Submitted:
06 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
| Cancer | Mean rate (cases/100,000/yr) | |
|---|---|---|
| Males | Females | |
| Bladder, urinary | 37 | 9 |
| Brain | 9 | 6 |
| Breast | 133 | |
| Colorectal | 42 | 32 |
| Corpus uteri | 28 | |
| Esophageal | 9 | 2 |
| Gastric | 7 | 3 |
| Hodgkin’s lymphoma | 3 | 3 |
| Laryngeal | 5 | 1 |
| Leukemia | 19 | 11 |
| Liver | 11 | 4 |
| Lung | 64 | 34 |
| Myeloma | 8 | 5 |
| Non-Hodgkin’s lymphoma | 24 | 16 |
| Oral cavity | 20 | 7 |
| Ovarian | 10 | |
| Pancreatic | 15 | 11 |
| prostate | 105 | |
| Renal | 24 | 12 |
3. Results
4. Discussion
4.1. Diet
4.2. Cigarette Smoking
4.3. Particulate Air Pollution
4.4. Solar UVB and Vitamin D
4.5. Changes in Cancer Incidence Rates
4.6. Public Health Implications
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2021. NCHS Data Brief 2022, 1–8. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef] [PubMed]
- Kulhanova, I.; Znaor, A.; Shield, K.D.; Arnold, M.; Vignat, J.; Charafeddine, M.; Fadhil, I.; Fouad, H.; Al-Omari, A.; Al-Zahrani, A.S.; et al. Proportion of cancers attributable to major lifestyle and environmental risk factors in the Eastern Mediterranean region. Int J Cancer 2020, 146, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002, 94, 1867–1875. [Google Scholar] [CrossRef]
- Grant, W.B.; Garland, C.F. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res 2006, 26, 2687–2699. [Google Scholar]
- Boscoe, F.P.; Schymura, M.J. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer 2006, 6, 264. [Google Scholar] [CrossRef]
- Chen, W.; Clements, M.; Rahman, B.; Zhang, S.; Qiao, Y.; Armstrong, B.K. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control 2010, 21, 1701–1709. [Google Scholar] [CrossRef]
- Borisenkov, M.F. Latitude of residence and position in time zone are predictors of cancer incidence, cancer mortality, and life expectancy at birth. Chronobiol Int 2011, 28, 155–162. [Google Scholar] [CrossRef]
- Grant, W.B. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermatoendocrinol 2012, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Leffell, D.J.; Brash, D.E. Sunlight and skin cancer. Sci Am 1996, 275, 52-53, 56-59. [Google Scholar] [CrossRef]
- Devesa, S.S.; Grauman, D.J.; Blot, W.J.; Pennello, G.A.; Hoover, R.N.; Fraumeni, J.F., Jr. Atlas of Cancer Mortality in the United States, 1950-94; National Institutes of Health; National Cancer Institue, Septgember 1999. [Google Scholar]
- Group, U.S.C.S.W. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). Availabe online: https://www.cdc.gov/cancer/dataviz (accessed on March 15, 2024).
- Data Visualizations Tool Technical Notes. Availabe online: https://www.cdc.gov/cancer/uscs/technical_notes/stat_methods/rates.htm (accessed on May 3, 2024).
- CDC. National Program of Cancer Registries. Availabe online: https://www.cdc.gov/cancer/npcr/index.htm (accessed on.
- National Cancer Institute, S., Epidemiology, and End Results (SEER) Program. Overview of the SEER Program. Availabe online: https://seer.cancer.gov/about/overview.html (accessed on April 12, 2024).
- Grant, W.B. Lower vitamin-D production from solar ultraviolet-B irradiance may explain some differences in cancer survival rates. J Natl Med Assoc 2006, 98, 357–364. [Google Scholar]
- Herman, J.R.; Krotkov, N.; Celarier, E.; Larko, D.; Lebow, G. Distribution of UV radiation at the Earth’s surface from TOMSmeasured UV-backscattered radiances. Journal of Geophysical Research 1999, 104, 12,059–012,076. [Google Scholar] [CrossRef]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007, 85, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr Dev Nutr 2019, 3, nzz087. [Google Scholar] [CrossRef] [PubMed]
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270. [Google Scholar] [CrossRef]
- Grant, W.B. Air pollution in relation to U.S. cancer mortality rates: an ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res 2009, 29, 3537–3545. [Google Scholar]
- Moore, J.X.; Akinyemiju, T.; Wang, H.E. Pollution and regional variations of lung cancer mortality in the United States. Cancer Epidemiol 2017, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Z.; Li, J.; Li, Z.; Han, J. A Healthy Dietary Pattern Reduces Lung Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- CDC. Diagnosed Diabetes - Non-Hispanic White (Race-Ethnicity), Adults Aged 18+ Years, Age-Adjusted Percentage, Natural Breaks, All States. Availabe online: https://gis.cdc.gov/grasp/diabetes/diabetesatlas-surveillance.html# (accessed on April 12, 2024).
- CDC. Behavioral Risk Factor Surveillance System. Availabe online: https://www.cdc.gov/brfss/index.html (accessed on April 12, 2024).
- CDC. Adult Obesity Prevalence Maps. Availabe online: https://www.cdc.gov/obesity/data/maps/2022/downloads/obesity-prevalence-map-by-race-ethnicity-2011-2021-508.pptx (accessed on April 12, 2024).
- USCensusBureau. State-level Urban and Rural Information for the 2020 Census and 2010 Census. Availabe online: https://www2.census.gov/geo/docs/reference/ua/ (accessed on April 12, 2024).
- The States That Drink the Most Alcohol in America, Mapped and Ranked, 2016. Availabe online: https://vividmaps.com/us-alcohol-consumption/ (accessed on April 1, 2024).
- Collaborators, U.S.B.o.D.; Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; et al. The State of US Health, 1990-2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef]
- Tu, H.; Heymach, J.V.; Wen, C.P.; Ye, Y.; Pierzynski, J.A.; Roth, J.A.; Wu, X. Different dietary patterns and reduction of lung cancer risk: A large case-control study in the U.S. Sci Rep 2016, 6, 26760. [Google Scholar] [CrossRef]
- Yu, D.; Zheng, W.; Johansson, M.; Lan, Q.; Park, Y.; White, E.; Matthews, C.E.; Sawada, N.; Gao, Y.T.; Robien, K.; et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. J Natl Cancer Inst 2018, 110, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.; Boeing, H.; Pischon, T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: implications for routine clinical practice. Dtsch Arztebl Int 2010, 107, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Dhokte, S.; Czaja, K. Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes. Nutrients 2024, 16, 1015. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Malek-Shahabi, T.; Malekshahi-Moghadam, A.; Shahbazi, R.; Esmaeili, S. Obesity as an important risk factor for certain types of cancer. Iran J Cancer Prev 2013, 6, 186–194. [Google Scholar]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int J Mol Sci 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol 2020, 49, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, V. Introduction to the dietary management of obesity in adults. Clin Med (Lond) 2023, 23, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, M.; Eliassen, A.H.; Wang, M.; Fung, T.T.; Clinton, S.K.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Tabung, F.K.; et al. Optimal dietary patterns for prevention of chronic disease. Nat Med 2023, 29, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Orlich, M.J.; Singh, P.N.; Sabate, J.; Fan, J.; Sveen, L.; Bennett, H.; Knutsen, S.F.; Beeson, W.L.; Jaceldo-Siegl, K.; Butler, T.L.; et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med 2015, 175, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Juan, W.J.; Yamini, S.; Britten, P. Food Intake Patterns of Self-identified Vegetarians among the U.S. Population, 2007-2010. Procedia Food Science 2015, 4, 86–93. [Google Scholar] [CrossRef]
- Zhang, J.; Dhakal, I.B.; Zhao, Z.; Li, L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur J Cancer Prev 2012, 21, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Trends in diet and Alzheimer's disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis 2014, 38, 611–620. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol 2019, 10, 1607. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Fierro, L.; Barrios, E.; Ronco, A. Tobacco, alcohol, diet and risk of non-Hodgkin's lymphoma: a case-control study in Uruguay. Leuk Res 1998, 22, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Aykan, N.F. Red Meat and Colorectal Cancer. Oncol Rev 2015, 9, 288. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghazaleh, N.; Chua, W.J.; Gopalan, V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J Gastroenterol Hepatol 2021, 36, 75–88. [Google Scholar] [CrossRef]
- Shikany, J.M.; Safford, M.M.; Newby, P.K.; Durant, R.W.; Brown, T.M.; Judd, S.E. Southern Dietary Pattern is Associated With Hazard of Acute Coronary Heart Disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation 2015, 132, 804–814. [Google Scholar] [CrossRef]
- Panigrahi, G.; Goodwin, S.M.; Staffier, K.L.; Karlsen, M. Remission of Type 2 Diabetes After Treatment With a High-Fiber, Low-Fat, Plant-Predominant Diet Intervention: A Case Series. Am J Lifestyle Med 2023, 17, 839–846. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef]
- Thomas, D. The mineral depletion of foods available to us as a nation (1940-2002)--a review of the 6th Edition of McCance and Widdowson. Nutr Health 2007, 19, 21–55. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stodard, J.L.; Weathers, K.C. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies. BioScience 2001, 51, 180–198. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. European Journal of Agronomy 2999, 31, 114–119. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci Total Environ 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Bueno de Mesquita, C.P.; Solon, A.J.; Barfield, A.; al., e. Adverse impacts of Roundup on soil bacteria, soil chemistry and mycorrhizal fungi during restoration of a Colorado grassland. Applied Soil Ecology 2023, 185, 104778. [CrossRef]
- Muka, T.; Kraja, B.; Ruiter, R.; Lahousse, L.; de Keyser, C.E.; Hofman, A.; Franco, O.H.; Brusselle, G.; Stricker, B.H.; Kiefte-de Jong, J.C. Dietary mineral intake and lung cancer risk: the Rotterdam Study. Eur J Nutr 2017, 56, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, S.; Um, C.Y.; Prizment, A.E.; Lazovich, D.; Bostick, R.M. Combined Mineral Intakes and Risk of Colorectal Cancer in Postmenopausal Women. Cancer Epidemiol Biomarkers Prev 2019, 28, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Leischner, C.; Helling, T.; Renner, O.; Burkard, M.; Marongiu, L. Minerals and Cancer: Overview of the Possible Diagnostic Value. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Visvanathan, K.; Mondul, A.M.; Zeleniuch-Jacquotte, A.; Wang, M.; Gail, M.H.; Yaun, S.S.; Weinstein, S.J.; McCullough, M.L.; Eliassen, A.H.; Cook, N.R.; et al. Circulating vitamin D and breast cancer risk: an international pooling project of 17 cohorts. Eur J Epidemiol 2023. [Google Scholar] [CrossRef]
- Xu, T.; Wan, S.; Shi, J.; Xu, T.; Wang, L.; Guan, Y.; Luo, J.; Luo, Y.; Sun, M.; An, P.; et al. Antioxidant Minerals Modified the Association between Iron and Type 2 Diabetes in a Chinese Population. Nutrients 2024, 16, 335. [Google Scholar] [CrossRef]
- Nanda, M.; Sharma, R.; Mubarik, S.; Aashima, A.; Zhang, K. Type-2 Diabetes Mellitus (T2DM): Spatial-temporal Patterns of Incidence, Mortality and Attributable Risk Factors from 1990 to 2019 among 21 World Regions. Endocrine 2022, 77, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Henley, S.J.; Calle, E.E. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene 2002, 21, 7307–7325. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2001, 10, 725–731. [Google Scholar]
- Dwyer-Lindgren, L.; Mokdad, A.H.; Srebotnjak, T.; Flaxman, A.D.; Hansen, G.M.; Murray, C.J. Cigarette smoking prevalence in US counties: 1996-2012. Popul Health Metr 2014, 12, 5. [Google Scholar] [CrossRef]
- Hu, Z. Spatial analysis of MODIS aerosol optical depth, PM2.5, and chronic coronary heart disease. Int J Health Geogr 2009, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Tamura-Wicks, H.; Parks, R.M.; Burnett, R.T.; Pope, C.A., 3rd; Bechle, M.J.; Marshall, J.D.; Danaei, G.; Ezzati, M. Particulate matter air pollution and national and county life expectancy loss in the USA: A spatiotemporal analysis. PLoS Med 2019, 16, e1002856. [Google Scholar] [CrossRef]
- Prada, D.; Baccarelli, A.A.; Terry, M.B.; Valdez, L.; Cabrera, P.; Just, A.; Kloog, I.; Caro, H.; Garcia-Cuellar, C.; Sanchez-Perez, Y.; et al. Long-term PM(2.5) exposure before diagnosis is associated with worse outcome in breast cancer. Breast Cancer Res Treat 2021, 188, 525–533. [Google Scholar] [CrossRef]
- Peller, S. Carcinogenesis as a means of reducing cancer mortality. Lancet 1936, 228, 552–556. [Google Scholar] [CrossRef]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-aging and Sunscreens: Paradigm Shift in Cosmetics. Adv Pharm Bull 2019, 9, 348–359. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D'Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Grant, W.B. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: case-control versus nested case-control studies. Anticancer Res 2015, 35, 1153–1160. [Google Scholar] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst 2019, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the Evidence from Observational Studies and Randomized Controlled Trials for Nonskeletal Health Effects of Vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; Group, D.d.R. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur J Med Chem 2020, 194, 112260. [Google Scholar] [CrossRef]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. JAMA Netw Open 2023, 6, e2328886. [Google Scholar] [CrossRef]
- Holick, M.F. The Death D-Fying Vitamin D3 for Digestive Tract Cancers-The p53 Antibody Connection. JAMA Netw Open 2023, 6, e2328883. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 microg) of Vitamin D for Adults in the General Population. Nutrients 2024, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Si, Y.; Li, X.; Hong, J.; Yu, C.; He, N. The relationship between tobacco and breast cancer incidence: A systematic review and meta-analysis of observational studies. Front Oncol 2022, 12, 961970. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D. Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Ann Epidemiol 2003, 13, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D. Rising trends in melanoma. An hypothesis concerning sunscreen effectiveness. Ann Epidemiol 1993, 3, 103–110. [Google Scholar] [CrossRef]
- Gorham, E.D.; Mohr, S.B.; Garland, C.F.; Chaplin, G.; Garland, F.C. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol 2007, 17, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Hoffman, R.M.; Slominski, A.T. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer Res 2020, 40, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Kos-Kudla, B.; Walczak, M.; Fal, A.; Zozulinska-Ziolkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewinski, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Cancer Risk and Prevention. Availabe online: https://www.cancer.org/cancer/risk-prevention.html (accessed on May 3, 2024).
- Kahwati, L.C.; LeBlanc, E.; Weber, R.P.; Giger, K.; Clark, R.; Suvada, K.; Guisinger, A.; Viswanathan, M. Screening for Vitamin D Deficiency in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1443–1463. [Google Scholar] [CrossRef]
- Grant, W.B.; Blake, S.M. Diet's Role in Modifying Risk of Alzheimer's Disease: History and Present Understanding. J Alzheimers Dis 2023, 96, 1353–1382. [Google Scholar] [CrossRef] [PubMed]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol 2017, 14, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.A.; Bardsley, J.K.; Cypress, M.; Funnell, M.M.; Harms, D.; Hess-Fischl, A.; Hooks, B.; Isaacs, D.; Mandel, E.D.; Maryniuk, M.D.; et al. Diabetes Self-management Education and Support in Adults With Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care 2020, 43, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int J Obes (Lond) 2009, 33, 621–628. [Google Scholar] [CrossRef]
- Temple, N.J. The Origins of the Obesity Epidemic in the USA-Lessons for Today. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Entezari, M.H.; Mohammadi, H.; Jayedi, A.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr 2023, 63, 249–260. [Google Scholar] [CrossRef]
- Moradi, S.; Hojjati Kermani, M.A.; Bagheri, R.; Mohammadi, H.; Jayedi, A.; Lane, M.M.; Asbaghi, O.; Mehrabani, S.; Suzuki, K. Ultra-Processed Food Consumption and Adult Diabetes Risk: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Mialon, M.; Serodio, P.M.; Crosbie, E.; Teicholz, N.; Naik, A.; Carriedo, A. Conflicts of interest for members of the US 2020 dietary guidelines advisory committee. Public Health Nutr 2022, 27, e69. [Google Scholar] [CrossRef]
- Holloway, T.; Miller, D.; Anenberg, S.; Diao, M.; Duncan, B.; Fiore, A.M.; Henze, D.K.; Hess, J.; Kinney, P.L.; Liu, Y.; et al. Satellite Monitoring for Air Quality and Health. Annu Rev Biomed Data Sci 2021, 4, 417–447. [Google Scholar] [CrossRef] [PubMed]
| State | UVB Dose (kJ/m2) |
|---|---|
| Alabama | 6.0 |
| Alaska | |
| Arkansas | 5.7 |
| Arizona | 9.0 |
| California | 7.5 |
| Colorado | 8.2 |
| Connecticut | 4.7 |
| Delaware | 4.7 |
| District of Columbia | 4.7 |
| Florida | 8.0 |
| Georgia | 7.2 |
| Hawaii | |
| Idaho | 6.0 |
| Illinois | 4.5 |
| Iowa | 4.7 |
| Indiana | 4.7 |
| Kansas | 6.3 |
| Kentucky | 5.8 |
| Louisiana | 7.5 |
| Massachusetts | 4.6 |
| Maine | 4.1 |
| Maryland | 4.7 |
| Michigan | 4.2 |
| Minnesota | 4.1 |
| Missouri | 6.5 |
| Mississippi | 7.0 |
| Montana | 4.7 |
| North Carolina | 6.6 |
| North Dakota | 6.2 |
| Nebraska | 5.1 |
| New Hampshire | 4.1 |
| New Jersey | 5.2 |
| New Mexico | 9.5 |
| Nevada | 8.5 |
| New York | 4.7 |
| Ohio | 4.7 |
| Oklahoma | 7.5 |
| Oregon | 5.2 |
| Pennsylvania | 4.5 |
| Rhode Island | 4.7 |
| South Carolina | 7.2 |
| South Dakota | 4.5 |
| Tennessee | 6.3 |
| Texas | 7.8 |
| Utah | 8.0 |
| Virginia | 6.0 |
| Vermont | 4.2 |
| Washington | 4.5 |
| Wisconsin | 4.5 |
| West Virginia | 5.2 |
| Wyoming | 6.0 |
| Factor | DM | LCF | LCM | Obs | UVB |
|---|---|---|---|---|---|
| Alcohol | 0.40, 0.14, 0.007 | 0.03 0.00, xx | 0.24, 0.03, 0.12 | 0.25, 0.04, 0.11 | 0.35, 0.10, 0.02 |
| Diabetes | 0.59, 0.33, * | 0.84, 0.69, * | 0.84, 0.69, * | 0.13, 0.00, -- | |
| Lung cancer, F | 0.88, 0.77, * | 0.54, 0.28, * | 0.14, 0.00, -- | ||
| Lung cancer, M | 0.75, 0.55, * | 0.39, 0.13, 0.008 | |||
| Obesity | 0.11, 0.00, -- |
| Cancer | Equation | r, adjusted r2, p (p) |
|---|---|---|
| All | 410 + (4.2 × Obs) − (7.3 × UVB) | 0.58, 0.30, 0.002, 0.02 |
| 350 + (4.8 × Obs) | 0.49, 0.23, <0.001 | |
| 549 − (9.5 × UVB) | 0.39, 0.13, 0.008 | |
| All less lung | 420 - (7.34 × UVB) + (0.77 * LCM) | 0.54, 0.26, 0.006, 0.01 |
| 480 - (8.3 × UVB) | 0.43, 0.16, 0.004 | |
| 370 + (0.89 × LCM) | 0.39, 0.13, 0.008 | |
| Bladder | 43 − (1.7 × UVB) + (1.5 × Alc) | 0.72, 0.50, <0.001, 0.09 |
| 48 − (1.9 × UVB) | 0.70, 0.47, <0.001 | |
| 29 + (3.1 × Alc) | 0.42, 0.16, 0.004 | |
| Brain | 8.5 − (0.14 × UVB) | 0.31, 0.08, 0.03 |
| Colorectal | 12 + (0.13 × LCM) + (0.70 × Obs) | 0.82, 0.65, <0.001 |
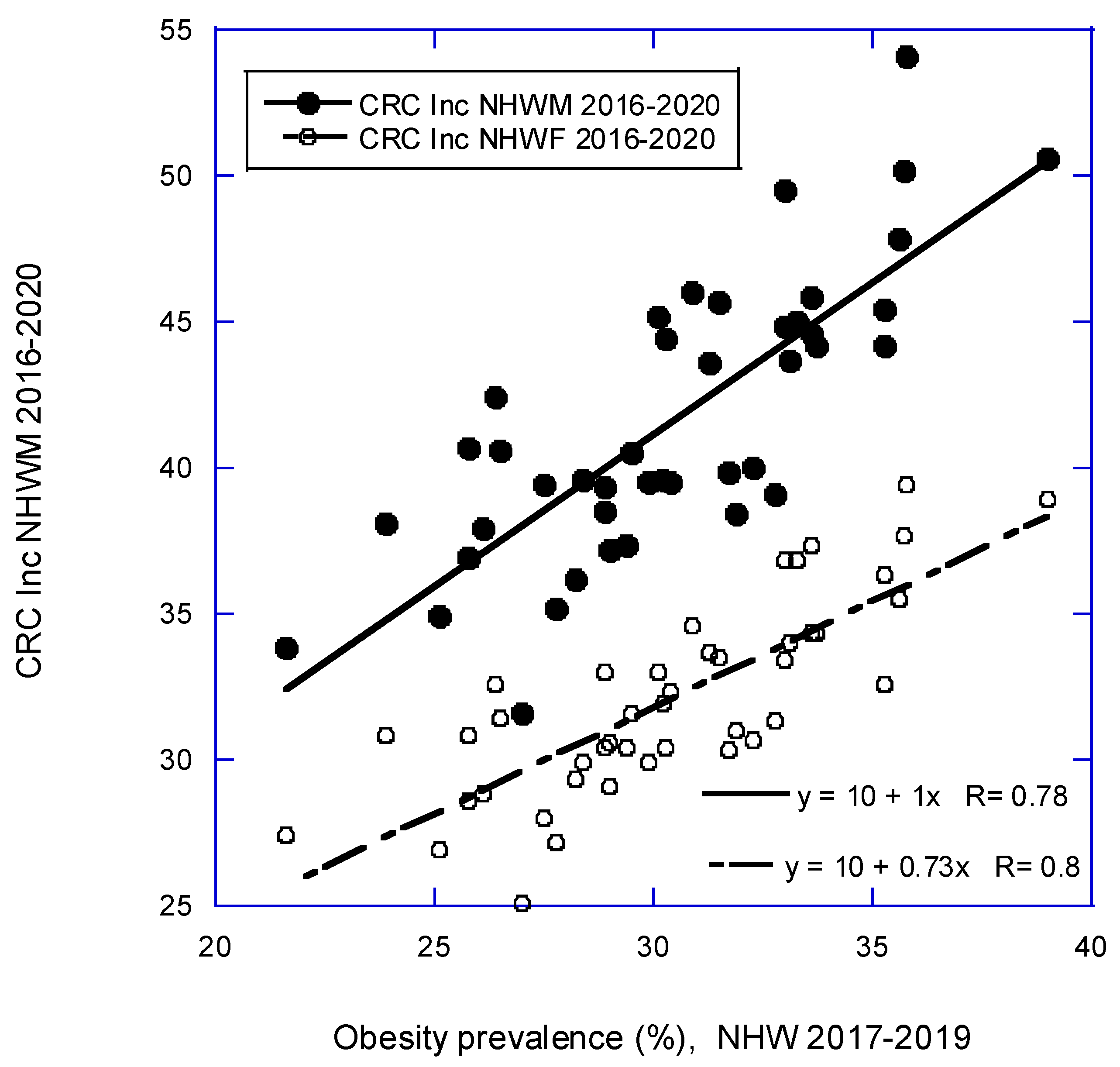
| 10 + (1.0 × Obs) | 0.78, 0.61, <0.001 | |
| 24 + (0.28 × LCM) | 0.73, 0.52, <0.001 | |
| Esophageal | 5.7+ (0.048 × LCM) − (0.29 × UVB) + (0.69 × Alc) | 0.77, 0.56, <0.001, 0.001, 0.006 |
| 8.5 − (0.39 × UVB) + (0.039 × LCM) | 0.71, 0.48, <0.001, <0.001 | |
| 11 − (0.44 × UVB) | 0.57, 0.31, <0.001 | |
| 7.1 + (0.69 × Alc) | 0.33, = 0.09, 0.03 | |
| Gastric | 9.0 − (0.36 × UVB) | 0.55, 0.29, <0.001 |
| Larynx | 1.3 + (0.058 × LCM) | 0.57, 0.31, <0.001 |
| Liver | 4.5 + (0.66 × DM) | 0.37, 0.12, 0.01 |
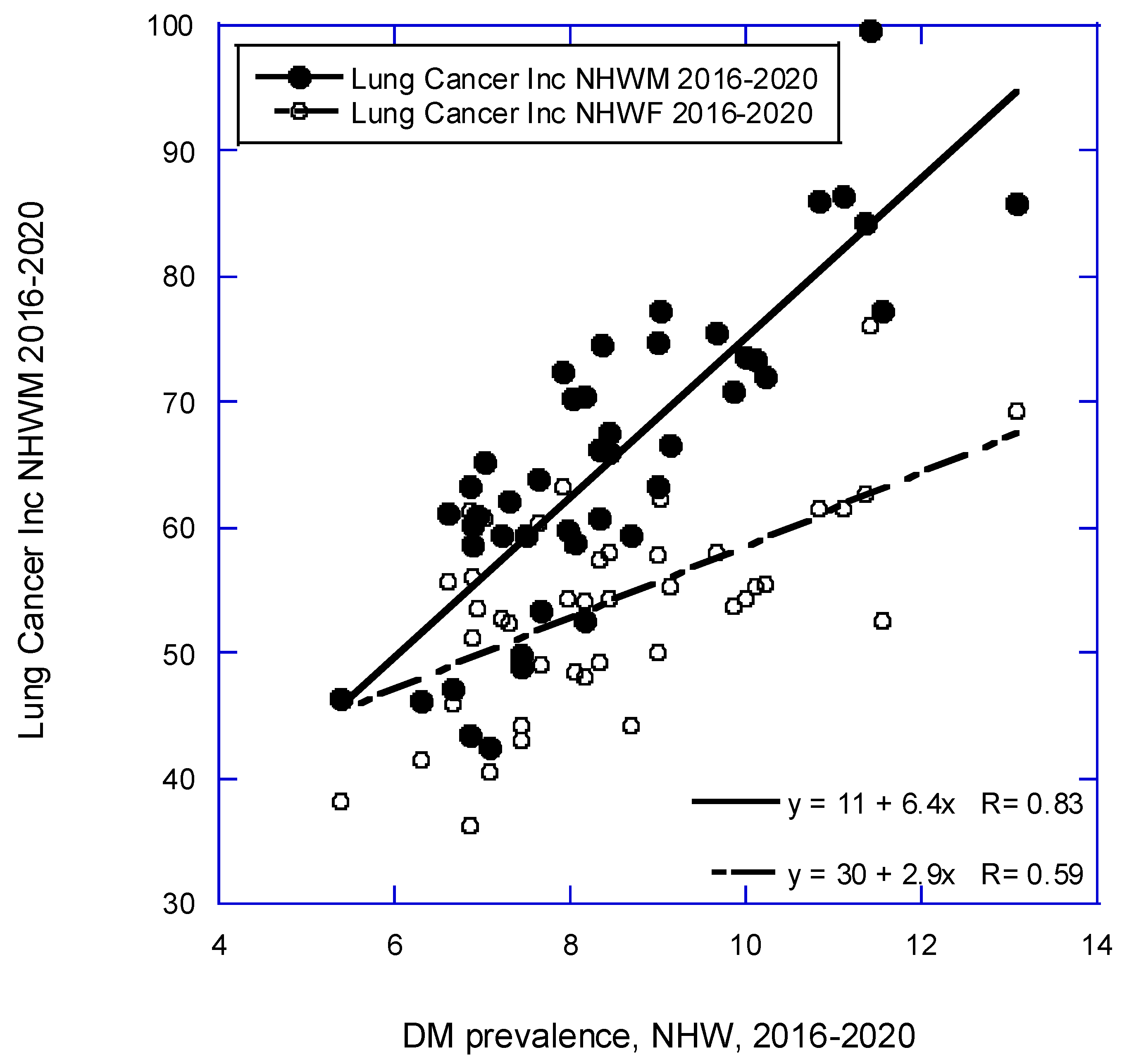
| Lung | 11 + (6.4 × DM) | 0.84, 0.69, <0.001 |
| 72 − (1.2 × UVB) | 0.14, 0.000 | |
| Non-Hodgkin’s lymphoma | 28 − (0.84 × UVB) | 0.56, 0.29, <0.001 |
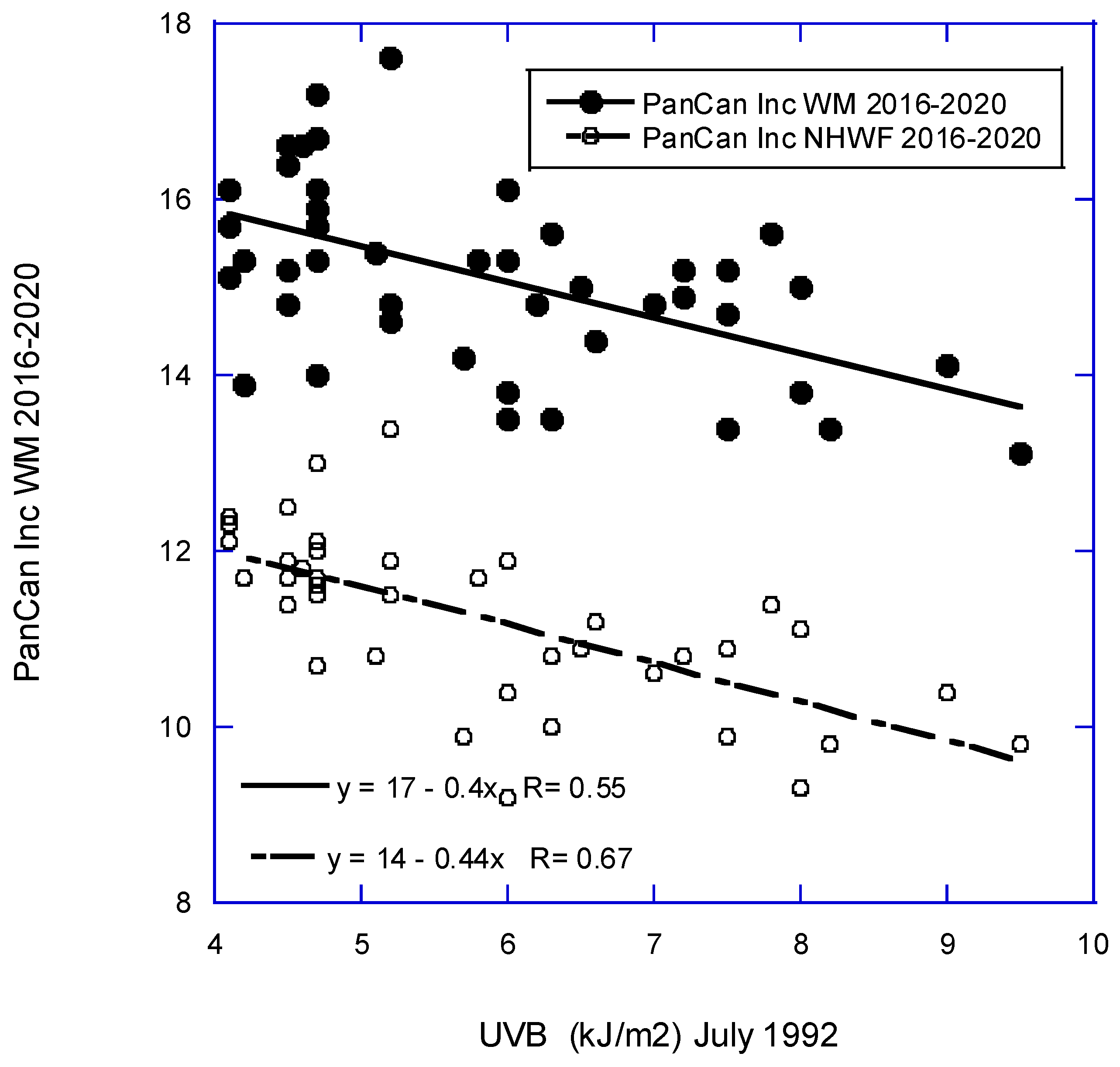
| Pancreatic | 18 − (0.40 × UVB) | 0.55, 0.29, <0.001 |
| Prostate | 130 − (3.4 × UVB) | 0.14, 0.15, 0.005 |
| Renal | 11 + (0.19 × LCM) | 0.75, 0.55, <0.001 |
| 3.6 + (0.66 × Obs) | 0.74, 0.53, <0.001 |
| Type | Equation | r, adjusted r2, p (p) |
|---|---|---|
| All | 440 − (9.8 × UVB)+ (1.7 × Obs) | 0.63, 0.37, <0.001, 0.06 |
| 430 − (7.7 × UVB) | 0.53, 0.26, <0.001 | |
| 360 + (2.5 × Obs) | 0.34, 0.09, 0.03 | |
| All less lung | 36 − (5.6 × UVB) + (0.94 × LCF) | 0.63, 0.36, 0.006, 0.009 |
| 430 − (7.7 × UVB) | 0.53, 0.26, <0.001 | |
| 310 + (1.3 × LCF) | 0.52, 0.25, <0.001 | |
| Bladder | 9.5 − (0.50 × UVB) + (1.0 × Alc) | 0.76, 0.56, <0.001, <0.001 |
| 13 − (0.64 × UVB) | 0.65, 0.41, <0.001 | |
| 5.5 + (1.5 × Alc) | 0.59, 0.33, <0.001 | |
| 5.2 + (0.074 × LCF) | 0.45, 0.18, 0.002 | |
| Breast | 160 − (2.3 × UVB) − (2.2 × DM) | 0.66, 0.41, <0.001, <0.001 |
| 180 − (2.8 × UVB) − (0.91 × Obs) | 0.65, 0.39, <0.001, <0.001 | |
| 150 − (2.4 × DM) | 0.51, 0.24, <0.001 | |
| 150 − (2.5 × UVB) | 0.47, 0.20, 0.001 | |
| 150 – (0.70 × Obs) | 0.34, 0.10, 0.02 | |
| Colorectal | 10 + (0.73 × Obs) | 0.80, 0.63, <0.001 |
| 21 + (0.21 × LCF) | 0.53, 0.27, <0.001 | |
| Corpus uteri | 38 − (1.9 × UVB) | 0.72, 0.50, <0.001 |
| Esophageal | 2.2 − (0.13 × UVB)+ (0.16 × Alc) | 0.72. 0.49, <0.001, 0.02 |
| 2.7 − (0.15 × UVB) | 0.67, 0.44, <0.001 | |
| 1.1 + (0.29 × Alc) | 0.47, 0.21, 0.001 | |
| 1.0 + (0.015 × LVF) | 0.41, 0.15, 0.006 | |
| Gastric | 4.1 − (0.14 × UVB) | 0.38, 0.12, 0.01 |
| Laryngeal | 0.19 + (0.021 × LCF) | 0.39, 0.13, 0.01 |
| Liver | 2.8 + (0.13 × DM) | 0.36, 0.11, 0.02 |
| Lung | 43 + (3.2 × DM) − (2.7 × UVB) | 0.75, 0.54, <0.001, <0.001 |
| 30 + (1.1 × Obs) − (1.8 × UVB) | 0.63, 0.36, <0.001, 0.02 | |
| 30 + (2.9 × DM) | 0.59, 0.33, <0.001 | |
| 67 − (2.2 × UVB) | 0.39, 0.13, 0.008 | |
| Non-Hodgkin’s lymphoma | 19 − (0.60 × UVB) | 0.51, 0.25, <0.001 |
| Pancreatic | 14 − (0.44 × UVB) | 0.67, 0.44, <0.001 |
| Renal | −2.0 + (0.46 × Obs) | 0.84, 0.69, <0.001 |
| 4.9 + (0.13 × LCF) | 0.55, 0.29, <0.001 |
| Cancer | UVB (2016–2020) | UVB, males [8]* |
UVB, females [8]* |
UVB (2006), males [7] |
UVB (2006), females [7] |
|---|---|---|---|---|---|
| Bladder | y | 1.13 | 1.15 | y | y |
| Brain | M only | 1.08 | 1.07 | ||
| Breast | y | 1.06 | y | y | |
| Cervical | 0.84 | n | |||
| Colon | 1.11 | 1.14 | y | y | |
| Colorectal | n | ||||
| Corpus uteri | y | 1.49 | y | ||
| Esophageal | y | 1.27 | 1.07 | y | y |
| Gastric | y | 1.42 | 1.27 | y | y |
| Hodgkin’s lymphoma | n | 1.16 | 1.19 | y | y |
| Laryngeal | n | 0.87 | 0.80 | y | y |
| Leukemia | n | 1.09 | 1.15 | n | n |
| Liver | n | 1.01 | 1.05 | n | n |
| Lung | F only | n | n | ||
| Myeloma | n | 1.19 | 1.22 | n | n |
| Non-Hodgkin’s lymphoma | y | 1.08 | 1.09 | y | y |
| Oral cavity | n | 0.77 | 0.83 | n | n |
| Ovarian | n | 1.03 | y | ||
| Pancreatic | y | 1.09 | 1.17 | y | n |
| Prostate | y | 1.20 | ? | ||
| Rectal | 1.27 | 1.14 | y | y | |
| Renal | n | 1.09 | 1.17 | y | y |
| Cancer site | M, Inc. rate*1982 | M, Inc. rate* 2018 | M Inc. rate*, ratio 2018 to 1982 | F, Inc. rate*, 1982 | F, Inc. rate* 2018 | F, Inc. rate* ratio 2018 to 1982 |
| All | 501 | 483 | 0.96 | 376 | 421 | 1.12 |
| Breast | 107 | 134 | 1.25 | |||
| Colorectum | 68 | 40 | 0.59 | 55 | 33 | 0.60 |
| Corpus uteri | 28 | 28 | 1.00 | |||
| Liver | 5 | 12 | 2.4 | 3 | 4 | 1.3 |
| Lung & bronchus | 94 | 51 | 0.54 | 36 | 43 | 1.2 |
| Melanoma | 12 | 34 | 2.8 | 11 | 20 | 1.8 |
| Prostate | 105 | 114 | 1.09 | |||
| Urinary bladder | 37 | 31 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





