1. Introduction
Sepsis, a life-threatening condition characterized by a dysregulated host response to infection, continues to be a significant challenge in critical care and emergency medicine[
1]. Despite advancements in understanding its pathophysiology and improvements in supportive care, sepsis and its severe manifestations, such as septic shock, remain leading causes of mortality and morbidity in intensive care units (ICUs) worldwide[
2]. The complexity of sepsis, marked by its heterogeneous presentation and progression, necessitates the identification and utilization of reliable prognostic markers to guide clinical decision-making and improve patient outcomes[
3].
Here is a concise and accurate response to the question, drawing from the given search results:
Severity scoring systems and prognostic models are important tools used in intensive care units (ICUs) to assess the severity of illness and predict patient outcomes. These systems enable comparative audits and evaluative research of ICUs[
1,
4].
The ideal severity scoring system should include easily measured, objective, and reproducible parameters collected during routine patient management. Some of the most commonly used severity scoring systems include the Acute Physiology and Chronic Health Evaluation (APACHE), Simplified Acute Physiology Score (SAPS), and Sequential Organ Failure Assessment (SOFA). These models aim to stratify patients based on severity of illness and predict outcomes like in-hospital mortality. They have been extensively validated, predominantly in high-income countries, and generally demonstrate good discrimination and calibration. However, the performance of these models may be limited in low- and middle-income country settings due to differences in case-mix, availability of predictor variables, and data collection challenges. Efforts are ongoing to develop and validate context-specific prognostic models for these settings[
5,
6].
Overall, severity scoring systems and prognostic models are essential tools for ICU care, but their appropriate application and interpretation is crucial to avoid misuse and ensure optimal utility in guiding patient management and resource allocation.[
1]
The Fibrosis 4 (FIB-4) index, originally developed to non-invasively assess liver fibrosis in patients with hepatitis C, has emerged as a potential prognostic marker in various clinical settings beyond liver disease[
7]. The FIB-4 index is calculated based on readily available laboratory parameters: age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count. This simplicity and non-invasiveness make the FIB-4 index an attractive tool for clinical use[
8]. Recent studies have suggested that the FIB-4 index may reflect not only liver fibrosis but also systemic inflammation and organ dysfunction, which are central to the pathophysiology of sepsis[
9].
The prognostic value of the FIB-4 index in sepsis patients remains an area of active investigation. Preliminary evidence indicates that elevated FIB-4 levels may be associated with worse outcomes in sepsis, including increased mortality, longer ICU stays, and higher rates of organ dysfunction. These associations are thought to arise from the FIB-4 index's ability to capture the extent of systemic inflammation and its impact on multiple organ systems, including the liver[
10]. Given the liver's pivotal role in modulating immune responses and its susceptibility to damage in the context of sepsis, the FIB-4 index could provide valuable insights into the severity and prognosis of septic patients.
This study aims to comprehensively evaluate the prognostic value of the FIB-4 index in patients with sepsis admitted to the ICU. By elucidating the relationship between FIB-4 levels and clinical outcomes in sepsis, we hope to contribute to the ongoing efforts to improve risk stratification, guide therapeutic interventions, and ultimately enhance the care of patients facing this formidable challenge.
2. Materials and Methods
2.1. Study Design and Population
This retrospective cohort study will include patients admitted to the Internal Medicine Intensive Care Unit and Anaesthesia Intensive Care Unit with a diagnosis of sepsis between December 12, 2021, and December 15, 2023.
Ethic Consideration: The study design conformed to the Declaration of Helsinki and was approved by Giresun Training and Research Hospital Ethics Committee on 25.12.2023 with the number number E-53593568-771-232700672
Inclusion Criteria: Patients diagnosed with sepsis upon admission or within the first 48 hours of hospitalization (community-acquired sepsis) or developing sepsis 48 hours after hospitalization (nosocomial sepsis), patients for whom complete medical records are available, including laboratory and clinical data necessary for the calculation of the FIB-4 index and APACHE II score.
Exclusion Criteria: Patients under 18 years of age, patients with incomplete medical records or missing data necessary for the analysis, patients who were discharged or transferred to another facility within 48 hours of admission.
2.2. Data Collection
Data was collected retrospectively from patient medical records. The following information was recorded for each patient: age, gender, body mass index (BMI), comorbidity status, sepsis status, focus of infection, microorganism produced, mean arterial pressure, C-reactive protein (CRP), procalcitonin, lactate, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total and direct bilirubin, urea, creatinine, white blood cell count, neutrophil count, and platelet count. Additionally, severity scores such as APACHE II score, Sequential Organ Failure Assessment (SOFA) score, inotrope requirement, duration of intensive care unit stay, and discharge or exit status were also recorded.
FIB-4 Index Calculation
FIB−4=Age(years)×AST(U/L)/Platelet count(×109/L)×ALT(U/L)1/2
2.3. Statistical Investigations
SPSS 26 (Statistical Package for the Social Sciences) program was used for statistical analysis. While evaluating the study data, quantitative variables were shown with mean, standard deviation, median, min and max values and qualitative variables were shown with descriptive statistical methods such as frequency and percentage. Shapiro Wilks test and Aox Plot graphs were used to evaluate the conformity of the data to normal distribution. Student's t-test was used for quantitative evaluations of two groups with normal distribution, and Mann Whitney-U test was used for evaluations of variables that did not show normal distribution according to two groups.
Chi-square test and Fisher's Exact test were used to compare qualitative data. Diagnostic screening tests and ROC analysis were used to determine the cut off of FIB-4 measurements according to mortality.
Logistic Regression analysis was used for multivariate evaluations of risk factors affecting early mortality. Results were evaluated at 95% confidence interval and significance was evaluated at p<0.05 level.
3. Results
The study was conducted in Hospital between ... dates with a total of 309 patients, 42.1% (n=130) of whom were female and 57.9% (n=179) of whom were male. The ages of the patients ranged between 27 and 98 years, with a mean age of 74.78±13.92 years(
Table 1).
When comorbidities were analyzed, HT was observed in 69.3% (n=214), DM in 29.1% (n=90), CRF in 37.9% (n=117), LVH in 15.5% (n=48), and CAD in 33% (n=102). Nasocomials infection were observed in 72.8% of cases (n=225). Of the study participants, 34% (n=105) had septic shock(
Table 2).
The site of reproductin was blood in 40.1% (n=124), catheter in 6.8% (n=21), tracheal aspirate culture (TAC)/sputum in 36.2% (n=112), urine in 30.1% (n=93), wound site in 6.5% (n=20) and other in 1.3% (n=4).
According to mortality, gender, nasocomial infection presence and causative agents of the cases did not show statistically significant difference (p>0.05).
The rate of DM was statistically significantly lower in those with mortality (p=0.009; p<0.01). The rate of septic shock was statistically significantly higher in those with mortality (p=0.001; p<0.01).
The rate of wound site of reproduction was statistically significantly lower in those with mortality (p=0.004; p<0.01)(
Table 2).
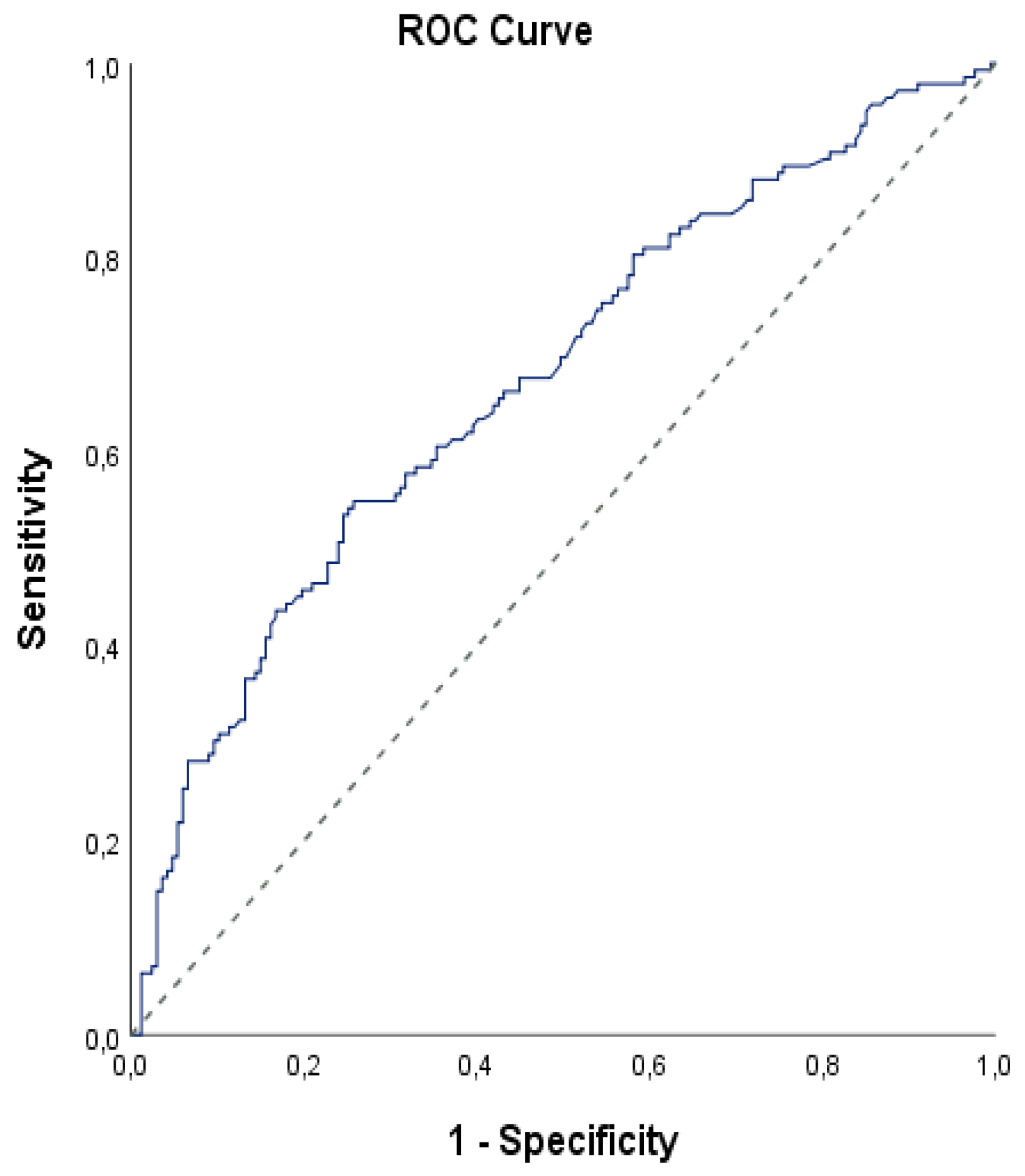
According to mortality, the ages and creatinine measurements of the patients did not show statistically significant difference (p>0.05). APACHE II measurements of those with mortality were statistically significantly higher (p=0.001; p<0.01). The SOFA measurements of those with mortality were statistically significantly higher (p=0.001; p<0.01). Lactate measurements of those with mortality were found to be statistically significantly higher (p=0.001; p<0.01). AST measurements of those with mortality were found to be statistically significantly higher (p=0.011; p<0.05). Total bilirubin measurements were statistically significantly higher in patients with mortality (p=0.001; p<0.01). Direct bilirubin measurements of those with mortality were found to be statistically significantly higher (p=0.001; p<0.01). Urea measurements of those with mortality were statistically significantly higher (p=0.001; p<0.01). FIB-4 measurements were statistically significantly higher in patients with mortality (p=0.001; p<0.01). Since the FIA-4 value was significant in relation to mortality, we determined a cut off point with ROC analysis, as it may be a marker for mortality(
Table 3).
According to the early mortality status, the cut off point for FIB-4 measurements was determined as 4.9 and above. For a cut-off value of 4.9 for the FIB-4 measurement; sensitivity was 54.92%, specificity was 74.25%, positive predictive value was 64.46%, and negative predictive value was 64.95%. In the ROC curve obtained, the underlying area was 67.5% with a standard error of 3.1% (
Table 4).
A statistically significant correlation was found between motility and the cut-off value of 4.9 of the FIB-4 level (p=0.001; p<0.05). The risk of disease was 3.52 times higher in patients with a FIB-4 level of 4.9 and above. The odds ratio for the FIB-4 measurement was 3.515 (95% CI: 2.177-5.675(
Table 4,
Figure 1)).
Logistic Regression Analysis
We evaluated the effects of DM, septic shock, wound site in reproduction, APACHE, SOFA, Lactate, AST, total and direct bilirubin, urea and FIB-4 on early mortality by logistic regression analysis.
When we evaluated the effects of DM, septic shock, wound site in reproduction, APACHE, SOFA, Lactate, AST, total and direct bilirubin, urea and FIB-4 on early mortality with Bacward Logistic regression analysis; the model was found to be significant (F=78.391; p=0.001; p<0.01) and the explanatory coefficient of the model (71.2%) was found to be at a good level. The effects of APACHE II, SOFA, direct bilirubin level and FIB-4 were statistically significant in the model (p<0.05). The effect of one unit increase in APACHE II score on early mortality was found to increase the odds ratio 1.101 (95% CI: 1.008-1.156) times; one unit increase in SOFA score increased the odds ratio 1.122 (95% CI: 1.007-1.251) times. One unit increase in direct bilirubin level increased early mortality by 1.228 (95% CI: 1.080-1.1497) times. The effect of FIB-4 cut of value of 4.9 and above on early mortality increased by 2.127 (95% CI: 1.237-3.659) times. Although DM, septic shock, wound site in reproduction, lactate, AST, total bilirubin and urea variables were significant in univariate evaluations, they were not significant in multivariate evaluation (p>0.05)(
Table 5).
APACHE II, SOFA, direct bilirubin level and FIB-4 (≥4.9) are independent risk factors for early mortality
4. Discussion
In our study, we planned to investigate whether there is a relationship between the severity of sepsis and mortality, the APACHEII score, and the FIB-4 score.
Our study highlights the prognostic significance of the Fibrosis-4 (FIB-4) index in sepsis patients admitted to the intensive care unit. The retrospective analysis, covering a cohort of 309 patients, revealed a statistically significant association between elevated FIB-4 levels and increased mortality rates. For example, an FIB-4 cut-off value of ≥4.9 had a sensitivity of 54.92% and a specificity of 74.25% for predicting death, with an odds ratio of 3.515. This meant that patients with a FIB-4 level of 4.9 or higher had a 3.52 times higher risk of dying early than those with lower FIB-4 levels.
Furthermore, our analysis found that APACHE II, SOFA, and direct bilirubin levels, along with FIB-4, are independent risk factors for early mortality in sepsis patients. The APACHE II and SOFA scores are well-established prognostic tools in critical care, reflecting the severity of illness and organ dysfunction, respectively. Adding FIB-4 as a separate risk factor to these established scores suggests that FIB-4's measurement of liver function is very important in determining the outcome of sepsis patients. This is particularly relevant given the liver's central role in the inflammatory response and its susceptibility to damage during sepsis.
Patients with conditions like viral hepatitis and non-alcoholic fatty liver disease (NAFLD) often use the Fibrosis-4 (FIB-4) index, a non-invasive scoring system, to evaluate liver fibrosis. Age, platelet count, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels are the basis for its calculation[
7,
11,
12]. The FIB-4 index has been found to be a useful tool not only in assessing liver fibrosis but also in predicting clinical outcomes in various patient populations, including those with sepsis and other critical illnesses[
13,
14,
15,
16,
17,
18].
Sepsis is a severe and life-threatening condition that arises when the body's response to an infection becomes uncontrolled, leading to widespread inflammation and potential organ failure. This condition can progress rapidly, making early recognition and treatment crucial[
19].
In septic patients, an elevated FIB-4 score has been associated with poor outcomes, such as increased mortality and the need for renal replacement therapy. The index serves as an independent short-term mortality scoring system, indicating that advanced stages of subclinical hepatic fibrosis can lead to worse outcomes in these patients. This association is also observed in non-septic critically ill patients, suggesting the generalizability of FIB-4 as a prognostic tool in critical care settings[
10].
External validation studies have shown that FIB-4 is a reliable tool for predicting outcomes in septic patients, with results that are similar to those from primary studies[
10]. This supports the use of FIB-4 as a supplementary tool alongside existing prognostic scoring systems to enhance the prediction of clinical outcomes [
1].
Beyond liver diseases, FIB-4 has been applied to other clinical scenarios, such as cardiovascular diseases and infections like COVID-19. For instance, it has been used to predict the need for mechanical ventilation in COVID-19 patients, with specific cutoff values providing significant predictive accuracy[
9,
20]
Studies have demonstrated that FIB-4 outperforms other liver fibrosis indices such as the NAFLD fibrosis score (NFS) and AST to Platelet Ratio Index (APRI). It has been particularly effective in differentiating the stages of liver fibrosis in patients with chronic viral hepatitis and NAFLD. Studies have demonstrated its utility in predicting long-term outcomes such as hepatocellular carcinoma incidence and mortality in these patient groups [
10,
21].
The study provided highlights the importance of the Fibrosis-4 (FIB-4) index as an independent predictor of short-term mortality in septic patients. This suggests the potential value of incorporating the FIB-4 index into existing prognostic tools in critical care settings for a more comprehensive assessment of patient outcomes.
The study comprehensively evaluated the prognostic value of the Fibrosis-4 (FIB-4) index in sepsis patients admitted to the intensive care unit. It examined the association between elevated FIB-4 levels and not only mortality rates, but also other important clinical outcomes such as the need for mechanical ventilation and renal replacement therapy.
There is limited research on the prognostic value of the FIB-4 index in the context of sepsis. This study adds to the growing body of evidence supporting the use of FIB-4 as a prognostic marker in critical illness, beyond its traditional application in chronic liver diseases. However, this is the first study in the literature comparing the FIB-4 index with the traditionally used SOFA scoring system and the APACHEE scoring system. In this study, similar to the study of Zhu, X., et al.[
10], an increase in the FIB-4 index is associated with unfavourable results. However, in our study, in addition to the FIB-4 index, SOFA score and APACHE scoring system were also studied and a comparison was also made between them.
This study emphasizes that elevated levels of the FIB-4 index are associated with increased mortality risk in septic patients, indicating a 3.52 times higher risk of early mortality for patients with a FIB-4 level of 4.9 and above. The FIB-4 index, initially designed to assess liver fibrosis in chronic liver diseases, has potential as a prognostic marker in sepsis, alongside established prognostic tools like APACHE II and SOFA scores. Elevated FIB-4 levels were associated with adverse outcomes in septic patients, including an increased need for invasive mechanical ventilation and renal replacement therapy[
6].
Study limitations.
Retrospective single-center design, lack of long-term follow-up, potential confounding factors, and relatively small sample size are the key limitations of this study that should be considered when interpreting the findings.
Conclusion
This study emphasizes the multifactorial nature of sepsis prognosis and the need for integrating clinical, demographic, and laboratory parameters to guide risk stratification and management decisions. Using the FIB-4 index, clinicians may be able to improve outcomes and reduce mortality in septic patients. Future prospective studies are necessary to validate these findings and explore the clinical utility of incorporating the FIB-4 index into sepsis management protocols.
Author Contributions
Conceptualization, B.Y, TA.; methodology, B.Y., TA.; software, B. Y.; validation, T.A., B.Y.; formal analysis, B.Y.; investigation, B.Y; resources, B. Y., T.A.; data curation, B.Y, TA.; writing—original draft preparation, T.A.; writing—review and editing, T.A. and B.Y. .; visualization, B.Y.; supervision, T.A. and B.Y.; project administration, B. Y., T.A.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study design conformed to the Declaration of Helsinki and was approved by Giresun Training and Research Hospital Ethics Committee on 25.12.2023 with the number number E-53593568-771-232700672.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data used in this study are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [CrossRef]
- Vincent, J.-L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit. Care 2019, 23, 1–11. [CrossRef]
- Tsalik, E.L.; Woods, C.W. Sepsis redefined: the search for surrogate markers. Int. J. Antimicrob. Agents 2009, 34, S16–S20. [CrossRef]
- Gullo, A., Intensive and Critical Care Medicine Reflections, Recommendations and Perspectives. 2005: Springer.
- Gunning, K.; Rowan, K. ABC of intensive care: Outcome data and scoring systems. BMJ 1999, 319, 241–244. [CrossRef]
- Kumar, S., et al., Comparison of the performance of APACHE II, SOFA, and mNUTRIC scoring systems in critically ill patients: a 2-year cross-sectional study. Indian Journal of Critical Care Medicine: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine, 2020. 24(11): p. 1057.
- Xu, X.-l., et al., The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? Journal of the Formosan Medical Association, 2022. 121(2): p. 454-466.
- Blanco-Grau, A.; Gabriel-Medina, P.; Rodriguez-Algarra, F.; Villena, Y.; Lopez-Martínez, R.; Augustín, S.; Pons, M.; Cruz, L.-M.; Rando-Segura, A.; Enfedaque, B.; et al. Assessing Liver Fibrosis Using the FIB4 Index in the Community Setting. Diagnostics 2021, 11, 2236. [CrossRef]
- Sterling, R.K.; Oakes, T.; Gal, T.S.; Stevens, M.P.; Dewit, M.; Sanyal, A.J. The Fibrosis-4 Index Is Associated With Need for Mechanical Ventilation and 30-Day Mortality in Patients Admitted With Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 1794–1797. [CrossRef]
- Zhu, X.; Hu, X.; Qin, X.; Pan, J.; Zhou, W. An elevated liver fibrosis index FIB 4 score is associated with poor clinical outcomes in patients with sepsis: an observational cohort study. Pol. Arch. Intern. Med. 2020, 130, 1064–1073. [CrossRef]
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Eguchi, Y.; Ono, M.; et al. FIB-4 First in the Diagnostic Algorithm of Metabolic-Dysfunction-Associated Fatty Liver Disease in the Era of the Global Metabodemic. Life 2021, 11, 143. [CrossRef]
- Houot, M.; Ngo, Y.; Munteanu, M.; Marque, S.; Poynard, T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment. Pharmacol. Ther. 2016, 43, 16–29. [CrossRef]
- Parajuli, P.; Sabo, R.; Alsaadawi, R.; Robinson, A.; French, E.; Sterling, R.K. Fibrosis-4 (FIB-4) Index as a Predictor for Mechanical Ventilation and 30-day Mortality Across COVID-19 Variants. J. Clin. Transl. Sci. 2023, 7, 1–25. [CrossRef]
- Park, J.G., et al., Fibrosis-4 index as a predictor for mortality in hospitalised patients with COVID-19: a retrospective multicentre cohort study. BMJ open, 2020. 10(11): p. e041989.
- Demir, N.; Yüzbasıoglu, B.; Calhan, T.; Ozturk, S. Prevalence and Prognostic Importance of High Fibrosis-4 Index in COVID-19 Patients. Int. J. Clin. Pr. 2022, 2022, 1–8. [CrossRef]
- Shibata, N.; Kondo, T.; Kazama, S.; Kimura, Y.; Oishi, H.; Arao, Y.; Kato, H.; Yamaguchi, S.; Kuwayama, T.; Hiraiwa, H.; et al. Impact of predictive value of Fibrosis-4 index in patients hospitalized for acute heart failure. Int. J. Cardiol. 2021, 324, 90–95. [CrossRef]
- Pranata, R.; Yonas, E.; Huang, I.; Lim, M.A.; Nasution, S.A.; Kuswardhani, R.A.T. Fibrosis-4 index and mortality in coronavirus disease 2019: a meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e368–e374. [CrossRef]
- Maeda, D.; Kanzaki, Y.; Sakane, K.; Tsuda, K.; Akamatsu, K.; Hourai, R.; Okuno, T.; Tokura, D.; Nakayama, S.; Hasegawa, H.; et al. Prognostic value of the liver fibrosis marker fibrosis-5 index in patients with acute heart failure. ESC Hear. Fail. 2022, 9, 1380–1387. [CrossRef]
- Font, M.D.; Thyagarajan, B.; Khanna, A.K. Sepsis and Septic Shock – Basics of diagnosis, pathophysiology and clinical decision making. Med Clin. North Am. 2020, 104, 573–585. [CrossRef]
- Li, Y., et al., Liver fibrosis index FIB-4 is associated with mortality in COVID-19. Hepatology communications, 2021. 5(3): p. 434-445.
- Peleg, N.; Issachar, A.; Sneh-Arbib, O.; Shlomai, A. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig. Liver Dis. 2017, 49, 1133–1138. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).