.50 ± 1.70cd |
| EPSH ITA | 81.30 ± 1.20c | 1.13 ± 0.06 a | 117.30 ± 2.00a | 121.20 ± 6.70ab |
| IPSH ITA | 82.30 ± 0.50bc | 1.01 ± 0.03 b | 110.70 ± 3.80ab | 111.70 ± 4.40bc |
| EPSH SRB | 83.10 ± 1.10ab | 0.99 ± 0.02 b | 104.00 ± 3.40bc | 122.60 ± 4.60ab |
| IPSH SRB | 84.40 ± 0.50a | 1.01 ± 0.04 b | 104.70 ± 2.10bc | 131.00 ± 3.20a |
| Drought conditions | ||||
| C | 72.70 ± 1.10bc | 1.00 ± 0.00 c | 100.00 ± 0.00d | 100.00 ± 0.00d |
| HP | 71.80 ± 0.60c | 1.16 ± 0.03 b | 105.20 ± 6.00d | 135.90 ± 8.80cd |
| EPSH ITA | 75.30 ± 0.20ab | 1.23 ± 0.01 a | 130.40 ± 0.80b | 167.20 ± 22.20bc |
| IPSH ITA | 74.20 ± 0.8 bc | 1.20 ± 0.01 ab | 118.60 ± 5.00c | 164.20 ± 18.10bc |
| EPSH SRB | 75.80 ± 1.90ab | 1.24 ± 0.01 a | 144.90 ± 2.60a | 224.80 ± 15.40a |
| IPSH SRB | 78.60 ± 1.80a | 1.19 ± 0.03 ab | 143.40 ± 4.30a | 204.50 ± 18.06 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





