Submitted:
18 April 2024
Posted:
19 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Vaccines in Cancer and Exosomes-Based Immunotherapy
2.1. Current Status of Anticancer Vaccines for Clinical Use
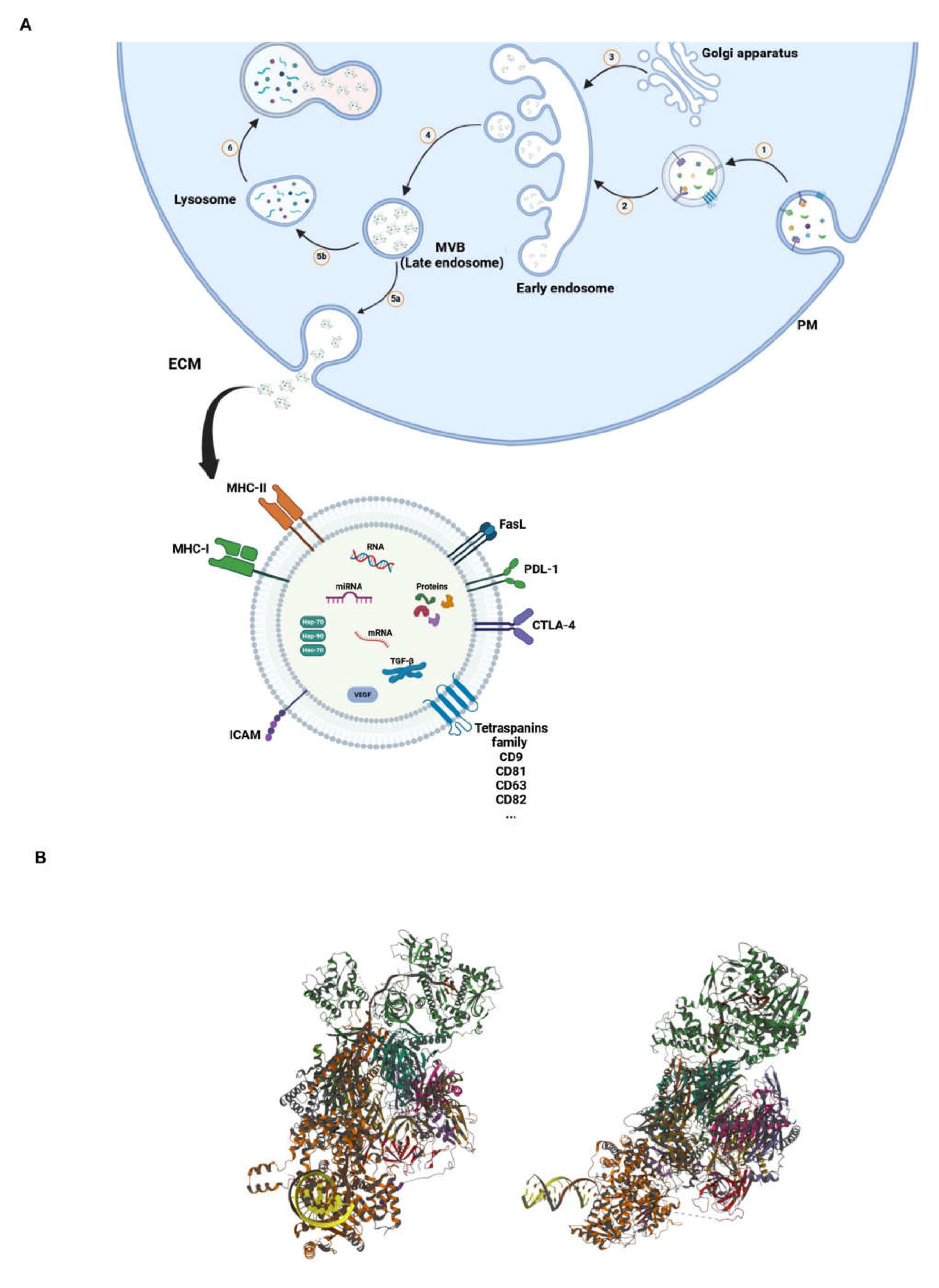
2.2. Exosomes: Definition, Composition and Biological Role
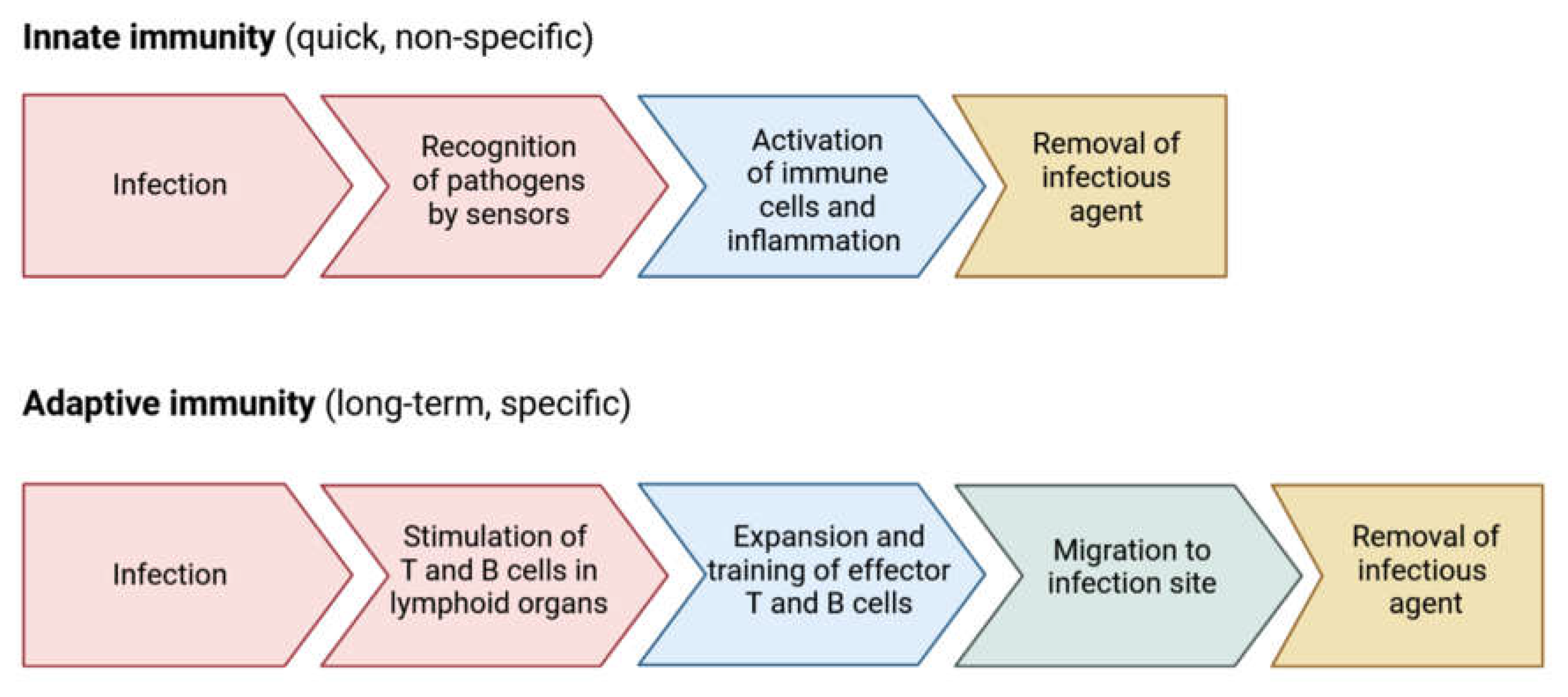
2.3. Immune Response Mechanisms
2.4. Exosomes’ Role in Cancer Immune Response
2.5. Exosomes as Intelligent Drug Carriers for Cancer Immunotherapy
2.6. Exosomes Based Vaccines and Anti-Tumor Immune Response
3. Exosomes-Based Vaccines in Solid Tumors Prevention and Treatment
3.1. Lung Cancer
3.2. Breast Cancer
3.3. Pancreatic Cancer
3.4. Ovarian Cancer
4. The Challenges and Prospects of Exosome-Based Vaccines
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Https://Gco.Iarc.Fr/Tomorrow/En/Dataviz/Isotype. (accessed on 16 February 2024).
- De Rosa, V.; Iommelli, F.; Monti, M.; Fonti, R.; Votta, G.; Stoppelli, M.P.; Del Vecchio, S. Reversal of Warburg Effect and Reactivation of Oxidative Phosphorylation by Differential Inhibition of EGFR Signaling Pathways in Non–Small Cell Lung Cancer. Clinical Cancer Research 2015, 21, 5110–5120. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, V.; Monti, M.; Terlizzi, C.; Fonti, R.; Del Vecchio, S.; Iommelli, F. Coordinate Modulation of Glycolytic Enzymes and OXPHOS by Imatinib in BCR-ABL Driven Chronic Myelogenous Leukemia Cells. Int J Mol Sci 2019, 20, 3134. [Google Scholar] [CrossRef]
- Iommelli, F.; De Rosa, V.; Gargiulo, S.; Panico, M.; Monti, M.; Greco, A.; Gramanzini, M.; Ortosecco, G.; Fonti, R.; Brunetti, A. Monitoring Reversal of MET-Mediated Resistance to EGFR Tyrosine Kinase Inhibitors in Non–Small Cell Lung Cancer Using 3′-Deoxy-3′-[18F]-Fluorothymidine Positron Emission Tomography. Clinical Cancer Research 2014, 20, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, C.; De Rosa, V.; Iommelli, F.; Pezone, A.; Altobelli, G.G.; Maddalena, M.; Dimitrov, J.; De Rosa, C.; Della Corte, C.M.; Avvedimento, V.E. ATM Inhibition Blocks Glucose Metabolism and Amplifies the Sensitivity of Resistant Lung Cancer Cell Lines to Oncogene Driver Inhibitors. Cancer Metab 2023, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, V.; Iommelli, F.; Terlizzi, C.; Leggiero, E.; Camerlingo, R.; Altobelli, G.G.; Fonti, R.; Pastore, L.; Del Vecchio, S. Non-Canonical Role of PDK1 as a Negative Regulator of Apoptosis through Macromolecular Complexes Assembly at the ER–Mitochondria Interface in Oncogene-Driven NSCLC. Cancers (Basel) 2021, 13, 4133. [Google Scholar] [CrossRef] [PubMed]
- Iommelli, F.; De Rosa, V.; Terlizzi, C.; Fonti, R.; Camerlingo, R.; Stoppelli, M.P.; Stewart, C.A.; Byers, L.A.; Piwnica-Worms, D.; Del Vecchio, S. A Reversible Shift of Driver Dependence from EGFR to Notch1 in Non-Small Cell Lung Cancer as a Cause of Resistance to Tyrosine Kinase Inhibitors. Cancers (Basel) 2021, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Iommelli, F.; De Rosa, V.; Terlizzi, C.; Monti, M.; Panico, M.; Fonti, R.; Del Vecchio, S. Inositol Trisphosphate Receptor Type 3-Mediated Enhancement of EGFR and MET Cotargeting Efficacy in Non–Small Cell Lung Cancer Detected by 18F-Fluorothymidine. Clinical Cancer Research 2018, 24, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Mulliri, S.; Laaksonen, A.; Spanu, P.; Farris, R.; Farci, M.; Mingoia, F.; Roviello, G.N.; Mocci, F. Spectroscopic and in Silico Studies on the Interaction of Substituted Pyrazolo [1, 2-a] Benzo [1, 2, 3, 4] Tetrazine-3-One Derivatives with c-Myc G4-DNA. Int J Mol Sci 2021, 22, 6028. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Spectroscopic and SEM Evidences for G4-DNA Binding by a Synthetic Alkyne-Containing Amino Acid with Anticancer Activity. Spectrochim Acta A Mol Biomol Spectrosc 2020, 229, 117884. [Google Scholar] [CrossRef]
- Greco, F.; Musumeci, D.; Borbone, N.; Falanga, A.P.; D’Errico, S.; Terracciano, M.; Piccialli, I.; Roviello, G.N.; Oliviero, G. Exploring the Parallel G-Quadruplex Nucleic Acid World: A Spectroscopic and Computational Investigation on the Binding of the c-Myc Oncogene Nhe III1 Region by the Phytochemical Polydatin. Molecules 2022, 27, 2997. [Google Scholar] [CrossRef]
- Marzano, M.; Falanga, A.P.; Marasco, D.; Borbone, N.; D’Errico, S.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Evaluation of an Analogue of the Marine ε-PLL Peptide as a Ligand of G-Quadruplex DNA Structures. Mar Drugs 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.P.; Terracciano, M.; Oliviero, G.; Roviello, G.N.; Borbone, N. Exploring the Relationship between G-Quadruplex Nucleic Acids and Plants: From Plant G-Quadruplex Function to Phytochemical G4 Ligands with Pharmaceutic Potential. Pharmaceutics 2022, 14, 2377. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer Vaccines. Bmj 2015, 350. [Google Scholar] [CrossRef] [PubMed]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-Specific Vaccines for Cancer Treatment. Hum Vaccin Immunother 2014, 10, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Baldin, A. V; Isayev, O.; Werner, J.; Zamyatnin Jr, A.A.; Bazhin, A. V Cancer Vaccines: Antigen Selection Strategy. Vaccines (Basel) 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Sun, H.; Sun, Z.; Liu, Y.; Wang, Q. Roles of Exosomes in Immunotherapy for Solid Cancers. Cell Death Dis 2024, 15, 106. [Google Scholar] [CrossRef]
- Plotkin, S.L.; Plotkin, S.A. A Short History of Vaccination. Vaccines (Basel) 2012, 4. [Google Scholar]
- Depelsenaire, A.C.I.; Kendall, M.A.F.; Young, P.R.; Muller, D.A. Introduction to Vaccines and Vaccination. In Micro and nanotechnology in vaccine development; Elsevier, 2017; pp. 47–62.
- Yadav, D.K.; Yadav, N.; Khurana, S.M.P. Vaccines: Present Status and Applications. In Animal biotechnology; Elsevier, 2020; pp. 523–542.
- Cunningham, A.L.; Garçon, N.; Leo, O.; Friedland, L.R.; Strugnell, R.; Laupèze, B.; Doherty, M.; Stern, P. Vaccine Development: From Concept to Early Clinical Testing. Vaccine 2016, 34, 6655–6664. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Vakili, B.; Nezafat, N. Exosome-Based Vaccines and Their Position in next Generation Vaccines. Int Immunopharmacol 2022, 113, 109265. [Google Scholar] [CrossRef]
- Kallerup, R.S.; Foged, C. Classification of Vaccines. In Subunit vaccine delivery; Springer, 2014; pp. 15–29.
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines (Basel) 2016, 4, 12. [Google Scholar] [CrossRef]
- Rahman, M.M.; Masum, M.H.U.; Talukder, A.; Akter, R. An in Silico Reverse Vaccinology Approach to Design a Novel Multiepitope Peptide Vaccine for Non-Small Cell Lung Cancers. Inform Med Unlocked 2023, 37, 101169. [Google Scholar] [CrossRef]
- Zhang, L. Multi-Epitope Vaccines: A Promising Strategy against Tumors and Viral Infections. Cell Mol Immunol 2018, 15, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Roy, S.; Saha, P.; Chatterjee, N.; Bishayee, A. Trends in Research on Exosomes in Cancer Progression and Anticancer Therapy. Cancers (Basel) 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.-B. HPV Vaccines: Today and in the Future. Journal of Adolescent Health 2008, 43, S26–S40. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J Infect Dis 2021, 224, S343–S351. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clinical Cancer Research 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- Https://Provenge.Com/.
- Lamm, D.L. Bacillus Calmette-Guerin Immunotherapy for Bladder Cancer. J Urol 1985, 134, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Nadofaragene Firadenovec: First Approval. Drugs 2023, 83, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Larocca, C.A.; LeBoeuf, N.R.; Silk, A.W.; Kaufman, H.L. An Update on the Role of Talimogene Laherparepvec (T-VEC) in the Treatment of Melanoma: Best Practices and Future Directions. Am J Clin Dermatol 2020, 21, 821–832. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A. Personalized RNA Neoantigen Vaccines Stimulate T Cells in Pancreatic Cancer. Nature 2023, 1–7. [Google Scholar] [CrossRef]
- Https://Classic.Clinicaltrials.Gov/Ct2/Show/NCT06007092.
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and Characterization of Extracellular Vesicle Subpopulations from Tissues. Nat Protoc 2021, 16, 1548–1580. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front Immunol 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Panwar, D.; Shrivastava, D.; Bhawal, S.; Gupta, L.K.; Kumar, N.S.S.; Chintagunta, A.D. Detection of Exosomes in Various Biological Fluids Utilizing Specific Epitopes and Directed Multiple Antigenic Peptide Antibodies. Rev Anal Chem 2023, 42, 20230056. [Google Scholar] [CrossRef]
- Xie, Q.-H.; Zheng, J.-Q.; Ding, J.-Y.; Wu, Y.-F.; Liu, L.; Yu, Z.-L.; Chen, G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells 2022, 11, 1946. [Google Scholar] [CrossRef]
- Joseph, J.; Rahmani, B.; Cole, Y.; Puttagunta, N.; Lin, E.; Khan, Z.K.; Jain, P. Can Soluble Immune Checkpoint Molecules on Exosomes Mediate Inflammation? Journal of Neuroimmune Pharmacology 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.W.A.; Jahangir, S.; Ghosh, B.; Yesmin, F.; Anis, A.; Satil, S.N.; Anwar, F.; Rashid, M.H. Exosomes for Regulation of Immune Responses and Immunotherapy. Journal of Nanotheranostics 2022, 3, 55–85. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Z.; Deng, X.; Tang, H.; Peng, C.; Zou, Y. Recent Advances in Exosome-Based Immunotherapy Applied to Cancer. Front Immunol 2023, 14, 1296857. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog Lipid Res 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Regimbeau, M.; Abrey, J.; Vautrot, V.; Causse, S.; Gobbo, J.; Garrido, C. Heat Shock Proteins and Exosomes in Cancer Theranostics. In Proceedings of the Seminars in cancer biology; Elsevier, 2022; Vol. 86; pp. 46–57. [Google Scholar]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-L.; Sun, P.; Li, Y.; Liu, S.-S.; Lu, Y. Exosomes as Critical Mediators of Cell-to-Cell Communication in Cancer Pathogenesis and Their Potential Clinical Application. Transl Cancer Res 2019, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes (Basel) 2013, 4, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-Created Drug Delivery Systems. Mol Cell Biochem 2019, 459, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Khatamianfar, S.; Mahdi Hassanian, S.; Nedaeinia, R.; Shafiee, M.; Maftouh, M.; Ghayour-Mobarhan, M.; ShahidSales, S.; Avan, A. Exosome-Encapsulated MicroRNAs as Potential Circulating Biomarkers in Colon Cancer. Curr Pharm Des 2017, 23, 1705–1709. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Nguyen, J. Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. Journal of Controlled Release 2016, 228, 179–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Gao, W.; Xie, N. Exosomes as Anticancer Drug Delivery Vehicles: Prospects and Challenges. Frontiers in Bioscience-Landmark 2022, 27, 293. [Google Scholar] [CrossRef]
- Felder, S.; Miller, K.; Moehren, G.; Ullrich, A.; Schlessinger, J.; Hopkins, C.R. Kinase Activity Controls the Sorting of the Epidermal Growth Factor Receptor within the Multivesicular Body. Cell 1990, 61, 623–634. [Google Scholar] [CrossRef]
- Futter, C.E.; Felder, S.; Schlessinger, J.; Ullrich, A.; Hopkins, C.R. Annexin I Is Phosphorylated in the Multivesicular Body during the Processing of the Epidermal Growth Factor Receptor. J Cell Biol 1993, 120, 77–83. [Google Scholar] [CrossRef]
- Krylova, S. V; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci 2023, 24, 1337. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.-S.; Chen, C.-A.; Zhou, Q.A. Exosomes─ Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef] [PubMed]
- Van Deurs, B.; Holm, P.K.; Kayser, L.; Sandvig, K.; Hansen, S.H. Multivesicular Bodies in HEp-2 Cells Are Maturing Endosomes. Eur J Cell Biol 1993, 61, 208–224. [Google Scholar] [PubMed]
- Gruenberg, J.; Maxfield, F.R. Membrane Transport in the Endocytic Pathway. Curr Opin Cell Biol 1995, 7, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Frydrychowicz, M.; Kolecka-Bednarczyk, A.; Madejczyk, M.; Yasar, S.; Dworacki, G. Exosomes–Structure, Biogenesis and Biological Role in Non-small-cell Lung Cancer. Scand J Immunol 2015, 81, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J. Life in the Lumen: The Multivesicular Endosome. Traffic 2020, 21, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers (Basel) 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.G.; Fedorko, M.E.; Cohn, Z.A. Vesicle Fusion and Formation at the Surface of Pinocytic Vacuoles in Macrophages. J Cell Biol 1968, 38, 629. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.-T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron Microscopic Evidence for Externalization of the Transferrin Receptor in Vesicular Form in Sheep Reticulocytes. J Cell Biol 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Mangeat, P.; Hoekstra, D. Aggregation Reroutes Molecules from a Recycling to a Vesicle-Mediated Secretion Pathway during Reticulocyte Maturation. J Cell Sci 1997, 110, 1867–1877. [Google Scholar] [CrossRef]
- Avgoulas, D.I.; Tasioulis, K.S.; Papi, R.M.; Pantazaki, A.A. Therapeutic and Diagnostic Potential of Exosomes as Drug Delivery Systems in Brain Cancer. Pharmaceutics 2023, 15, 1439. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Dupuy, A.; Mai, H. le; Sailliet, N.; Logé, C.; Robert, J.-M.H.; Brouard, S. Exosomes as New Biomarkers and Drug Delivery Tools for the Prevention and Treatment of Various Diseases: Current Perspectives. Int J Mol Sci 2021, 22, 7763. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barcelo, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time Has Come. Adv Anat Pathol 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chengalvala, V.; Hu, H.; Sun, D. Tumor-Derived Exosomes: Nanovesicles Made by Cancer Cells to Promote Cancer Metastasis. Acta Pharm Sin B 2021, 11, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Varshney, A.; Bajaj, R.; Pokharkar, V. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules 2022, 27, 7289. [Google Scholar] [CrossRef] [PubMed]
- Peate, I. The Immune System. British Journal of Healthcare Assistants 2021, 15, 492–497. [Google Scholar] [CrossRef]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate Immunity in Vertebrates: An Overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Stewart, J.; Weir, D.M. Innate and Acquired Immunity. Medical Microbiology, edited by Greenwood D. New York: Churchill Livingstone 2012, 109–135.
- Mukherjee, J.; Das, P.K.; Banerjee, D.; Mukherjee, A. Immune System. In Textbook of Veterinary Physiology; Springer, 2023; pp. 89–110.
- Vickers, P.S. The Immune System. Fundamentals of Anatomy and Physiology for Student Nurses, 2nd edn, John Wiley & Sons Ltd., Chichester 2017, 557–594.
- Sá-Nunes, A. Overview of Immune Responses. Essential Aspects of Immunometabolism in Health and Disease 2022, 1–11. [Google Scholar]
- LeBien, T.W.; Tedder, T.F. B Lymphocytes: How They Develop and Function. Blood, The Journal of the American Society of Hematology 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B Lymphocytes. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press, 2013.
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small Vesicles with Big Roles in Cancer, Vaccine Development, and Therapeutics. Bioact Mater 2022, 10, 281–294. [Google Scholar] [CrossRef]
- Essola, J.M.; Zhang, M.; Yang, H.; Li, F.; Xia, B.; Mavoungou, J.F.; Hussain, A.; Huang, Y. Exosome Regulation of Immune Response Mechanism: Pros and Cons in Immunotherapy. Bioact Mater 2024, 32, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S. Macrophage Microvesicles Induce Macrophage Differentiation and MiR-223 Transfer. Blood, The Journal of the American Society of Hematology 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjöstrand, M.; Olsson, B.; Jernås, M.; Lötvall, J. Exosomes Communicate Protective Messages during Oxidative Stress; Possible Role of Exosomal Shuttle RNA. PLoS One 2010, 5, e15353. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Shin, S.; Baek, M.-C.; Yea, K. Modification of Immune Cell-Derived Exosomes for Enhanced Cancer Immunotherapy: Current Advances and Therapeutic Applications. Exp Mol Med 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Majidpoor, J.; Mortezaee, K. Extracellular Vesicle–Based Drug Delivery in Cancer Immunotherapy. Drug Deliv Transl Res 2023, 1–17. [Google Scholar]
- Wang, Z.; Qin, F.; Chen, J. Exosomes: A Promising Avenue for Cancer Diagnosis Beyond Treatment. Front Cell Dev Biol 12, 1344705.
- Harvey, B.T.; Fu, X.; Li, L.; Neupane, K.R.; Anand, N.; Kolesar, J.M.; Richards, C.I. Dendritic Cell Membrane-Derived Nanovesicles for Targeted T Cell Activation. ACS Omega 2022, 7, 46222–46233. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Immune Modulation Using Extracellular Vesicles Encapsulated with MicroRNAs as Novel Drug Delivery Systems. Int J Mol Sci 2022, 23, 5658. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Berger, A.C.; Roche, P.A. T Cell-induced Secretion of MHC Class II–Peptide Complexes on B Cell Exosomes. EMBO J 2007, 26, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-Derived Extracellular Vesicles: Diverse Mediators of Pathology and Therapeutics in Multiple Diseases. Cell Death Dis 2020, 11, 924. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a pro-Inflammatory Microenvironment in the Lymph Node. Molecular Therapy 2017, 25, 1665–1675. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Sun, M.; Cui, Y.; Xing, J.; Teng, L.; Xi, Z.; Yang, Z. Exosomes as Smart Drug Delivery Vehicles for Cancer Immunotherapy. Front Immunol 2023, 13, 1093607. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Nam, G.; Choi, Y.; Kim, G.B.; Kim, S.; Kim, S.A.; Kim, I. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Advanced Materials 2020, 32, 2002440. [Google Scholar] [CrossRef]
- Ji, P.; Yang, Z.; Li, H.; Wei, M.; Yang, G.; Xing, H.; Li, Q. Smart Exosomes with Lymph Node Homing and Immune-Amplifying Capacities for Enhanced Immunotherapy of Metastatic Breast Cancer. Molecular Therapy-Nucleic Acids 2021, 26, 987–996. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell Derived Exosomes. Nat Med 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Muller, L.; Mitsuhashi, M.; Simms, P.; Gooding, W.E.; Whiteside, T.L. Tumor-Derived Exosomes Regulate Expression of Immune Function-Related Genes in Human T Cell Subsets. Sci Rep 2016, 6, 20254. [Google Scholar] [CrossRef]
- Naseri, M.; Bozorgmehr, M.; Zöller, M.; Ranaei Pirmardan, E.; Madjd, Z. Tumor-Derived Exosomes: The next Generation of Promising Cell-Free Vaccines in Cancer Immunotherapy. Oncoimmunology 2020, 9, 1779991. [Google Scholar] [CrossRef]
- Gupta, M.; Wahi, A.; Sharma, P.; Nagpal, R.; Raina, N.; Kaurav, M.; Bhattacharya, J.; Rodrigues Oliveira, S.M.; Dolma, K.G.; Paul, A.K. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines (Basel) 2022, 10, 2011. [Google Scholar] [CrossRef] [PubMed]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; McArdle, S.E.B. Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments. Front Immunol 2021, 11, 615240. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A. Exosomes-Based Cell-Free Cancer Therapy: A Novel Strategy for Targeted Therapy. Immunol Med 2021, 44, 116–123. [Google Scholar] [CrossRef]
- Https://Gco.Iarc.Fr/Today/En/Dataviz/Tables?Mode=population&cancers=15&types=0.
- Castillo-Peña, A.; Molina-Pinelo, S. Landscape of Tumor and Immune System Cells-Derived Exosomes in Lung Cancer: Mediators of Antitumor Immunity Regulation. Front Immunol 2023, 14. [Google Scholar] [CrossRef]
- Raniszewska, A.; Kwiecień, I.; Rutkowska, E.; Rzepecki, P.; Domagała-Kulawik, J. Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies. Cancers (Basel) 2021, 13, 2996. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Mitchell, R.A.; Putty, K.; Willer, S.; Sharma, R.K.; Yan, J.; Bodduluri, H.; Eaton, J.W. Vaccination with Embryonic Stem Cells Protects against Lung Cancer: Is a Broad-Spectrum Prophylactic Vaccine against Cancer Possible? 2012.
- Meng, S.; Whitt, A.G.; Stamp, B.F.; Eaton, J.W.; Li, C.; Yaddanapudi, K. Exosome-Based Cancer Vaccine for Prevention of Lung Cancer. Stem Cell Investig 2023, 10. [Google Scholar] [CrossRef]
- Burke, E.E.; Kodumudi, K.; Ramamoorthi, G.; Czerniecki, B.J. Vaccine Therapies for Breast Cancer. Surgical Oncology Clinics 2019, 28, 353–367. [Google Scholar] [CrossRef]
- Li, R.; Chibbar, R.; Xiang, J. Novel EXO-T Vaccine Using Polyclonal CD4+ T Cells Armed with HER2-Specific Exosomes for HER2-Positive Breast Cancer. Onco Targets Ther 2018, 7089–7093. [Google Scholar] [CrossRef]
- Barok, M.; Puhka, M.; Vereb, G.; Szollosi, J.; Isola, J.; Joensuu, H. Cancer-Derived Exosomes from HER2-Positive Cancer Cells Carry Trastuzumab-Emtansine into Cancer Cells Leading to Growth Inhibition and Caspase Activation. BMC Cancer 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Ahmed, K.A.; Ahmed, S.; Sami, A.; Chibbar, R.; Xu, Q.; Kane, S.E.; Hao, S.; Mulligan, S.J. Exosomal PMHC-I Complex Targets T Cell-Based Vaccine to Directly Stimulate CTL Responses Leading to Antitumor Immunity in Transgenic FVBneuN and HLA-A2/HER2 Mice and Eradicating Trastuzumab-Resistant Tumor in Athymic Nude Mice. Breast Cancer Res Treat 2013, 140, 273–284. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Xu, A.; Ahmeqd, S.; Sami, A.; Chibbar, R.; Freywald, A.; Zheng, C.; Xiang, J. Heterologous Human/Rat HER2-Specific Exosome-Targeted T Cell Vaccine Stimulates Potent Humoral and CTL Responses Leading to Enhanced Circumvention of HER2 Tolerance in Double Transgenic HLA-A2/HER2 Mice. Vaccine 2018, 36, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C. Engineered Exosomes as an in Situ DC-Primed Vaccine to Boost Antitumor Immunity in Breast Cancer. Mol Cancer 2022, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Schwarz, L.; Borbath, I.; Henry, A.; Van Laethem, J.-L.; Malka, D.; Ducreux, M.; Conroy, T. An Update on Treatment Options for Pancreatic Adenocarcinoma. Ther Adv Med Oncol 2019, 11, 1758835919875568. [Google Scholar] [CrossRef]
- Xiao, L.; Erb, U.; Zhao, K.; Hackert, T.; Zöller, M. Efficacy of Vaccination with Tumor-Exosome Loaded Dendritic Cells Combined with Cytotoxic Drug Treatment in Pancreatic Cancer. Oncoimmunology 2017, 6, e1319044. [Google Scholar] [CrossRef]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-Associated Fibroblast Exosomes Regulate Survival and Proliferation of Pancreatic Cancer Cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef]
- Batista, I.A.; Melo, S.A. Exosomes and the Future of Immunotherapy in Pancreatic Cancer. Int J Mol Sci 2019, 20, 567. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Su, M.-J.; Aldawsari, H.; Amiji, M. Pancreatic Cancer Cell Exosome-Mediated Macrophage Reprogramming and the Role of MicroRNAs 155 and 125b2 Transfection Using Nanoparticle Delivery Systems. Sci Rep 2016, 6, 30110. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, T.; Liu, G. The Roles of Exosomes in Ovarian Cancer Chemo-Resistance. J Cancer 2023, 14, 2128. [Google Scholar] [CrossRef]
- Li, X.; Wang, X. The Emerging Roles and Therapeutic Potential of Exosomes in Epithelial Ovarian Cancer. Mol Cancer 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Reza, A.M.M.T.; Choi, Y.-J.; Yasuda, H.; Kim, J.-H. Human Adipose Mesenchymal Stem Cell-Derived Exosomal-MiRNAs Are Critical Factors for Inducing Anti-Proliferation Signalling to A2780 and SKOV-3 Ovarian Cancer Cells. Sci Rep 2016, 6, 38498. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Bu, N.; Yu, Y.-C.; Hua, W.; Xin, X.-Y. Exvivo Experiments of Human Ovarian Cancer Ascites-Derived Exosomes Presented by Dendritic Cells Derived from Umbilical Cord Blood for Immunotherapy Treatment. Clin Med Oncol 2008, 2, CMO–S776. [Google Scholar] [CrossRef] [PubMed]
- Odunsi, K. Immunotherapy in Ovarian Cancer. Annals of oncology 2017, 28, viii1–viii7. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
| Type of Vaccines | Availability | Name | Vaccine strategy | Type of antigens | Adjuvant | Type of cancer | Ref. |
|---|---|---|---|---|---|---|---|
| Preventive | FDA approved | HPV (Gardasil Gardasil9 Cervarix) |
Multiepitope VLP |
Protein | yes | Cervical, Head and Neck, Anal, Penile, Vaginal, Vulvar | [28] |
| HBV | Subunit VLP |
Protein | yes | Liver | [29] | ||
| Therapeutic |
FDA approved |
Sipuleucel-T (Provenge) | Autologous | Protein Prostatic acid phosphatase (PAP) |
Yes | Prostate | [30,31] |
| BCG | Classical | PAMPs and DAMPs | No | Bladder | [32] | ||
| Nadofaragene firadonevec (Adstiladrin) | DNA vector | BCG | No | Bladder | [33] | ||
| T-VEC (Imlygic) | Classical | Live attenuated HSV-1 | Yes | Melanoma | [34] | ||
| Clinical Trials | Phase II clinical | Multiepitope Nanoparticle |
mRNA and checkpoint inhibitors |
Yes | Pancreatic | [35] | |
| Phase II clinical | Multiepitope |
HPV (Protein based) | Yes | Oropharyngeal | [36] |
| Content | Molecular Type | References |
|---|---|---|
| Membrane Markers | Tetraspanins family (CD9, CD81, CD63, CD82) | [40,41] |
| Immunomodulatory (MHC-I, MHC-II, PDL-1) | [42,43,44,45] | |
| Lipid raft (PS, Sphingolipids, Cholesterol, Ceramide) | [46] | |
| Luminal Space | Chaperones (HSP-70, HSP-90, HSC-70) | [47] |
| MVB-Associated Proteins (ALIX, TSG101, Clathrin, Flotillin-1) | [48] | |
| Signaling proteins (HIF-α, TGF-β, cdc-42, VEGF, ARF-1) | [49,50] |
| Exosome types | Membrane antigens | Lumen components | Applications |
|---|---|---|---|
| Cancer-derived exosomes | CD63, CD81, CD9 | miRNA, mRNA, proteins | cancer diagnostics, prognostics, and therapy monitoring |
| Immune cell exosomes | CD81, HSP70 | various cytokines | immunomodulation and autoimmune disease therapy |
| Neural cell exosomes | CD9, CD81, HSP90 | neurotrophic factors | neurodegenerative disorder diagnostics and therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





