Submitted:
18 April 2024
Posted:
22 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Host’s Immune Response, Tolerance, and Resistance
1.2. Sickness Behavior and Microglial Response
1.3. Microglia and Toxoplasmosis
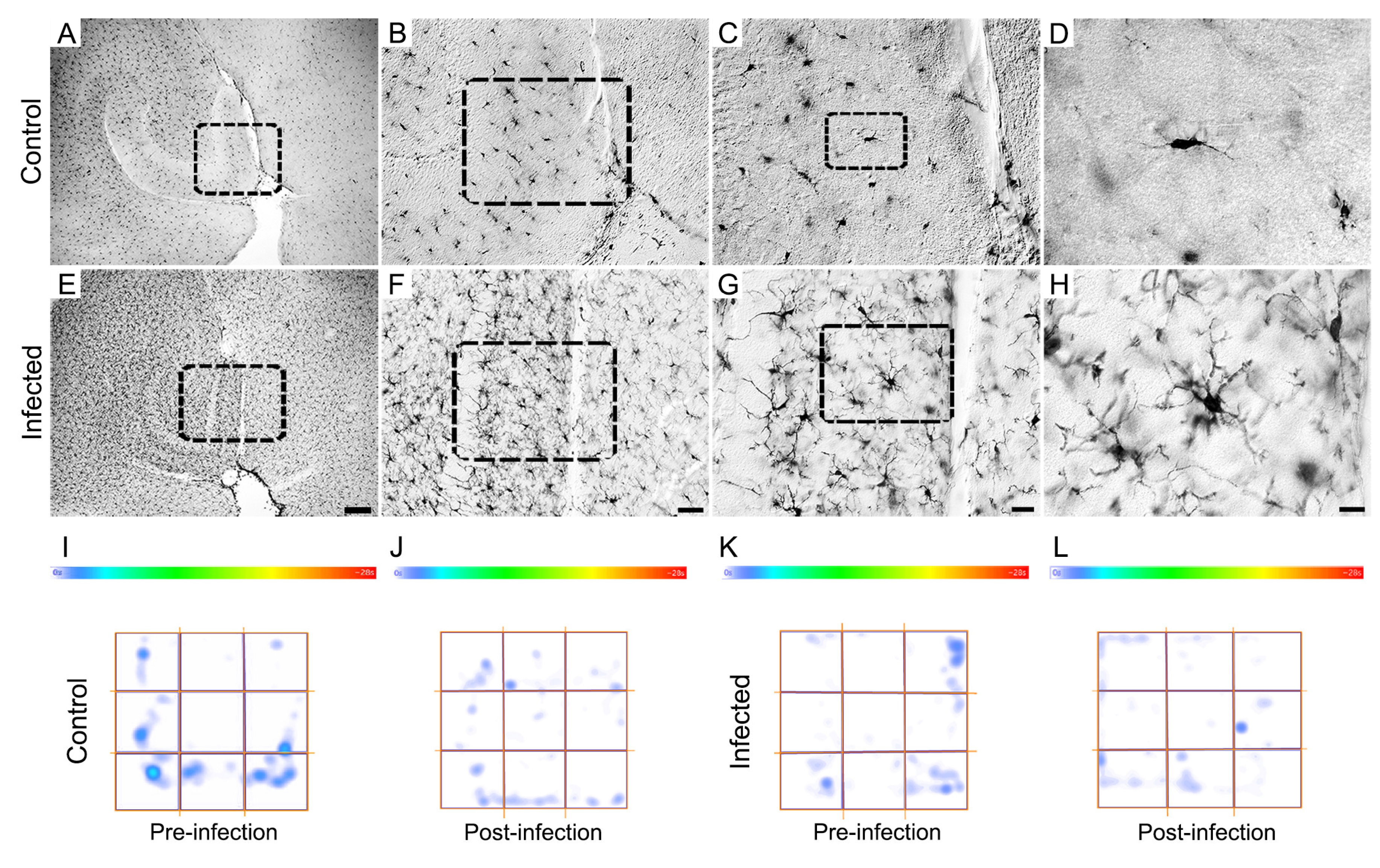
2. Results
2.1. Comparative Microglial Numerical Changes in control and T. gondii Infected Mice
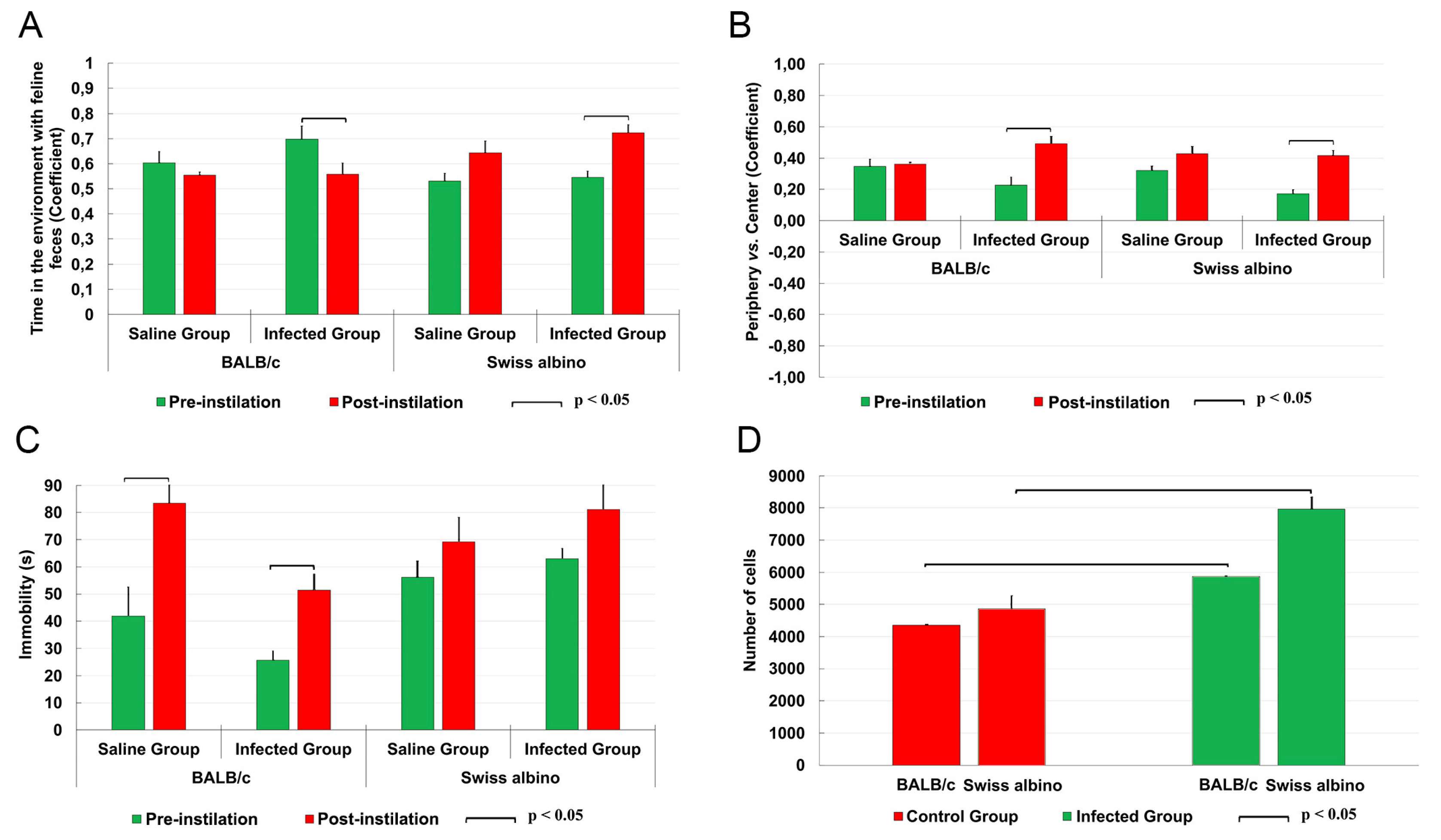
2.2. Behavioral Changes
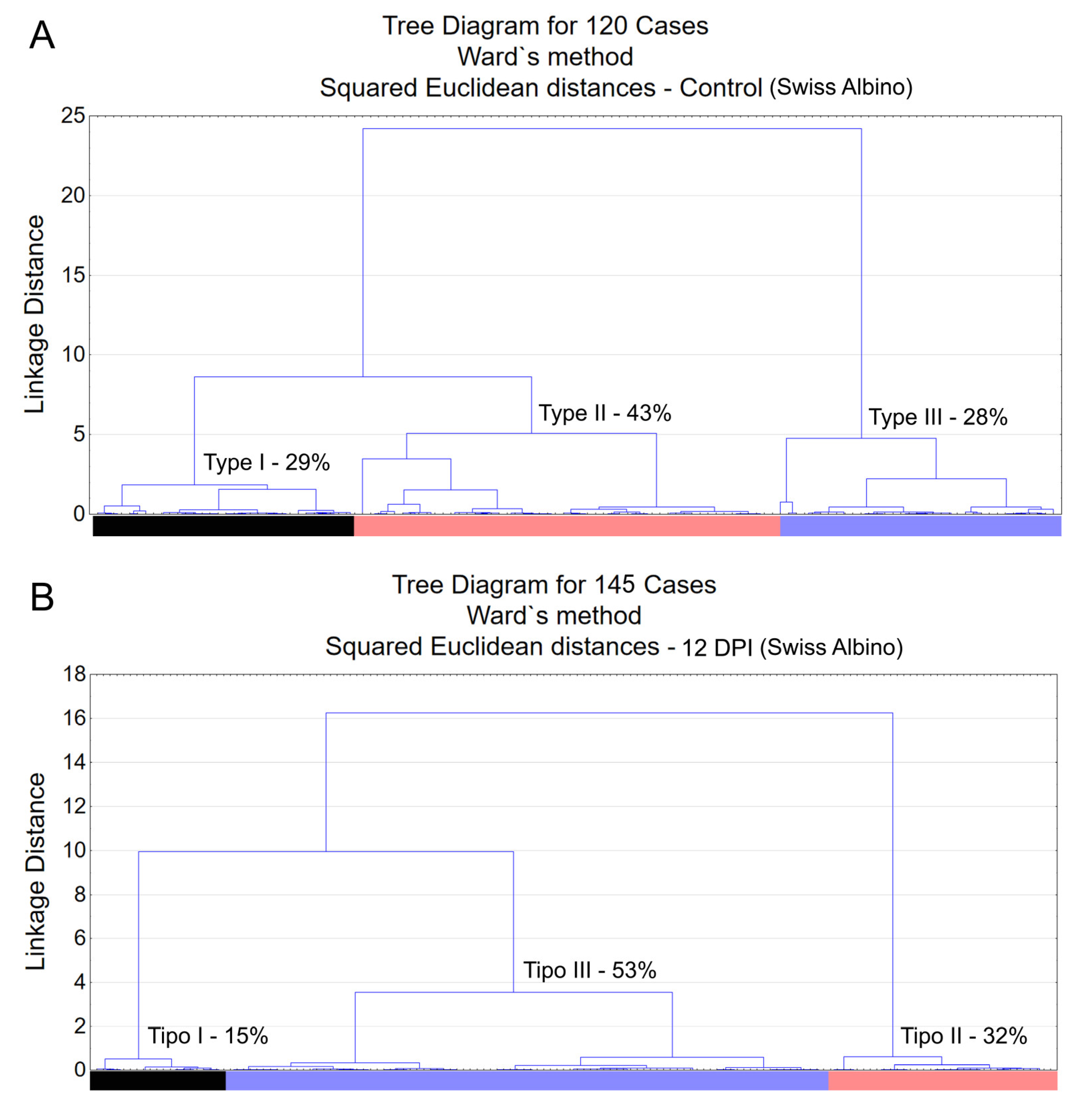
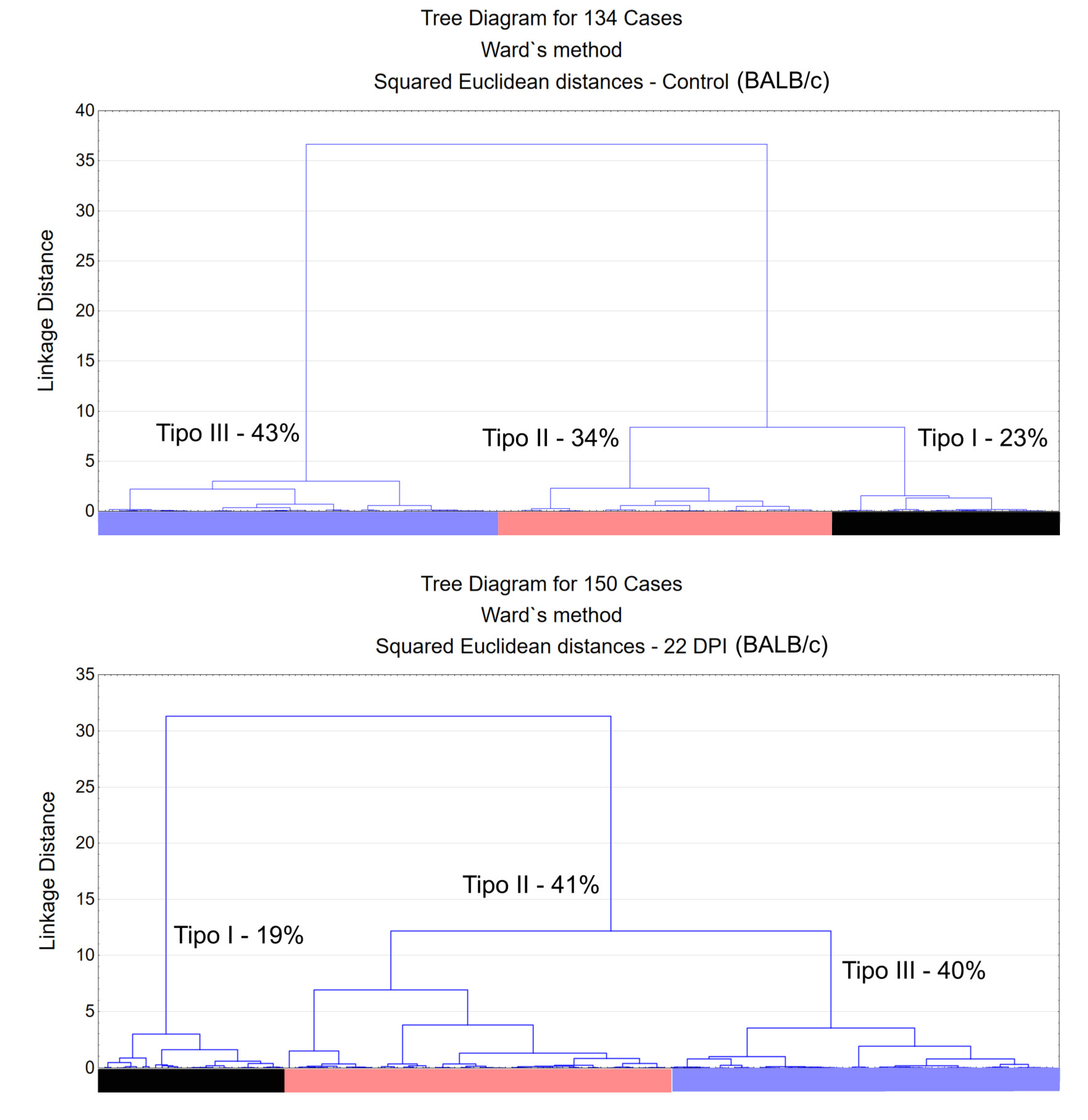
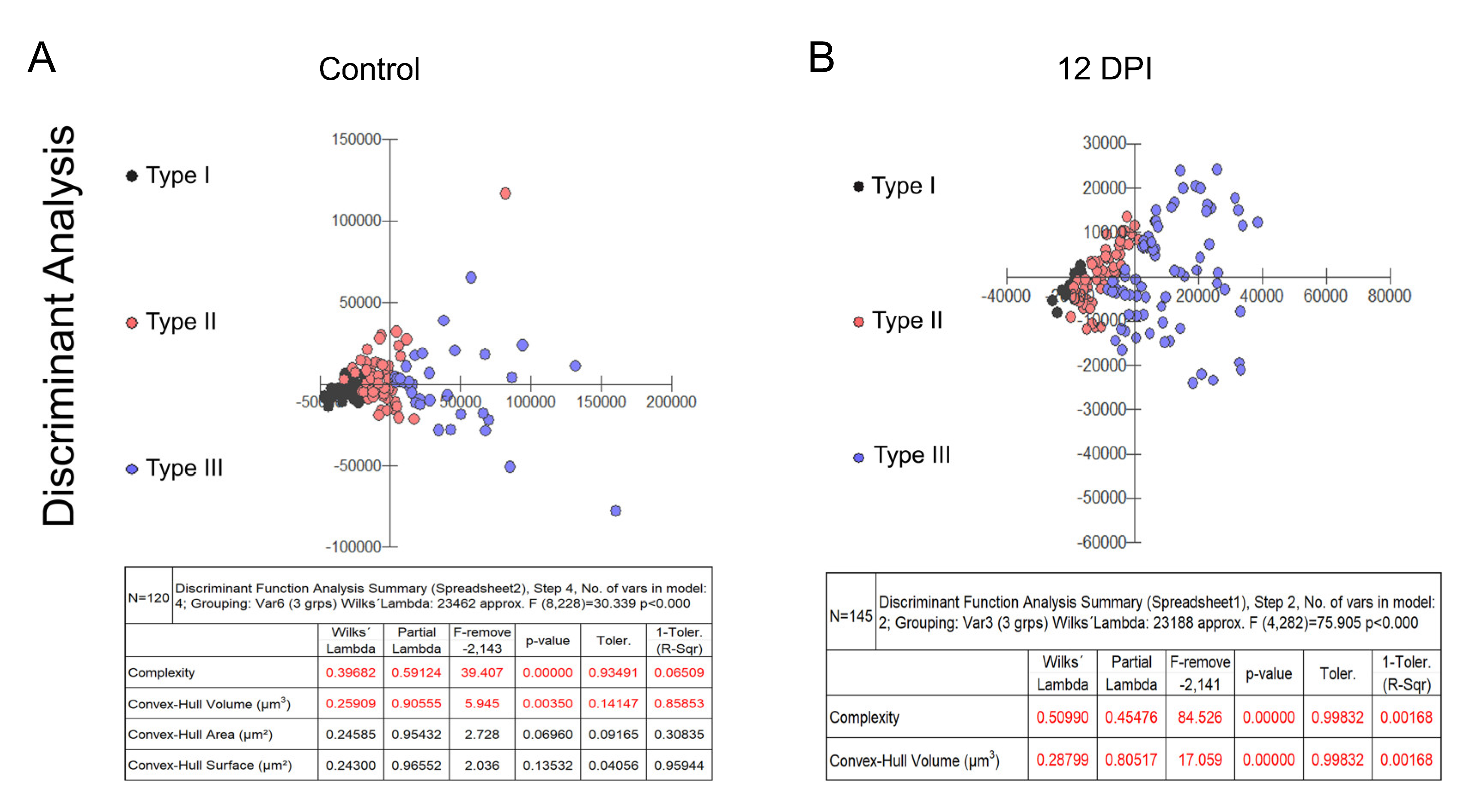
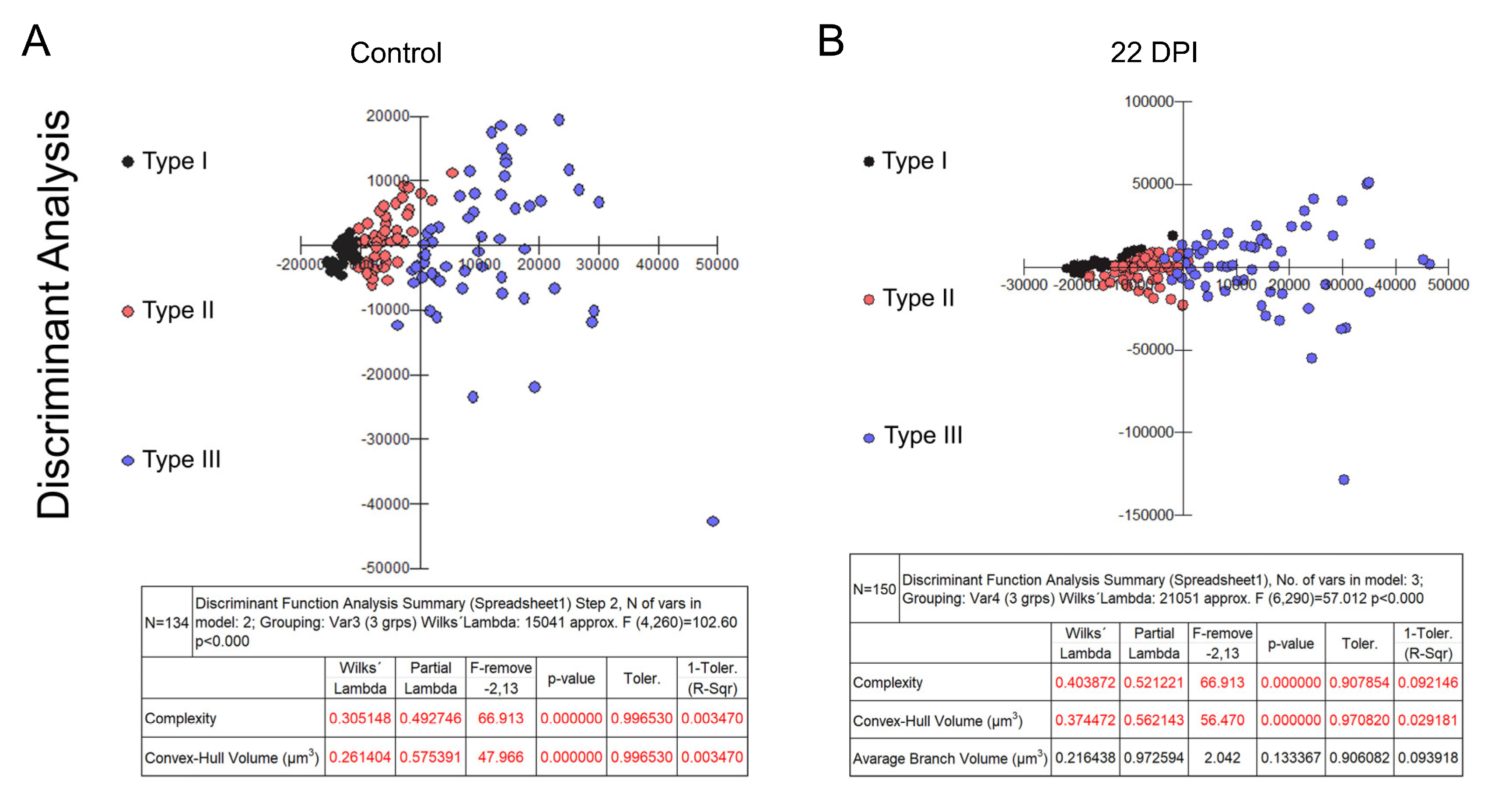
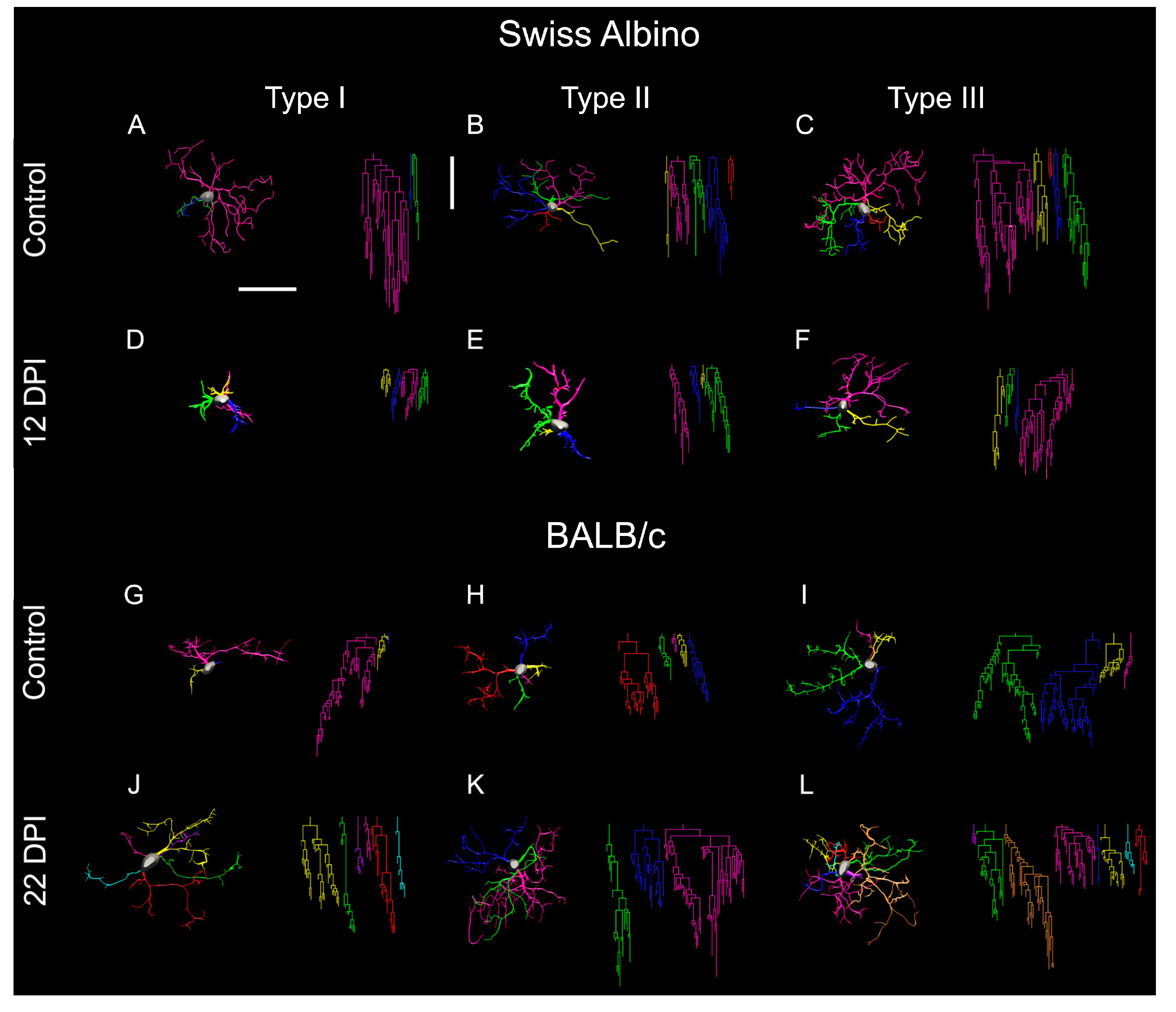
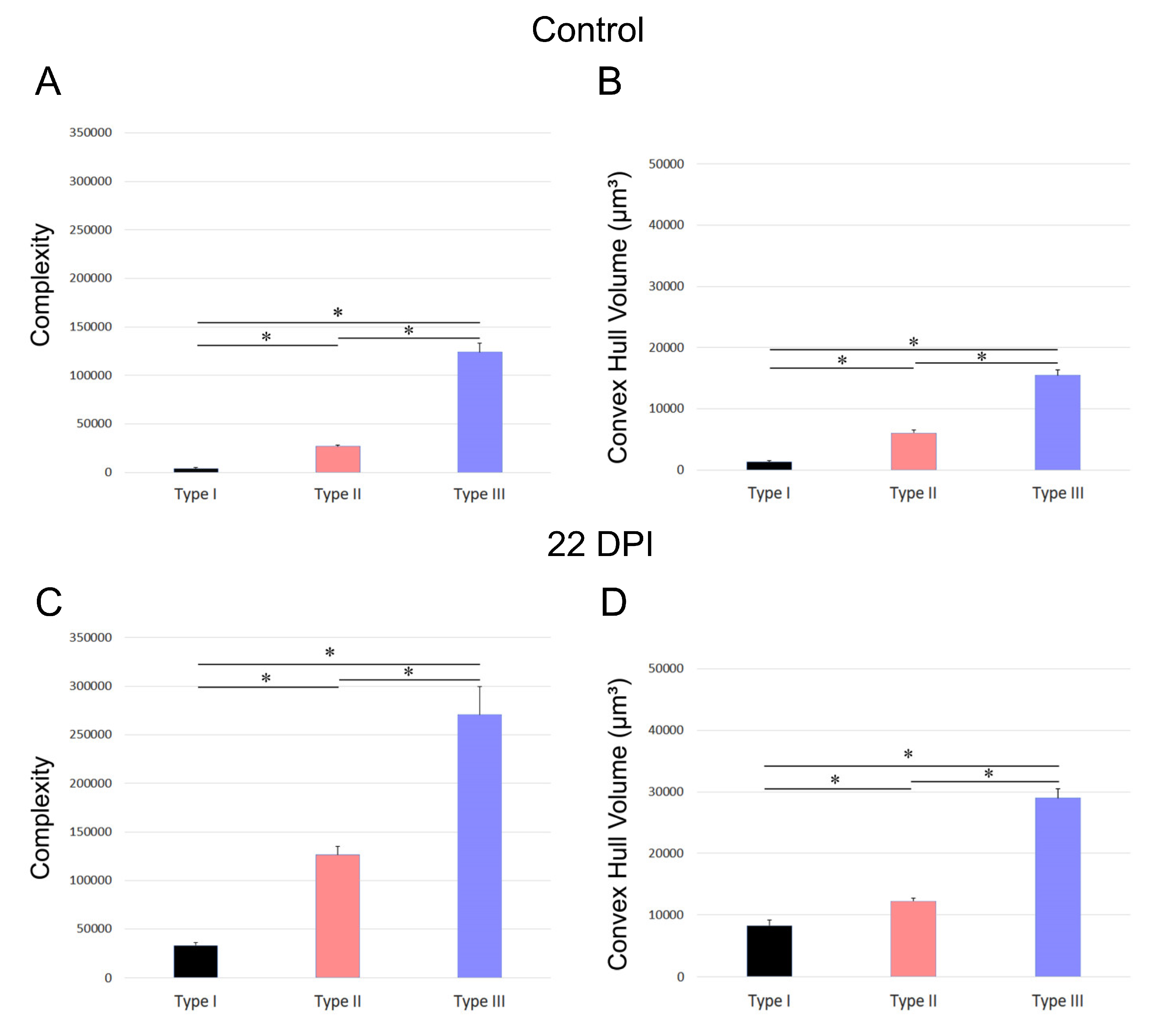
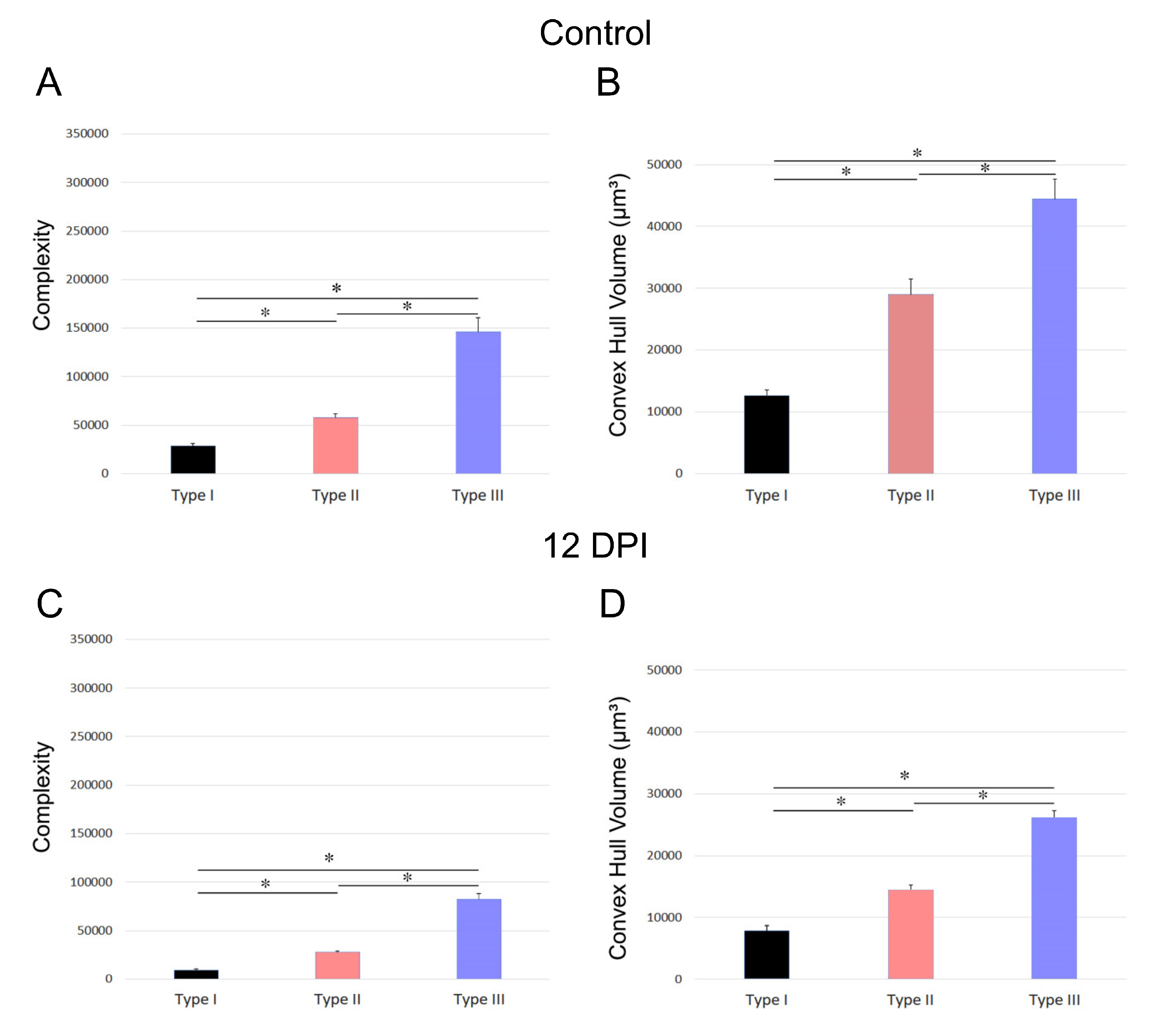
2.3. Microglial Morphotypes in T. gondii Infected and Control Mice
3. Discussion
4. Materials and Methods
4.1. Animal Handling, Anesthesia, and the Instillation of T. gondii into the Eye
4.2. Behavioral Testing: Open Field and Olfactory Discrimination
4.3. Histological and Immunohistochemical Procedures
4.4. Stereological Counting Procedures
4.5. Three-Dimensional Reconstructions
4.6. Stereological Sampling Approach and Hierarchical Cluster Analysis to Classify Microglia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019, 36, 898–925. [Google Scholar]
- Ben-Harari, R.R.; Connolly, M.P. High burden and low awareness of toxoplasmosis in the United States. Postgrad. Med. 2019, 131, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, Y.; Pan, Z.; Wang, H.; Yuan, M.; Lin, H. Seroprevalence of Toxoplasma gondii infection in blood donors in mainland China: a systematic review and meta-analysis. Parasite 2018, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Dommey, A.; Pogreba-Brown, K.; Villa-Zapata, L. Seroprevalence of Toxoplasma gondii in the U.S.: Evidence from a representative cross-sectional survey. Parasitol. Int. 2020, 79, 102175. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Mehra, V.; Groll, A.H.; Martino, R.; Lagrou, K.; Robin, C.; Perruccio, K.; Blijlevens, N.; Nucci, M.; Slavin, M.; et al. Guidelines for the management of Toxoplasma gondii infection and disease in patients with haematological malignancies and after haematopoietic stem-cell transplantation: guidelines from the 9th European Conference on Infections in Leukaemia, 2022. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease Tolerance as a Defense Strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef]
- Rivas, F.V.; Chervonsky, A.V.; Medzhitov, R. ART and immunology. Trends Immunol. 2014, 35, 451. [Google Scholar] [CrossRef]
- Chen, W.; Gullett, J.M.; Tweedell, R.E.; Kanneganti, T. Innate immune inflammatory cell death: PANoptosis and PANoptosomes in host defense and disease. Eur. J. Immunol. 2023, 53, e2250235. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Babaie, F.; Kazemi, T.; Mehrani Moghaddam, S.; Moghimi, A.; Hosseinzadeh, R.; et al. Overview of Apoptosis, Autophagy, and Inflammatory Processes in. Pathogens. 2023, 12. [Google Scholar]
- Johnson, H.J.; Koshy, A.A. Latent Toxoplasmosis Effects on Rodents and Humans: How Much is Real and How Much is Media Hype? mBio. 2020, 11. [Google Scholar] [CrossRef]
- Tong, W.H.; Pavey, C.; O’handley, R.; Vyas, A. Behavioral biology of Toxoplasma gondii infection. Parasites Vectors 2021, 14, 1–6. [Google Scholar] [CrossRef]
- Suzuki, Y. The immune system utilizes two distinct effector mechanisms of T cells depending on two different life cycle stages of a single pathogen, Toxoplasma gondii, to control its cerebral infection. Parasitol. Int. 2020, 76, 102030–102030. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.F.; Davis, P.H. Systematic review and meta-analysis of variation in Toxoplasma gondii cyst burden in the murine model. Exp. Parasitol. 2019, 196, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Melchor, S.J.; Ewald, S.E. Disease Tolerance in. Front Cell Infect Microbiol. 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Melchor, S.J.; Saunders, C.M.; Sanders, I.; Hatter, J.A.; Byrnes, K.A.; Coutermarsh-Ott, S.; Ewald, S.E. IL-1R Regulates Disease Tolerance and Cachexia in Toxoplasma gondii Infection. J. Immunol. 2020, 204, 3329–3338. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Travassos, L.H.; Paiva, C.N.; Bozza, M.T. Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance. PLOS Pathog. 2020, 16, e1008599. [Google Scholar] [CrossRef]
- Melo, M.B.; Jensen, K.D.; Saeij, J.P. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Piotti, P.; Pierantoni, L.; Albertini, M.; Pirrone, F. Inflammation and Behavior Changes in Dogs and Cats. Veter- Clin. North Am. Small Anim. Pr. 2024, 54, 1–16. [Google Scholar] [CrossRef]
- Kealy, J.; Murray, C.; Griffin, E.W.; Lopez-Rodriguez, A.B.; Healy, D.; Tortorelli, L.S.; Lowry, J.P.; Watne, L.O.; Cunningham, C. Acute Inflammation Alters Brain Energy Metabolism in Mice and Humans: Role in Suppressed Spontaneous Activity, Impaired Cognition, and Delirium. J. Neurosci. 2020, 40, 5681–5696. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fereig, R.; Nishikawa, Y. Involvement of Host Defense Mechanisms against Toxoplasma gondii Infection in Anhedonic and Despair-Like Behaviors in Mice. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Estato, V.; Stipursky, J.; Gomes, F.; Mergener, T.C.; Frazão-Teixeira, E.; Allodi, S.; Tibiriçá, E.; Barbosa, H.S.; Adesse, D. The Neurotropic Parasite Toxoplasma gondii Induces Sustained Neuroinflammation with Microvascular Dysfunction in Infected Mice. Am. J. Pathol. 2018, 188, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Barrios, L.C.; Pinheiro, A.P.D.S.; Gibaldi, D.; Silva, A.A.; e Silva, P.M.R.; Roffê, E.; Santiago, H.d.C.; Gazzinelli, R.T.; Mineo, J.R.; Silva, N.M.; et al. Behavioral alterations in long-term Toxoplasma gondii infection of C57BL/6 mice are associated with neuroinflammation and disruption of the blood brain barrier. PLOS ONE 2021, 16, e0258199. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.E.; Medeiros, M.; Porfirio, J.; Tavares, W.; Pessôa, L.; Grinberg, L.; Leite, R.E.; Ferretti-Rebustini, R.E.L.; Suemoto, C.K.; Filho, W.J.; et al. Similar Microglial Cell Densities across Brain Structures and Mammalian Species: Implications for Brain Tissue Function. J. Neurosci. 2020, 40, 4622–4643. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef]
- E Hirbec, H.; Noristani, H.N.; Perrin, F.E. Microglia Responses in Acute and Chronic Neurological Diseases: What Microglia-Specific Transcriptomic Studies Taught (and did Not Teach) Us. Front Aging Neurosci. 2017, 9, 227. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Collins, K.E.; Patel, R.; Wellman, C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLOS ONE 2017, 12, e0187631–e0187631. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Hart, A.D.; Wyttenbach, A.; Perry, V.H.; Teeling, J.L. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain, Behav. Immun. 2012, 26, 754–765. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Gorse, K.M.; Lafrenaye, A.D. The Importance of Inter-Species Variation in Traumatic Brain Injury-Induced Alterations of Microglial-Axonal Interactions. Front. Neurol. 2018, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.-A.; et al. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 2019, 179, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Stratoulias, V.; Venero, J.L.; Tremblay, M.-È.; Joseph, B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019, 38, e101997. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.C.; Carrier, M.; Tremblay, M.-. Ève Morphology of Microglia Across Contexts of Health and Disease. Methods Mol. Biol. 2019, 2034, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglial Morphometric Parameters Correlate With the Expression Level of IL-1β, and Allow Identifying Different Activated Morphotypes. Front Cell Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235–235. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.C.; St-Pierre, M.-K.; Hui, C.W.; Tremblay, M.-E. Microglial Ultrastructure in the Hippocampus of a Lipopolysaccharide-Induced Sickness Mouse Model. Front. Neurosci. 2019, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Johnson, S.K.; Johnson, P.T. Toxoplasmosis: Recent Advances in Understanding the Link Between Infection and Host Behavior. Annu. Rev. Anim. Biosci. 2021, 9, 249–264. [Google Scholar] [CrossRef]
- Sasai, M.; Pradipta, A.; Yamamoto, M. Host immune responses toToxoplasma gondii. Int. Immunol. 2018, 30, 113–119. [Google Scholar] [CrossRef]
- Suzuki, Y.; Orellana, M.A.; Schreiber, R.D.; Remington, J.S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988, 240, 516–518. [Google Scholar] [CrossRef]
- Jeffers, V.; Tampaki, Z.; Kim, K.; Sullivan, W.J. A latent ability to persist: differentiation in Toxoplasma gondii. Cell. Mol. Life Sci. 2018, 75, 2355–2373. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Prandovszky, E.; Kannan, G.; Viscidi, R.P.; Pletnikov, M.V.; Yolken, R.H. Behavioral Abnormalities in a Mouse Model of Chronic Toxoplasmosis Are Associated with MAG1 Antibody Levels and Cyst Burden. PLOS Neglected Trop. Dis. 2016, 10, e0004674–e0004674. [Google Scholar] [CrossRef]
- Afonso, C.; Paixão, V.B.; Costa, R.M. Chronic Toxoplasma Infection Modifies the Structure and the Risk of Host Behavior. PLOS ONE 2012, 7, e32489. [Google Scholar] [CrossRef]
- Shen, B.; Yuan, Y.; Cheng, J.; Pan, M.; Xia, N.; Zhang, W.; Wang, Y.; Zhou, Y.; Zhao, J. Activation of chronic toxoplasmosis by transportation stress in a mouse model. Oncotarget 2016, 7, 87351–87360. [Google Scholar] [CrossRef]
- Sullivan, W.J.; Jeffers, V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012, 36, 717–733. [Google Scholar] [CrossRef]
- Suzuki, Y.; Claflin, J.; Wang, X.; Lengi, A.; Kikuchi, T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol. 2005, 35, 83–90. [Google Scholar] [CrossRef]
- Soares, G.L.d.S.; de Leão, E.R.L.P.; Freitas, S.F.; Alves, R.M.C.; Tavares, N.d.P.; Costa, M.V.N.; de Menezes, G.C.; de Oliveira, J.H.P.; Guerreiro, L.C.F.; de Assis, A.C.L.; et al. Behavioral and Neuropathological Changes After Toxoplasma gondii Ocular Conjunctival Infection in BALB/c Mice. Front. Cell. Infect. Microbiol. 2022, 12, 812152. [Google Scholar] [CrossRef]
- de Sousa, A.A.; dos Reis, R.R.; de Lima, C.M.; de Oliveira, M.A.; Fernandes, T.N.; Gomes, G.F.; et al. Three-dimensional morphometric analysis of microglial changes in a mouse model of virus encephalitis: age and environmental influences. European Journal of Neuroscience. 2015, 42, 2036–2050. [Google Scholar] [CrossRef]
- Rosso, M.; Wirz, R.; Loretan, A.V.; Sutter, N.A.; da Cunha, C.T.P.; Jaric, I.; Würbel, H.; Voelkl, B. Reliability of common mouse behavioural tests of anxiety: A systematic review and meta-analysis on the effects of anxiolytics. Neurosci. Biobehav. Rev. 2022, 143, 104928. [Google Scholar] [CrossRef]
- Ennaceur, A. Tests of unconditioned anxiety — Pitfalls and disappointments. Physiol. Behav. 2014, 135, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Ju, G.; Fan, L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 1988, 85, 169–171. [Google Scholar] [CrossRef]
- Saper, C.B.; Sawchenko, P.E. Magic peptides, magic antibodies: Guidelines for appropriate controls for immunohistochemistry. J. Comp. Neurol. 2003, 465, 161–163. [Google Scholar] [CrossRef]
- West, M.J. Design-based stereological methods for counting neurons. Prog Brain Res. 2002, 135, 43–51. [Google Scholar]
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef]
- Gundersen, H.J.G.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction*. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef]
- Glaser, E.M.; Wilson, P.D. The coefficient of error of optical fractionator population size estimates: a computer simulation comparing three estimators. J. Microsc. 1998, 192, 163–171. [Google Scholar] [CrossRef]
- West, M.J. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999, 22, 51–61. [Google Scholar] [CrossRef]
- Slomianka, L.; West, M. Estimators of the precision of stereological estimates: An example based on the CA1 pyramidal cell layer of rats. Neuroscience 2005, 136, 757–767. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2019, 25, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Bohlen, C.J.; York, E.M.; Choi, H.B.; Kamyabi, A.; Dissing-Olesen, L.; et al. Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep. 2019, 27, 2895–2908e4. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Creão, L.S.d.S.; Neto, J.B.T.; de Lima, C.M.; dos Reis, R.R.; de Sousa, A.A.; dos Santos, Z.A.; Diniz, J.A.P.; Diniz, D.G.; Diniz, C.W.P. Microglial Metamorphosis in Three Dimensions in Virus Limbic Encephalitis: An Unbiased Pictorial Representation Based on a Stereological Sampling Approach of Surveillant and Reactive Microglia. Brain Sci. 2021, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Paulo, D. ; JB T, C SF, TCG O, AA dS, RR R, et al. Microglial morphology across distantly related species: phylogenetic, environmental and age influences on microglia reactive and surveillance states. Frontiers in Immunology [Internet]. 2021, 12:[12, 683026 p.].
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflammation 2014, 11, 12–12. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, I.E.; Fetani, A.F.; Lewen, A.; Heinemann, U.; Kann, O. Widespread activation of microglial cells in the hippocampus of chronic epileptic rats correlates only partially with neurodegeneration. Anat. Embryol. 2014, 220, 2423–2439. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Jinno, S. Novel objective classification of reactive microglia following hypoglossal axotomy using hierarchical cluster analysis. J. Comp. Neurol. 2012, 521, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, L.; Renehan, W. The use of cluster analysis for cell typing. Brain Res. Protoc. 1997, 1, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.G.; de Oliveira, M.A.; de Lima, C.M.; Fôro, C.A.R.; Sosthenes, M.C.K.; Bento-Torres, J.; Vasconcelos, P.F.d.C.; Anthony, D.C.; Diniz, C.W.P. Age, environment, object recognition and morphological diversity of GFAP-immunolabeled astrocytes. Behav. Brain Funct. 2016, 12, 1–19. [Google Scholar] [CrossRef]
- Kolb, H.; Fernandez, E.; Schouten, J.; Ahnelt, P.; Linberg, K.A.; Fisher, S.K. Are there three types of horizontal cell in the human retina? J Comp Neurol. 1994, 343, 370–386. [Google Scholar] [CrossRef]
- Råberg, L.; Graham, A.L.; Read, A.F. Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. B: Biol. Sci. 2008, 364, 37–49. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zheng, H.; Huang, S.; Lu, F. Association of TREM-1, IL-1β, IL-33/ST2, and TLR Expressions With the Pathogenesis of Ocular Toxoplasmosis in Mouse Models on Different Genetic Backgrounds. Front. Microbiol. 2019, 10, 2264. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yuk, J.-M.; Cha, G.-H.; Lee, Y.-H. Expression of cytokines and co-stimulatory molecules in the Toxoplasma gondii-infected dendritic cells of C57BL/6 and BALB/c mice. Parasites, Hosts Dis. 2023, 61, 138–146. [Google Scholar] [CrossRef]
- Bergersen, K.V.; Barnes, A.; Worth, D.; David, C.; Wilson, E.H. Targeted Transcriptomic Analysis of C57BL/6 and BALB/c Mice During Progressive Chronic Toxoplasma gondii Infection Reveals Changes in Host and Parasite Gene Expression Relating to Neuropathology and Resolution. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bruder, D.; Wolf, S.A.; Jeron, A.; Mack, M.; Heimesaat, M.M.; et al. Ly6C(high) monocytes control cerebral toxoplasmosis. J Immunol. 2015, 194, 3223–3235. [Google Scholar] [CrossRef]
- Zhao, Y.; Reyes, J.; Rovira-Diaz, E.; Fox, B.A.; Bzik, D.J.; Yap, G.S. Cutting Edge: CD36 Mediates Phagocyte Tropism and Avirulence of Toxoplasma gondii. J. Immunol. 2021, 207, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Shin, J.-H.; Yang, J.-P.; Jung, B.-K.; Lee, S.H.; Shin, E.-H. Characteristics of Infection Immunity Regulated by Toxoplasma gondii to Maintain Chronic Infection in the Brain. Front. Immunol. 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Dunay, I.R.; Schlüter, D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. 2015, 37, 150–158. [Google Scholar] [CrossRef]
- Bezerra, E.C.M.; dos Santos, S.V.; dos Santos, T.C.C.; de Andrade, H.F.; Meireles, L.R. Behavioral evaluation of BALB/c (Mus musculus) mice infected with genetically distinct strains of Toxoplasma gondii. Microb. Pathog. 2018, 126, 279–286. [Google Scholar] [CrossRef]
- Dubey, J.P.; Ferreira, L.R.; Martins, J.; McLeod, R. Oral oocyst-induced mouse model of toxoplasmosis: effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out-bred, in-bred) on pathogenesis and mortality. Parasitology. 2012, 139, 1–13. [Google Scholar] [CrossRef]
- Saeij, J.P.; Boyle, J.P.; Boothroyd, J.C. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Fernández-Escobar, M.; Katzer, F.; Su, C.; Ortega-Mora, L.M. Unifying Virulence Evaluation in Toxoplasma gondii: A Timely Task. Front. Cell. Infect. Microbiol. 2022, 12, 868727. [Google Scholar] [CrossRef] [PubMed]
- Desmettre, T. Toxoplasmosis and behavioural changes. 2020, 43, e89–e93. [CrossRef]
- Pittman, K.J.; Aliota, M.T.; Knoll, L.J. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genom. 2014, 15, 806. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.B.; Worth, D.; Hong, D.; Huang, S.; Sullivan, W.J.; Wilson, E.H.; White, M.W. Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLOS Pathog. 2018, 14, e1007035. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, K.V.; Ramirez, A.D.; Kavvathas, B.; Mercer, F.; Wilson, E.H. Human neutrophil-like cells demonstrate antimicrobial responses to the chronic cyst form of Toxoplasma gondii. Parasite Immunol. 2023, 45, e13011. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Lutshumba, J.; Chen, K.C.; Abdelaziz, M.H.; Sa, Q.; Ochiai, E. IFN-γ production by brain-resident cells activates cerebral mRNA expression of a wide spectrum of molecules critical for both innate and T cell-mediated protective immunity to control reactivation of chronic infection with Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2023, 13, 1110508. [Google Scholar] [CrossRef]
- Carlo, C.; Stevens, C. Analysis of differential shrinkage in frozen brain sections and its implications for the use of guard zones in stereology. J. Comp. Neurol. 2011, 519, 2803–2810. [Google Scholar] [CrossRef]
| Animal | Total Number | Thickness (µm) | CE Scheaffer |
| Control | |||
| Animal 6 | 4448 | 41.20 | 0.042 |
| Animal 7 | 4243 | 40.50 | 0.043 |
| Animal 12M | 6018 | 34.80 | 0.046 |
| Animal 6M | 5688 | 35.20 | 0.048 |
| Animal 7M | 3913 | 22.50 | 0.043 |
| Mean | 4862 | 34.84 | 0.043 |
| SD | 931.9 | 7.498 | 0.044 |
| CV | 0.191 | 0.215 | |
| CV² | 0.036 | 0.046 | |
| CE2 | 0.001 | 0.001 | |
| CE² / CV² | 0.027 | 0.021 | |
| CV² - CE² | 0.035 | 0.045 | |
| CVB² (%) | 97.22 | 97.82 | |
| 12 DPI | |||
| Animal 18 | 6392 | 34.80 | 0.032 |
| Animal 22 | 7355 | 41.00 | 0.033 |
| Animal 37 | 8793 | 51.50 | 0.033 |
| Animal 41 | 8640 | 37.40 | 0.029 |
| Animal 42 | 8347 | 38.10 | 0.029 |
| Animal 43 | 8229 | 51.00 | 0.037 |
| Mean | 7959 | 42.30 | 0.032 |
| SD | 916.8 | ||
| CV | 0.115 | 0.170 | |
| CV² | 0.013 | 0.020 | |
| CE2 | 0.001 | 0.001 | |
| CE² / CV² | 0.076 | 0.050 | |
| CV² - CE² | 0.012 | 0.019 | |
| CVB² (%) | 92.30 | 95.00 | |
| Animal | Thickness (µm) | No. of Sections | No. of Probes | Probe (µm) | Grid (µm) | Dissector height (µm) | Interval |
| Control | |||||||
| Animal 6 | 41.2 | 6 | 721 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 7 | 40.5 | 6 | 706 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 12M | 34.8 | 5 | 492 | 50x50 | 70x70 | 15 | 1/5 |
| Animal 6M | 35.2 | 5 | 438 | 50x50 | 70x70 | 15 | 1/5 |
| Animal 7M | 22.5 | 5 | 420 | 50x50 | 70x70 | 15 | 1/5 |
| 12 DPI | |||||||
| Animal 18 | 34.8 | 5 | 638 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 22 | 41.0 | 6 | 810 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 37 | 51.5 | 6 | 797 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 41 | 37.4 | 6 | 907 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 42 | 38.1 | 5 | 808 | 50x50 | 70x70 | 15 | 1/3 |
| Animal 43 | 51.0 | 6 | 661 | 50x50 | 70x70 | 15 | 1/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





