Submitted:
19 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
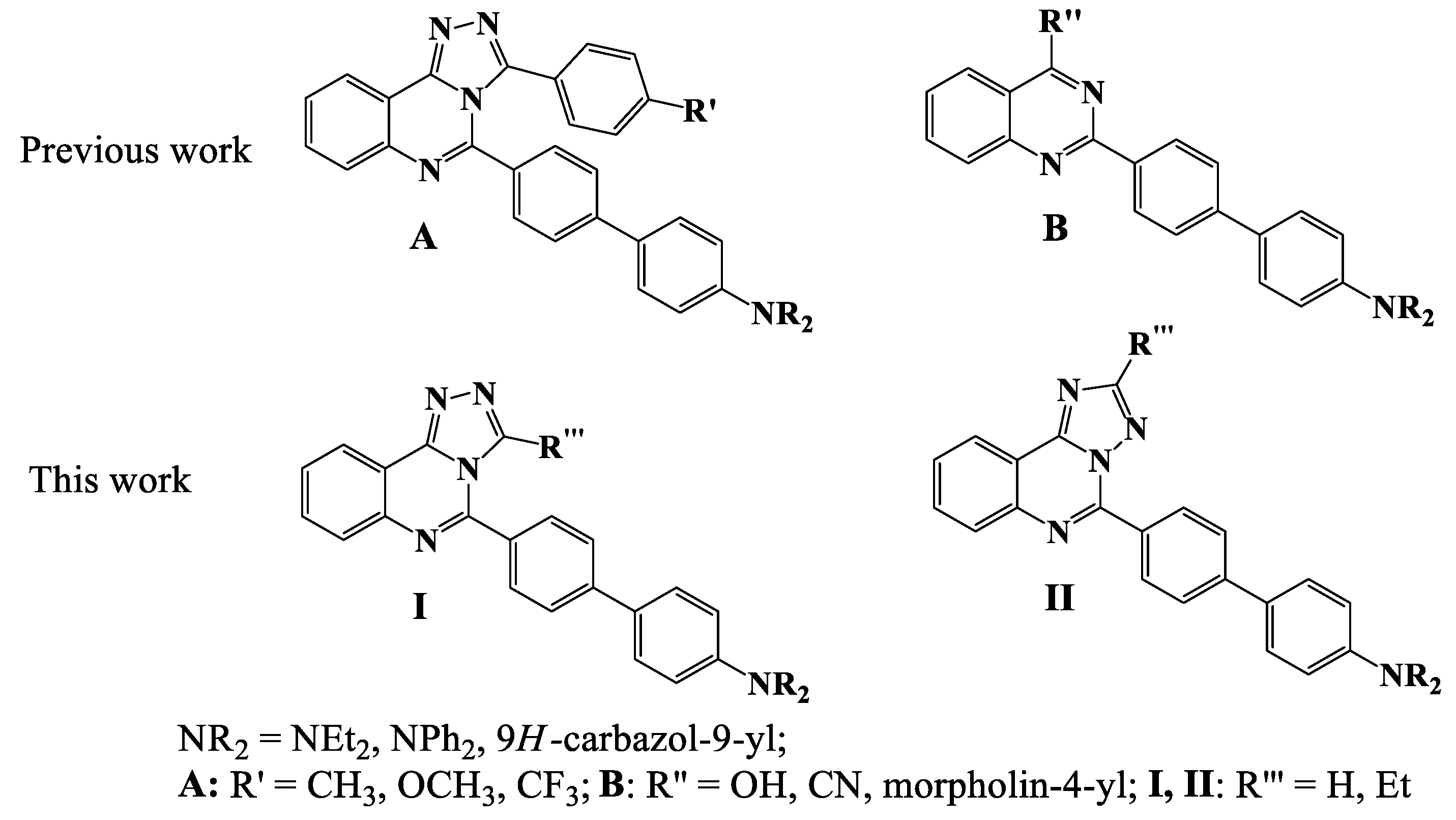
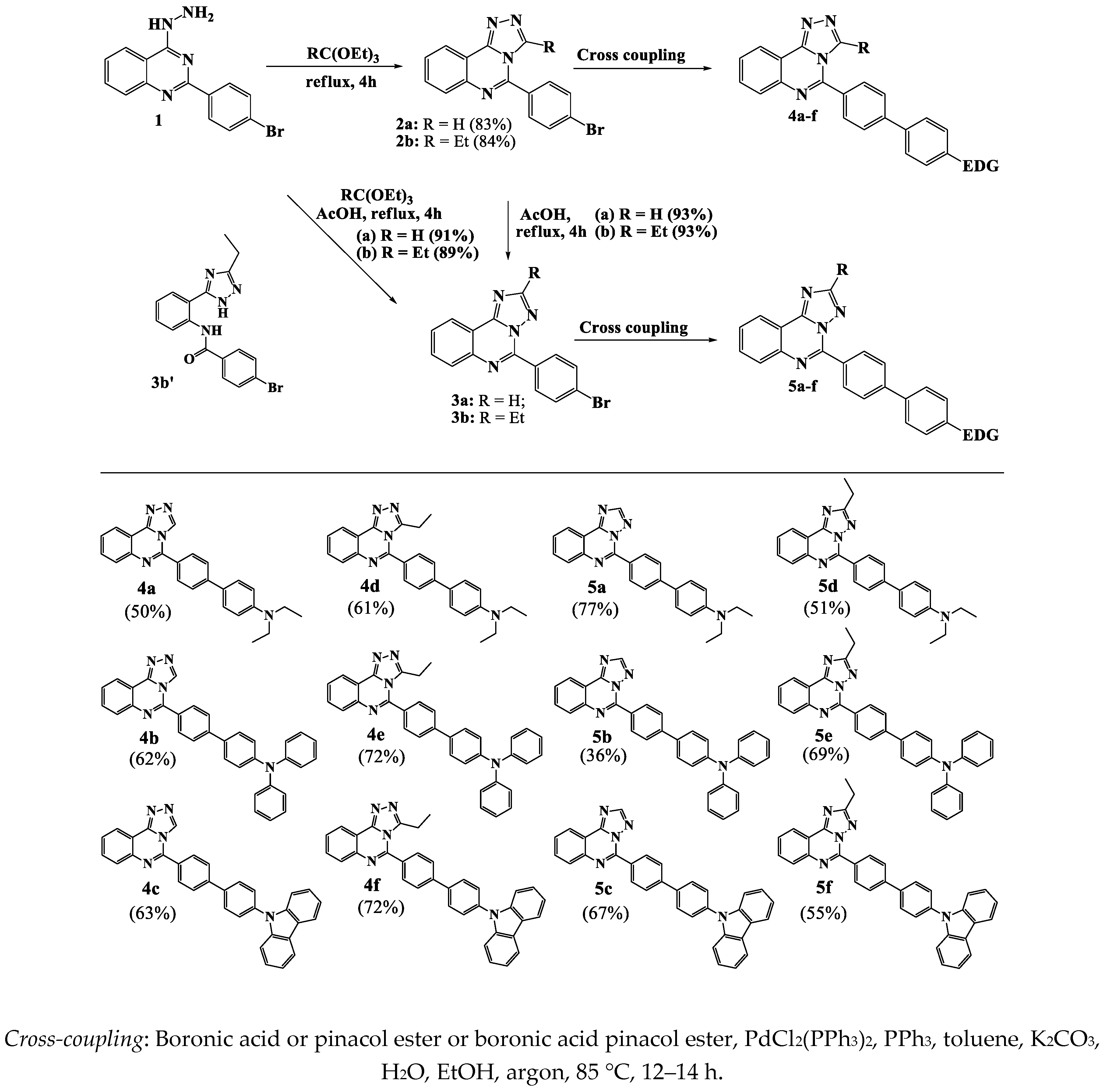
2.1. Synthesis
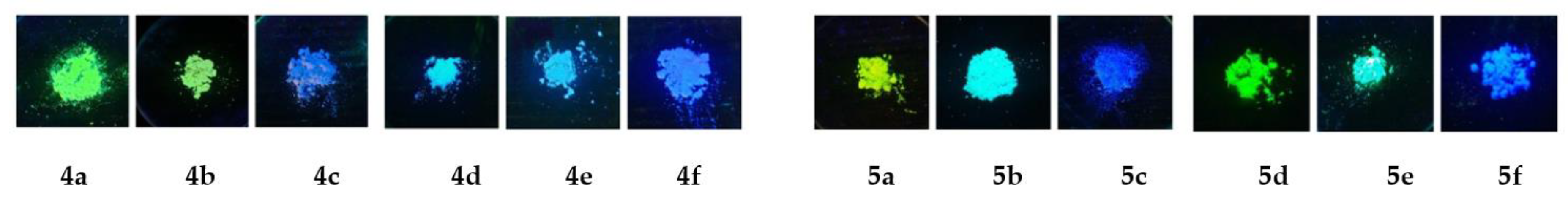
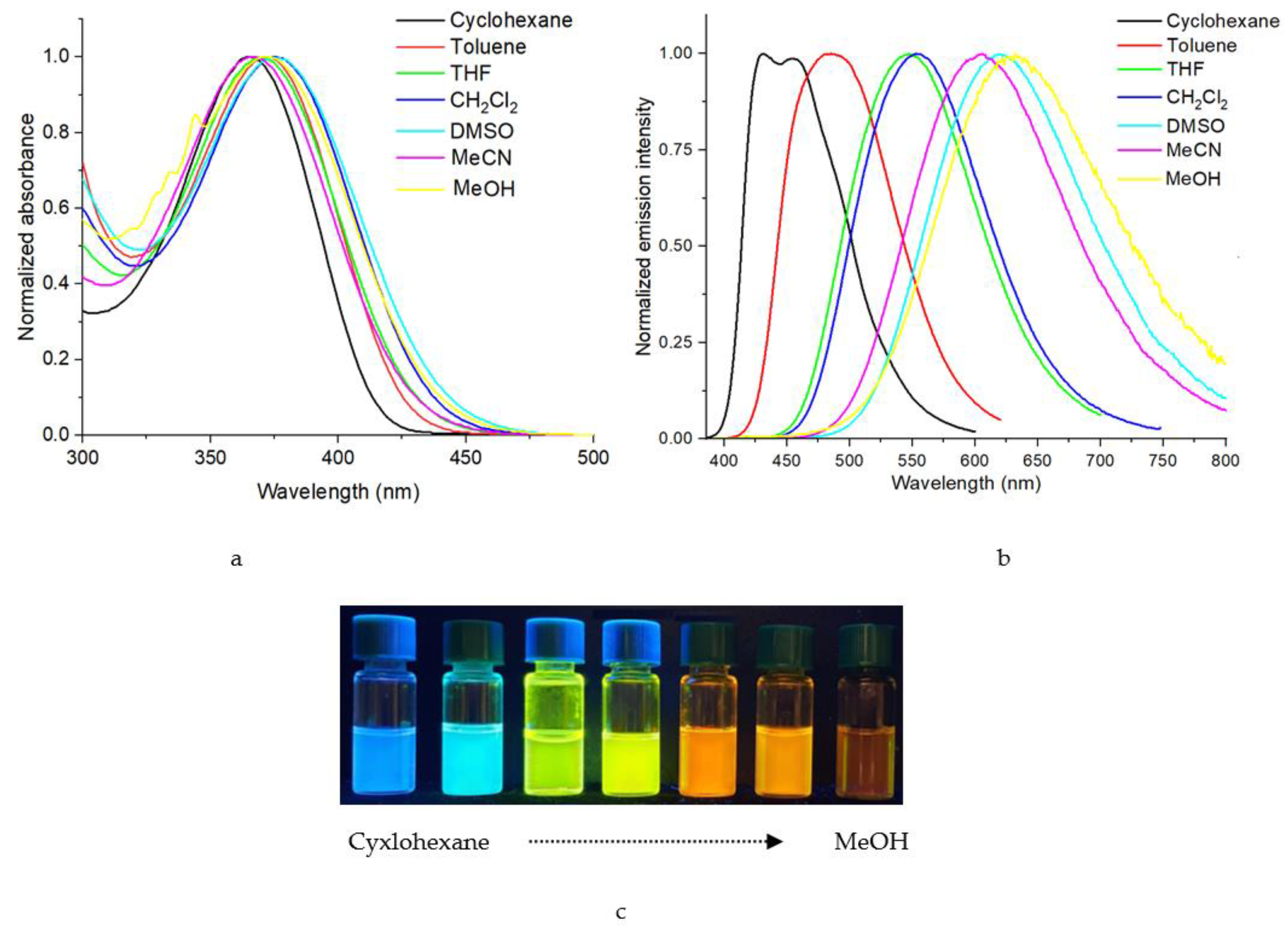
2.2. UV/Vis and Fluorescence Spectroscopy
2.3. Effects of Solvent Polarity for Compounds 4 and 5
2.4. Electrochemical Studies of [1,2,4]Triazoloquinazolines
2.5. Quantum-Chemical Calculations
3. Experimental Methods
3.1. General Information
3.2. Photophysical Characterization
3.3. Electrochemical Studies
3.4. Quantum-Chemical Calculations
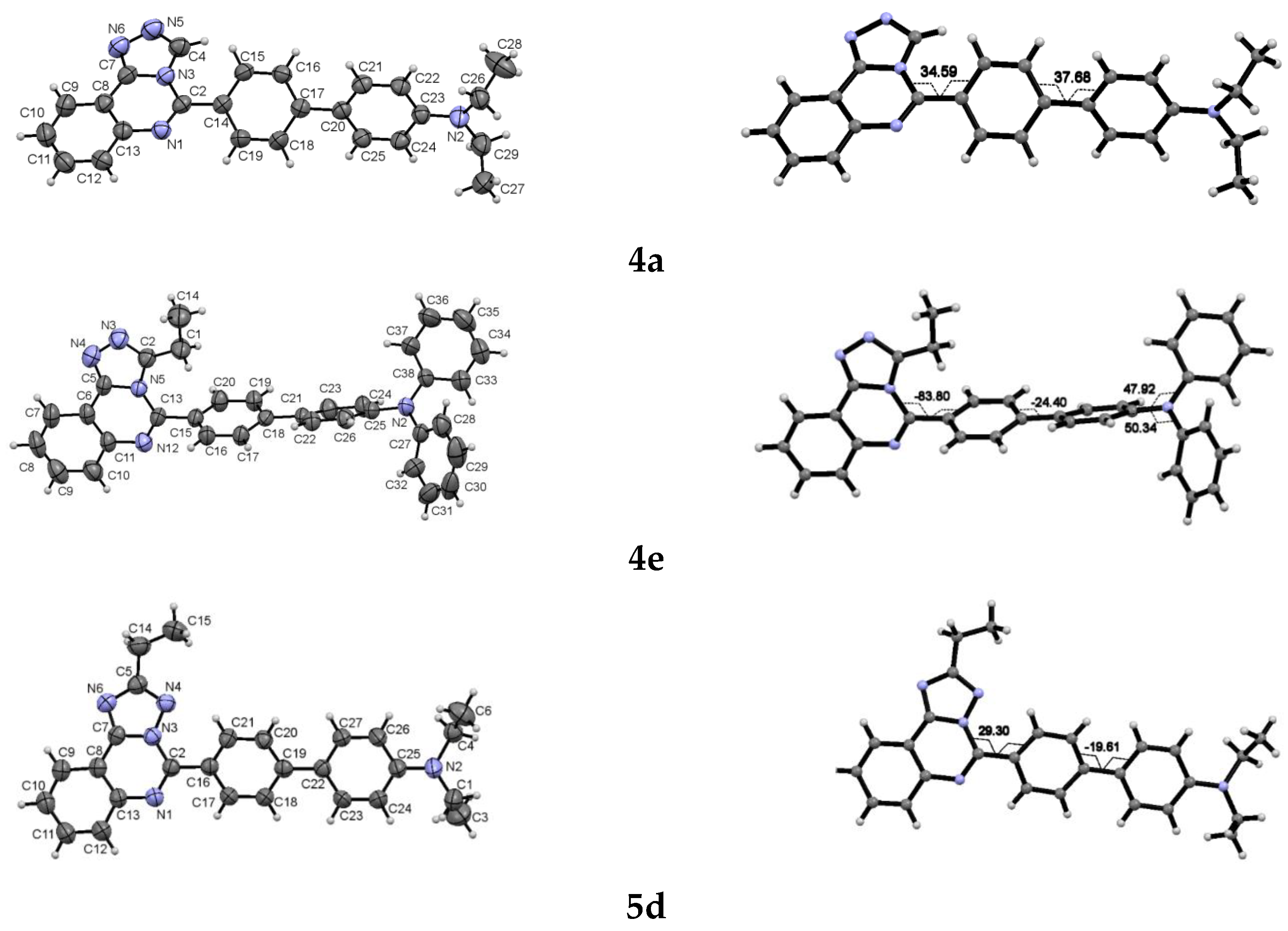
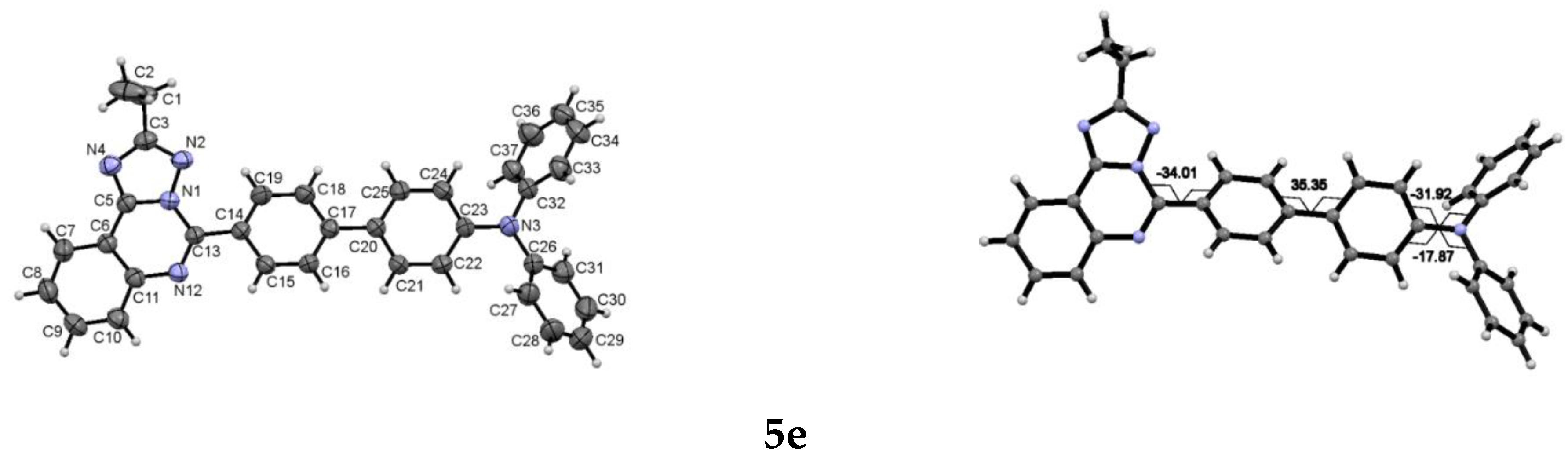
3.5. Crystallography
3.6. Synthesis of Compounds 2a,b, 3a,b, 4a-f and 5a-f
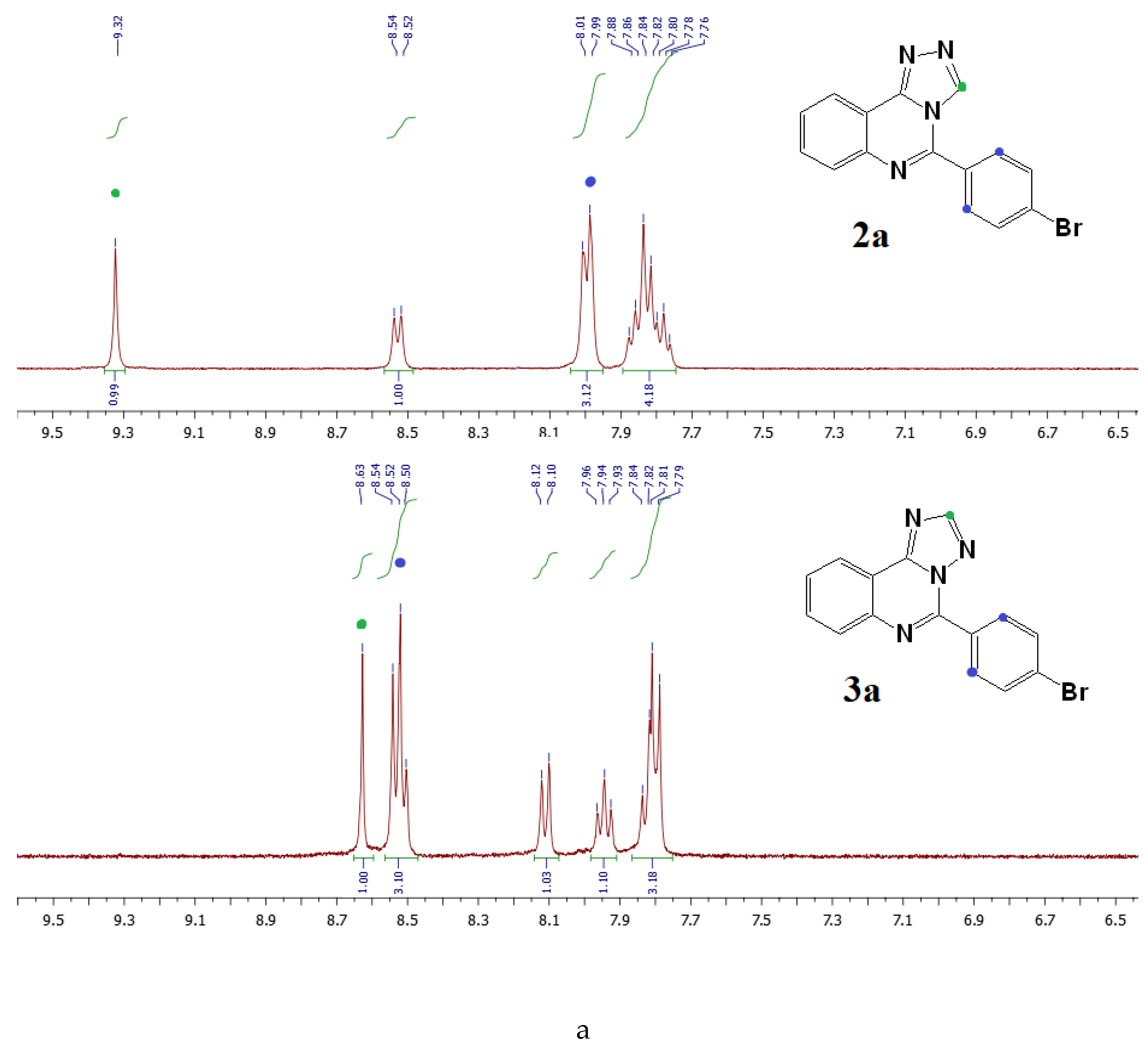
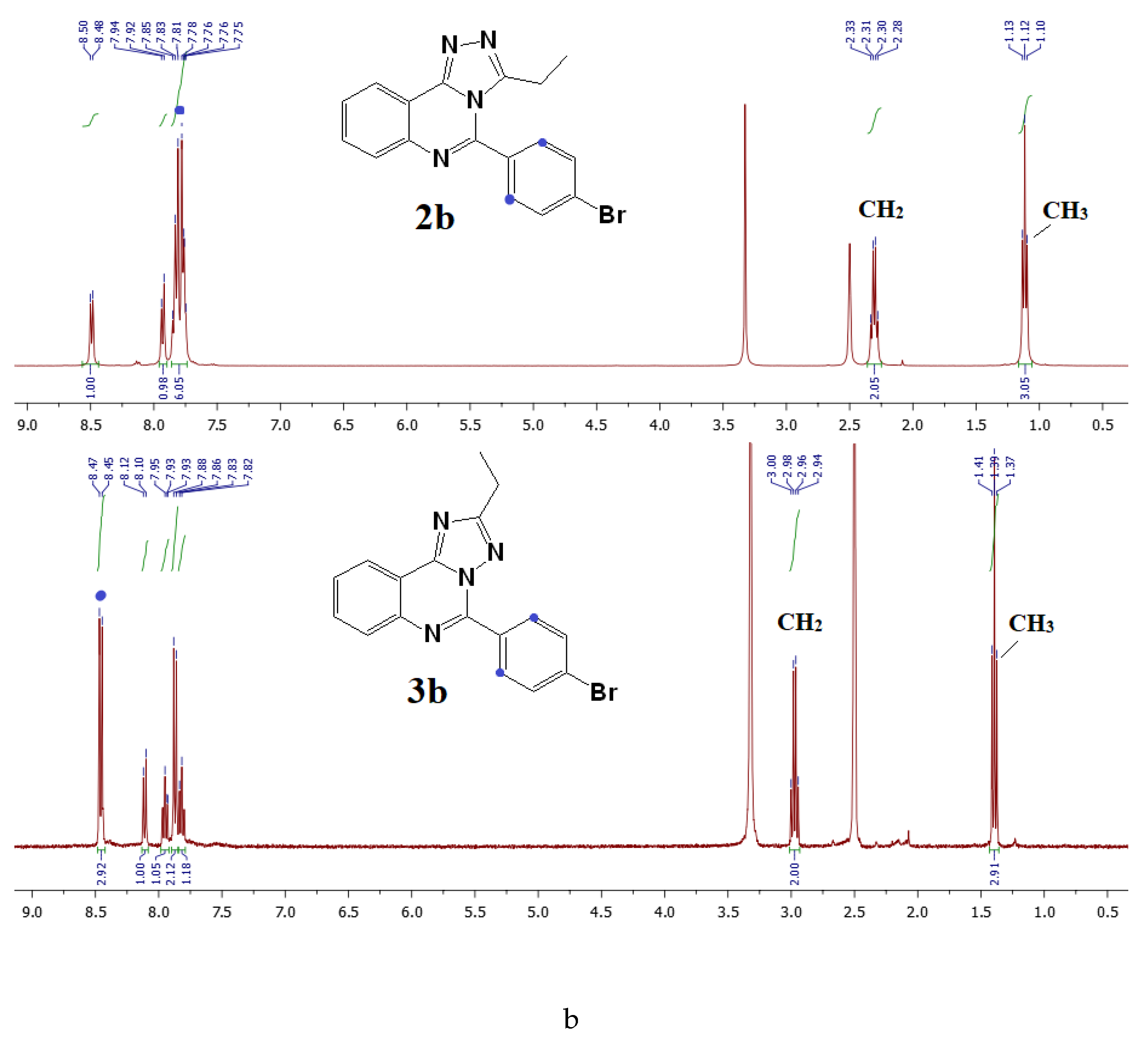
3.6.1. General Procedure for the Synthesis of [1,2,4]triazolo[4,3-c]quinazolines (2a,b)
3.6.2. General Procedure for the Synthesis of [1,2,4]triazolo[1,5-c]quinazolines (3a,b)
3.6.3. General Procedures for the Synthesis of target products 4a-f and 5a-f
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jabeen, T.; Aslam, S.; Ahmad, M.; ul Haq, A.; A. Al-Hussain, S.; E.A. Zaki, M. Triazoloquinazoline: Synthetic Strategies and Medicinal Importance. In Recent Advances on Quinazoline [Working Title]; IntechOpen, 2023.
- Abuelizz, H.A.; Al-Salahi, R. An Overview of Triazoloquinazolines: Pharmacological Significance and Recent Developments. Bioorg. Chem. 2021, 115, 105263. [Google Scholar] [CrossRef]
- Motoyama, M.; Doan, T.H.; Hibner-Kulicka, P.; Otake, R.; Lukarska, M.; Lohier, J.F.; Ozawa, K.; Nanbu, S.; Alayrac, C.; Suzuki, Y.; et al. Synthesis and Structure-Photophysics Evaluation of 2-N-Amino-Quinazolines: Small Molecule Fluorophores for Solution and Solid State. Chem. - An Asian J. 2021, 16, 2087–2099. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, X.; Zhu, B.; Wang, J.; Wu, G.; Yin, Y.; Song, Q. Comparative Studies of Organic Dyes with a Quinazoline or Quinoline Chromophore as π-Conjugated Bridges for Dye-Sensitized Solar Cells. Dye. Pigment. 2016, 124, 72–81. [Google Scholar] [CrossRef]
- Bonnaud, T.; Scaviner, M.; Robin-le Guen, F.; Achelle, S. 4-substituted Push-pull Quinazoline Chromophores with Extended π-conjugated Linker. J. Heterocycl. Chem. 2024, 61, 358–364. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E. V.; Charushin, V.N.; Chupakhin, O.N. Functionalized Quinazolines and Pyrimidines for Optoelectronic Materials. Curr. Org. Synth. 2018, 15, 793–814. [Google Scholar] [CrossRef]
- Li, P.; Xiang, Y.; Gong, S.; Lee, W.K.; Huang, Y.H.; Wang, C.Y.; Yang, C.; Wu, C.C. Quinazoline-Based Thermally Activated Delayed Fluorescence Emitters for High-Performance Organic Light-Emitting Diodes with External Quantum Efficiencies about 28%. J. Mater. Chem. C 2021, 9, 12633–12641. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Su, S.; Guo, F.; Cao, Y.; Zhang, Y. Quinazoline-Based Thermally Activated Delayed Fluorecence for High-Performance OLEDs with External Quantum Efficiencies Exceeding 20%. Adv. Opt. Mater. 2019, 7, 1801496. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Wang, H.; Shen, B.; Zhang, J.; Hao, J.; Cao, J.; Wang, Z. Synthesis, Photophysical and Optoelectronic Properties of Quinazoline-Centered Dyes and Their Applications in Organic Light-Emitting Diodes. Dye. Pigment. 2016, 125, 299–308. [Google Scholar] [CrossRef]
- Plaza-Pedroche, R.; Georgiou, D.; Fakis, M.; Fihey, A.; Katan, C.; Guen, F.R. le; Achelle, S.; Rodríguez-López, J. Effect of Protonation on the Photophysical Properties of 4-Substituted and 4,7-Disubstituted Quinazoline Push-Pull Chromophores. Dye. Pigment. 2021, 185, 108948. [Google Scholar] [CrossRef]
- Plaza-Pedroche, R.; Georgiou, D.; Fakis, M.; Fihey, A.; Katan, C.; Guen, F.R. le; Achelle, S.; Rodríguez-López, J. Effect of Protonation on the Photophysical Properties of 4-Substituted and 4,7-Disubstituted Quinazoline Push-Pull Chromophores. Dye. Pigment. 2021, 185, 108948. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar Singh, N.; Mukhopadhyay, S.; Shankar Pandey, D. AIE Active Quinazoline Based Probes for Selective Detection of Fe3+ and Acidochromism. Inorganica Chim. Acta 2023, 546, 121294. [Google Scholar] [CrossRef]
- Dwivedi, B.K.; Singh, V.D.; Paitandi, R.P.; Pandey, D.S. Substituent-Directed ESIPT-Coupled Aggregation-Induced Emission in Near-Infrared-Emitting Quinazoline Derivatives. ChemPhysChem 2018, 19, 2672–2682. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, G.; Wong, W.Y. Functionalization of Phosphorescent Emitters and Their Host Materials by Main-Group Elements for Phosphorescent Organic Light-Emitting Devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Ao, L.; Zhong, C.; Yang, C.; Qin, J.; Ma, D. Highly Efficient Phosphorescent Organic Light-Emitting Diodes Hosted by 1,2,4-Triazole-Cored Triphenylamine Derivatives: Relationship between Structure and Optoelectronic Properties. J. Phys. Chem. C 2010, 114, 601–609. [Google Scholar] [CrossRef]
- Lee, J.; Shizu, K.; Tanaka, H.; Nomura, H.; Yasuda, T.; Adachi, C. Oxadiazole- and Triazole-Based Highly-Efficient Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1, 4599. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Światkowski, M. Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials (Basel). 2020, 13, 1–21. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-Triazole Derivatives by Suzuki Cross-Coupling Reactions and Their Luminescence Properties. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, A.; Wang, L.; Zhang, Z.; Feng, Y.; Zhao, Y.; Zhang, M. Novel Triazole-Based AIE Materials: Dual-Functional, Highly Sensitive and Selective Fluorescence Probe. Dye. Pigment. 2020, 174, 108050. [Google Scholar] [CrossRef]
- Wu, J.; You, Q.; Lan, J.; Guo, Q.; Li, X.; Xue, Y.; You, J. Cu-Catalysed Direct C-H (Hetero)Arylation of [1,2,4]Triazolo[4,3-a]Pyridine to Construct Deep-Blue-Emitting Luminophores. Org. Biomol. Chem. 2015, 13, 5372–5375. [Google Scholar] [CrossRef]
- Vadagaonkar, K.S.; Yang, C.J.; Zeng, W.H.; Chen, J.H.; Patil, B.N.; Chetti, P.; Chen, L.Y.; Chaskar, A.C. Triazolopyridine Hybrids as Bipolar Host Materials for Green Phosphorescent Organic Light-Emitting Diodes (OLEDs). Dye. Pigment. 2019, 160, 301–314. [Google Scholar] [CrossRef]
- Cao, C.; Chen, W.C.; Tian, S.; Chen, J.X.; Wang, Z.Y.; Zheng, X.H.; Ding, C.W.; Li, J.H.; Zhu, J.J.; Zhu, Z.L.; et al. A Novel D-π-A Blue Fluorophore Based on [1,2,4]Triazolo[1,5-a] Pyridine as an Electron Acceptor and Its Application in Organic Light-Emitting Diodes. Mater. Chem. Front. 2019, 3, 1071–1079. [Google Scholar] [CrossRef]
- Song, W.; Shi, L.; Gao, L.; Hu, P.; Mu, H.; Xia, Z.; Huang, J.; Su, J. Triazolo[1,5-a]Pyridine as Building Blocks for Universal Host Materials for High-Performance Red, Green, Blue and White Phosphorescent Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 5714–5722. [Google Scholar] [CrossRef]
- Sun, E.; Fang, R.; Liu, S. Organic Compound for Light-Emitting Device, and Organic Light-Emitting Device Comprising the Same. Patent WO2022078250. 2022.
- Sun, E.; Fang, R.; Liu, S. Organic Compound for Light-Emitting Device, Application of Organic Compound and Organic Light-Emitting Device. Patent CN112174968A. 2021.
- Sun, E.; Zeng, L.; Liu, S.; Fang, R.; Wu, J. Preparation of Spiro[Acridine-Fluorene]-Derivative Luminescent Material for Organic Electroluminescent Devices. Patent CN112442037. 2021.
- Sun, E.; Liu, S.; Li, Z.; Zhang, X. Compound and Organic Electroluminescent Device Using Same. Patent CN109824672. 2019.
- Sun, E.; Liu, S.; Wu, J.; Feng, J. Organic Electroluminescent Material and Device. Patent WO2019206242. 2019.
- Sun, E.; Liu, S.; Wu, J.; Shao, S. Organic Electroluminescent Material and Device. Patent CN110407838. 2019.
- Li, Z.-P.; Zhao, H.; Zhang, Z.-H.; Qiu, Z.-X.; Li, X.-F.; Huang, L.-J. A Novel [1, 2, 4]Triazolo[5,1-b]Quinazoline Derivative as a Fluorescent Probe for Highly Selective Detection of Fe3+ Ions. J. Asian Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kopotilova, A.E.; Moshkina, T.N.; Nosova, E. V.; Lipunova, G.N.; Starnovskaya, E.S.; Kopchuk, D.S.; Kim, G.A.; Gaviko, V.S.; Slepukhin, P.A.; Charushin, V.N. 3-Aryl-5-Aminobiphenyl Substituted [1,2,4]Triazolo[4,3-c]Quinazolines: Synthesis and Photophysical Properties. Molecules 2023, 28, 1937. [Google Scholar] [CrossRef] [PubMed]
- Moshkina, T.N.; Nosova, E. V; Permyakova, J. V; Lipunova, G.N.; Valova, M.S.; Slepukhin, P.A.; Sadieva, L.K.; Charushin, V.N. Synthesis and Photophysical Properties of 2-Aryl-4-(Morpholin-4-yl)Quinazoline Chromophores: The Effect of π-Linker Moiety. Dye. Pigment. 2022, 206, 110592. [Google Scholar] [CrossRef]
- Moshkina, T.N.; Nosova, E. V.; Permyakova, J. V.; Lipunova, G.N.; Zhilina, E.F.; Kim, G.A.; Slepukhin, P.A.; Charushin, V.N. Push-Pull Structures Based on 2-Aryl/Thienyl Substituted Quinazolin-4(3H)-Ones and 4-Cyanoquinazolines. Molecules 2022, 27, 7156. [Google Scholar] [CrossRef] [PubMed]
- Nosova, E.V.; Kopotilova, A.E.; Ivan’kina, M.A.; Moshkina, T.N.; Kopchuk, D.S. Synthesis of 5-(4-Bromophenyl)- and 5-(5-Bromothiophen-2-yl)-Substituted 3-Aryl[1,2,4]Triazolo[4,3-c]Quinazolines. Russ. Chem. Bull. 2022, 71, 1483–1487. [Google Scholar] [CrossRef]
- Postovskii, I. Ya.; Vereshchagina, N.N.; Mertsalov, S.L. Researches on benzodiazines VI. Synthesis of 2-R-4-hydrazinoquinazolines, 5-R-[8,4-c]-s-triazolo-and 5-R-[1,5-c]tetrazoloquinazolines. Chem. Heterocycl. Compd. 1966; 2, 94–97. [Google Scholar]
- Mamedov, V.А.; Zhukova, N.А.; Kadyrova, M.S. The Dimroth Rearrangement in the Synthesis of Condensed Pyrimidines – Structural Analogs of Antiviral Compounds. Chem. Heterocycl. Compd. 2021, 57, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Geronikaki, A.; Spinelli, D.; Hovakimyan, A.A.; Noravyan, A.S. Synthesis and Structure of Condensed Triazolo- and Tetrazolopyrimidines. Tetrahedron 2013, 69, 10637–10643. [Google Scholar] [CrossRef]
- Vorob’ev, E.V. Kletskii, M.E., Krasnikov, V.V. et al. Studies on mechanisms of the rearrangement of thieno[3,2-e][1,2,4]triazolo[4,3-c]pyrimidines into thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines. Russ Chem Bull. 2006, 55, 2247–2255. [Google Scholar] [CrossRef]
- Moshkina, T.N.; Le Poul, P.; Barsella, A.; Pytela, O.; Bureš, F.; Robin-Le Guen, F.; Achelle, S.; Nosova, E. V; Lipunova, G.N.; Charushin, V.N. Electron-Withdrawing Substituted Quinazoline Push-Pull Chromophores: Synthesis, Electrochemical, Photophysical and Second-Order Nonlinear Optical Properties. European J. Org. Chem. 2020, 2020, 5445–5454. [Google Scholar] [CrossRef]
- Starnovskaya, E.S.; Valieva, M.I.; Aluru, R.; Kopchuk, D.S.; Khasanov, A.F.; Taniya, O.S.; Novikov, A.S.; Kalinichev, A.A.; Santra, S.; Zyryanov, G. V.; Ranu, B.C. Carbazole/Fluorene-Substituted 5-Phenyl-2,2′-Bipyridine D-π-A Fluorophores: Photophysical Data, Hyperpolarizability and CT-Indices. New J. Chem. 2023, 47, 12393–12402. [Google Scholar] [CrossRef]
- Porrès, L.; Holland, A.; Pålsson, L.O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, I.S.; Taniya, O.S.; Sadieva, L.K.; Volkova, N.N.; Minin, A.S.; Grzhegorzhevskii, K. V.; Gorbunov, E.B.; Zyryanov, G. V.; Chupakhin, O.N.; Charushin, V.N.; Tsurkan, M.V. Bola-Type PAH-Based Fluorophores/Chemosensors: Synthesis via an Unusual Clemmensen Reduction and Photophysical Studies. J. Photochem. Photobiol. A Chem. 2021, 420, 113466. [Google Scholar] [CrossRef]
- Kournoutas, F.; Fihey, A.; Malval, J.P.; Spangenberg, A.; Fecková, M.; Le Poul, P.; Katan, C.; Robin-Le Guen, F.; Bureš, F.; Achelle, S.; Fakis, M. Branching Effect on the Linear and Nonlinear Optical Properties of Styrylpyrimidines. Phys. Chem. Chem. Phys. 2020, 22, 4165–4176. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Peng, Z.; Wang, Z.; Wang, Y.; Lu, P. Preparation and Photophysical Properties of Quinazoline-Based Fluorophores. RSC Adv. 2020, 10, 30297–30303. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd, ed.; Lakowicz, J.R., Ed.; Springer US: Boston, MA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Lippert, E. Spektroskopische Bestimmung Des Dipolmomentes Aromatischer Verbindungen Im Ersten Angeregten Singulettzustand. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für Phys. Chemie. 1957, 61, 962–975. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Fast Calculation of van Der Waals Volume as a Sum of Atomic and Bond Contributions and Its Application to Drug Compounds. J. Org. Chem. 2003, 68, 7368–7373. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Maka, V.K.; Moorthy, J.N. Remarkable Influence of ‘Phane Effect’ on the Excited-State Properties of Cofacially Oriented Coumarins. Phys. Chem. Chem. Phys. 2017, 19, 4758–4767. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, T.; Chen, Q. An Sp-Hybridized All-Carboatomic Ring, Cyclo[18]Carbon: Electronic Structure, Electronic Spectrum, and Optical Nonlinearity. Carbon N. Y. 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Alegre-Requena, J. V.; Sowndarya S. V., S.; Pérez-Soto, R.; Alturaifi, T.M.; Paton, R.S. AQME: Automated Quantum Mechanical Environments for Researchers and Educators. WIREs Comput. Mol. Sci. 2023, 13. [Google Scholar] [CrossRef]
- Dolomanov, O. V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
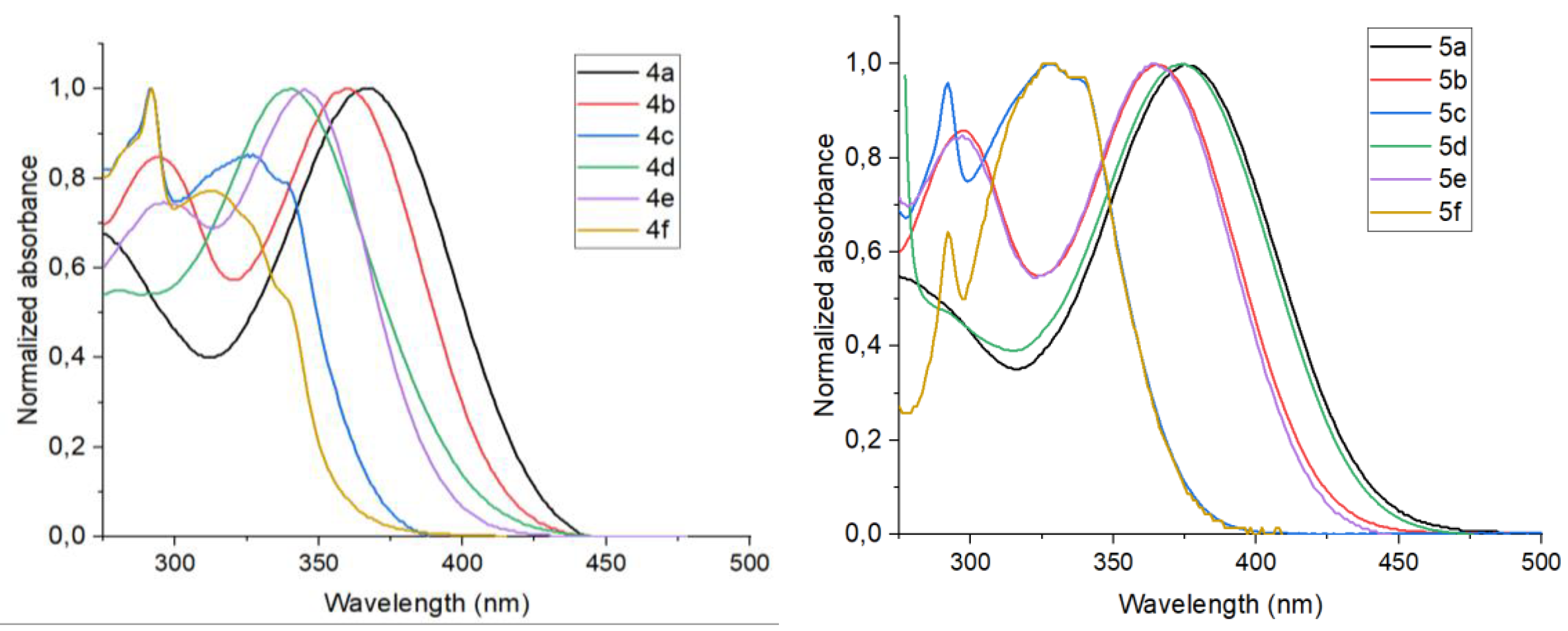

| Compound | Solvent | λabs, nm (ε, 104 M-1cm-1) | λem, nm | ΔνSta, cm-1 | ΦFb, % | |
|---|---|---|---|---|---|---|
| 4a | Toluene | 371 (1.93) | 476 | 5946 | 90 | |
| MeCN | 276 sh (1.77), 366 (2.41) | 605 | 10793 | 29 | ||
| Solid | - | 500 | - | 8 | ||
| 4b | Toluene | 370 (2.27) | 469 | 5705 | 97 | |
| MeCN | 295 (2.67), 359 (3.08) | 609 | 11435 | 14 | ||
| Solid | - | 509 | - | 17 | ||
| 4c | Toluene | 329 (3.61), 341 (3.31) | 422 | 5629 | 13 | |
| MeCN | 291 (1.84), 325 (1.63), 337 sh (1.54) |
548 | 12521 | 43 | ||
| 4d | Toluene | 343 (2.82) | 481 | 8364 | 16 | |
| MeCN | 340 (4.26) | 608 | 12964 | 41 | ||
| Solid | - | 455 | - | 32 | ||
| 4e | Toluene | 355 (1.53) | 467 | 6756 | 11 | |
| MeCN | 296 (1.72), 345 (2.30) | 609 | 12565 | 25 | ||
| Solid | - | 468 | - | 10 | ||
| 4f | Toluene | 327 (3.92), 339 sh (3.53) | 423 | 6940 | < 1 | |
| MeCN | 292 (3.18), 312 (2.54) | 538 | 13464 | 3 | ||
| Solid | - | 432 | - | 14 | ||
| 5a | Toluene | 298 (0.55), 383 (1.47) | 479 | 5233 | 90 | |
| MeCN | 375 (3.01) | 598 | 9944 | 34 | ||
| Solid | - | 510 | - | 3 | ||
| 5b | Toluene | 377 (2.78) | 472 | 5339 | >98 | |
| MeCN | 298 (3.25), 365 (3.73) | 603 | 10814 | 24 | ||
| Solid | - | 479 | - | 42 | ||
| 5c | Toluene | 294 (-)c, 342 (-) | 441 | 6564 | 75 | |
| MeCN | 292, 328, 337 (sh) | 537 | 11257 | 57 | ||
| 5d | Toluene | 297 (-), 380 (-) | 486 | 5740 | >98 | |
| MeCN | 253 (-), 291 (sh) (-), 374 (-) | 579 | 9467 | 90 | ||
| Solid | - | 517 | - | 8 | ||
| 5e | Toluene | 287 (3.76), 375 (2.41) | 465 | 5161 | >98 | |
| MeCN | 297 (1.35), 363 (1.59) | 593 | 10684 | 39 | ||
| Solid | - | 481 | - | 28 | ||
| 5f | Toluene | 293 (-), 342 (-) | 420 | 5430 | >98 | |
| MeCN | 292 (-), 328 (-), 339 (sh) (-) | 530 | 10630 | 96 | ||
| Solid | - | 427 | - | 31 |
| Comp. | τav, ns | kra , 107 s-1 | knra , 109 s-1 |
|---|---|---|---|
| 4a | 1.73 | 52.02 | 5.78 |
| 4b | 1.85 | 52.43 | 1.62 |
| 4c | 0.49 | 26.53 | 177.55 |
| 4d | 0.22 | 72.73 | 381.82 |
| 4e | 1.47 | 7.48 | 60.54 |
| 5a | 1.68 | 53.57 | 5.95 |
| 5b | 1.74 | 56.32 | 1.15 |
| 5c | 1.26 | 59.52 | 19.84 |
| 5d | 1.60 | 61.25 | 1.25 |
| 5e | 1.66 | 59.04 | 1.20 |
| 5f | 1.11 | 88.29 | 1.80 |
| Comp. | Slopes | R2 | a1a, Å | Δµ1, D | a2b, Å | Δµ2, D | ΔµDFT, D |
|---|---|---|---|---|---|---|---|
| 4a | 17676 | 0.97 | 4.36 | 12.08 | 9.11 | 36.47 | 20.67 |
| 4d | 18798 | 0.93 | 4.50 | 13.06 | 9.36 | 39.12 | 15.59 |
| 5a | 16405 | 0.97 | 4.36 | 11.64 | 9.10 | 35.08 | 21.26 |
| 5d | 13456 | 0.94 | 4.50 | 11.05 | 9.35 | 33.08 | 20.55 |
| 5e | 18364 | 0.97 | 4.79 | 14.14 | 9.94 | 42.36 | 26.93 |
| 5f | 19123 | 0.92 | 4.70 | 14.06 | 9.88 | 42.81 | 30.69 |
| Compound | EOxonset, Va | EHOMOel, eVb | EHOMODFT, eV | ELUMODFT, eV | EgDFT, eV |
|---|---|---|---|---|---|
| 4a | 0.31 | -5.41 | -5.61 | -1.87 | 3.74 |
| 4b | 0.47 | -5.57 | -5.60 | -2.02 | 3.58 |
| 4c | 0.77 | -5.87 | -5.98 | -2.21 | 3.77 |
| 4d | 0.31 | -5.41 | -5.58 | -1.64 | 3.94 |
| 4e | 0.46 | -5.56 | -5.58 | -1.82 | 3.76 |
| 4f | 0.81 | -5.91 | -5.96 | -2.01 | 3.95 |
| 5a | 0.27 | -5.37 | -5.44 | -1.84 | 3.60 |
| 5b | 0.43 | -5.53 | -5.47 | -2.00 | 3.47 |
| 5c | 0.77 | -5.87 | -5.86 | -2.15 | 3.71 |
| 5d | 0.28 | -5.38 | -5.41 | -1.75 | 3.66 |
| 5e | 0.47 | -5.57 | -5.45 | -1.92 | 3.53 |
| 5f | 0.78 | -5.88 | -5.85 | -2.07 | 3.78 |
| Comp | Sr(a.u.)a | D-index(Å)b | H-index(Å)c | t-index(Å)d | Ece |
|---|---|---|---|---|---|
| 4a | 0.50544 | 6.310 | 3.719 | 2.895 | 3.08 |
| 4b | 0.50926 | 6.850 | 4.106 | 3.163 | 2.76 |
| 4c | 0.39654 | 7.830 | 3.934 | 4.350 | 2.42 |
| 4d | 0.50724 | 6.356 | 3.755 | 2.951 | 2.98 |
| 4e | 0.52832 | 6.586 | 4.149 | 2.880 | 2.79 |
| 4f | 0.45366 | 6.982 | 4.116 | 3.333 | 2.66 |
| 5a | 0.49229 | 6.546 | 3.698 | 3.176 | 2.94 |
| 5b | 0.48785 | 7.272 | 4.074 | 3.620 | 2.63 |
| 5c | 0.40714 | 8.170 | 3.994 | 4.616 | 2.34 |
| 5d | 0.51262 | 6.317 | 3.748 | 3.026 | 2.85 |
| 5e | 0.50296 | 7.018 | 4.104 | 3.406 | 2.61 |
| 5f | 0.42992 | 7.809 | 4.068 | 4.233 | 2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





