1. Introduction
The exceptional properties of titanium alloys, which include high corrosion resistance in the tissue environment, biocompatibility, high relative strength Rm/ρ, as well as a low value of Young's modulus, the closest of all metallic materials to Young’s modulus of bone, determine their usefulness in implant applications, particularly in orthopedics [
1]. The most commonly used titanium alloys in medicine are Ti6Al4V and Ti6Al7Nb.
Titanium alloys have the ability to self-passivation, which is responsible for their high corrosion resistance. It results in the spontaneous formation of a passive oxide layer with a thickness of a few nanometers, which is characterized by low electronic conductivity and consists of amorphous titanium dioxide (TiO
2), which forms its outer part, and non-stoichiometric TiO oxides
2-x in the inner part [1-3]. In addition, it consists of a small volume of oxides of aluminum and vanadium or niobium, depending on the chemical composition of the alloy [
4]. Their presence in the surface layer can cause adverse effects in the body, such as vanadium-induced cytotoxic reactions or bone softening, which is affected by aluminum [5-6]. To eliminate the adverse effects of vanadium, the vanadium-free Ti6Al7Nb alloy is increasingly being used.
The undesirable effects of the contribution of aluminum ions to the passive layer are one of the reasons for the widely developing field of surface modification of alloys to improve their biocompatibility, as well as their physicochemical properties. The surface treatment technologies used can be most broadly divided into mechanical, chemical and physical methods [7-9]. Depending on the surface modification method, the following can be improved: mechanical contact between the implant and bone tissue, abrasion resistance, corrosion resistance, biocompatibility and bioactivity [
8]. Ultimately, this can contribute to the success of the treatment process. Considering the desired way in which the surface of the material interacts with the bone tissue, two distinct goals of the modifications can be distinguished: improving osteoconductivity or improving osteoinductivity [10-11].
The purpose of using the mechanical method of modification is to obtain a surface with the desired topography, which determines the adhesive properties of the layer to the substrate [
8]. The required surface topography is determined primarily by the intended use of the product. For example, in the case of implants used in orthopedics, in order to allow bone tissue adhesion, it is necessary to ensure adequate surface roughness [
12], conversely for implants for blood contact, where the surface should be as smooth as possible [
13]. This goal can be achieved by grinding, polishing or sandblasting (abrasive blasting) [
7,
14]. One of the most popular methods used in biomedical engineering is sandblasting with silicon carbide or oxide particles, aluminum oxide, biphasic calcium phosphates (BCPs), and hydroxyapatite particles with calcium phosphates [
8,
15]. Sandblasting the surface of titanium alloys causes a change in the chemical composition of the surface layer, an increase in surface roughness and a strengthening of the surface, which affects a decrease in resistance to pitting corrosion and an increase in the amount of ions penetrating from the surface [
16]. Chemical methods of surface modification are based on reactions occurring at the interface between titanium alloys and the solution [
15]. They lead to improved biocompatibility, bioactivity and osteoconductivity, as well as corrosion resistance, and may remove contaminants on the surface. Depending on the medium used, treatment with acids, alkalis or hydrogen peroxide can be distinguished [
8].
One of the most popular methods of surface modification is anodic oxidation. Such a process involves the formation of an oxide layer on the surface of the anode, which is either a metal or its alloy. The reactions occurring between the anode and cathode are initiated by the flow of current from an external power source [
17]. Anodization can be carried out in three ways: under potentiostatic conditions, under galvanostatic conditions, and by a combined method [
18].
Obtaining a passive layer with controlled properties is possible by controlling the process parameters, which include the chemical composition of the electrolyte, pH of the electrolyte, temperature, current voltage, current density, hydrodynamic conditions and process duration [
18]. The chemical composition of the bath used determines the composition of the resulting passive layer, which allows the bioactivity to be improved, or vice versa, depending on the components selected [
2]. Its thickness increases faster in acidic than in basic electrolytes [
19]. After the anodic oxidation process, there is an improvement in pitting corrosion resistance. However, the fatigue strength of the material decreases [
2]. The value of the voltage used during the process affects the thickness of the layer, as well as its morphology. To obtain a homogeneous layer that inherits the substrate topography, the process should be carried out at medium voltage values. In this voltage range, a color effect is observed, which depends on the thickness of the oxide layer obtained. The use of a voltage lower than 40 V leads to a layer of nanotubes, which is favorable for good adhesion of hydroxyapatite particles to the substrate. This results in a much more strongly bonded and stable layer, which can extend the life of the implant. Spark anodic oxidation and plasma electrolytic oxidation methods are also promising. When conducting the plasma process, it is possible to use a current of more than 150 V, while in the case of spark oxidation, a pulsed voltage in the range of 200 - 500 V is used. As a result of both processes, porous layers are obtained with pore sizes that vary depending on the conditions of the processes [
2,
8,
20].
Despite the extensive use of anodic oxidation of titanium implants in orthopedics, there are few works focused on a detailed analysis of the chemical composition of the surface layers. In addition, the effect of treatments before anodic oxidation on the properties of the produced layer has not been analyzed. Thus, the purpose of this study was to analyze in detail the chemical composition of the passive layer of Ti6Al7Nb alloy obtained by anodic oxidation preceded by sandblasting. Moreover, the physicochemical properties of the analyzed surface affecting biocompatibility were determined. For this purpose, surface observation, wettability and roughness tests, microhardness tests, electrochemical tests and phase composition analysis were carried out. In addition, the preclinical suitability, of the surface modified in this way, was evaluated by cytotoxicity tests.
4. Discussion
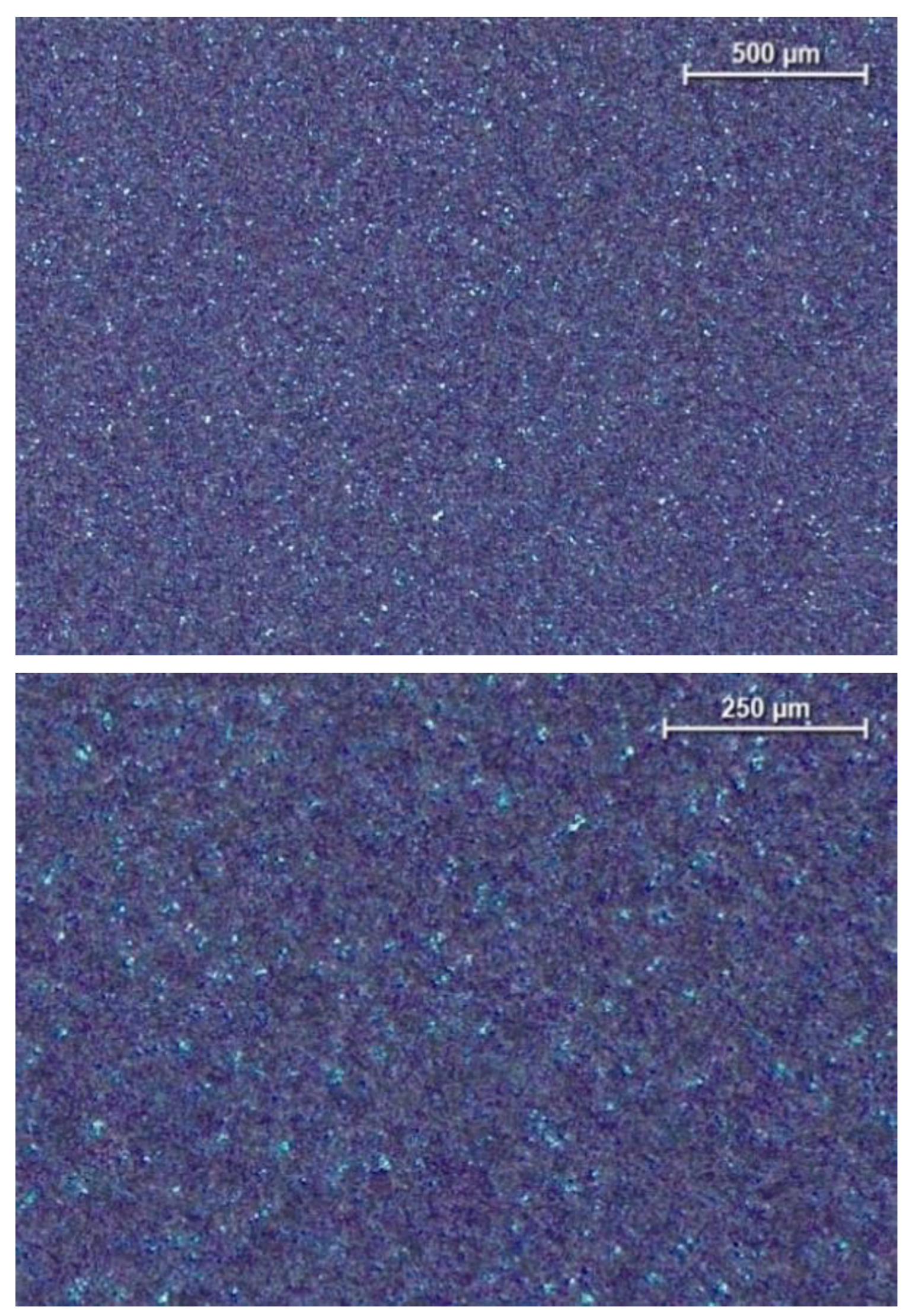
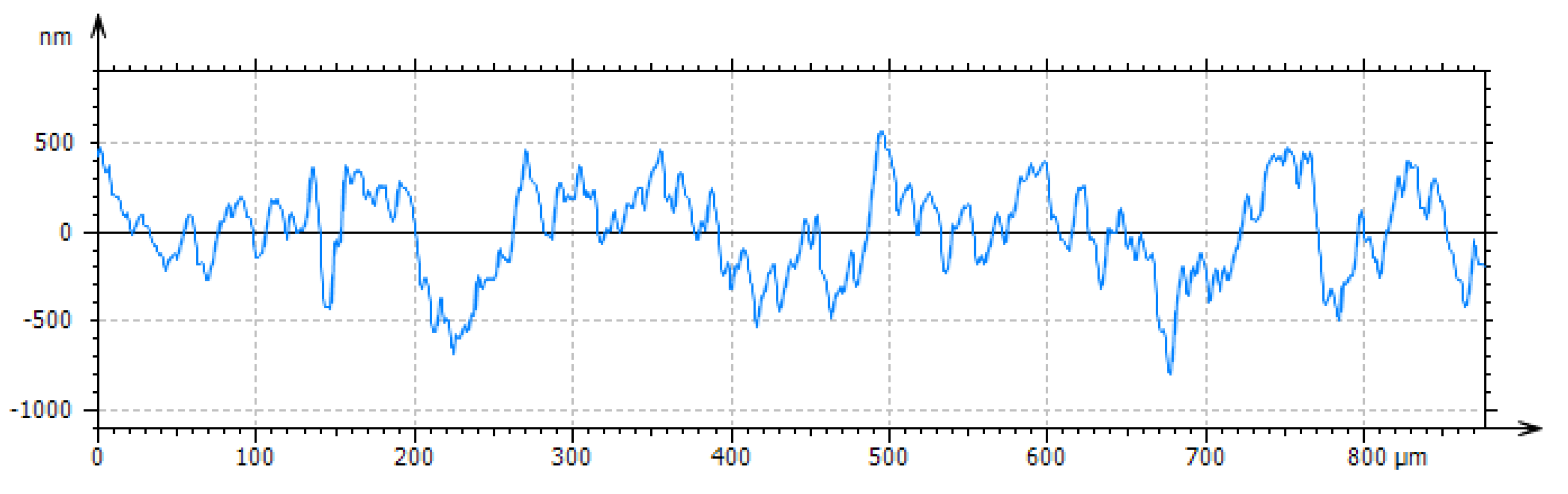
The surface of the samples was characterized by traces typical of sandblasting. The surface was characterized by high homogeneity, and its roughness was Sa = 0.396(84) μm and Ra = 0.343(97) μm. The uneven surface coloring is the result of differential light interference after the anodic oxidation process caused by its development (
Figure 1-2). Previously conducted research [
21] indicates that the topography of the surface of anodically oxidized samples depends on the modification procedures carried out before. Its thickness obtained at an oxidation voltage of 97V is about 200 nm. The obtained value of the wetting angle (Θ = 54⁰) indicates the hydrophilic properties of the surface. Based on the study of Yamamoto et al., it can be concluded that the analyzed surface of Ti6Al7Nb titanium alloy has wettability and surface roughness that should provide good osteoconductive properties [
22].
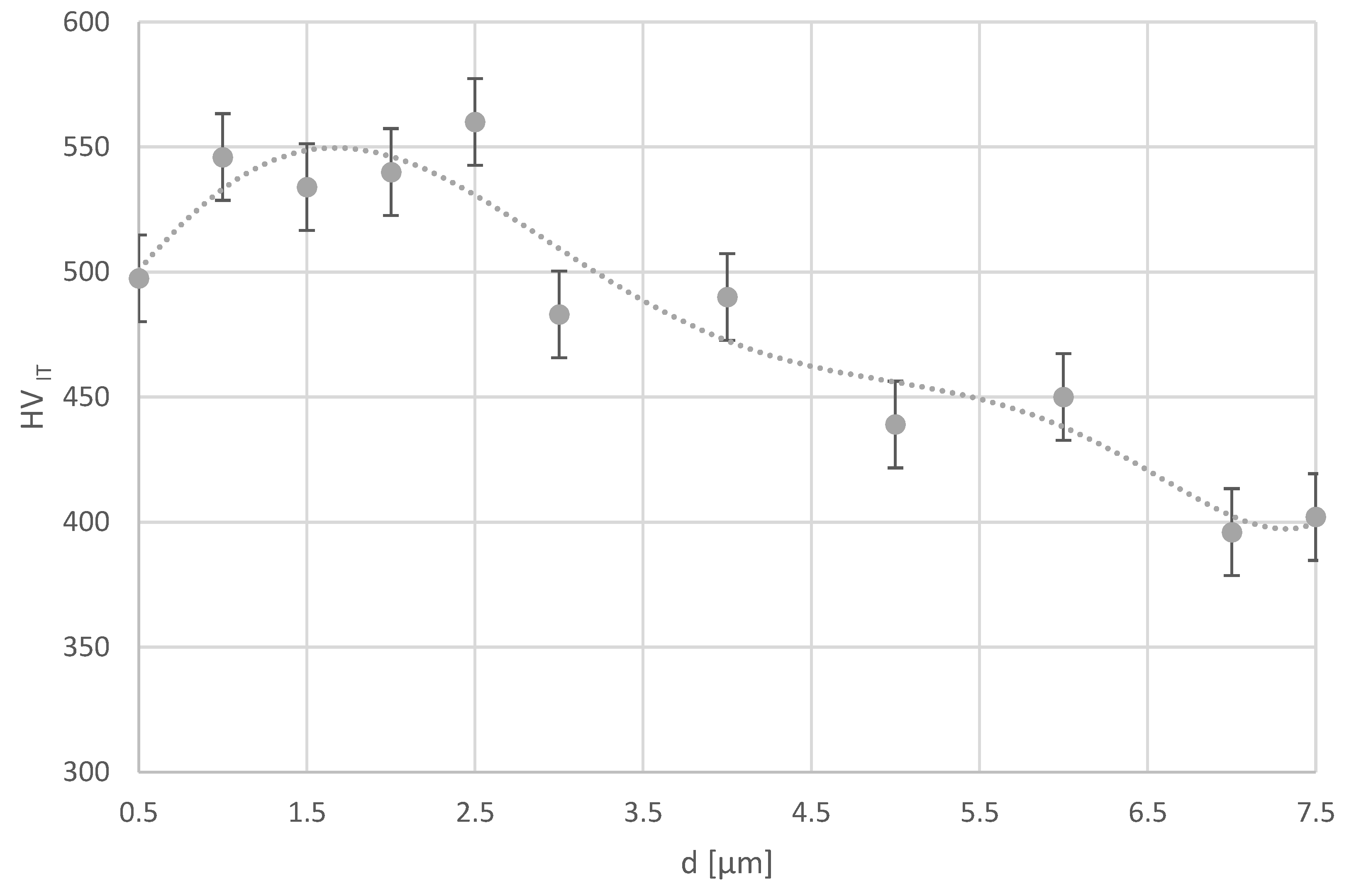
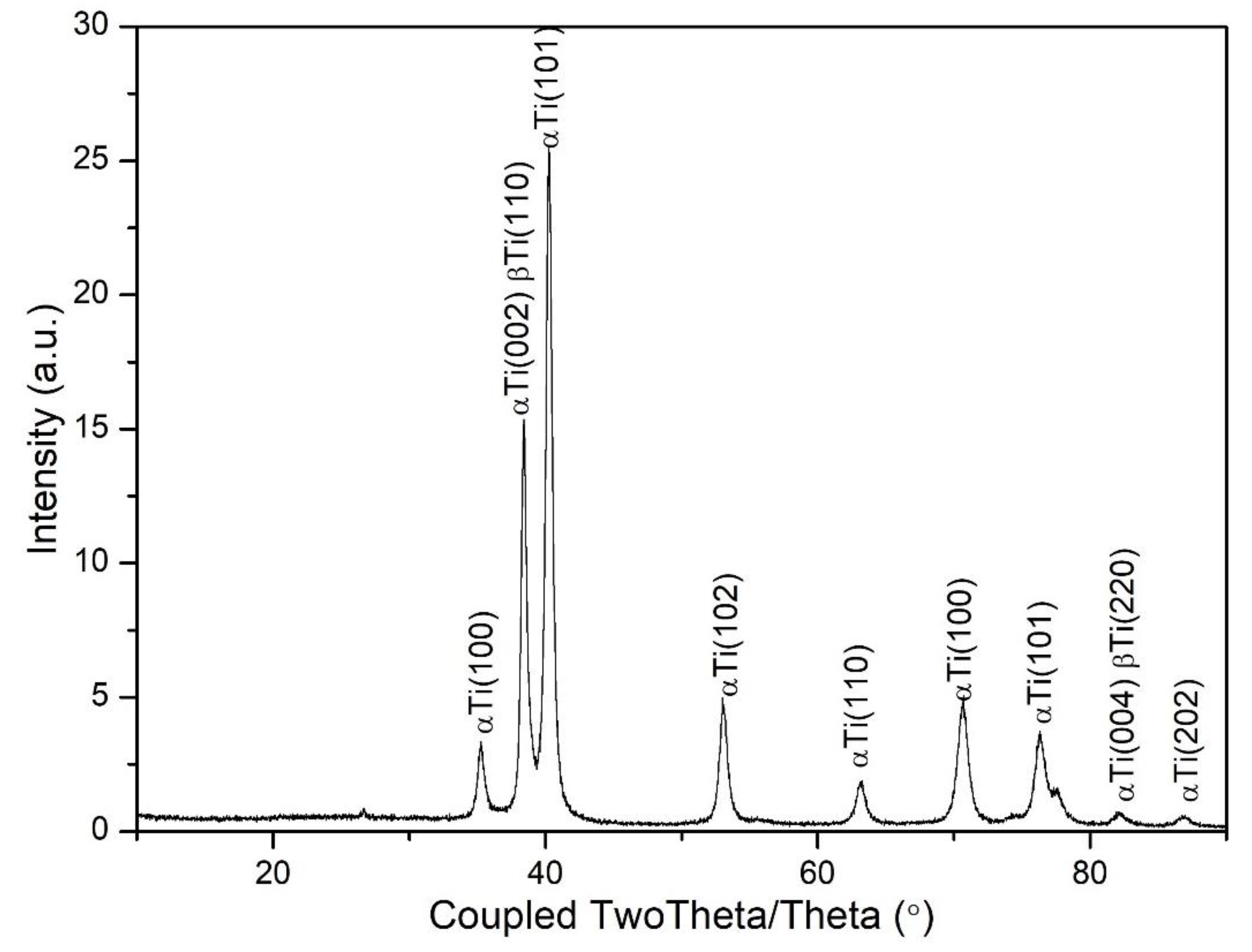
Based on the diffractogram obtained by X-ray phase analysis of the examined Ti6Al7Nb alloy, αTi and βTi phases were identified in the surface layer. Moreover, it was found that there is stress in the layer, and the obtained hardness profile is correlated with the XRD results, indicating strengthening in the subsurface zone. This may confirm that the sandblasting process of the titanium alloy under study strengthens its surface.
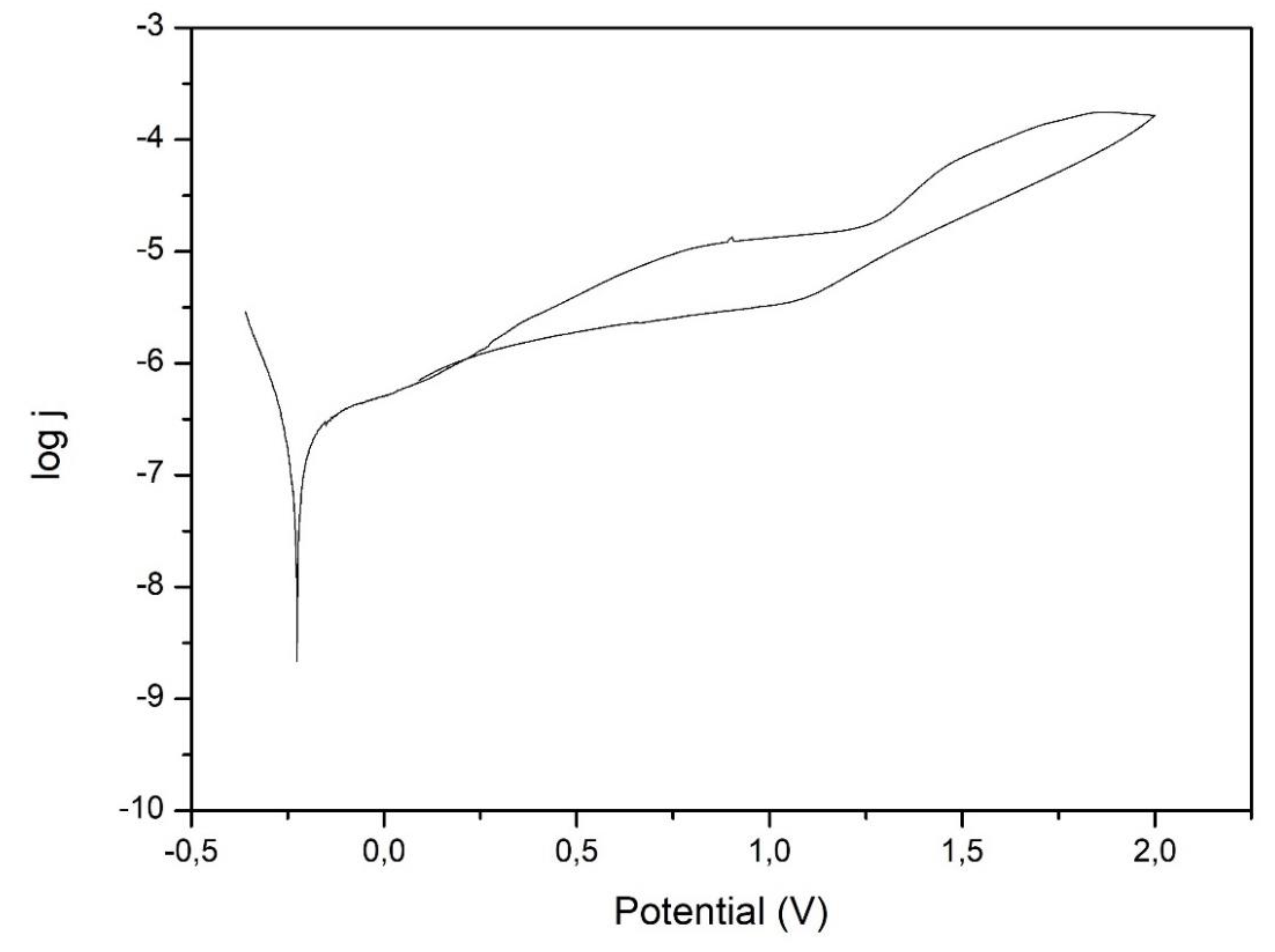
The analysis of the potentiodynamic curves did not show any breakdown potential, and the return curve is below the original curve, which demonstrates the resistance of Ti6Al7Nb alloy after anodic oxidation preceded by grinding and sandblasting to pitting corrosion. Moreover, the anodizing of titanium forms an oxide coating that is rather resistant to the attack of chloride ions present in PBS solution [
23]. The authors' extensive study [
24] of the effect of treatment prior to anodic oxidation on the corrosion resistance of Ti6Al7Nb alloy showed that the layers obtained after sandblasting had the best resistance. The study analyzed the corrosion resistance of the alloy in the initial state as well as after 28-day exposure to Ringer's solution. Complementary tests were carried out on the penetration of metal ions into the corrosive environment.
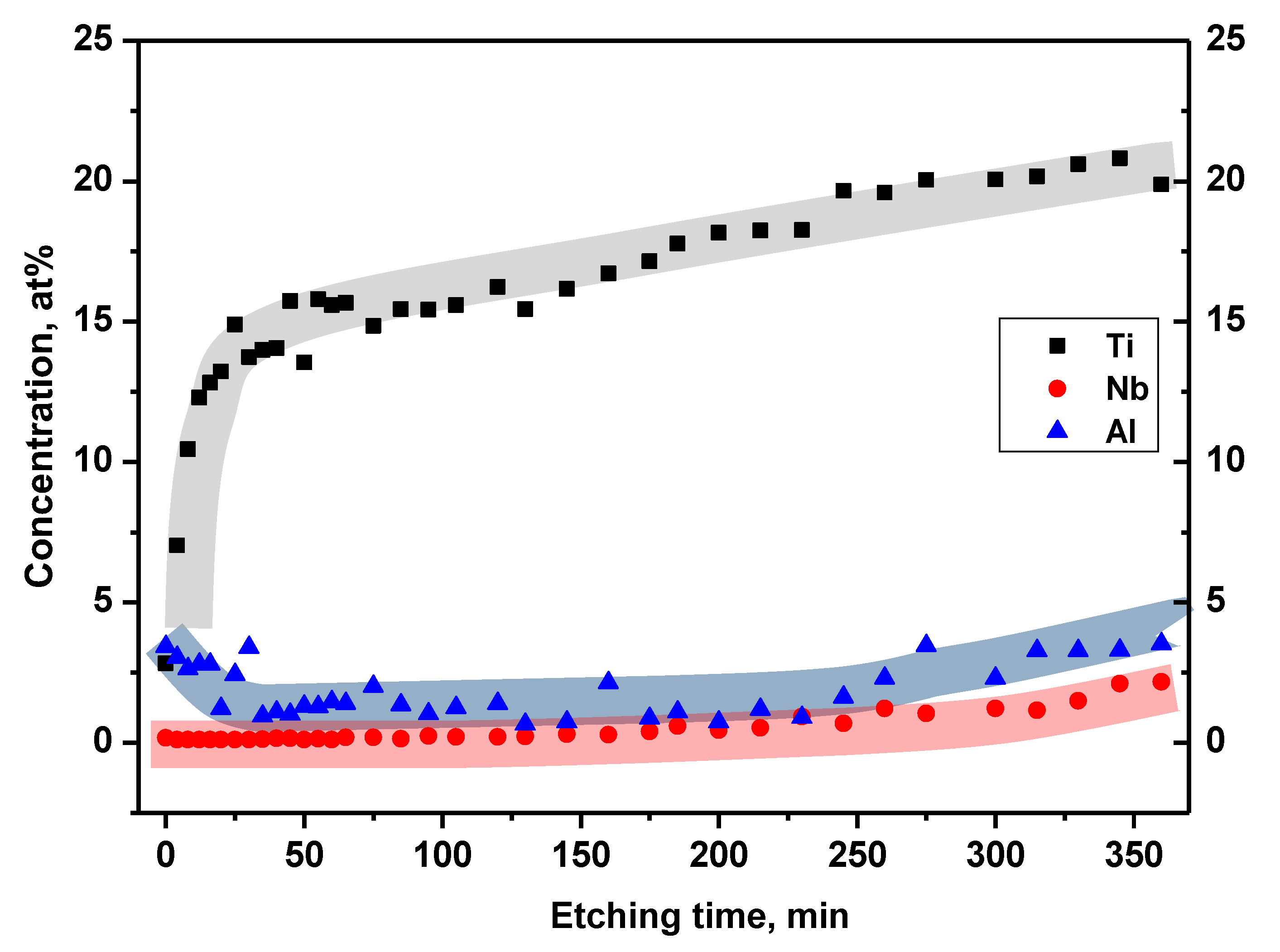
Analysis of the Ti profile (
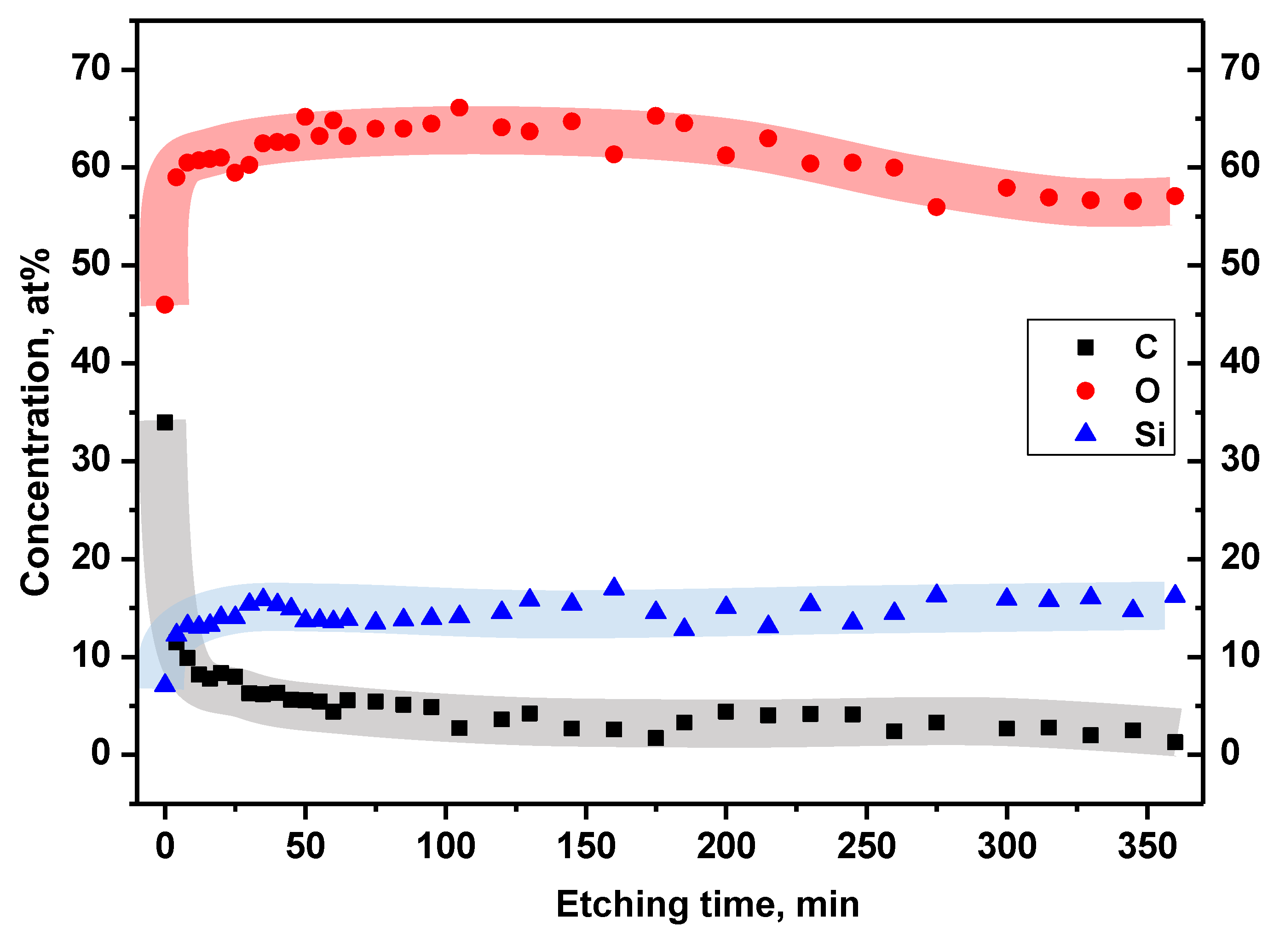
Figure 8) showed that the main metallic component of the alloy is not dominant on the native surface of the sample (0 min. etching time). There, titanium reached a concentration of about 3% similar to the surface concentration of Al. However, with the penetration depth into the layer, the Ti contribution increased exponentially achieving a stable value of about 15% for the Ar+ etching time in the range 25-130 min. Further depth penetration showed only a gradual increase of titanium content up to a concentration of about 20%. On the other hand, during the robust increase of the Ti content, the aluminum concentration decreased. After 25 min of etching, the Al concentration oscillated around 1-2%. Then, again grew to about 3.5% in depth corresponding to etching of 260-360 min. The niobium concentration was found to be minor compared to the other main alloy components. It remained constant at a value of 0.2% from the native surface to the depth obtained after 160 min etching. For longer etching, Nb content started to gradually increase reaching ~2% for maximal etching time equaled 360 min.
Oxygen was revealed as the main component of the passive layer reaching a concentration of about 60-65% for a depth corresponding to the interval of 4-260 min (
Figure 9) Ar+ etching. This value decreased slightly by a few percent in the next 100 min of ion interaction. Only the native surface was characterized by a lower oxygen content (about 45%) caused probably by the presence of excess carbon. The carbon content on the native surface reached almost 34%. This value drastically dropped after only a few minutes of ion etching (to ~10%) and then systematically decreased to about 2% for a maximum etching time of 360 min. The signal from silicon, like from oxygen, showed a lower concentration at the native surface (about 7%) than in the deeper layers of the alloy. It remains constant at around 15% for the entire etching time range.
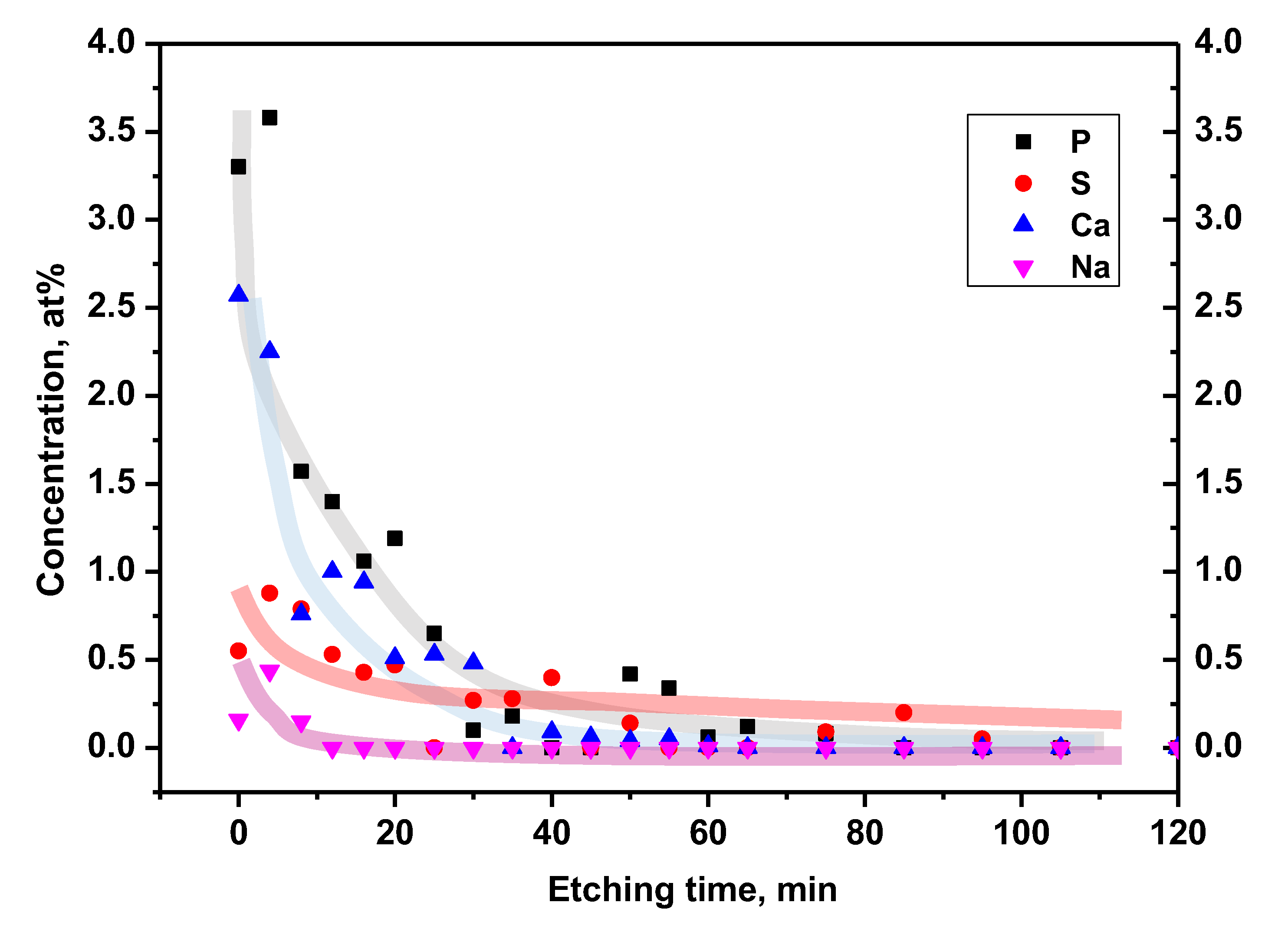
Phosphorus and calcium showed relatively the highest concentration on the native surface, around 3.5% and 2.5%, respectively (
Figure 10). The contribution of these elements gradually disappeared to a depth corresponding to 30 min. of etching. The sulfur profile behaved similarly. It reached a maximum value of about 1% on the native surface and gradually vanished after 35 min. of etching. Sodium was residual at a concentration of about 0.4% on the surface and disappeared after 12 min. of etching. Phosphorus, calcium, sulfur and sodium are residues of the anodic oxidation process (they are present in the electrolyte used). Their presence in the passive layer may promote surface bioactivation by stimulating the formation of hydroxyapatite on the alloy surface [
25].
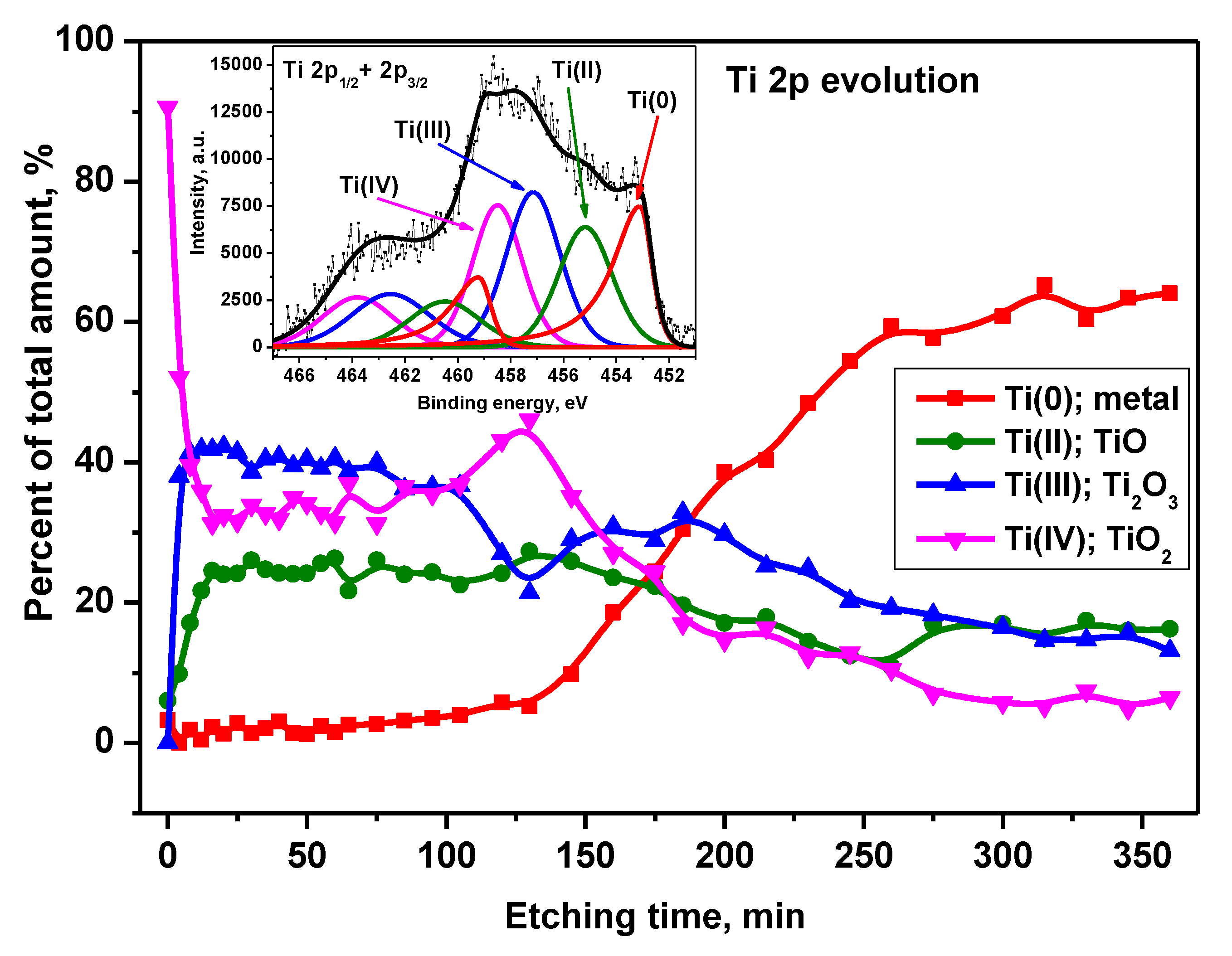
The decomposition of the Ti 2p XPS peak (exemplary window spectrum: inset to
Figure 11) revealed the existence of Ti components in metallic form and the 2nd, 3rd, and 4th oxidation states including the spin-orbit splitting of 2p transition (2p3/2 and 2p1/2) equaled 6.1 eV (
Figure 11). The asymmetric Ti 2p3/2 component at position 453.1 eV was assigned to Ti(0): metal. The subsequent symmetric Ti 2p3/2 components at BE of 455.2 eV, 457.1 eV, and 458, 5 eV were assigned to Ti(II) in the form of TiO, Ti(III) - Ti2O3 and Ti(IV) as TiO
2, respectively [
26]. Generally, on the alloy native surface, there was 90% titanium in the form of TiO
2. This situation changed drastically within the first minutes of etching when the amount of TiO
2 dropped to about 35% of the total titanium content. There, the Ti
2O
3 and TiO fractions also appeared in 40% and 25% of Ti, respectively. Above 150 min. of etching, an increase in the contribution of titanium in metallic form became noticeable. It reached a value of about 60% of the Ti signal in the 260-360 min. etching range. For this period TiO and Ti
2O
3 showed a signal of ~15% Ti, while TiO2 had a contribution of ~6%.
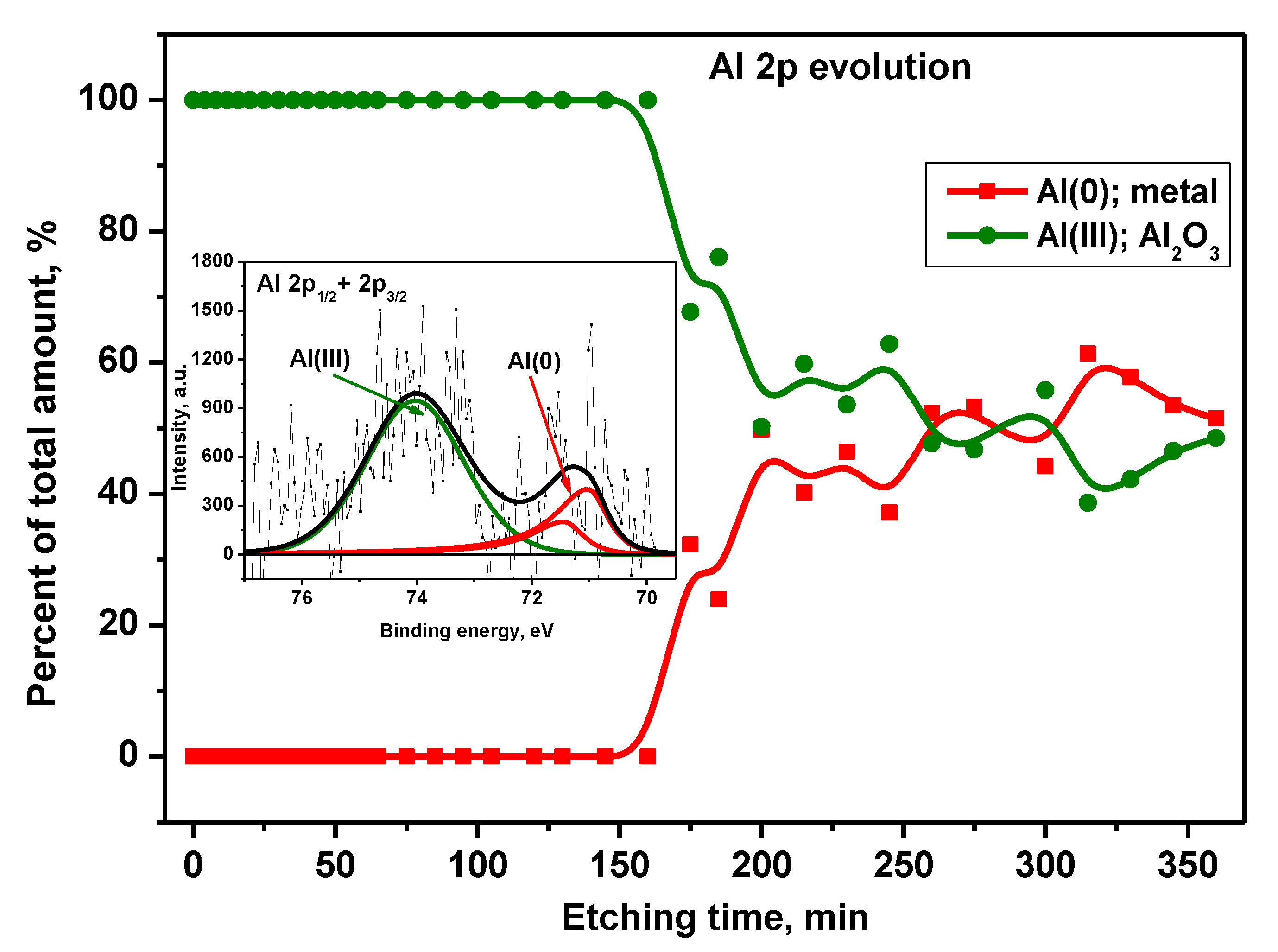
Peak Al 2p decomposition revealed the existence of Al components in metallic form (with spin-orbit splitting equaled 0,42 eV [
27]) and in 3rd oxidation states (
Figure 12). The asymmetric Al 2p3/2 component located at 71.0 eV was attributed to Al(0): metal, whereas the symmetric, wide one at 74.0 eV was assigned to Al(III): Al
2O
3 [28-29]. From the alloy's native surface to the depth corresponding to ~150 min. of etching, the aluminum exists only in the form of Al
2O
3. Then, an abrupt decrease of oxide content was observed reaching a level of ~50% of the total Al signal after 200 min. The next 50% was related to Al in the metallic form. The situation was stable up to 360 min of Ar+ sputtering.
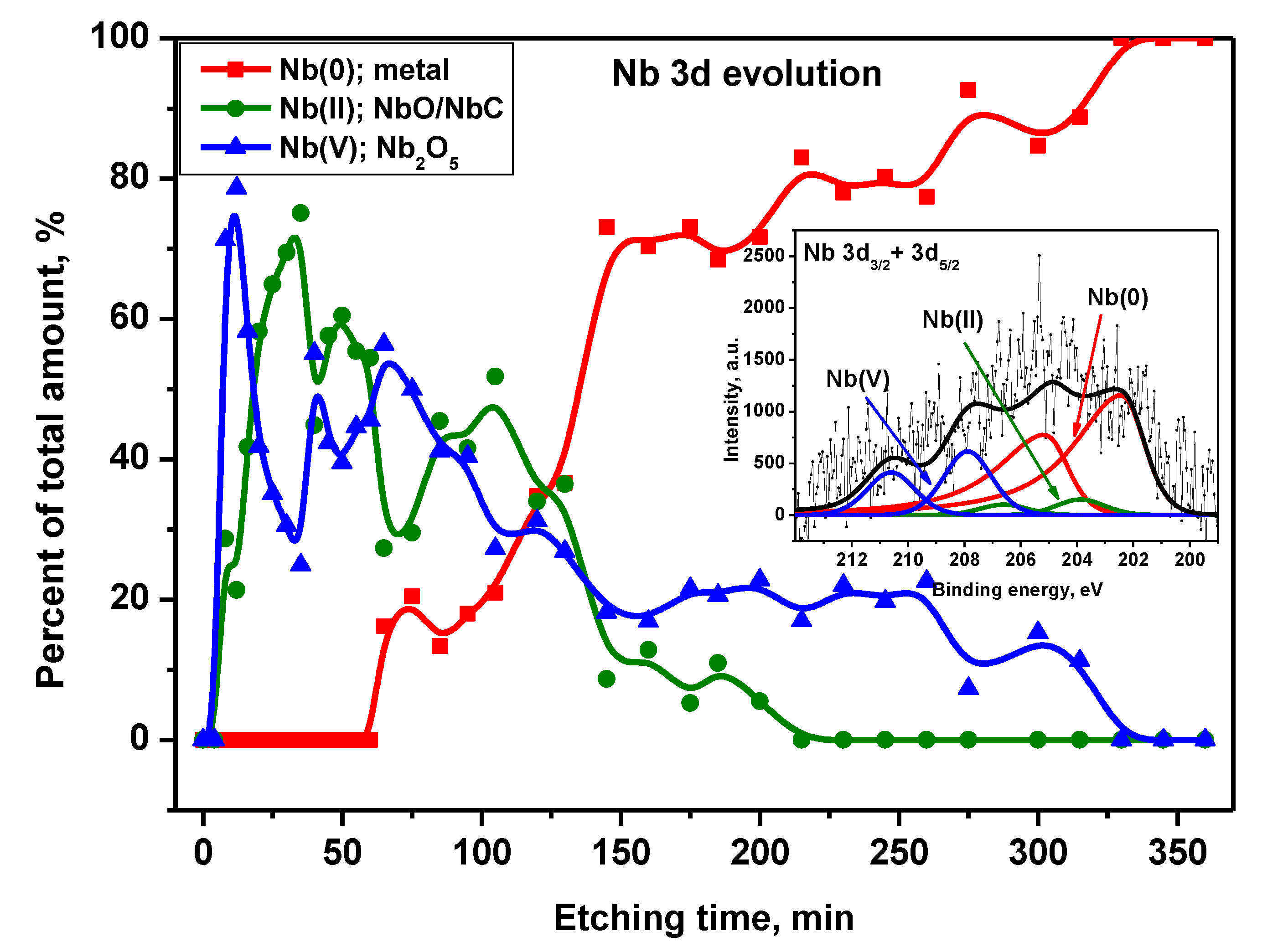
Peak Nb 3d decomposition showed the existence of Nb components in metallic form and the 2nd and 5th oxidation states with the spin-orbit splitting of 3d transition (3d5/2 and 3d3/2) equaled 2.7 eV (
Figure 13) [
27]. The asymmetric Nb 3d5/2 component located at 202.4 eV was attributed to Nb(0): metal [
30]. The next symmetric Nb 3d5/2 components at 203.8 eV and 207.9 eV were assigned to Nb(II) in the form of NbO and/or NbC, Nb(V) - Nb
2O
5, correspondingly [
30]. On the alloy native surface and for the very first minute of argon sputtering the niobium signal was on noise level likely due to surface contaminations. For more than 8 min. of etching, the phases of NbO/NbC and Nb
2O
5 appeared and their amount oscillated around 50% of total Nb content. After 60 min. of Ar treatment, the metallic component appeared. It started to grow up gradually reaching ~70% after 145 min. At the same time, the niobium oxides components’ contribution continuously decreased. Finally, the NbO/NbC input to the Nb signal disappeared after 215 min, Nb
2O
5 – after 315 min of etching.
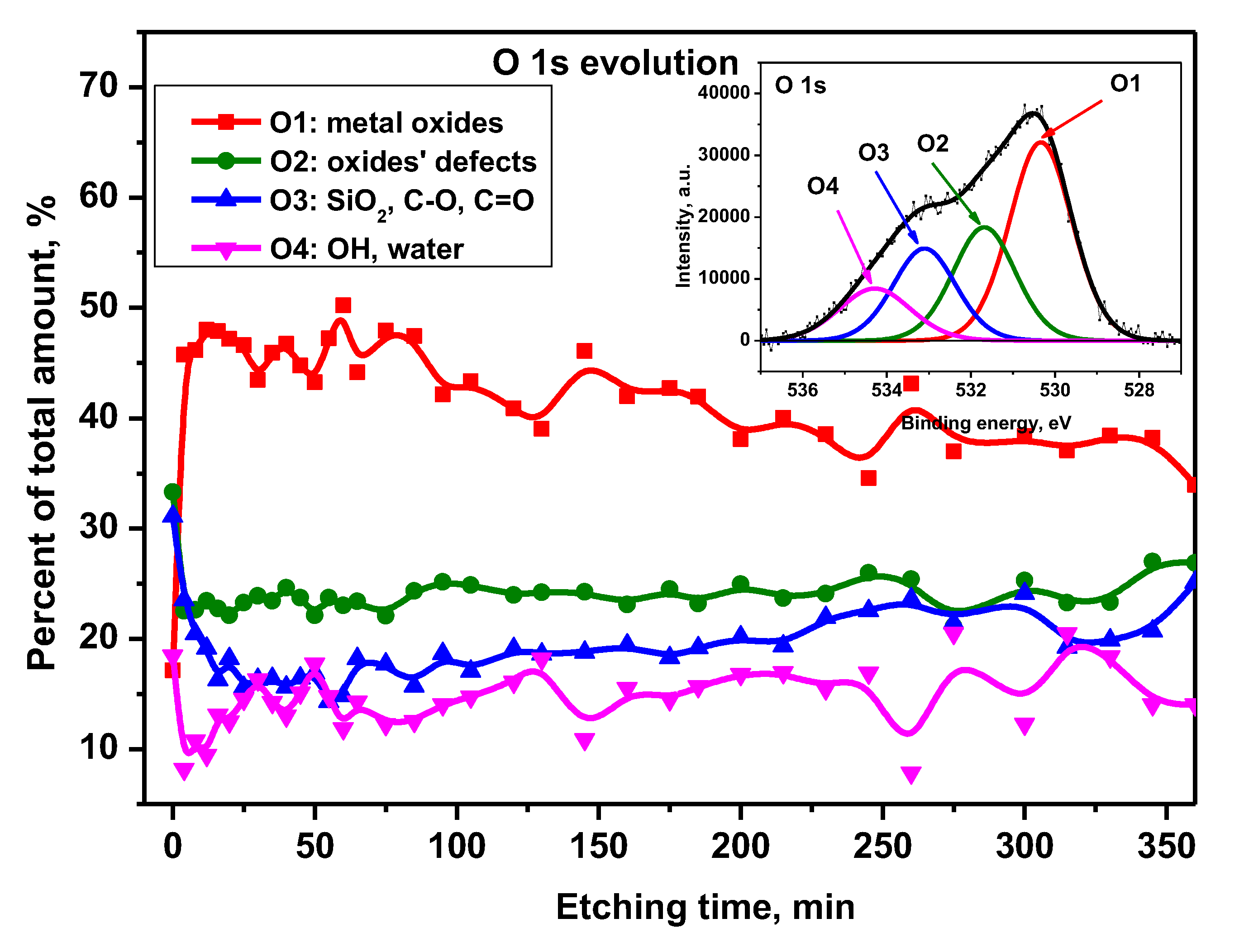
The decomposition of the O 1s XPS peak manifested the appearance of four oxygen components (
Figure 15). The O1 positioned at 530.3 eV was attributed to metal oxides, and the O2 – at BE = 531.6 eV was assigned to metal oxides’ defects [31-32]. The next one (O3), located at 533.0 eV, originated from carbon-related species C-O, C=O and probably from SiO
2 oxide [11,12 ]. The fourth component (O4) at 534.1 eV was related to OH/water bonds [33-34]. On alloy native surface dominates signals from O2 and O3 species (both ~32% of total oxygen content). It corroborated with a significant amount of carbon detected on the alloy surface. There, metal oxides, as well as water phases (O1 and O4, respectively), were evenly on the level of ~18%. But, after 4 min of etching, metal oxide contribution increased suddenly up to ~50% and stayed stable till 85 min of Ar+ sputtering. Then, O1 tended to fall slowly reaching a final value of ~40% for maximum etching time. A similar effect of oxide content decrease within the corresponding alloy depth was observed for titanium, aluminum and niobium (
Figure 12-14). The other oxygen components, O2, O3 and O4, within the argon sputtering period of 85-360 min remained stable at the level of approximately 25%, 20% and 15%, respectively.
Tests of the samples showed that the passive layer consists mainly of oxides of alloying elements Ti, Al, Nb, the contribution of which decreases over time as the contribution of metallic elements increases.
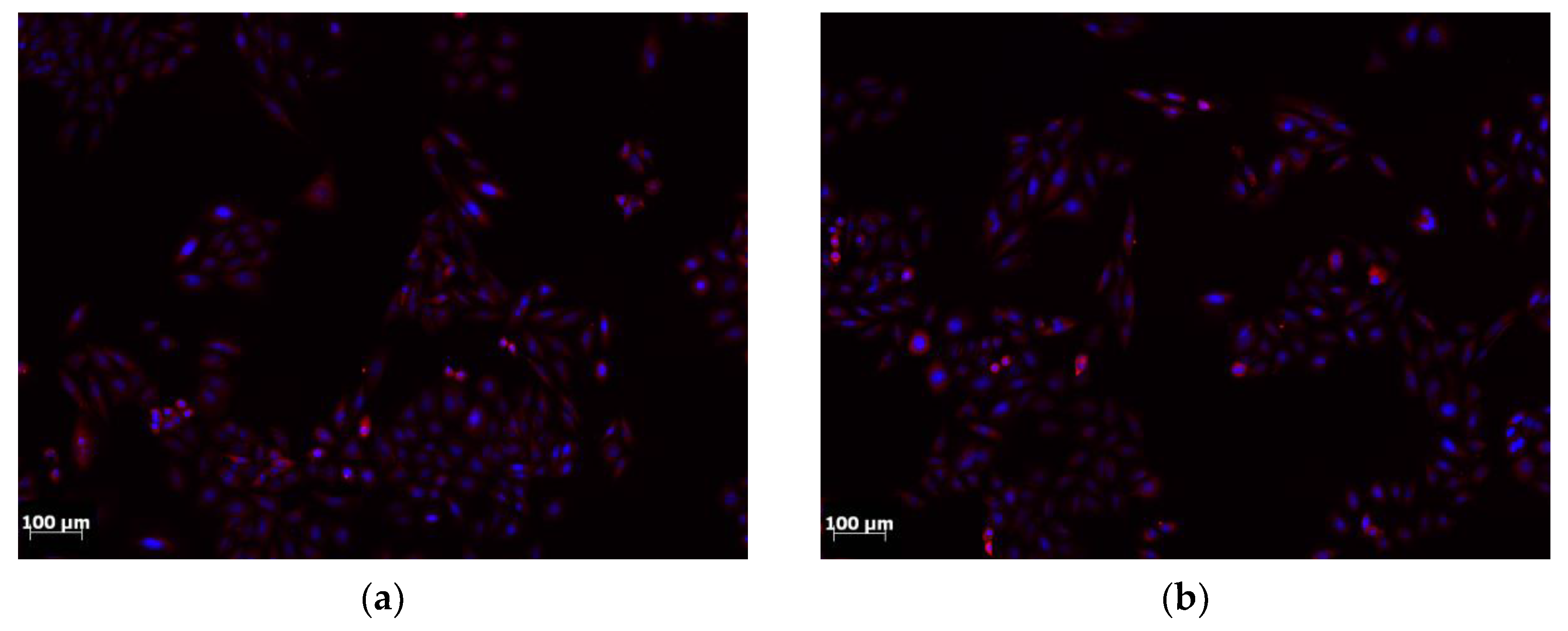
Biocompatibility tests allowed to conclude that the analyzed titanium alloy surface does not cause any cytotoxic effect. Moreover, cells of the SaOS-2 line, both in the control group and after incubation with Ti6Al7Nb alloy extracts, did not show any changes in their morphology and organization (
Figure 16).
Figure 1.
Surface of Ti6Al7Nb alloy after grinding, sandblasting and anodic oxidation, light microscope.
Figure 1.
Surface of Ti6Al7Nb alloy after grinding, sandblasting and anodic oxidation, light microscope.
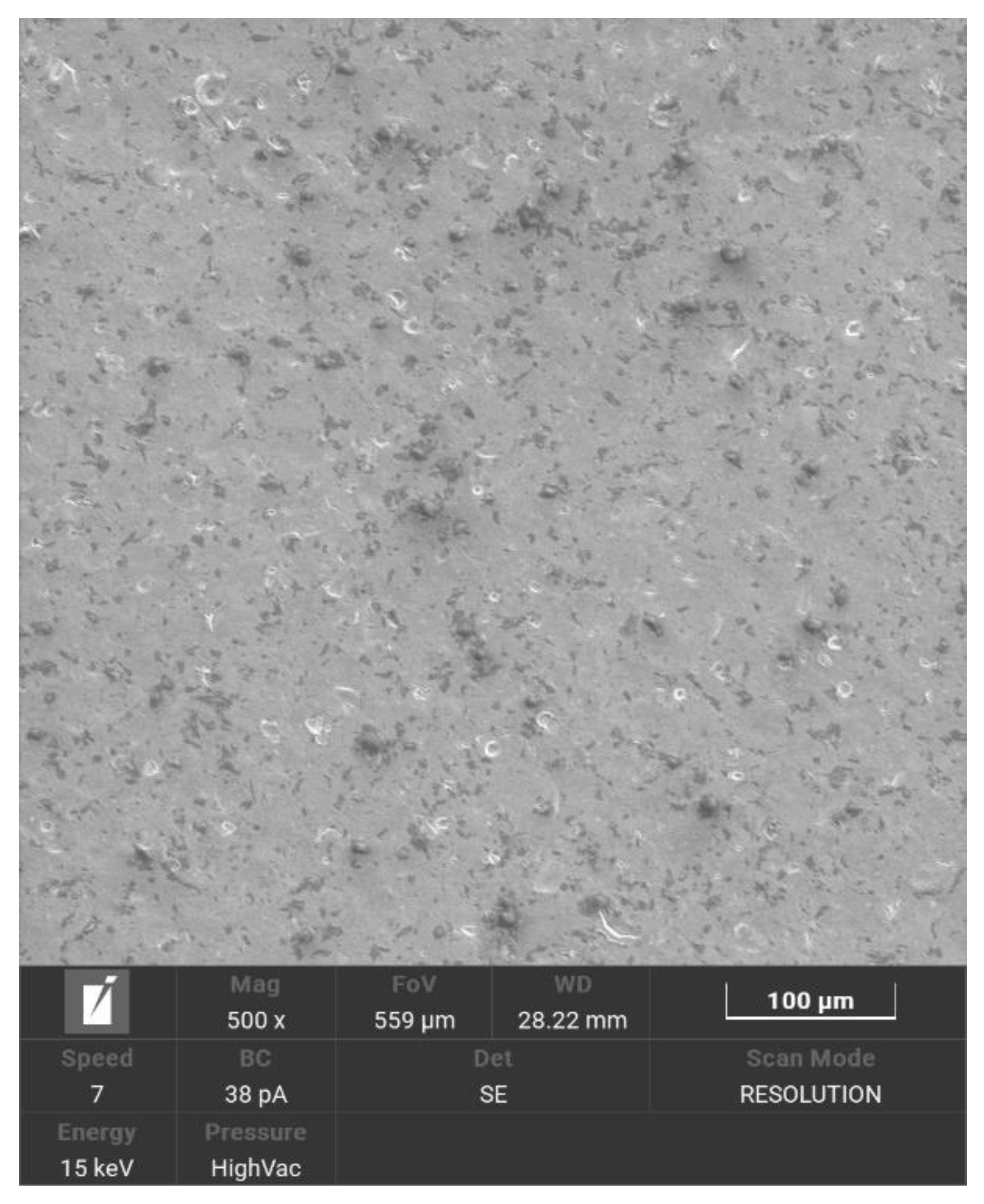
Figure 2.
The surface of Ti6Al7Nb alloy after grinding, sandblasting and anodic oxidation, SEM.
Figure 2.
The surface of Ti6Al7Nb alloy after grinding, sandblasting and anodic oxidation, SEM.
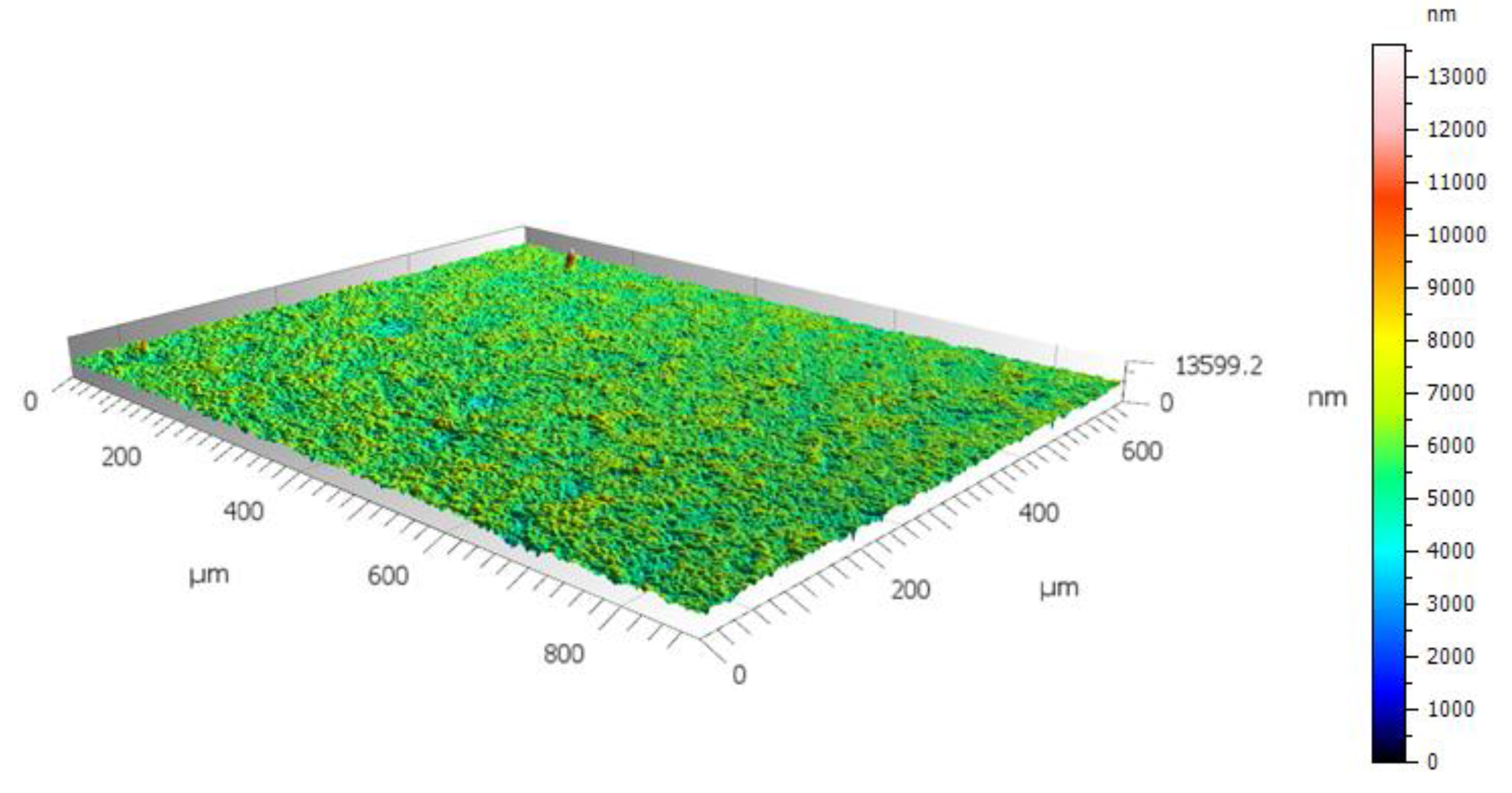
Figure 3.
Topography of sandblasted and anodic oxidized Ti6Al7Nb alloy surface.
Figure 3.
Topography of sandblasted and anodic oxidized Ti6Al7Nb alloy surface.
Figure 4.
Ti6Al7Nb alloy surface roughness profile.
Figure 4.
Ti6Al7Nb alloy surface roughness profile.
Figure 5.
A drop of water on the surface of the Ti6Al7Nb alloy
Figure 5.
A drop of water on the surface of the Ti6Al7Nb alloy
Figure 7.
XRD pattern obtained for Ti6Al7Nb alloy.
Figure 7.
XRD pattern obtained for Ti6Al7Nb alloy.
Figure 8.
Example of potentiodynamic curves obtained for Ti6Al7Nb alloy.
Figure 8.
Example of potentiodynamic curves obtained for Ti6Al7Nb alloy.
Figure 9.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for titanium, aluminum and niobium.
Figure 9.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for titanium, aluminum and niobium.
Figure 10.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for carbon, oxygen and silicon.
Figure 10.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for carbon, oxygen and silicon.
Figure 11.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for potassium, sulfur, calcium and sodium.
Figure 11.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for potassium, sulfur, calcium and sodium.
Figure 12.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for potassium, sulfur, calcium and sodium.
Figure 12.
Concentration profile of Ti6Al7Nb surface as a function of Ar+ etching time for potassium, sulfur, calcium and sodium.
Figure 13.
Al 2p signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative Al 2p spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 13.
Al 2p signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative Al 2p spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 14.
Nb 3d signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative Nb 3d spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 14.
Nb 3d signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative Nb 3d spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 15.
O 1s signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative O 1s spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 15.
O 1s signal components’ evolution as a function of Ar+ etching time. Inset: XPS representative O 1s spectrum with peak fitting for Ti6Al7Nb surface after 175 min. of etching.
Figure 16.
Image of cells: (a) control group; (b) cells subjected to incubation with extracts from Ti6Al7Nb alloy.
Figure 16.
Image of cells: (a) control group; (b) cells subjected to incubation with extracts from Ti6Al7Nb alloy.
Table 1.
Selected structural parameters determined using Rietveld refinement.
Table 1.
Selected structural parameters determined using Rietveld refinement.
| Lattice parameters, ICDD, Å |
Lattice parameters, calculated, Å |
Crystallite size, nm |
Lattice strain, % |
Compressive stress, MPa |
a = 2.951,
c= 4.683 |
a=2.950
c=4.695 |
30 |
0.89 |
-544(21) |