Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Study Area
3. Methods
3.1. Plant Materials
3.2. Floristic Analysis
3.3. Genetically Analysis
3.4. Statistical Processing
3.5. Results
Floristic Analysis of C. ambigua Populations
3.6. Age Composition of C. ambigua in Populations
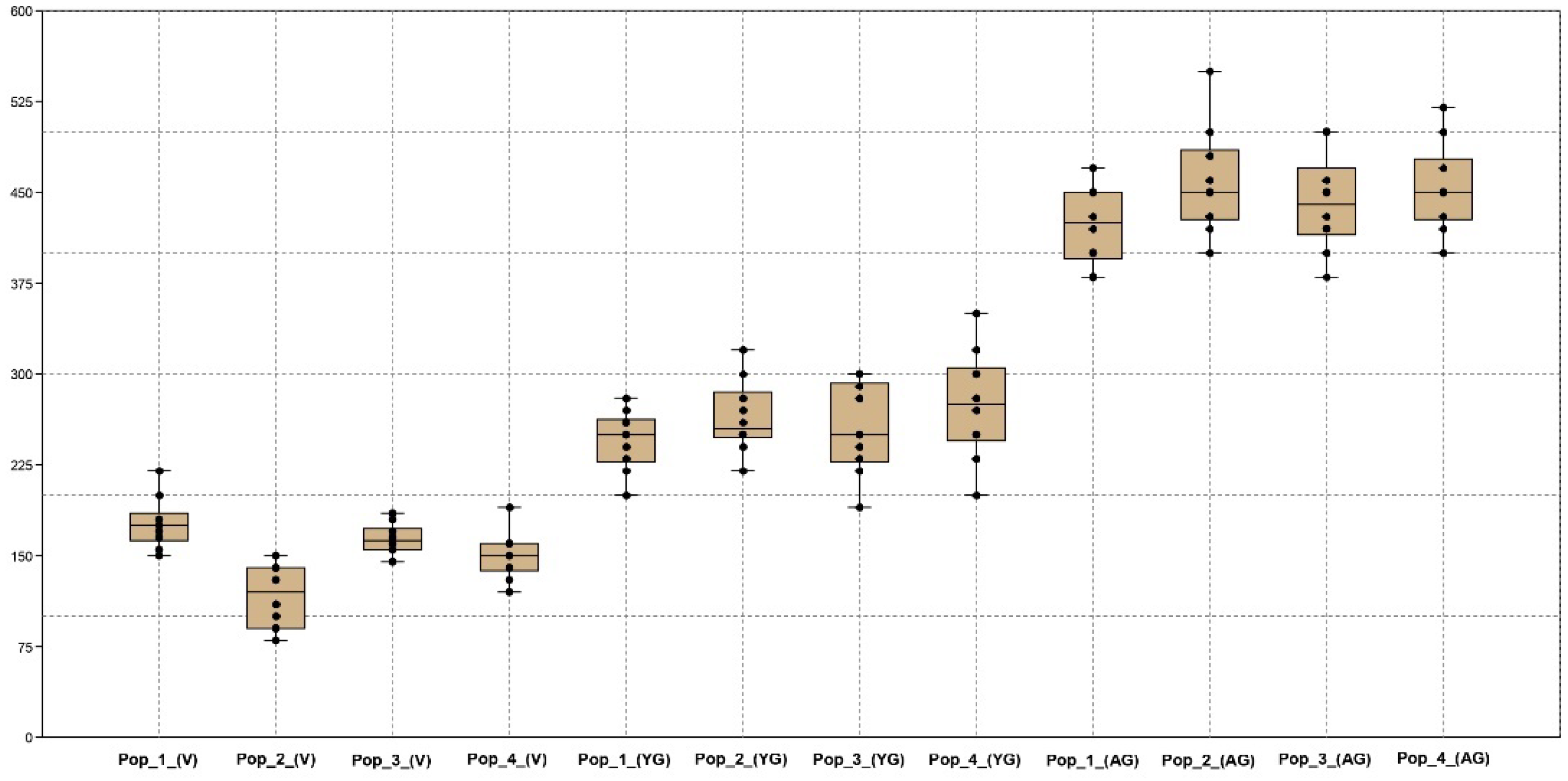
3.7. Morphological Structure of C. ambigua Populations
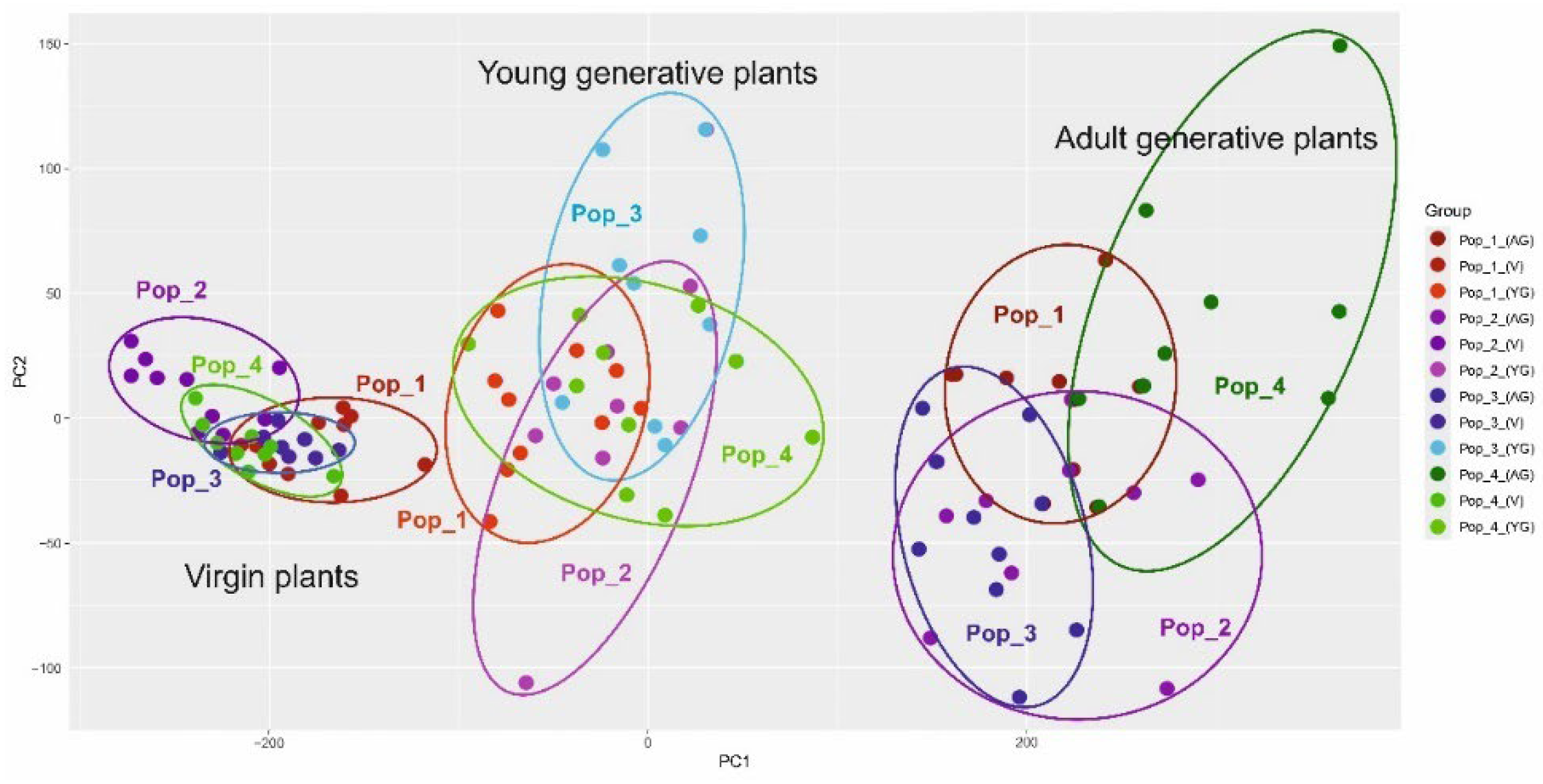
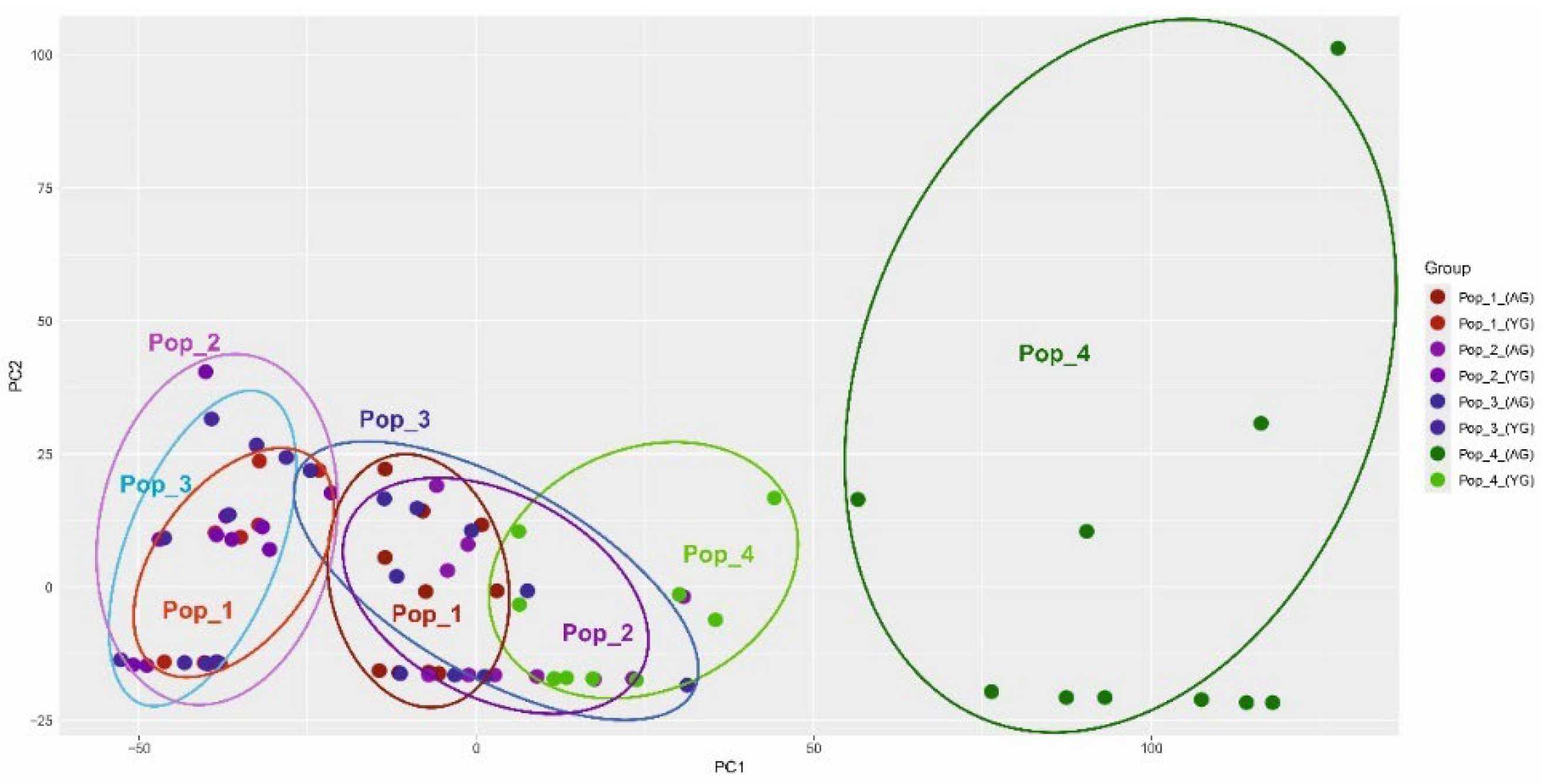
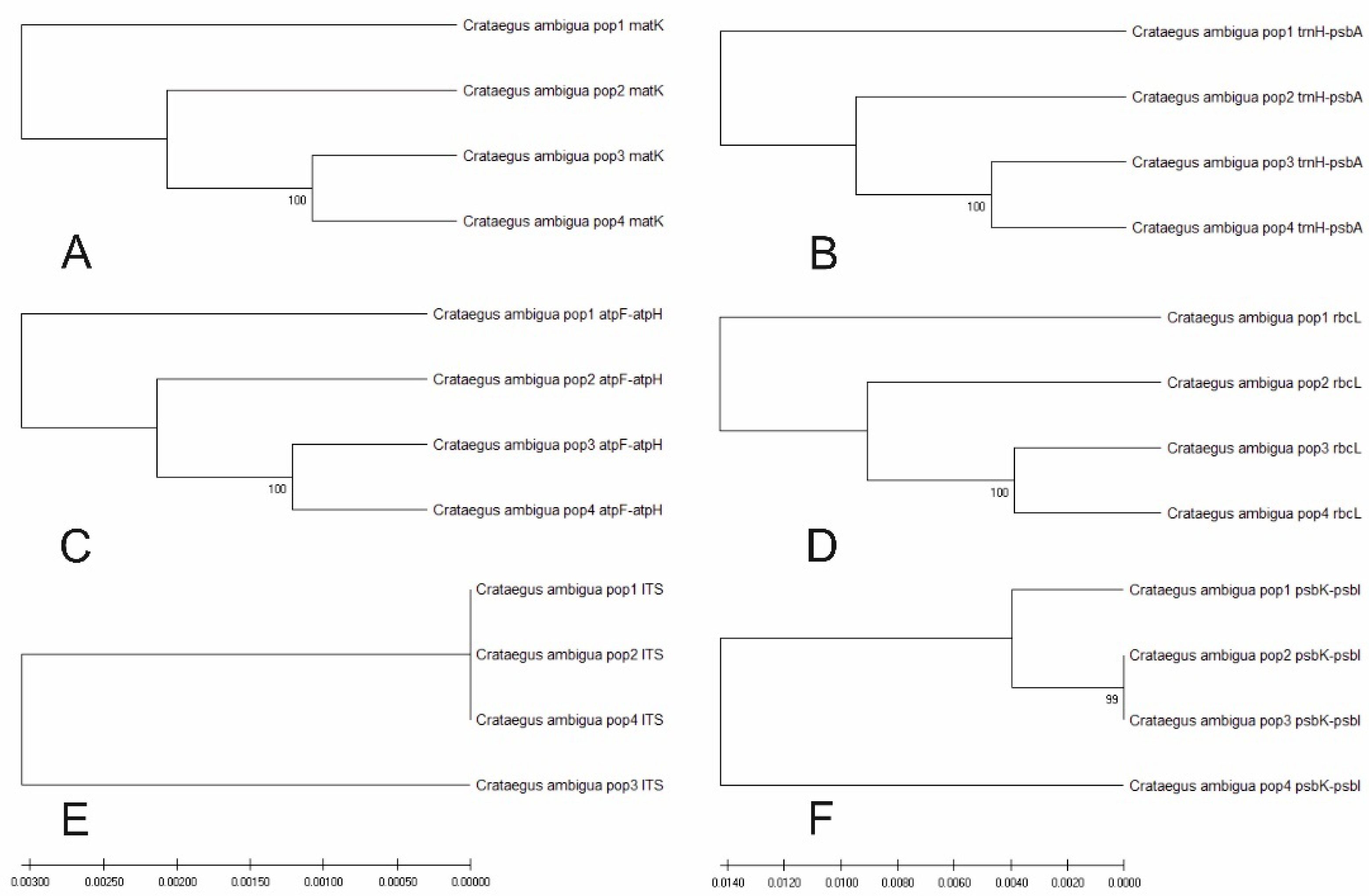
3.8. Genetic Analysis of C. ambigua Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Letukhova, V.; Potapenko, I.; Fedoronchuk, M. Taxonomic analysis of some species of the genus Crataegus (Rosaceae) from the flora of Crimea. Ukr. Bot. J. 2014, 71, 182–187. [Google Scholar] [CrossRef]
- Poletiko, O.M. Hawthorn - Crataegus L // In the book: Trees and shrubs of the USSR.-M.; L.: USSR Academy of Sciences, 1954. [Google Scholar]
- Rusanov, F. N. (1965). Introduced hawthorns of the botanical garden of the Academy of Sciences of the UzSSR. Dendrology of Uzbekistan, 1, 8-254 (In Russian).
- Chang, Q. , Zuo, Z. Hawthorn. The Journal of Clinical Pharmacology 42(6), 605–612.
- Kristafovich, A.N. , Paleobotany. - L.: Science, 1957. -594s.
- Alekhin, V.V. , Kudryasheva L.V., Govorukhin V.S. Geography of plants with basics of botany. - M.: Uchpedgiz, 1957. -519 pp.
- Zhukovsky, P.M. Cultivated plants and their relatives. -L.: Kolos, 1964. -792 p.
- Wulf, E.V. Introduction to the historical geography of plants. - M.; L.: USSR Academy of Sciences, 1933. [Google Scholar]
- Rusanov, F.N. History of the development of the river Crataegus L // B book: Introduction and acclimation of plants. -Tashkent: Fan, 1970.- Issue 6.- pp. 20-28.
- Tsinovskis, R.E. Hawthorns of the Balkhash region. - Riga: Zinatne, 1971.-384 p.
- Boboreko, E.Z. Hawthorn. -Minsk: Science and Technology, 1974. -222 s.
- Baytenov, M.S. Flora of Kazakhstan. – Almaty: Gylym, 2001.- 280 p.
- Sokolov, S.Ya. , Svyazeva O.A. Geography of woody plants USSR.-M.; L.: Nauka, 1965. [Google Scholar]
- Kriissmann, G. Die Laubgeholze Aiifgabe. - Berlin: Verlag Parey, 1951.-С.118-125.
- Komorov, VL. 1939. Flora SSSR. M. 9. 416-468 (in Russian).
- Flora of Kazakhstan. – Alma-Ata, 1961. - T. 4. – 410 p.
- Baytenov, M.S. Flora of Kazakhstan. – Almaty: Gylym, 2001.- 280 p.
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised checklist of endemic vascular plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Red Book of Kazakhstan. – Almaty: Science, 1981. – T. 2. – 99 p.
- Aralbay, N.K. , Kudabaeva G.M., Imanbaeva A.A. and others. Catalog of rare and endangered plant species of the Mangystau region (Red Book). – Aktau, 2006. -32 p.
- Safronova, I.N. Deserts of Mangyshlak (essay on vegetation) // Proceedings of Bot. Inta RAS. - 1996. – Issue 18. – 211 p.
- Kisykov, U.K. Materials on the flora of mountain Mangyshlak // Tr. Institute of Botany An KazSSR. - 1955. – T.1. – pp. 84-117.
- Sumbembayev, A.; Abugalieva, S.; Danilova, A.; Matveyeva, E.; Szlachetko, D. Flower morphometry of members of the genus Dactylorhiza Necker ex Nevski (Orchidaceae) from the Altai Mountains of Kazakhstan. Biodiversitas J. Biol. Divers. 2021, 22. [Google Scholar] [CrossRef]
- Romanovich, V.V. Towards the use of elements of wild flora for landscaping industrial centers and settlements of the Mangyshlak Peninsula. // Tr. Institute of Regional Pathology. - Alma-Ata: Science, 1969. – T.18. – 187 p.
- Cherepanov, S.K. Vascular plants of the USSR. - Leningrad: Science, 1981. – 509 p.
- Lyubimov, V.B. On the question of taxonomy of Сrataegus trancaspica A.Pojark. // Bulletin of the Main bot. garden – M.: Nauka, 1989. – Issue 151. – P. 47-50.
- Imanbaeva. A.; Garden, R.O.K.M.E.B.; Ishmuratova, M.Y.; Tuyakova, A.T. Screening of Mangystau Flora for Wild Relatives of Cultivated Plants. Central Eur. J. Bot. 2015, 1, 12–20. [Google Scholar] [CrossRef]
- Pavlov, N.B. Flora Kazahstana [Flora of Kazakhstan]. 1956. Vol 1. Alma-Ata: AS Kazakh SSR, 347 p.
- Duysenova N., I. et al. The age composition of populations of Crataegus ambigua in the natural conditions of Mangyshlak //Вестник Карагандинскoгo университета. Серия: Биoлoгия. Медицина. Геoграфия. – 2017. – №. 4. – С. 29-34.
- DÖNMEZ, A. A. (2004). The genus Crataegus L. (Rosaceae) with special reference to hybridisation and biodiversity in Turkey. Turkish Journal of Botany, 28(1), 29-37.
- Zargar, M.; Dyussibayeva, E.; Orazov, A.; Zeinullina, A.; Zhirnova, I.; Yessenbekova, G.; Rysbekova, A. Microsatellite-Based Genetic Diversity Analysis and Population Structure of Proso Millet (Panicum miliaceum L.) in Kazakhstan. Agronomy 2023, 13, 2514. [Google Scholar] [CrossRef]
- Zeinullina, A. , Zargar, M., Dyussibayeva, E., Orazov, A., Zhirnova, I., Yessenbekova, G.,... & Hu, Y. G. (2023). Agro-Morphological Traits and Molecular Diversity of Proso Millet (Panicum miliaceum L.) Affected by Various Colchicine Treatments. Agronomy, 13(12), 2973.
- Orazov, A. , Yermagambetova, M., Myrzagaliyeva, A., Mukhitdinov, N., Tustubayeva, S., Turuspekov, Y., & Almerekova, S. (2024). Plant height variation and genetic diversity between Prunus ledebouriana (Schlecht.) YY Yao and Prunus tenella Batsch based on using SSR markers in East Kazakhstan. PeerJ, 12, e16735.
- Hebert P., D. , Cywinska A., Ball S. L., deWaard J. R. Biological identifications through DNA barcodes // Proc Biol Sci. ‒ 2003. ‒ Vol. 270, № 1512. ‒ C. 313-321.
- Kress W., J. , Erickson D. L. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region // PLoS One. ‒ 2007. ‒ Vol. 2, № 6. ‒ C. e508.
- Kress W., J. , Erickson D. L. DNA barcodes: methods and protocols // Methods Mol Biol. ‒ 2012. ‒ Vol. 858. ‒ C. 3-8.
- Chase M., W. , Salamin N., Wilkinson M., Dunwell J. M., Kesanakurthi R. P., Haider N., Savolainen V. Land plants and DNA barcodes: short-term and long-term goals // Philos Trans R Soc Lond B Biol Sci. ‒ 2005. ‒ Vol. 360, № 1462. ‒ C. 1889-95.
- Lahaye, R. , van der Bank M., Bogarin D., Warner J., Pupulin F., Gigot G., Maurin O., Duthoit S., Barraclough T. G., Savolainen V. DNA barcoding the floras of biodiversity hotspots // Proc Natl Acad Sci U S A. ‒ 2008. ‒ Vol. 105, № 8. ‒ C. 2923-8.
- Chen, S. , Yao H., Han J., Liu C., Song J., Shi L., Zhu Y., Ma X., Gao T., Pang X., Luo K., Li Y., Li X., Jia X., Lin Y., Leon C. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species // PLoS One. ‒ 2010. ‒ Vol. 5, № 1. ‒ C. e8613.
- Vol. 9, №1. – С.32-43.
- Nieto Feliner, G. , Rosselló J. A. Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants // Mol Phylogenet Evol. ‒ 2007. ‒ Vol. 44, № 2. ‒ C. 911-9.
- Schultz, J. , Müller T., Achtziger M., Seibel P. N., Dandekar T., Wolf M. The internal transcribed spacer 2 database--a web server for (not only) low level phylogenetic analyses // Nucleic Acids Res. ‒ 2006. ‒ Vol. 34, № Web Server issue. ‒ C. W704-7.
- Patwardhan, A. , Ray S., Roy A. Molecular Markers in Phylogenetic Studies-A Review // J. Phylogen. Evolution. Biol. – 2014, – Vol.2, №2. - Р.1-9.
- Orazov, A.; Myrzagaliyeva, A.; Mukhitdinov, N.; Tustubayeva, S. Callus induction with 6-BAP and IBA as a way to preserve Prunus ledebouriana (Rosaceae), and endemic plant of Altai and Tarbagatai, East Kazakhstan. Biodiversitas J. Biol. Divers. 2022, 23. [Google Scholar] [CrossRef]
- Кoshim, A.G.; Sergeyeva, A.M.; Bexeitova, R.T.; Aktymbayeva, A.S. LANDSCAPE OF THE MANGYSTAU REGION IN KAZAKHSTAN AS A GEOMORPHOTOURISM DESTINATION: A GEOGRAPHICAL REVIEW. Geoj. Tour. Geosites 2020, 29, 385–397. [Google Scholar] [CrossRef]
- Sagyndykova, M.; Imanbayeva, A.; Gassanova, G.; Ishmuratova, M. Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity 2024, 16, 219. [Google Scholar] [CrossRef]
- Smirnova, O.V.; Zaugolnova, L.B.; Ermakova, I.M. Cenopopulation of Plants; Science Publishing House: Moscow, Russia, 1976; p. 217. [Google Scholar]
- Pavlov, N.B. Flora Kazahstana [Flora of Kazakhstan]; Publishing House of the Kazakh Academy of Sciences: Almaty, Kazakhstan, 1956; Volume 1, p. 347. [Google Scholar]
- Goloskokov, V.P. (Ed.) Illiustrirovannyi Opredelitel Rastenii Kazakhstana [Illustrated Determinant of Plants of Kazakhstan]; Nauka: Almaty, Kazakhstan, 1972; Volume 2. (In Russian) [Google Scholar]
- Kamelin, R.V. Key to Plants of Central Asia. A Critical Abstract of Flora; Science Publishing House: Leningrad, Russia, 2015; Volume 11. [Google Scholar]
- International Plant Names Index. Available online: www.ipni.org (accessed on 30 January 2024).
- Serebryakov, I.G. Ecological Morphology of Plants. Life Forms of the Overgrowths and Conifers; High School: Moscow, Russia, 1982; 380p. (In Russian) [Google Scholar]
- Shay, J.E.; Pennington, L.K.; Montiel-Molina, J.A.M.; Toews, D.J.; Hendrickson, B.T.; Sexton, J.P. Rules of Plant Species Ranges: Applications for Conservation Strategies. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Kew Royal Botanical Garden. Plants of the World Online. Available online: www.powo.science.kew.org (accessed on 30 January 2024).
- Komarov, A.; Palenova, M.; Smirnova, O. The concept of discrete description of plant ontogenesis and cellular automata models of plant populations. Ecol. Model. 2003, 170, 427–439. [Google Scholar] [CrossRef]
- Fedorova, S.V. Methodological Approaches in Population Botany and Plant Ecology. Am. J. Biosci. 2020, 8, 73. [Google Scholar] [CrossRef]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15.
- Maniatis, T. , Fritsch E.E., Sambrook J. Molecular cloning. A laboratory manual. – New York: Cold Spring Harbor Laboratory, 1982. – P.545.
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Available online:. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 7 November 2021).
- Kuziev, R.K.; Sektimenko, V.E. Soils of Uzbekistan; Extremum Press Publishing House: Tashkent, Uzbekistan, 2009; p. 351. (In Russian) [Google Scholar]
- Kuziev, R.K.; Yuldashev, G.Y.U.; Akramov, I.A. Bonitization of Soils; The Way of Science Publishing House: Tashkent, Uzbekistan, 2004; p. 127. [Google Scholar]
- Joshi, S.P.; Gupta, V.S.; Aggarwal, R.K.; Ranjekar, P.K.; Brar, D.S. Genetic diversity and phylogenetic relationship revealed by intersimple sequence repeat (ISSR) polymorphism in the genus Oryza. Theor. Appl. Genet. 2000, 100, 1311–1320. [Google Scholar] [CrossRef]
- Rohlf, F. NTSYSpc: Numerical Taxonomy and Multivariate Analysis System, Version 2.02; Exeter Software: Setauket, NY, USA, 1998. [Google Scholar]
- Tuyakova, A. T. , Imanbayeva, A. A., Duysenova, N. I., & Ishmuratova, M. Y. (2016). Anatomical structure study of aerial organs of Crataegus ambigua CA Mey. Biosciences Biotechnology Research Asia, 13(3), 1303-1309. [CrossRef]
- Phipps, J.B. Biogeographic, Taxonomic, and Cladistic Relationships Between East Asiatic and North American Crataegus. Ann. Mo. Bot. Gard. 1983, 70, 667. [Google Scholar] [CrossRef]
- Duysenova, N. I. , Imanbaeva, А. А., Tuyakova, А. Т., & Kopbaeva, G. B. (2017). The age composition of populations of Crataegus ambigua in the natural conditions of Mangyshlak. Вестник Карагандинскoгo университета. Серия: Биoлoгия. Медицина. Геoграфия, (4), 29-34.
- Duisenova, N. I. , Imanbayeva, A. A., & Ishmuratova, M. Y. (2016). The study of the state and structure of populations of rare plant of mangyshlak crataegus ambigua CA Mey ex A. Beck. Ecology, Environment and Conservation, 22(4), 2087-2093.
- Hamzeh'Ee, B.; Attar, F.; Assareh, M.H.; Maassoumi, A.A.; Osaloo, S.K.; Christensen, K.I. Taxonomic notes on Crataegus, ser. Crataegus, subser. Erianthae (Rosaceae), new species and record, using morphology and micromorphological evidence. Nord. J. Bot. 2014, 32, 26–37. [Google Scholar] [CrossRef]
- Sharifnia, F. , Seyedipour, N., Mehregan, I., & Salimpour, F. (2013). Phylogenetic study some of Crataegus L.(Rosaceae, Pyreae) species in Iran. Journal of Biodiversity and Environmental Sciences, 3, 1-11. ( 3, 1–11.
- Yermagambetova, M. , Almerekova, S., Turginov, O., Sultangaziev, O., Abugalieva, S., & Turuspekov, Y. (2023). Genetic Diversity and Population Structure of Juniperus seravschanica Kom. Collected in Central Asia. Plants, 12(16), 2961. [CrossRef]
- Anar, M. , Ainur, S., Manar, T., Saule, M., Zhumagul, M. Z., Zheksenbaevna, N. A.,... & Zharakovich, M. M. (2023). Morphological variability of the rare species Linaria cretacea in the conditions of the chalk hills in North-Western Kazakhstan. Caspian Journal of Environmental Sciences, 21(5), 1273-1278. [CrossRef]
- Sagyndykova, M. , Imanbayeva, A. Gassanova, G., & Ishmuratova, M. (2024). Current Status and Resources of Alhagi pseudalhagi (Fabaceae) in the Atyrau Region, Western Kazakhstan. Diversity, 16(4), 219. ( 16(4), 219. [CrossRef]
- Baibagyssov, A. , Thevs, N., Nurtazin, S., Waldhardt, R., Beckmann, V., & Salmurzauly, R. (2020). Biomass resources of Phragmites australis in Kazakhstan: Historical developments, utilization, and prospects. Resources, 9(6), 74. [CrossRef]
- Zhang, Y.; Tariq, A.; Hughes, A.C.; Hong, D.; Wei, F.; Sun, H.; Sardans, J.; Peñuelas, J.; Perry, G.; Qiao, J.; et al. Challenges and solutions to biodiversity conservation in arid lands. Sci. Total. Environ. 2023, 857, 159695. [Google Scholar] [CrossRef]
- Orazov, A. , Tustubayeva, S., Alemseytova, J., Mukhitdinov, N., Myrzagaliyeva, A., Turuspekov, Y., & Sramko, G. (2021). Flora accompanying Prunus ledebouriana (Schltdl.) YY Yao in the Tarbagatai State National Park in Kazakhstan. International Journal of Biology and Chemistry, 14(1), 21-34. [CrossRef]
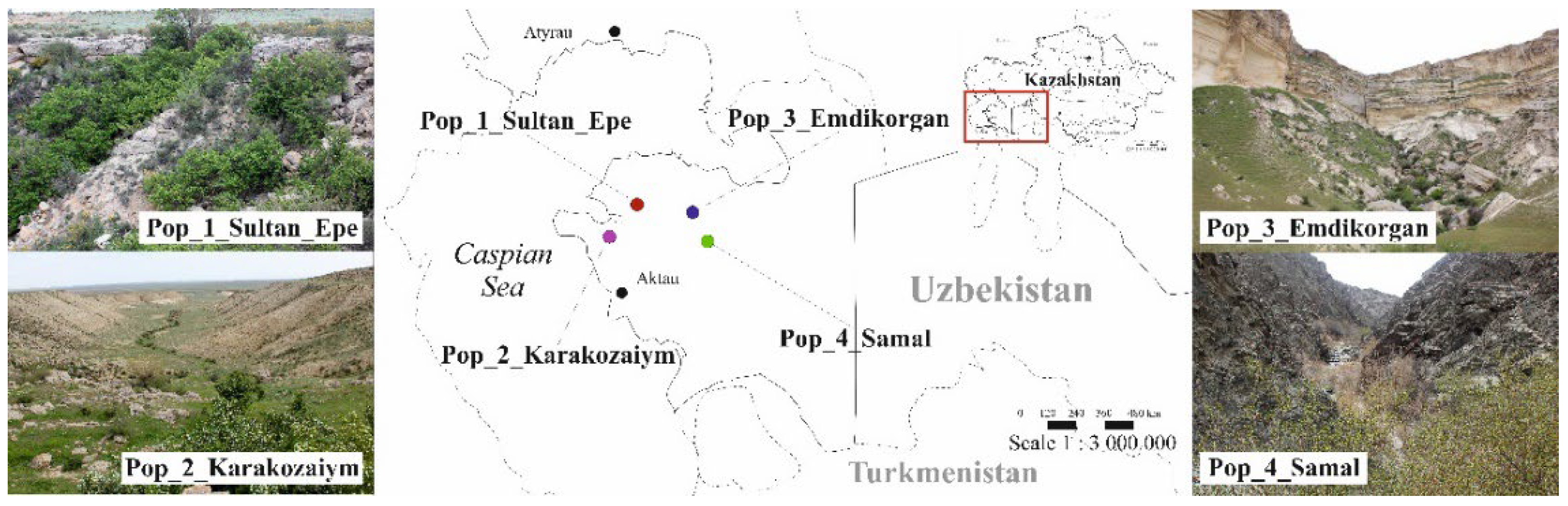


| Populations | Name | Geographical location | Coordinates | Altitude |
|---|---|---|---|---|
| Pop 1 | Sultan Epe | Sultan Epe Gorge (Tyubkaragan Peninsula) | 44°25’85.7”N 50°58’30.7”E |
172 |
| Pop 2 | Karakozaiym | Karakozayim Gorge (Tyubkaragan Peninsula) | 44°27’41.8”N 50.37’71.5”E |
136 |
| Pop 3 | Emdikorgan | Emdikorgan Gorge (Northern Aktau Ridge) | 44°28’62.8” N 51°25’25.4”E |
35 |
| Pop 4 | Samal | Samal Gorge (Western Karatau Ridge) | 44°07’43.6”N 51°35’41.8”E |
247 |
| Name | Sequence 5’ – 3’ | Locus for barcode |
|---|---|---|
| atpF | ACTCGCACACACTCCCTTTCC | atpF-atpH |
| atpH | GCTTTTATGGAAGCTTTAACAAT | atpF-atpH |
| ITS4 | TCCTCCGCTTATTGATATGC | ITS1 and ITS2 |
| ITS5 | GGAAGTAAAAGTCGTAACAAG | ITS1 and ITS2 |
| 3F_KIMf | CGTACAGTACTTTTGTGTTTACGAG | matK |
| 1R_KIMr | ACCCCATTCATCTGGAAATCTTGGTTC | matK |
| psbK | TTAGCCTTTGTTTGGCAAG | psbK-psbI |
| psbI | AGAGTTTGAGAGTAAGCAT | psbK-psbI |
| rbcLa_F | ATGTCACCACAAACAGAGACTAAAGC | rbcL |
| rbcLa_R | GTAAAATCAAGTCCACCRCG | rbcL |
| psbA3f | GTTATGCATGAACGTAATGCTC | trnH-psbA |
| trnHf_05 | CGCGCATGGTGGATTCACAATCC | trnH-psbA |
| Populations | Total copies, pcs. | Age condition | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Juveniles | Virginia | Young generative | Adult generative | ||||||
| pcs. | % | pcs. | % | pcs. | % | pcs. | % | ||
| Pop 1 | 415 | 89 | 21,4 | 34 | 8,2 | 175 | 42,2 | 117 | 28,2 |
| Pop 2 | 130 | 35 | 26,9 | 3 | 2,3 | 88 | 67,7 | 4 | 3,1 |
| Pop 3 | 55 | 15 | 27,27 | 11 | 20,00 | 22 | 40,00 | 7 | 12,73 |
| Pop 4 | 104 | 16 | 15,3 | 12 | 11,5 | 44 | 42,3 | 32 | 30,9 |
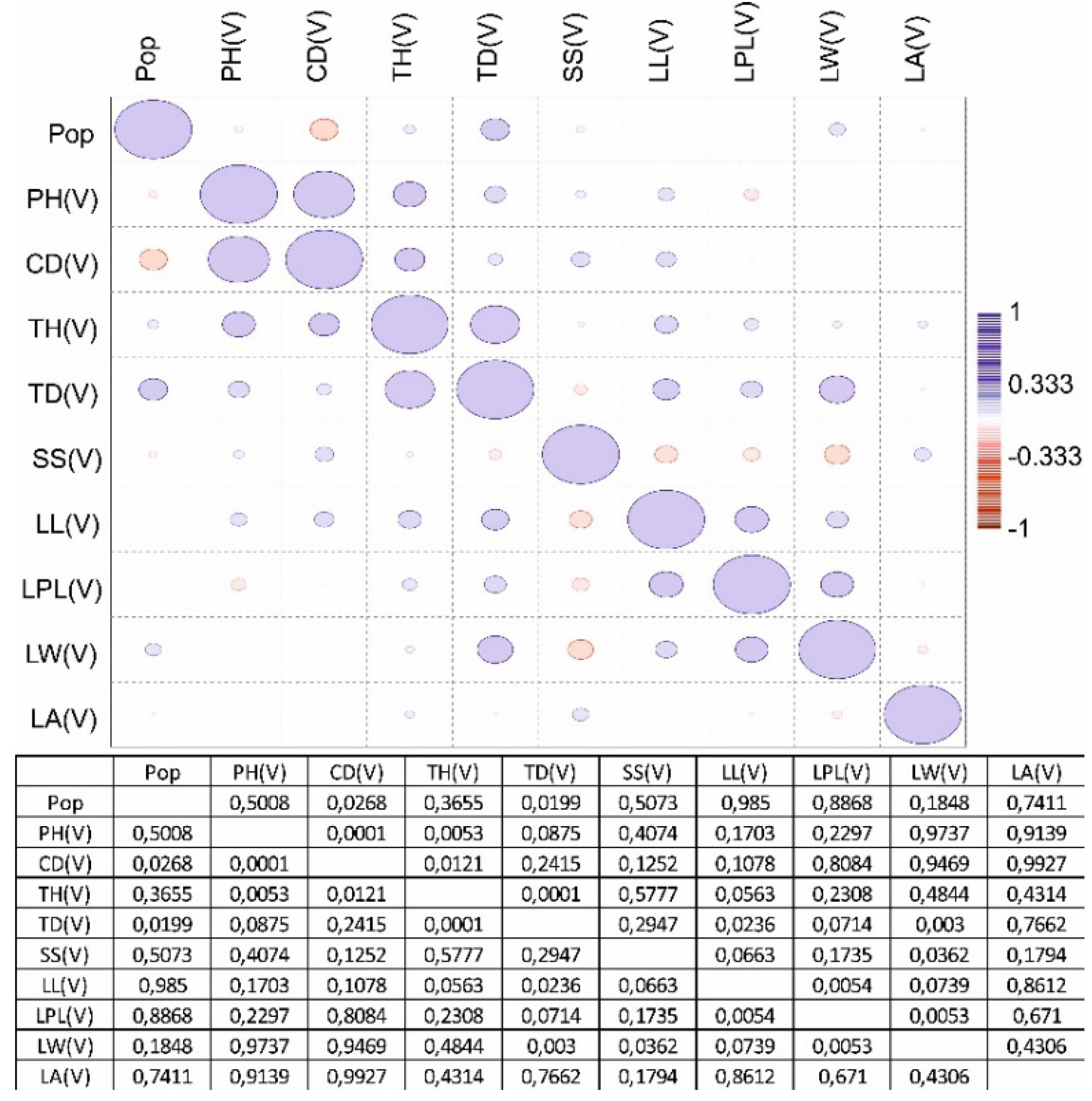
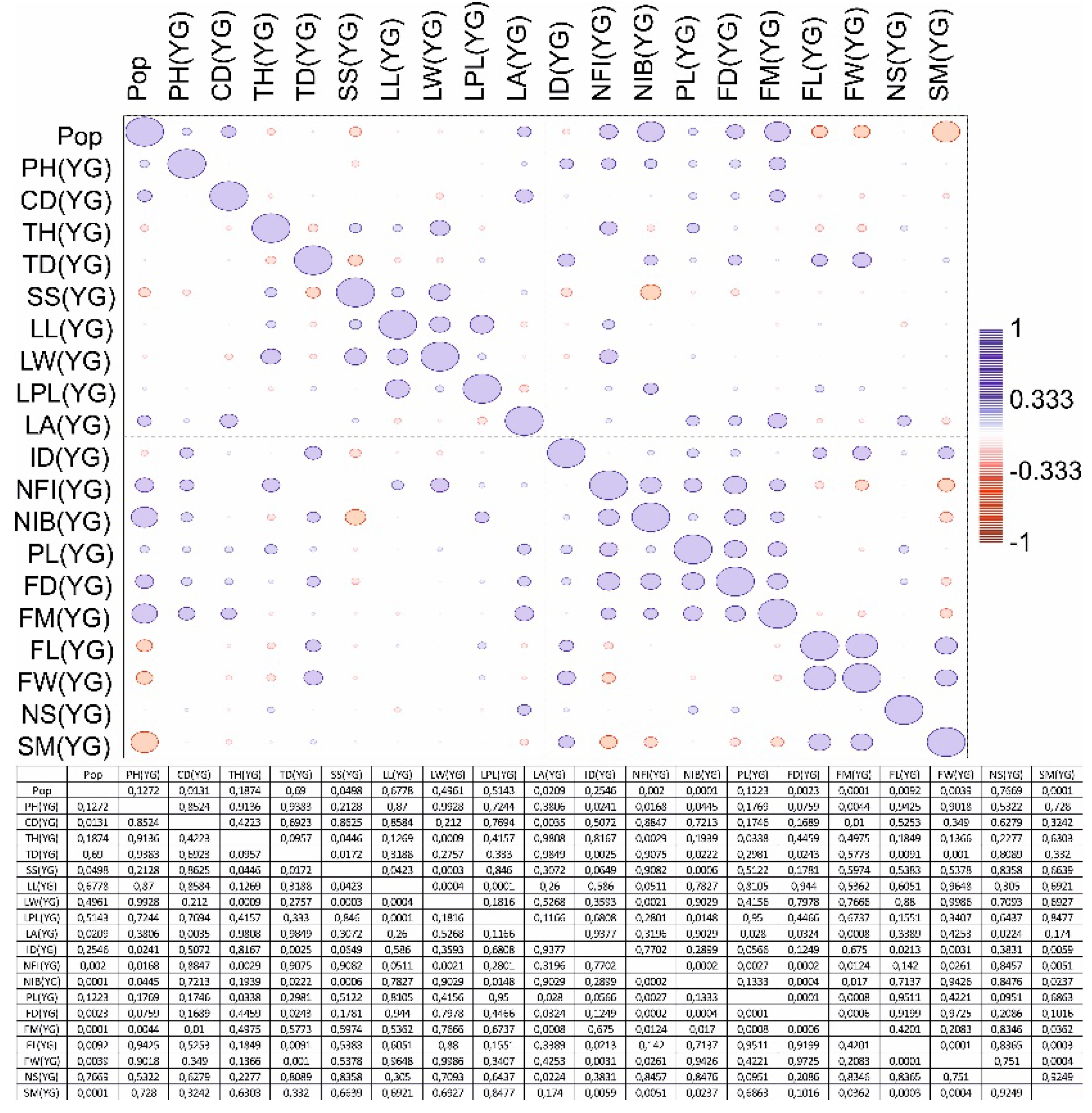
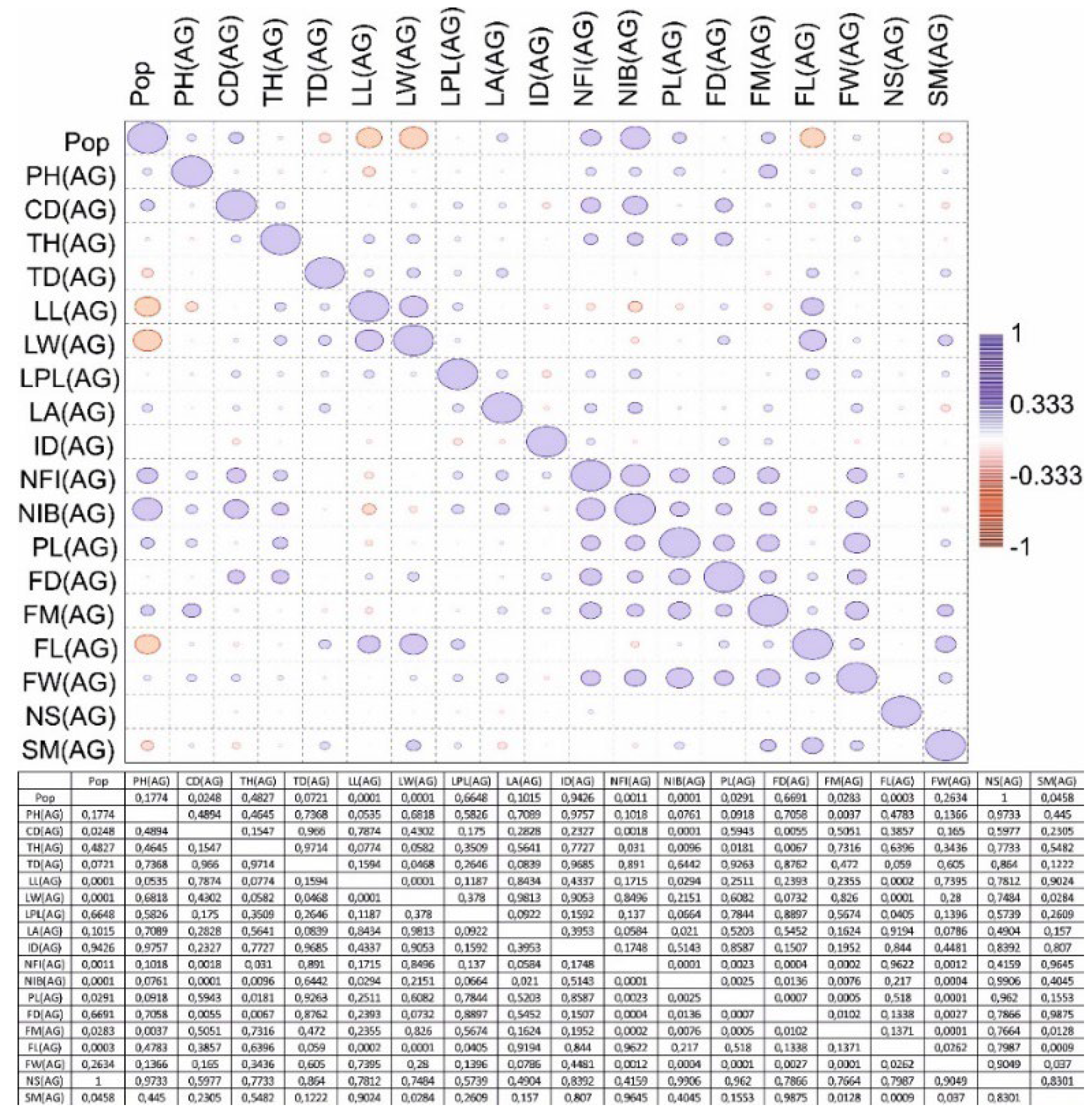
| Morphological parameters | Pop 1 (Mean/SD/CV) |
Pop 2 (Mean/SD/CV) |
Pop 3 (Mean/SD/CV) |
Pop 4 (Mean/SD/CV) |
|---|---|---|---|---|
| Plant height (V) | 185/13,2//0,07 | 126,7/25,2/0,20 | 170/10/0,06 | 136,7/15,3/0,1 |
| Plant height (YG) | 246,7/25,2/0,10 | 260/10/0,04 | 273,3/37,9/0,14 | 250/20/0,08 |
| Plant height (AG) | 400/20/0,05 | 426,7/5,8/0,01 | 450/50/0,11 | 456,7/11,5/0,03 |
| Plant crown diameter (V) | 126,7/14,4/0,11 | 80/17,3/0,22 | 101,7/7,6/0,08 | 81,7/7,6/0,09 |
| Plant crown diameter (YG) | 206,7/20,8/0,10 | 263,3/32,1/0,12 | 276,7/25,2/0,09 | 260/36,1/0,14 |
| Plant crown diameter (AG) | 423,3/45,1/0,11 | 373,3/41,6/0,11 | 363,3/15,3/0,04 | 450/50/0,11 |
| Plant trunk height (V) | 30/5/0,17 | 35/8,7/0,25 | 31/3,6/0,12 | 33,3/12,6/0,38 |
| Plant trunk height (YG) | 54,3/4,04/0,07 | 73,3/7,6/0,10 | 53,3/3,51/0,07 | 56,3/5,5/0,10 |
| Plant trunk height (AG) | 80,7/9,02/0,11 | 64,3/6,03/0,09 | 71/3,61/0,05 | 90/5/0,06 |
| Plant trunk diameter (V) | 10/0,5/0,05 | 9,3/0,58/0,06 | 9,5/1,32/0,14 | 11,7/4,16/0,36 |
| Plant trunk diameter (YG) | 15,1/0,32/0,02 | 12,2/1,26/0,10 | 13,3/1,26/0,09 | 15,3/1,5/0,10 |
| Plant trunk diameter (AG) | 19,3/3,51/0,18 | 16,3/1,53/0,09 | 14,2/0,76/0,05 | 14,3/2,08/0,15 |
| Spike size (V) | 1,07/0,21/0,20 | 0,87/0,15/0,18 | 0,9/0,1/0,11 | 0,9/0,1/0,11 |
| Spike size (YG) | 1,1/0,1/0,09 | 1,07/0,15/0,14 | 1,1/0,3/0,27 | 0,93/0,15/0,16 |
| Leaf length (V) | 3,53/0,72/0,21 | 3,53/0,61/0,17 | 3,15/0,58/0,18 | 3,66/0,69/0,19 |
| Leaf length (YG) | 3,46/0,68/0,20 | 4,15/0,76/0,18 | 3,07/0,48/0,16 | 3,67/0,60/0,16 |
| Leaf length (AG) | 5,11/0,57/0,11 | 4,02/0,42/0,10 | 3,47/0,48/0,14 | 3,68/0,84/0,23 |
| Leaf width (V) | 3,11/0,40/0,13 | 3,01/0,41/0,14 | 2,4/0,75/0,31 | 3,77/0,62/0,16 |
| Leaf width (YG) | 2,91/0,41/0,14 | 3,75/0,76/0,20 | 2,52/0,57/0,23 | 3,09/0,41/0,13 |
| Leaf width (AG) | 4,84/0,54/0,11 | 3,85/0,37/0,10 | 2,35/0,70/0,30 | 3,13/0,49/0,16 |
| Leaf petiole length (V) | 1,62/0,58/0,35 | 2,01/0,39/0,19 | 1,21/0,28/0,23 | 1,92/0,34/0,17 |
| Leaf petiole length (YG) | 1,7/0,45/0,27 | 1,6/0,39/0,25 | 1,38/0,23/0,17 | 1,91/0,54/0,28 |
| Leaf petiole length (AG) | 1,82/0,70/0,39 | 1,77/0,41/0,23 | 1,42/0,34/0,24 | 2,05/0,58/0,28 |
| Leaf area (V) | 9,7/1,26/0,13 | 10,38/1,45/0,14 | 10,971/1,34/0,12 | 9,79/1,82/0,19 |
| Leaf area (YG) | 11,1/1,53/0,14 | 12,81/2,37/0,19 | 14,95/3,15/0,21 | 13,21/1,93/0,15 |
| Leaf area (AG) | 13,9/1,72/0,12 | 14,24/3,14/0,22 | 13,8/2,19/0,16 | 15,87/1,44/0,09 |
| Morphological parameters | Pop 1 (Mean/SD/CV) |
Pop 2 (Mean/SD/CV) |
Pop 3 (Mean/SD/CV) |
Pop 4 (Mean/SD/CV) |
|---|---|---|---|---|
| Inflorescence diameter (YG) | 4,97/0,52/0,10 | 4,39/0,80/0,18 | 4,39/0,73/0,17 | 4,52/1,13/0,25 |
| Inflorescence diameter (AG) | 5,83/0,48/0,08 | 5,3/0,78/0,15 | 6,21/0,75/0,12 | 5,5/0,63/0,11 |
| Number of flowers on 1 inflorescence (YG) | 7,6/1,26/0,17 | 14,8/0,79/0,05 | 8,2/1,81/0,22 | 15,6/2,37/0,15 |
| Number of flowers on 1 inflorescence (AG) | 14,4/2,22/0,15 | 14,4/1,78/0,12 | 14,3/3,20/0,22 | 19,1/2,13/0,11 |
| Number of inflorescences on 1 branch (YG) | 14,4/1,71/0,12 | 11,3/3,71/0,33 | 12,2/2,86/0,23 | 42,8/6,94/0,16 |
| Number of inflorescences on 1 branch (AG) | 27,7/3,59/0,13 | 35,6/7,41/0,21 | 29,6/8,77/0,30 | 83,5/13,24/0,16 |
| Pedicel length (YG) | 4,46/1,00/0,22 | 6,35/1,97/0,31 | 5,49/1,12/0,20 | 5,92/1,76/0,30 |
| Pedicel length (AG) | 5,12/0,60/0,12 | 6,52/2,20/0,34 | 5,46/1,32/0,24 | 7,33/2,14/0,29 |
| Flower diameter (YG) | 13,45/1,45/0,11 | 14,58/1,47/0,10 | 14,29/1,79/0,13 | 15,93/1,23/0,08 |
| Flower diameter (AG) | 16,21/1,31/0,08 | 15,01/1,52/0,10 | 14,83/1,42/0,10 | 16,58/1,08/0,06 |
| Fruit weight (YG) | 0,672/0,20/0,30 | 0,943/0,21/0,23 | 1,102/0,17/0,16 | 1,122/0,14/0,13 |
| Fruit weight (AG) | 0,977/0,21/0,21 | 1,102/0,19/0,17 | 1,07/0,14/0,13 | 1,169/0,13/0,11 |
| Fruit length (YG) | 1,408/0,27/0,19 | 1,071/0,10/0,10 | 1,06/0,08/0,08 | 1,163/0,06/0,05 |
| Fruit length (AG) | 1,503/0,28/0,18 | 1,435/0,21/0,14 | 1,068/0,13/0,12 | 1,14/0,11/0,09 |
| Fruit width (YG) | 1,532/0,36/0,24 | 0,958/0,14/0,14 | 1,082/0,12/0,12 | 1,11/0,08/0,07 |
| Fruit width (AG) | 1,07/0,05/0,04 | 1,202/0,08/0,07 | 1,002/0,12/0,12 | 1,20/0,08/0,07 |
| Number of seeds (YG) | 1,6/0,52/0,32 | 1,6/0,52/0,32 | 1,8/0,63/0,35 | 1,6/0,52/0,32 |
| Number of seeds (AG) | 1,6/0,52/0,32 | 1,6/0,52/0,32 | 1,6/0,52/0,32 | 1,6/0,52/0,32 |
| Seed mass (YG) | 0,255/0,06/0,22 | 0,173/0,05/0,29 | 0,152/0,05/0,31 | 0,116/0,03/0,25 |
| Seed mass (AG) | 0,149/0,06/0,39 | 0,183/0,05/0,28 | 0,132/0,03/0,25 | 0,121/0,03/0,23 |
| Genetic markers | Length | Conserved sites | Variable sites | Singleton |
|---|---|---|---|---|
| atpF-atpH | 414 | 408 | 0 | 0 |
| ITS | 332 | 328 | 2 | 2 |
| matK | 570 | 570 | 0 | 0 |
| psbK-psbI | 193 | 185 | 7 | 6 |
| rbcL | 497 | 493 | 0 | 0 |
| trnH-psbA | 270 | 270 | 0 | 0 |
| Nucleotide | pop1 | pop2 | pop3 | pop4 | Avg. |
|---|---|---|---|---|---|
| atpF-atpH | |||||
| T(U) | 37.7 | 37.3 | 37.5 | 37.5 | 37.5 |
| C | 12.8 | 12.3 | 12.7 | 12.7 | 12.7 |
| A | 34.0 | 33.9 | 33.8 | 33.8 | 33.9 |
| G | 15.5 | 16.5 | 15.9 | 15.9 | 16.0 |
| Total | 406 | 413 | 408 | 408 | 408.8 |
| ITS | |||||
| T(U) | 19.4 | 19.4 | 19.8 | 19.7 | 19.6 |
| C | 32.4 | 32.4 | 32.8 | 32.4 | 32.5 |
| A | 18.5 | 18.5 | 18.2 | 18.5 | 18.4 |
| G | 29.7 | 29.7 | 29.2 | 29.4 | 29.5 |
| Total | 330 | 330 | 329 | 330 | 329.8 |
| matK | |||||
| T(U) | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 |
| C | 18.1 | 18.1 | 18.1 | 18.1 | 18.1 |
| A | 35.3 | 35.3 | 35.3 | 35.3 | 35.3 |
| G | 16.3 | 16.3 | 16.3 | 16.3 | 16.3 |
| Total | 570 | 570 | 570 | 570 | 570 |
| psbK-psbI | |||||
| T(U) | 43.5 | 43.8 | 44.0 | 44.0 | 43.8 |
| C | 13.5 | 14.1 | 13.6 | 13.6 | 13.7 |
| A | 27.5 | 27.6 | 27.7 | 27.7 | 27.6 |
| G | 15.5 | 14.6 | 14.7 | 14.7 | 14.9 |
| Total | 193 | 192 | 191 | 191 | 191.8 |
| rbcL | |||||
| T(U) | 29.6 | 29.4 | 29.6 | 29.6 | 29.6 |
| C | 21.3 | 21.1 | 21.3 | 21.3 | 21.3 |
| A | 26.6 | 27.0 | 26.6 | 26.6 | 26.7 |
| G | 22.5 | 22.5 | 22.5 | 22.5 | 22.5 |
| Total | 493 | 497 | 493 | 493 | 494 |
| trnH-psbA | |||||
| T(U) | 33.3 | 33.2 | 33.2 | 33.3 | 33.3 |
| C | 13.3 | 13.4 | 13.4 | 13.3 | 13.4 |
| A | 46.7 | 47.0 | 47.0 | 46.7 | 46.8 |
| G | 6.7 | 6.3 | 6.3 | 6.7 | 6.5 |
| Total | 270 | 268 | 268 | 270 | 269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





