Submitted:
19 April 2024
Posted:
22 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
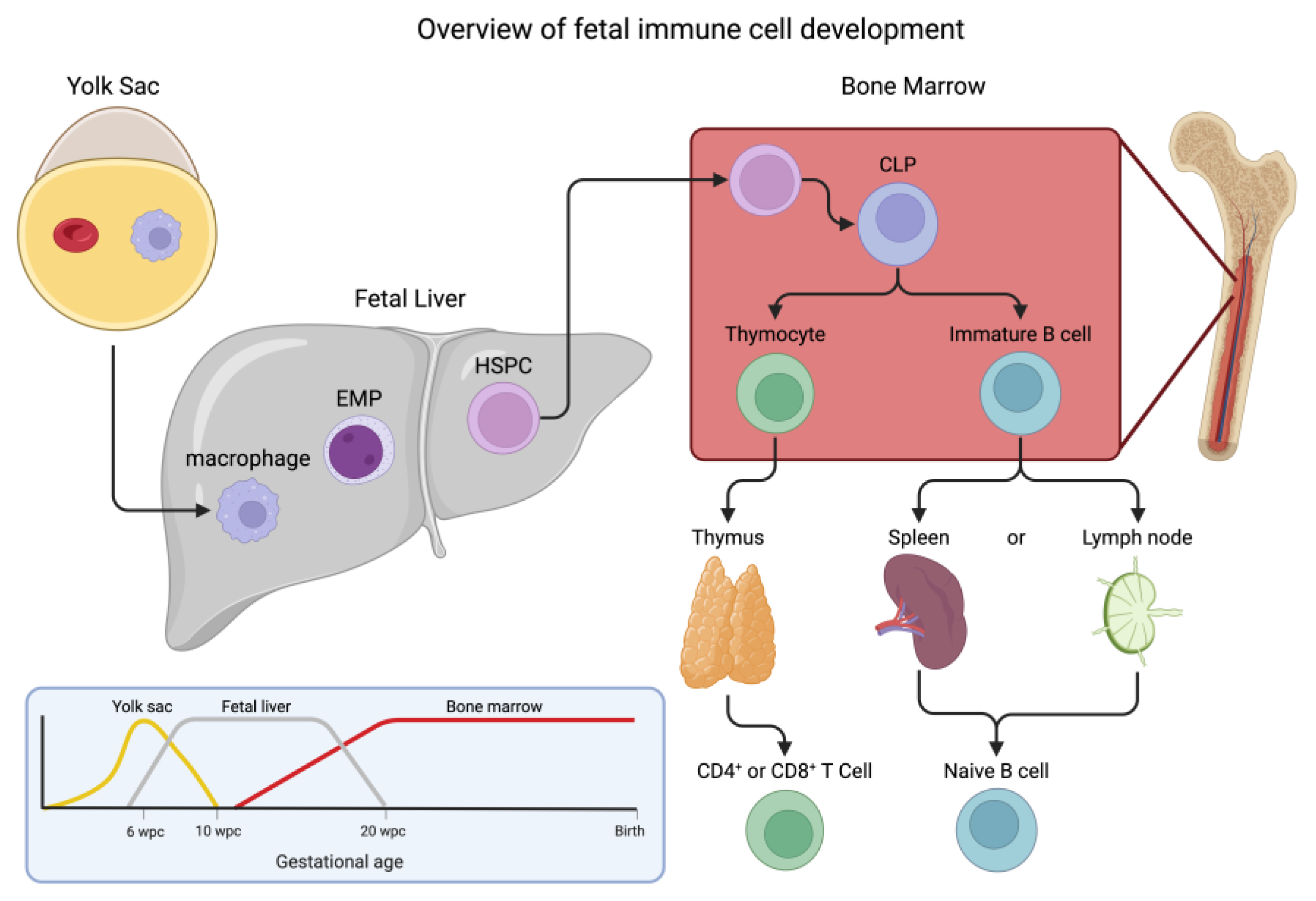
2. Immune Cell Development: Hematopoiesis
3. Impact of Maternal Obesity/WSD on Maternal and Placental Immune Function
3.1. Maternal Obesity Changes Placental Immunity
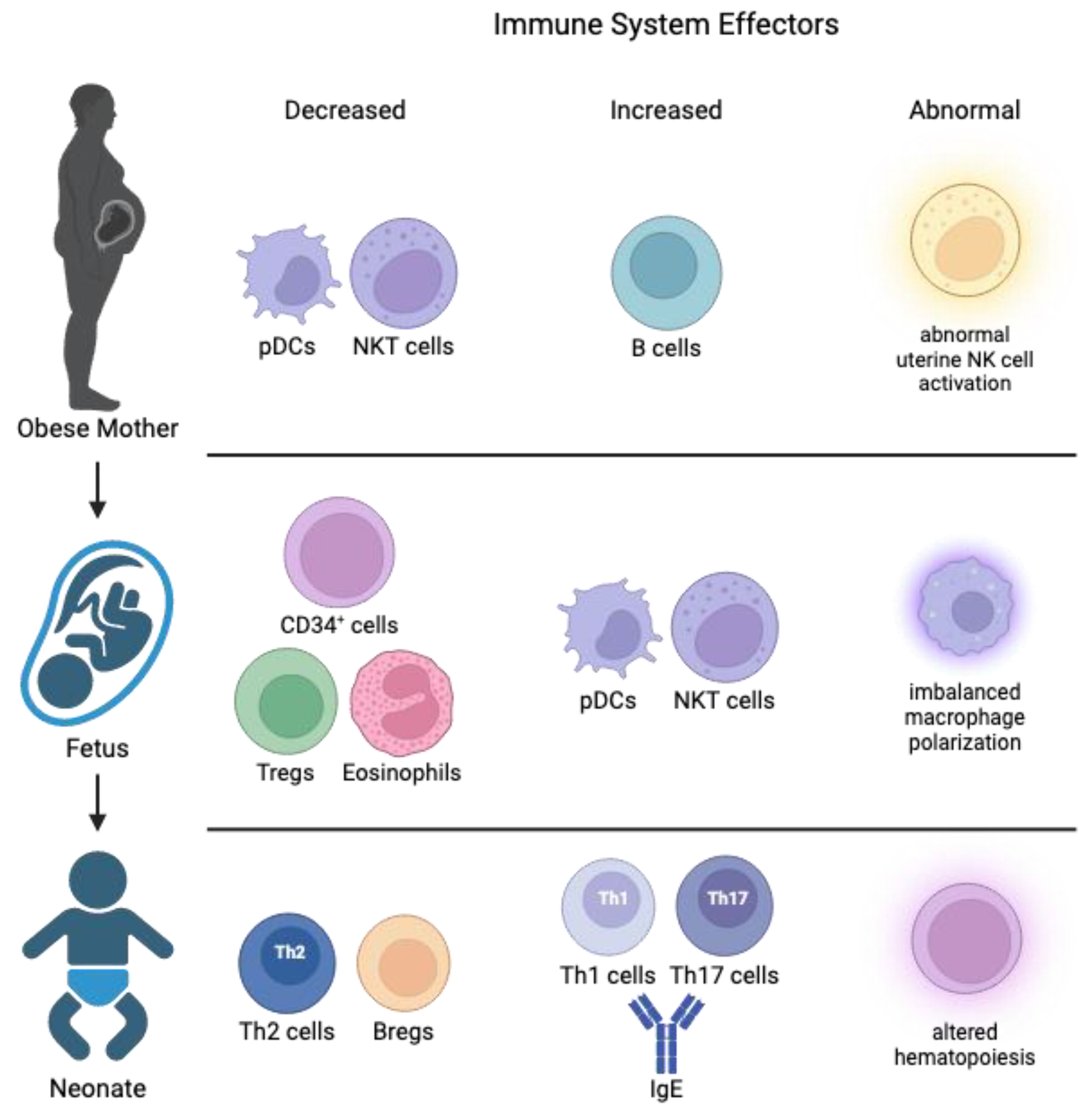
3.2. Cord Blood Changes in Offspring from Obese Pregnancies
3.3. Maternal Obesity Impacts Adaptive and Innate Immunity in the Neonate
4. Impact of Maternal Obesity on the Fetal Liver and Offspring Immunity
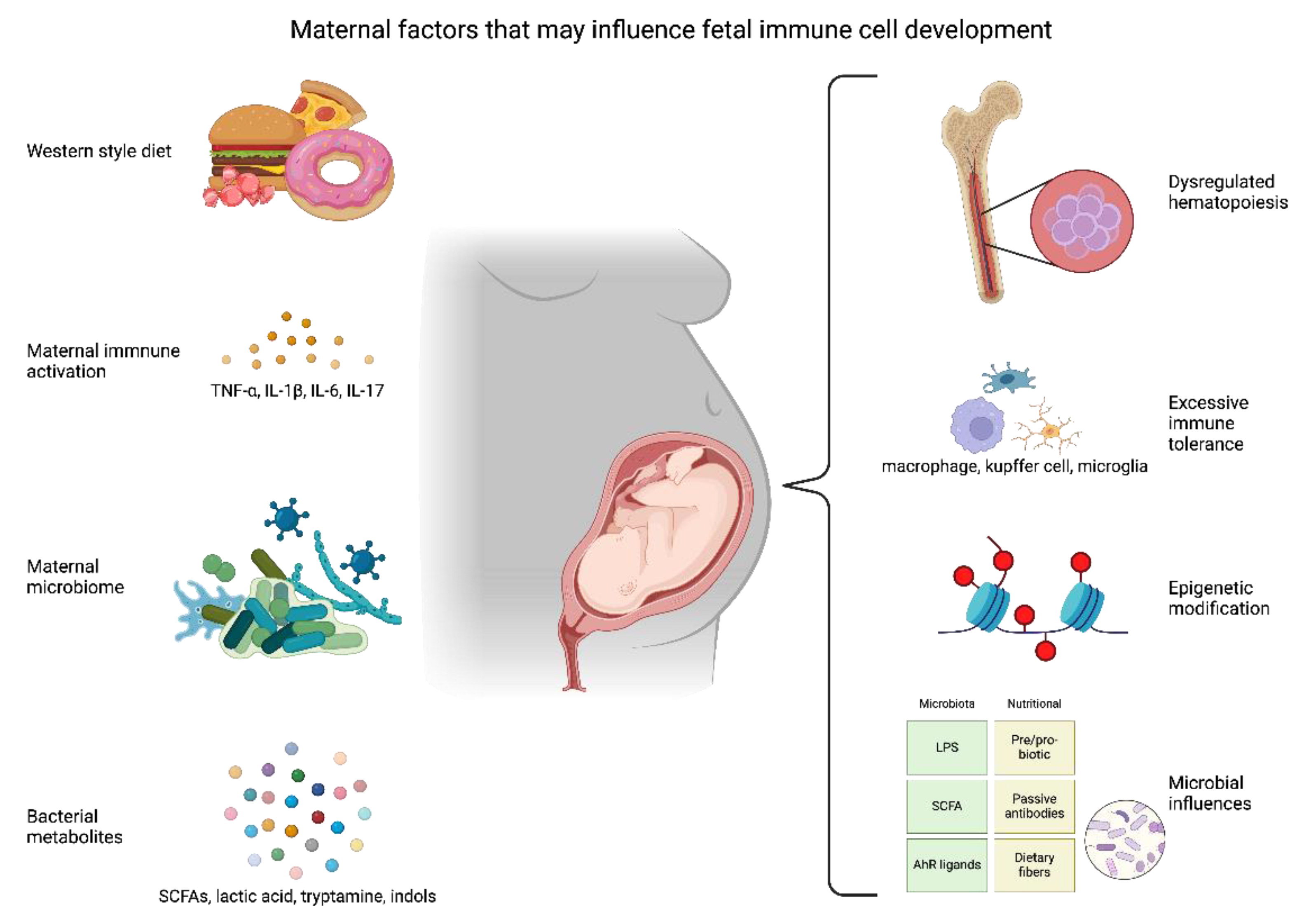
4.1. Causal Factors for Maternal WSD Remodeling of Fetal and Neonatal Immunity
5. Microglia Activation by a Maternal WSD
6. Maternal Obesity Influences the Fetal Microbiome and Immune Development
7. Metabolites Regulate Fetal Metabolism and Immune Development
8. Role of Epigenetic Programming in Immune Cells from Offspring Exposed to WSD
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Brines, J.; Rigourd, V.; Billeaud, C. The First 1000 Days of Infant. Healthcare (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Likhar, A.; Patil, M.S. Importance of Maternal Nutrition in the First 1,000 Days of Life and Its Effects on Child Development: A Narrative Review. Cureus 2022, 14, e30083. [Google Scholar] [CrossRef] [PubMed]
- Simione, M.; Moreno-Galarraga, L.; Perkins, M.; Price, S.N.; Luo, M.; Kotelchuck, M.; Blake-Lamb, T.L.; Taveras, E.M. Effects of the First 1000 Days Program, a systems-change intervention, on obesity risk factors during pregnancy. BMC Pregnancy Childbirth 2021, 21, 729. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.J.; Dobrinskikh, E.; Soderborg, T.K.; Janssen, R.C.; Takahashi, D.L.; Dean, T.A.; Varlamov, O.; Hennebold, J.D.; Gannon, M.; Aagaard, K.M.; et al. Maternal diet alters long-term innate immune cell memory in fetal and juvenile hematopoietic stem and progenitor cells in nonhuman primate offspring. Cell Rep 2023, 42, 112393. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Doratt, B.M.; Mendza, N.; Varlamov, O.; Rincon, M.; Marshall, N.E.; Messaoudi, I. Maternal obesity blunts antimicrobial responses in fetal monocytes. Elife 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yoshimoto, M. Multiple waves of fetal-derived immune cells constitute adult immune system. Immunol Rev 2023, 315, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Gentek, R. Fetal-Derived Immune Cells at the Roots of Lifelong Pathophysiology. Front Cell Dev Biol 2021, 9, 648313. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat Rev Immunol 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Brittain, T. Molecular aspects of embryonic hemoglobin function. Mol Aspects Med 2002, 23, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Kapoor, S.; Carvalho, C.; Bajenoff, M.; Gentek, R. Mast cell ontogeny: From fetal development to life-long health and disease. Immunol Rev 2023, 315, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Tober, J.; Koniski, A.; McGrath, K.E.; Vemishetti, R.; Emerson, R.; de Mesy-Bentley, K.K.; Waugh, R.; Palis, J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 2007, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.E.; Frame, J.M.; Fegan, K.H.; Bowen, J.R.; Conway, S.J.; Catherman, S.C.; Kingsley, P.D.; Koniski, A.D.; Palis, J. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep 2015, 11, 1892–1904. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 2018, 49, 640–653e645. [Google Scholar] [CrossRef]
- Calvanese, V.; Mikkola, H.K.A. The genesis of human hematopoietic stem cells. Blood 2023, 142, 519–532. [Google Scholar] [CrossRef]
- Hall, T.; Sriram, P.; McKinney-Freeman, S. Murine Fetal Bone Marrow HSPCs Undergo a Dramatic Shift in Frequency at Birth. Blood 2019, 134, 2471–2471. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F. The evolving views of hematopoiesis: from embryo to adulthood and from in vivo to in vitro. J Genet Genomics 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wijanarko, K.; Liani, O.; Strumila, K.; Ng, E.S.; Elefanty, A.G.; Stanley, E.G. Lymphoid cell development from fetal hematopoietic progenitors and human pluripotent stem cells. Immunol Rev 2023, 315, 154–170. [Google Scholar] [CrossRef]
- O'Byrne, S.; Elliott, N.; Rice, S.; Buck, G.; Fordham, N.; Garnett, C.; Godfrey, L.; Crump, N.T.; Wright, G.; Inglott, S.; et al. Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs. Blood 2019, 134, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Porayette, P.; Glosson, N.L.; Conway, S.J.; Carlesso, N.; Cardoso, A.A.; Kaplan, M.H.; Yoder, M.C. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 2012, 119, 5706–5714. [Google Scholar] [CrossRef] [PubMed]
- Luis, T.C.; Luc, S.; Mizukami, T.; Boukarabila, H.; Thongjuea, S.; Woll, P.S.; Azzoni, E.; Giustacchini, A.; Lutteropp, M.; Bouriez-Jones, T.; et al. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nat Immunol 2016, 17, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Krangel, M.S. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol 2009, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.R.; Ling, R.E.; Roy, A. The Origin of B-cells: Human Fetal B Cell Development and Implications for the Pathogenesis of Childhood Acute Lymphoblastic Leukemia. Front Immunol 2021, 12, 637975. [Google Scholar] [CrossRef]
- Pabst, O.; Nowosad, C.R. B cells and the intestinal microbiome in time, space and place. Semin Immunol 2023, 69, 101806. [Google Scholar] [CrossRef]
- Dorshkind, K.; Crooks, G. Layered immune system development in mice and humans. Immunol Rev 2023, 315, 5–10. [Google Scholar] [CrossRef]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol 2014, 72, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci Immunol 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Moffett-King, A. Natural killer cells and pregnancy. Nat Rev Immunol 2002, 2, 656–663. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Schust, D.J. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol 2010, 63, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, H.; Morozov, P.; Straus, A.; Sahasrabudhe, N.; Max, K.E.A.; Garzia, A.; Kustagi, M.; Tuschl, T.; Williams, Z. A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 2018, 4, eaau4788. [Google Scholar] [CrossRef]
- Burke, S.D.; Barrette, V.F.; Gravel, J.; Carter, A.L.; Hatta, K.; Zhang, J.; Chen, Z.; Leno-Duran, E.; Bianco, J.; Leonard, S.; et al. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am J Reprod Immunol 2010, 63, 472–481. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol 2019, 10, 792. [Google Scholar] [CrossRef]
- Wanaditya, G.K.; Putra, I.W.A.; Aryana, M.B.D.; Mulyana, R.S. Obesity in Pregnant Women and Its Impact on Maternal and Neonatal Morbidity. European Journal of Medical and Health Sciences 2023, 5, 17–21. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Marshall, N.E.; Wilson, R.M.; Barr, T.; Rais, M.; Purnell, J.Q.; Thornburg, K.L.; Messaoudi, I. Inflammatory Determinants of Pregravid Obesity in Placenta and Peripheral Blood. Frontiers in Physiology 2018, 9. [Google Scholar] [CrossRef]
- Sen, S.; Iyer, C.; Klebenov, D.; Histed, A.; Aviles, J.A.; Meydani, S.N. Obesity impairs cell-mediated immunity during the second trimester of pregnancy. American Journal of Obstetrics and Gynecology 2013, 208, 139–e131.e138. [Google Scholar] [CrossRef]
- Bravo-Flores, E.; Mancilla-Herrera, I.; Espino, Y.S.S.; Ortiz-Ramirez, M.; Flores-Rueda, V.; Ibarguengoitia-Ochoa, F.; Ibanez, C.A.; Zambrano, E.; Solis-Paredes, M.; Perichart-Perera, O.; et al. Macrophage Populations in Visceral Adipose Tissue from Pregnant Women: Potential Role of Obesity in Maternal Inflammation. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Pendeloski, K.P.T.; Ono, E.; Torloni, M.R.; Mattar, R.; Daher, S. Maternal obesity and inflammatory mediators: A controversial association. Am J Reprod Immunol 2017, 77. [Google Scholar] [CrossRef] [PubMed]
- Tinius, R.A.; Blankenship, M.M.; Furgal, K.E.; Cade, W.T.; Pearson, K.J.; Rowland, N.S.; Pearson, R.C.; Hoover, D.L.; Maples, J.M. Metabolic flexibility is impaired in women who are pregnant and overweight/obese and related to insulin resistance and inflammation. Metabolism 2020, 104, 154142. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.; Sultana, Z.; Fisher, J.J.; Maiti, K.; Smith, R. Extracellular vesicles- crucial players in human pregnancy. Placenta 2023, 140, 30–38. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; O'Connor, S.M.; Lam, G.K.; Taylor, D.D. Shed membrane fragment modulation of CD3-zeta during pregnancy: link with induction of apoptosis. J Reprod Immunol 2002, 56, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles From Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J Clin Endocrinol Metab 2019, 104, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Kandzija, N.; Zhang, W.; Motta-Mejia, C.; Mhlomi, V.; McGowan-Downey, J.; James, T.; Cerdeira, A.S.; Tannetta, D.; Sargent, I.; Redman, C.W.; et al. Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. J Extracell Vesicles 2019, 8, 1617000. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Huang, X.; Chen, P.; Deng, L.; Li, S.; Lin, S.; Wang, Z.; Liu, B. Plasma Exosomal miRNAs Associated With Metabolism as Early Predictor of Gestational Diabetes Mellitus. Diabetes 2022, 71, 2272–2283. [Google Scholar] [CrossRef]
- Sacks, G.P.; Studena, K.; Sargent, K.; Redman, C.W. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998, 179, 80–86. [Google Scholar] [CrossRef]
- Batorsky, R.; Ceasrine, A.M.; Shook, L.L.; Kislal, S.; Bordt, E.A.; Devlin, B.A.; Perlis, R.H.; Slonim, D.K.; Bilbo, S.D.; Edlow, A.G. Hofbauer cells and fetal brain microglia share transcriptional profiles and responses to maternal diet-induced obesity. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Castellana, B.; Perdu, S.; Kim, Y.; Chan, K.; Atif, J.; Marziali, M.; Beristain, A.G. Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunol Cell Biol 2018, 96, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Funamizu, A.; Yokota, M.; Yamada, K.; Nakamua, R.; Fukuhara, R.; Kimura, H.; Mizunuma, H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol 2011, 90, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Zuniga, F.; Arroyo-Jousse, V.; Soto-Carrasco, G.; Casanello, P.; Uauy, R.; Krause, B.J.; Castro-Rodriguez, J.A. IL-10 expression in macrophages from neonates born from obese mothers is suppressed by IL-4 and LPS/INFgamma. J Cell Physiol 2017, 232, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Marshall, N.E.; Jeske, D.R.; Purnell, J.Q.; Thornburg, K.; Messaoudi, I. Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol 2015, 26, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Haghiac, M.; Surace, P.; Challier, J.C.; Guerre-Millo, M.; Singh, K.; Waters, T.; Minium, J.; Presley, L.; Catalano, P.M.; et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Espinosa, L.O.; Montiel-Cervantes, L.A.; Guerra-Marquez, A.; Penaflor-Juarez, K.; Reyes-Maldonado, E.; Vela-Ojeda, J. Maternal obesity associated with increase in natural killer T cells and CD8+ regulatory T cells in cord blood units. Transfusion 2016, 56, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Mendoza, N.; Jankeel, A.; Wilson, R.M.; Marshall, N.E.; Messaoudi, I. Phenotypic and Epigenetic Adaptations of Cord Blood CD4+ T Cells to Maternal Obesity. Front Immunol 2021, 12, 617592. [Google Scholar] [CrossRef]
- Dosch, N.C.; Guslits, E.F.; Weber, M.B.; Murray, S.E.; Ha, B.; Coe, C.L.; Auger, A.P.; Kling, P.J. Maternal Obesity Affects Inflammatory and Iron Indices in Umbilical Cord Blood. J Pediatr 2016, 172, 20–28. [Google Scholar] [CrossRef]
- McCloskey, K.; Ponsonby, A.L.; Collier, F.; Allen, K.; Tang, M.L.K.; Carlin, J.B.; Saffery, R.; Skilton, M.R.; Cheung, M.; Ranganathan, S.; et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes 2018, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Marshall, N.E.; Messaoudi, I. Impact of pregravid obesity on maternal and fetal immunity: Fertile grounds for reprogramming. J Leukoc Biol 2019, 106, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Sole, A.; Luo, Y.; Vlagea, A.; Deya-Martinez, A.; Yague, J.; Plaza-Martin, A.M.; Juan, M.; Alsina, L. B Regulatory Cells: Players in Pregnancy and Early Life. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 2013, 5, 173ra123. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Messaoudi, I. The impact of maternal obesity during pregnancy on offspring immunity. Mol Cell Endocrinol 2015, 418 Pt 2, 134–142. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.M.; Bettini, M.L. Early-life microbiota-immune homeostasis. Front Immunol 2023, 14, 1266876. [Google Scholar] [CrossRef] [PubMed]
- Denizli, M.; Capitano, M.L.; Kua, K.L. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol 2022, 12, 940937. [Google Scholar] [CrossRef]
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A., Jr. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 2016, 71, 1295–1304. [Google Scholar] [CrossRef]
- Harpsoe, M.C.; Basit, S.; Bager, P.; Wohlfahrt, J.; Benn, C.S.; Nohr, E.A.; Linneberg, A.; Jess, T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol 2013, 131, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Lindell, N.; Carlsson, A.; Josefsson, A.; Samuelsson, U. Maternal obesity as a risk factor for early childhood type 1 diabetes: a nationwide, prospective, population-based case-control study. Diabetologia 2018, 61, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Odaka, Y.; Nakano, M.; Tanaka, T.; Kaburagi, T.; Yoshino, H.; Sato-Mito, N.; Sato, K. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring) 2010, 18, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Physiol Regul Integr Comp Physiol 2014, 307, R26–34. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.M.; Gomez-Lopez, J.; Medina-Bravo, P.; Juarez-Sanchez, F.; Contreras-Ramos, A.; Galicia-Esquivel, M.; Sanchez-Urbina, R.; Klunder-Klunder, M. Maternal obesity increases oxidative stress in the newborn. Obesity (Silver Spring) 2015, 23, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.E.; Bishop, J.M.; Williams, S.M.; Grayson, B.E.; Smith, M.S.; Friedman, J.E.; Grove, K.L. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009, 119, 323–335. [Google Scholar] [CrossRef]
- Mouralidarane, A.; Soeda, J.; Visconti-Pugmire, C.; Samuelsson, A.M.; Pombo, J.; Maragkoudaki, X.; Butt, A.; Saraswati, R.; Novelli, M.; Fusai, G.; et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 2013, 58, 128–138. [Google Scholar] [CrossRef]
- Cohen, C.C.; Francis, E.C.; Perng, W.; Sauder, K.A.; Scherzinger, A.; Sundaram, S.S.; Shankar, K.; Dabelea, D. Exposure to maternal fuels during pregnancy and offspring hepatic fat in early childhood: The healthy start study. Pediatr Obes 2022, 17, e12902. [Google Scholar] [CrossRef]
- Luci, C.; Bourinet, M.; Leclere, P.S.; Anty, R.; Gual, P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front Endocrinol (Lausanne) 2020, 11, 597648. [Google Scholar] [CrossRef]
- Platek, A.E.; Szymanska, A. Metabolic dysfunction-associated steatotic liver disease as a cardiovascular risk factor. Clin Exp Hepatol 2023, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Doskey, L.C.; Malhi, H. The Role of Endoplasmic Reticulum in Lipotoxicity during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Pathogenesis. Am J Pathol 2023, 193, 1887–1899. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Albano, E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol 2020, 17, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014, 14, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Jindal, A.; Locatelli, I.; Vacchiano, M.; Gigliotti, L.; Bozzola, C.; Albano, E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014, 59, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Takahara, T.; Kawai, K.; Fujino, M.; Sugiyama, T.; Tsuneyama, K.; Tsukada, K.; Nakae, S.; Zhong, L.; Li, X.K. IFN-gamma deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am J Physiol Gastrointest Liver Physiol 2013, 305, G891–899. [Google Scholar] [CrossRef]
- Ferreyra Solari, N.E.; Inzaugarat, M.E.; Baz, P.; De Matteo, E.; Lezama, C.; Galoppo, M.; Galoppo, C.; Chernavsky, A.C. The role of innate cells is coupled to a Th1-polarized immune response in pediatric nonalcoholic steatohepatitis. J Clin Immunol 2012, 32, 611–621. [Google Scholar] [CrossRef]
- Inzaugarat, M.E.; Ferreyra Solari, N.E.; Billordo, L.A.; Abecasis, R.; Gadano, A.C.; Chernavsky, A.C. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol 2011, 31, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Chan, C.N.; Robino, J.J.; Parmelee, L.K.; Nash, M.J.; Wesolowski, S.R.; Pietras, E.M.; Friedman, J.E.; Takahashi, D.; Shen, W.; et al. Maternal Western-style diet remodels the transcriptional landscape of fetal hematopoietic stem and progenitor cells in rhesus macaques. Stem Cell Reports 2022, 17, 2595–2609. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O'Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Akdis, M.; Akdis, C.A. Influence of Innate Immunity on Immune Tolerance. Acta Med Acad 2020, 49, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Weis, S.; Netea, M.G.; Wetzker, R. Remembering Pathogen Dose: Long-Term Adaptation in Innate Immunity. Trends Immunol 2018, 39, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Iredale, J.P. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J. Hepatol. 2012, 56, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.A.; MacKinnon, A.C.; Ramachandran, P.; Dhaliwal, K.; Duffin, R.; Phythian-Adams, A.T.; van Rooijen, N.; Haslett, C.; Howie, S.E.; Simpson, A.J.; et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med 2011, 184, 569–581. [Google Scholar] [CrossRef]
- Li, M.; Riddle, S.; Zhang, H.; D'Alessandro, A.; Flockton, A.; Serkova, N.J.; Hansen, K.C.; Moldvan, R.; McKeon, B.A.; Frid, M.; et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional co-repressor C-terminal binding protein-1. Circulation 2016, 134, 1105–1121. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Stenmark, K.R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol 2015, 27, 267–275. [Google Scholar] [CrossRef] [PubMed]
- El Kasmi, K.C.; Pugliese, S.C.; Riddle, S.R.; Poth, J.M.; Anderson, A.L.; Frid, M.G.; Li, M.; Pullamsetti, S.S.; Savai, R.; Nagel, M.A.; et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J. Immunol. 2014, 193, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Sureshchandra, S.; Marshall, N.E.; Mendoza, N.; Jankeel, A.; Zulu, M.Z.; Messaoudi, I. Functional and genomic adaptations of blood monocytes to pregravid obesity during pregnancy. iScience 2021, 24, 102690. [Google Scholar] [CrossRef]
- Xu, E.; Pereira, M.M.A.; Karakasilioti, I.; Theurich, S.; Al-Maarri, M.; Rappl, G.; Waisman, A.; Wunderlich, F.T.; Bruning, J.C. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun 2017, 8, 14803. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; McFadden, T.; Link, V.M.; Han, S.J.; Karlsson, R.M.; Stacy, A.; Farley, T.K.; Lima-Junior, D.S.; Harrison, O.J.; Desai, J.V.; et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 2021, 373. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef]
- Ghazarian, M.; Revelo, X.S.; Nohr, M.K.; Luck, H.; Zeng, K.; Lei, H.; Tsai, S.; Schroer, S.A.; Park, Y.J.; Chng, M.H.Y.; et al. Type I Interferon Responses Drive Intrahepatic T cells to Promote Metabolic Syndrome. Sci Immunol 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, M.; Wiede, F.; Dodd, G.T.; Gurzov, E.N.; Ooi, G.J.; Butt, T.; Rasmiena, A.A.; Kaur, S.; Gulati, T.; Goh, P.K.; et al. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell 2018, 175, 1289–1306 e1220. [Google Scholar] [CrossRef]
- Thapa, M.; Chinnadurai, R.; Velazquez, V.M.; Tedesco, D.; Elrod, E.; Han, J.H.; Sharma, P.; Ibegbu, C.; Gewirtz, A.; Anania, F.; et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology 2015, 61, 2067–2079. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Sage, A.P.; Mallat, Z.; Binder, C.J. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol 2015, 35, 296–302. [Google Scholar] [CrossRef]
- Magalhaes, M.S.; Potter, H.G.; Ahlback, A.; Gentek, R. Developmental programming of macrophages by early life adversity. Int Rev Cell Mol Biol 2022, 368, 213–259. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 2015, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Thapa, N.; Facchini, S.; Stubbs, B.; Fornaro, M.; Carvalho, A.F.; Correll, C.U. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr 2017, 22, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Mitchell, A.J.; Selby, M.; Fair, D.A.; Gustafsson, H.C.; Sullivan, E.L. Maternal diet and obesity shape offspring central and peripheral inflammatory outcomes in juvenile non-human primates. Brain Behav Immun 2022, 102, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Khambadkone, S.G.; Dunn, G.; Bagley, J.; Tamashiro, K.L.K.; Fair, D.; Gustafsson, H.; Sullivan, E.L. Maternal Western-style diet reduces social engagement and increases idiosyncratic behavior in Japanese macaque offspring. Brain Behav Immun 2022, 105, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, S.; Thompson, J.R.; Feczko, E.; Miranda-Dominguez, O.; Dunn, G.A.; Selby, M.; Mitchell, A.J.; Sullivan, E.L.; Fair, D.A. Perinatal Western-style diet exposure associated with decreased microglial counts throughout the arcuate nucleus of the hypothalamus in Japanese macaques. J Neurophysiol 2024, 131, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J Clin Invest 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Tome, S.; Takei, Y. Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Mol Brain 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.; N, A.D. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res Rev 2021, 68, 101323. [Google Scholar] [CrossRef]
- Nichols, R.G.; Davenport, E.R. The relationship between the gut microbiome and host gene expression: a review. Hum Genet 2021, 140, 747–760. [Google Scholar] [CrossRef]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig Dis Sci 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017, 15, 73. [Google Scholar] [CrossRef]
- Koren, O.; Konnikova, L.; Brodin, P.; Mysorekar, I.U.; Collado, M.C. The maternal gut microbiome in pregnancy: implications for the developing immune system. Nat Rev Gastroenterol Hepatol 2024, 21, 35–45. [Google Scholar] [CrossRef]
- Jennison, E.; Byrne, C.D. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin Mol Hepatol 2021, 27, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A maternal higher-complex carbohydrate diet increases bifidobacteria and alters early life acquisition of the infant microbiome in women with gestational diabetes mellitus. Front Endocrinol (Lausanne) 2022, 13, 921464. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Csaszar, A.; Bodis, J.; Kovacs, K. The Maternal-Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hamalainen, A.M.; Harkonen, T.; Ryhanen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016, 8, 343ra381. [Google Scholar] [CrossRef]
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal Vertical Transmission Affecting Early-life Microbiota Development. Trends Microbiol 2020, 28, 28–45. [Google Scholar] [CrossRef]
- Li, W.; Tapiainen, T.; Brinkac, L.; Lorenzi, H.A.; Moncera, K.; Tejesvi, M.V.; Salo, J.; Nelson, K.E. Vertical Transmission of Gut Microbiome and Antimicrobial Resistance Genes in Infants Exposed to Antibiotics at Birth. J Infect Dis 2021, 224, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Bourdet-Sicard, R.; Brandtzaeg, P.; Gill, H.S.; McGuirk, P.; van Eden, W.; Versalovic, J.; Weinstock, J.V.; Rook, G.A. Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol 2006, 3, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Soderborg, T.K.; Clark, S.E.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 2018, 9, 4462. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Medzhitov, R. On developmental programming of the immune system. Trends Immunol 2023, 44, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, Y.; Zhang, H.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Intestinal 'Infant-Type' Bifidobacteria Mediate Immune System Development in the First 1000 Days of Life. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 2012, 7, e36957. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; van Sinderen, D.; Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int J Food Microbiol 2011, 149, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.T.; Roesch, L.F.W.; Ordberg, M.; Ilonen, J.; Atkinson, M.A.; Schatz, D.A.; Triplett, E.W.; Ludvigsson, J. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat Commun 2019, 10, 3621. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d'Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hamalainen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Minichino, D.; Biswas, C.; Chu, N.; Lee, J.J.; Bittinger, K.; Albeituni, S.; Nichols, K.E.; Behrens, E.M. Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Soderborg, T.K.; Carpenter, C.M.; Janssen, R.C.; Weir, T.L.; Robertson, C.E.; Ir, D.; Young, B.E.; Krebs, N.F.; Hernandez, T.L.; Barbour, L.A.; et al. Gestational Diabetes Is Uniquely Associated With Altered Early Seeding of the Infant Gut Microbiota. Front Endocrinol (Lausanne) 2020, 11, 603021. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, Y.; Chen, M.; Zhang, Y.; Wang, X.; Dong, T.; Chen, G.; Sun, X.; Lu, T.; White, R.A., 3rd; et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med 2021, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Reinke, S.N.; Mousavi-Derazmahalleh, M.; Garssen, J.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Keelan, J.; Prescott, S.L.; Palmer, D.J.; et al. Maternal prebiotic supplementation during pregnancy and lactation modifies the microbiome and short chain fatty acid profile of both mother and infant. Clin Nutr 2024, 43, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Barman, P.K.; Goodridge, H.S. Microbial Sensing by Hematopoietic Stem and Progenitor Cells. Stem Cells 2022, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Paucar Iza, Y.A.; Brown, C.C. Early life imprinting of intestinal immune tolerance and tissue homeostasis. Immunol Rev 2024. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Delbauve, S.; Smout, J.; Torres, D.; Flamand, V. Very early-life exposure to microbiota-induced TNF drives the maturation of neonatal pre-cDC1. Gut 2021, 70, 511–521. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Belkaid, Y. Early-life imprinting of unconventional T cells and tissue homeostasis. Science 2021, 374, eabf0095. [Google Scholar] [CrossRef]
- Sefik, E.; Geva-Zatorsky, N.; Oh, S.; Konnikova, L.; Zemmour, D.; McGuire, A.M.; Burzyn, D.; Ortiz-Lopez, A.; Lobera, M.; Yang, J.; et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015, 349, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, E.M.; Shao, T.Y.; Woo, V.; Rice, T.; Engleman, L.; Didriksen, B.J.; Whitt, J.; Haslam, D.B.; Way, S.S.; Alenghat, T. Intestinal epithelial HDAC3 and MHC class II coordinate microbiota-specific immunity. J Clin Invest 2023, 133. [Google Scholar] [CrossRef] [PubMed]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Dejardin, F.; Sparwasser, T.; Berard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288 e1275. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Lai, G.C.; Yao, L.J.; Aung, T.T.; Shental, N.; Rotter-Maskowitz, A.; Shepherdson, E.; Singh, G.S.N.; Pai, R.; Shanti, A.; et al. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184, 3394–3409 e3320. [Google Scholar] [CrossRef]
- Hu, M.; Eviston, D.; Hsu, P.; Marino, E.; Chidgey, A.; Santner-Nanan, B.; Wong, K.; Richards, J.L.; Yap, Y.A.; Collier, F.; et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun 2019, 10, 3031. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Rager, S.L.; Carrow, H.C.; Ananthanarayanan, A.; Callaghan, R.; Hart, L.R.; Li, T.; Ravisankar, P.; Brown, J.A.; Amir, M.; et al. Gut bacteria-derived serotonin promotes immune tolerance in early life. Sci Immunol 2024, 9, eadj4775. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yanez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Josefsdottir, K.S.; Baldridge, M.T.; Kadmon, C.S.; King, K.Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 2017, 129, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Staffas, A.; Burgos da Silva, M.; Slingerland, A.E.; Lazrak, A.; Bare, C.J.; Holman, C.D.; Docampo, M.D.; Shono, Y.; Durham, B.; Pickard, A.J.; et al. Nutritional Support from the Intestinal Microbiota Improves Hematopoietic Reconstitution after Bone Marrow Transplantation in Mice. Cell Host Microbe 2018, 23, 447–457 e444. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Yanez, A.; Coetzee, S.G.; Olsson, A.; Muench, D.E.; Berman, B.P.; Hazelett, D.J.; Salomonis, N.; Grimes, H.L.; Goodridge, H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47, 890–902 e894. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.; Melandri, D.; Anselmi, G.; Perchet, T.; Loschko, J.; Dubrot, J.; Patel, R.; Gautier, E.L.; Hugues, S.; Longhi, M.P.; et al. The Heterogeneity of Ly6C(hi) Monocytes Controls Their Differentiation into iNOS(+) Macrophages or Monocyte-Derived Dendritic Cells. Immunity 2016, 45, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, K.R.; Abrams, J.; Friedman, J.E. Maternal Diet Alters Trained Immunity in the Pathogenesis of Pediatric NAFLD. J Cell Immunol 2020, 2, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, G.L.; Hannemann, N.; Ipseiz, N.; Kronke, G.; Bauerle, T.; Munos, L.; Wirtz, S.; Schett, G.; Bozec, A. Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metab 2015, 22, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Bermick, J.; Schaller, M. Chorioamnionitis exposure dampens the preterm monocyte response to subsequent challenges. Immunol Cell Biol 2018, 96, 789–791. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Hancock, D.G.; Wells, C.; Richmond, P.; Simmer, K.; Burgner, D.; Strunk, T.; Currie, A.J. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylococcus epidermidis. Immunol Cell Biol 2018, 96, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol 2016, 7, 185. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Developmental Programming and Reprogramming of Hypertension and Kidney Disease: Impact of Tryptophan Metabolism. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Patil, N.Y.; Friedman, J.E.; Joshi, A.D. Role of Hepatic Aryl Hydrocarbon Receptor in Non-Alcoholic Fatty Liver Disease. Receptors (Basel) 2023, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 2002, 1, 287–299. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 2010, 285, 27601–27608. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Gunther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front Immunol 2021, 12, 678360. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with Sodium Butyrate Modulates the Composition of the Gut Microbiota and Ameliorates High-Fat Diet-Induced Obesity in Mice. J Nutr 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.Y.; Li, S.P.; Zhao, H.M.; Jiang, F.J.; Wu, Y.X.; Tong, Y.; Pang, Q.F. Maternal propionate supplementation ameliorates glucose and lipid metabolic disturbance in hypoxia-induced fetal growth restriction. Food Funct 2022, 13, 10724–10736. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Lischka, J.; Schanzer, A.; Baumgartner, M.; de Gier, C.; Greber-Platzer, S.; Zeyda, M. Tryptophan Metabolism Is Associated with BMI and Adipose Tissue Mass and Linked to Metabolic Disease in Pediatric Obesity. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep 2018, 23, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, Y.; Park, S.; Lee, K.W.; Park, T. Indole-3-carbinol prevents diet-induced obesity through modulation of multiple genes related to adipogenesis, thermogenesis or inflammation in the visceral adipose tissue of mice. J Nutr Biochem 2012, 23, 1732–1739. [Google Scholar] [CrossRef]
- Shinde, R.; McGaha, T.L. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol 2018, 39, 1005–1020. [Google Scholar] [CrossRef]
- Bock, K.W. Aryl hydrocarbon receptor (AHR), integrating energy metabolism and microbial or obesity-mediated inflammation. Biochem Pharmacol 2021, 184, 114346. [Google Scholar] [CrossRef] [PubMed]
- Ghiboub, M.; Verburgt, C.M.; Sovran, B.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy to Modulate Tryptophan Metabolism and Aryl Hydrocarbon-Receptor Signaling Activation in Human Diseases. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014, 5, 3611. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Morbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Molgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol 2021, 6, 1367–1382. [Google Scholar] [CrossRef]
- Wang, J.P.; Yoo, J.S.; Lee, J.H.; Jang, H.D.; Kim, H.J.; Shin, S.O.; Seong, S.I.; Kim, I.H. Effects of phenyllactic acid on growth performance, nutrient digestibility, microbial shedding, and blood profile in pigs. J Anim Sci 2009, 87, 3235–3243. [Google Scholar] [CrossRef]
- Zugasti, O.; Bose, N.; Squiban, B.; Belougne, J.; Kurz, C.L.; Schroeder, F.C.; Pujol, N.; Ewbank, J.J. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nat Immunol 2014, 15, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, S.W.; Niemiec, S.; Wood, C.; Gyllenhammer, L.E.; Jansson, T.; Friedman, J.E.; Tryggestad, J.B.; Borengasser, S.J.; Davidson, E.J.; Yang, I.V.; et al. Cord blood DNA methylation of immune and lipid metabolism genes is associated with maternal triglycerides and child adiposity. Obesity (Silver Spring) 2024, 32, 187–199. [Google Scholar] [CrossRef]
- van der Heijden, C.; Noz, M.P.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and Trained Immunity. Antioxid Redox Signal 2018, 29, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Dominguez-Andres, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef]
- Suter, M.; Bocock, P.; Showalter, L.; Hu, M.; Shope, C.; McKnight, R.; Grove, K.; Lane, R.; Aagaard-Tillery, K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J 2011, 25, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.A.; Takahashi, D.; Grove, K.L.; Aagaard, K.M. Postweaning exposure to a high-fat diet is associated with alterations to the hepatic histone code in Japanese macaques. Pediatr Res 2013, 74, 252–258. [Google Scholar] [CrossRef]
- Harmancioglu, B.; Kabaran, S. Maternal high fat diets: impacts on offspring obesity and epigenetic hypothalamic programming. Front Genet 2023, 14, 1158089. [Google Scholar] [CrossRef] [PubMed]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Segovia, S.A.; Vickers, M.H. Experimental Models of Maternal Obesity and Neuroendocrine Programming of Metabolic Disorders in Offspring. Front Endocrinol (Lausanne) 2017, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.G.; Desai, M. Developmental programming of appetite/satiety. Ann Nutr Metab 2014, 64 Suppl 1, 36–44. [Google Scholar] [CrossRef]
- Chen, H.; Simar, D.; Morris, M.J. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PLoS One 2009, 4, e6259. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 2000, 279, E83–87. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Sardinha, F.L.; Telles, M.M.; Albuquerque, K.T.; Oyama, L.M.; Guimaraes, P.A.; Santos, O.F.; Ribeiro, E.B. Gender difference in the effect of intrauterine malnutrition on the central anorexigenic action of insulin in adult rats. Nutrition 2006, 22, 1152–1161. [Google Scholar] [CrossRef]
- Galjaard, S.; Devlieger, R.; Van Assche, F.A. Fetal growth and developmental programming. J Perinat Med 2013, 41, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gali Ramamoorthy, T.; Allen, T.J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int J Obes (Lond) 2018, 42, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A.; Harder, T.; Brunn, M.; Harder, A.; Roepke, K.; Wittrock-Staar, M.; Ziska, T.; Schellong, K.; Rodekamp, E.; Melchior, K.; et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol 2009, 587, 4963–4976. [Google Scholar] [CrossRef]
- Suter, M.A.; Chen, A.; Burdine, M.S.; Choudhury, M.; Harris, R.A.; Lane, R.H.; Friedman, J.E.; Grove, K.L.; Tackett, A.J.; Aagaard, K.M. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J 2012, 26, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Funato, H.; Oda, S.; Yokofujita, J.; Igarashi, H.; Kuroda, M. Fasting and high-fat diet alter histone deacetylase expression in the medial hypothalamus. PLoS One 2011, 6, e18950. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-alpha on appetite-regulating NPY neurons. Int J Obes (Lond) 2017, 41, 149–158. [Google Scholar] [CrossRef]
- Grayson, B.E.; Levasseur, P.R.; Williams, S.M.; Smith, M.S.; Marks, D.L.; Grove, K.L. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010, 151, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, W.; Jin, H.; Chen, X. A Review of Single-Cell RNA-Seq Annotation, Integration, and Cell-Cell Communication. Cells 2023, 12. [Google Scholar] [CrossRef]
- Chen, B.; de Launoit, E.; Renier, N.; Schneeberger, M. Maternal nutritional programming shapes the cerebral landscape. Trends Endocrinol Metab 2023. [Google Scholar] [CrossRef]
- Wu, R.; Prachyathipsakul, T.; Zhuang, J.; Liu, H.; Han, Y.; Liu, B.; Gong, S.; Qiu, J.; Wong, S.; Ribbe, A.; et al. Conferring liver selectivity to a thyromimetic using a novel nanoparticle increases therapeutic efficacy in a diet-induced obesity animal model. PNAS Nexus 2023, 2, pgad252. [Google Scholar] [CrossRef]
- Ayyangar, U.; Karkhanis, A.; Tay, H.; Afandi, A.F.B.; Bhattacharjee, O.; Ks, L.; Lee, S.H.; Chan, J.; Raghavan, S. Metabolic rewiring of macrophages by epidermal-derived lactate promotes sterile inflammation in the murine skin. EMBO J 2024, 43, 1113–1134. [Google Scholar] [CrossRef] [PubMed]
| Subjects (year) | Physiological outcomes | Programming effects | Reference |
| 222 human infants and NOD mice (2016) |
|
|
[150] |
| Germ-free mice colonized with microbes of infants from obese or NW mothers (2018) |
|
|
[143] |
| 17,055 neonates from the general population (2019) |
|
|
[149] |
| Antibiotic-treated and germ-free mice (2019) |
|
|
[152] |
| 46 full-term neonates from obese mother with and without GDM (2020) |
|
|
[153] |
| 418 mothers and their neonates (2021) |
|
|
[154] |
| 394 pregnant women in the first trimester and germ-free mice (2023) |
|
|
[155] |
| 65 mother-infant pairs, half receiving a prebiotic (2024) |
|
|
[156] |
| Metabolite | Mechanism | Effect | Reference |
|---|---|---|---|
| Acetate | FFAR2 and acetyl-CoA carboxylase | Promoted lipid metabolism and reduced appetite | [203,204] |
| Propionate | FFAR2 and FFAR3 | Promoted glucose metabolism | [203] |
| Butyrate | FFAR3 | Anti-inflammatory effects and promoted lipid metabolism | [203] |
| Indolelactic Acid | AHR and HCA3 | Improved gut health and immune responses | [205] |
| Pheyllactic Acid | Mineral utilization | Increased lymphoid cell count without infection | [206] |
| 4-Hydroxyphenyllactic Acid | DCAR-1 | Triggered increased innate immune responses | [207] |
| Tryptamine | AHR | Suppressed inflammation | [197] |
| Indole-3-acetate | AHR | Suppressed inflammation | [197] |
| Indole-3-ethanol | MyoIIA, ezrin | Maintained gut permeability | [198] |
| Indole-3-pyruvate | MyoIIA, ezrin | Maintained gut permeability | [198] |
| Indole-3-aldehye | MyoIIA, ezrin | Maintained gut permeability | [198] |
| Indole-3-carbinol | UCPs, PPAR, sirtuin-1, leptin, aP2 | Decreased adipogenesis, thermogenesis, and inflammation | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





