Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
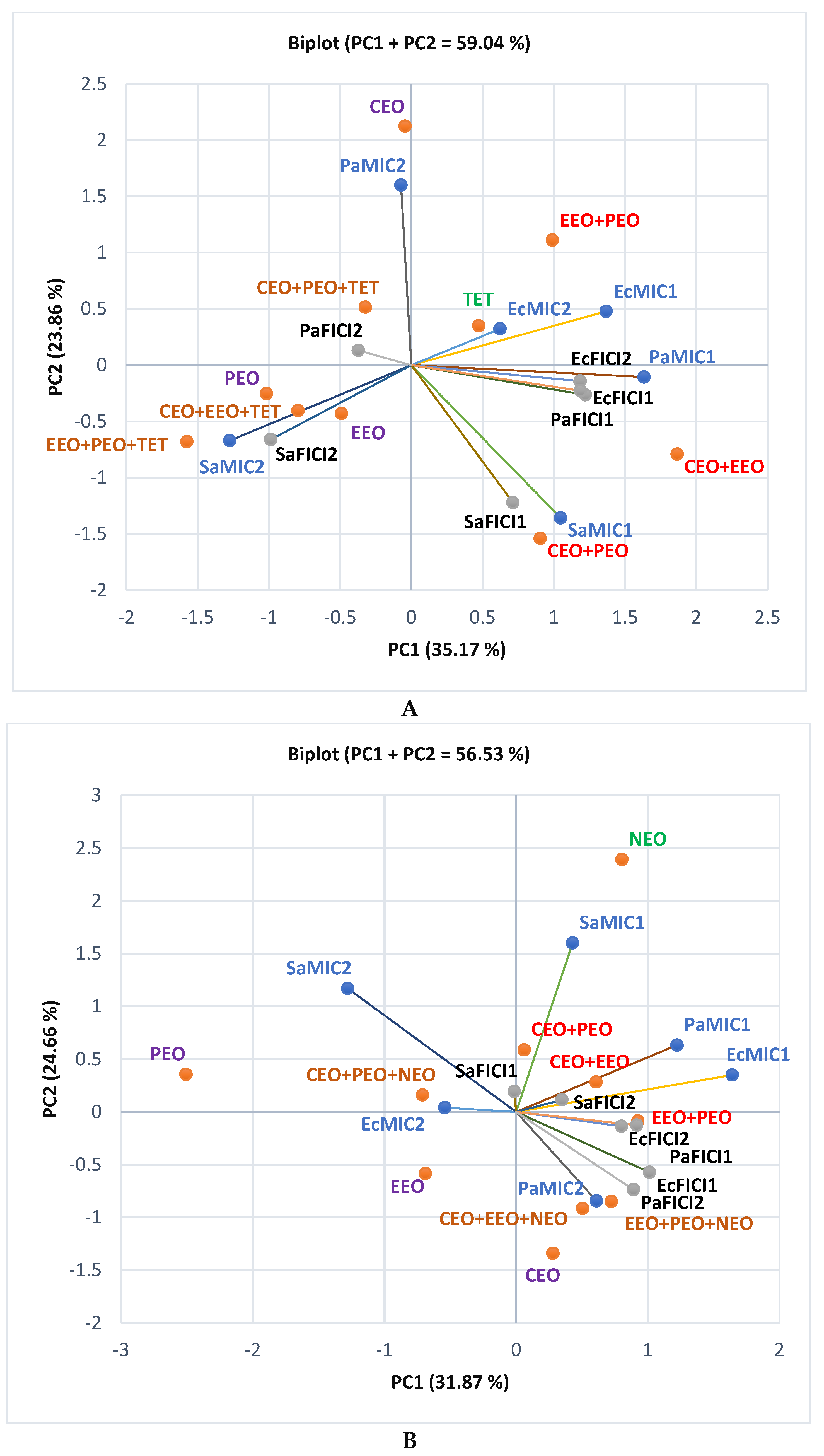
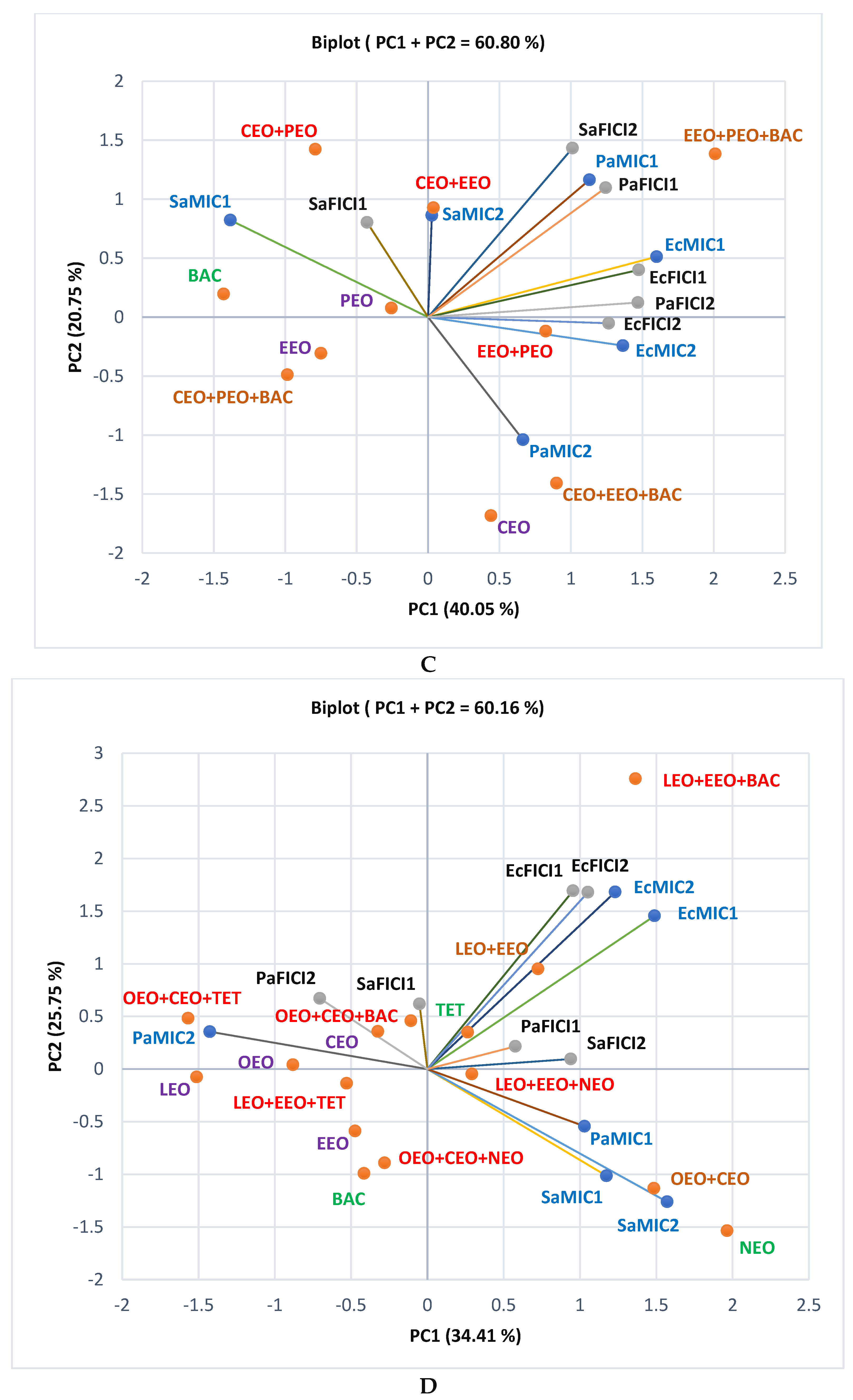
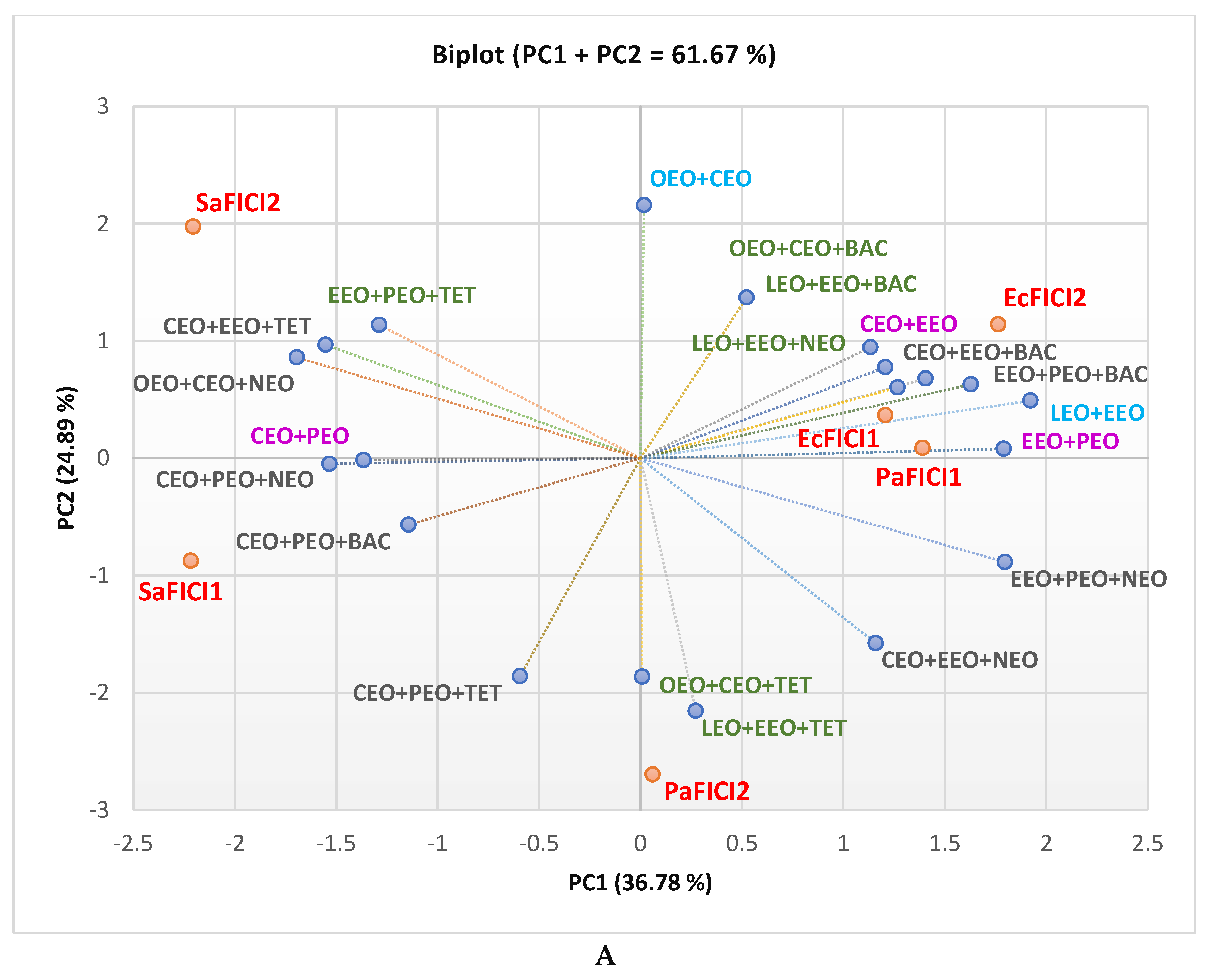
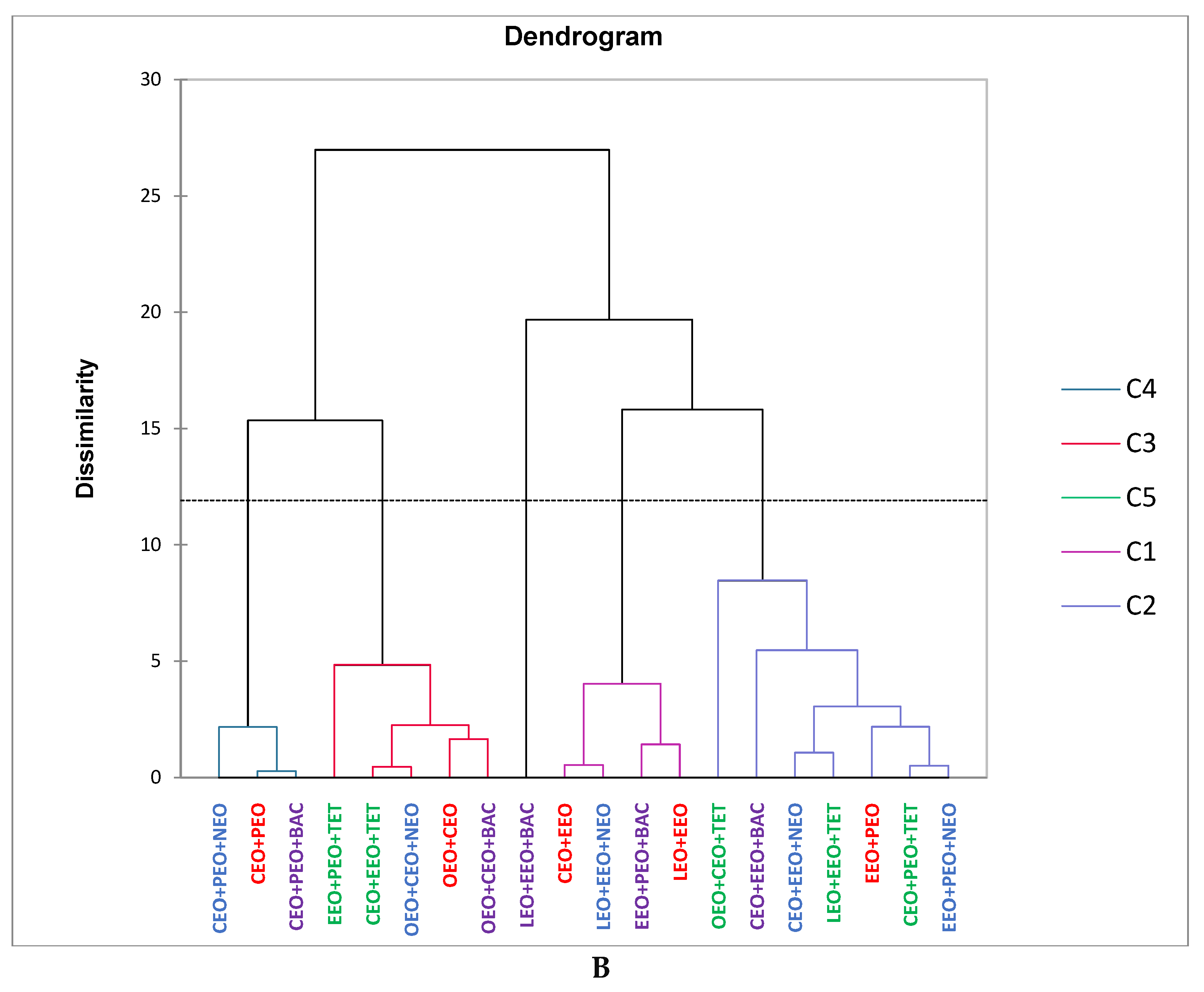
2.1. Gas Chromatography-Mass Spectrometry Analysis
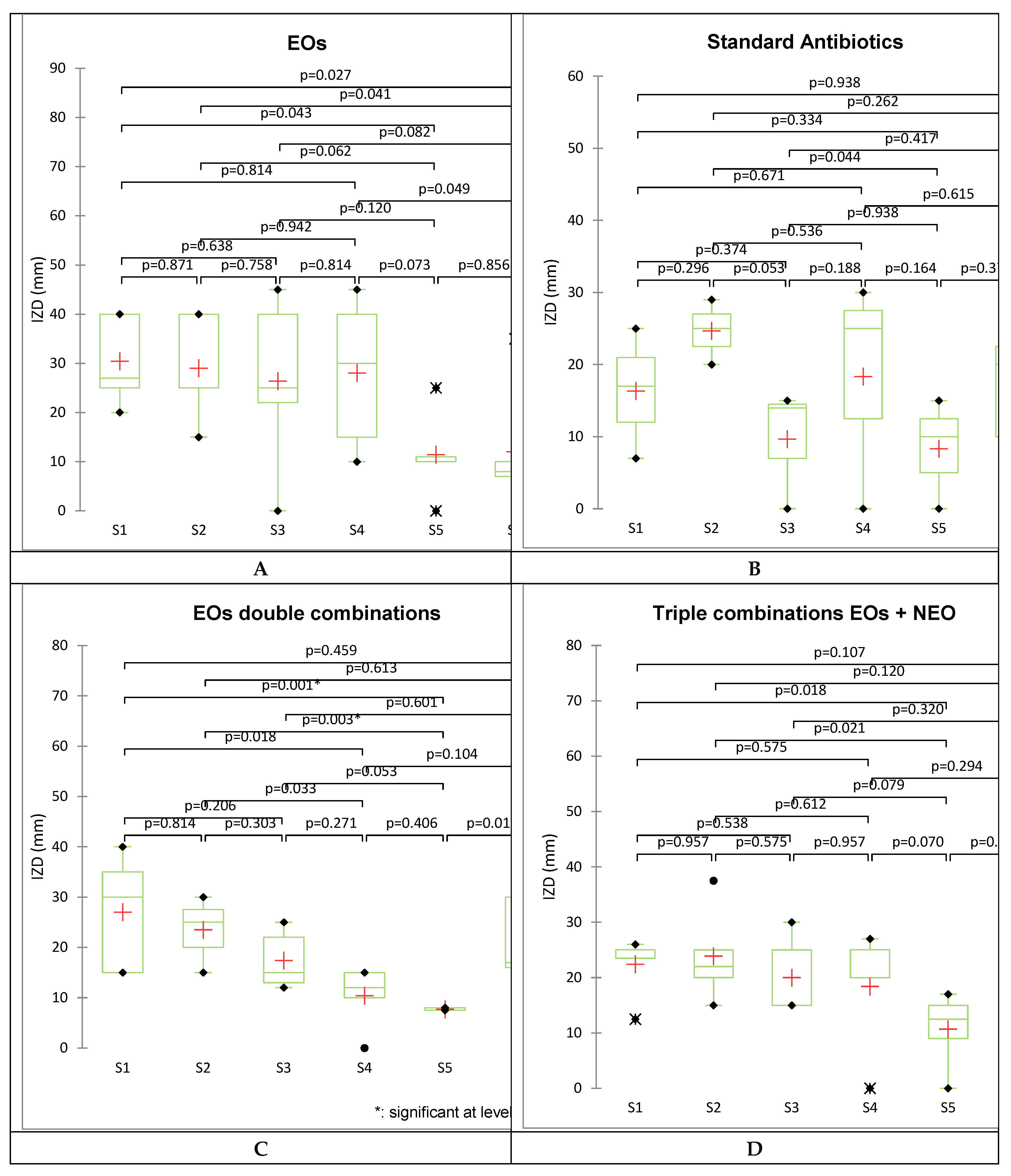
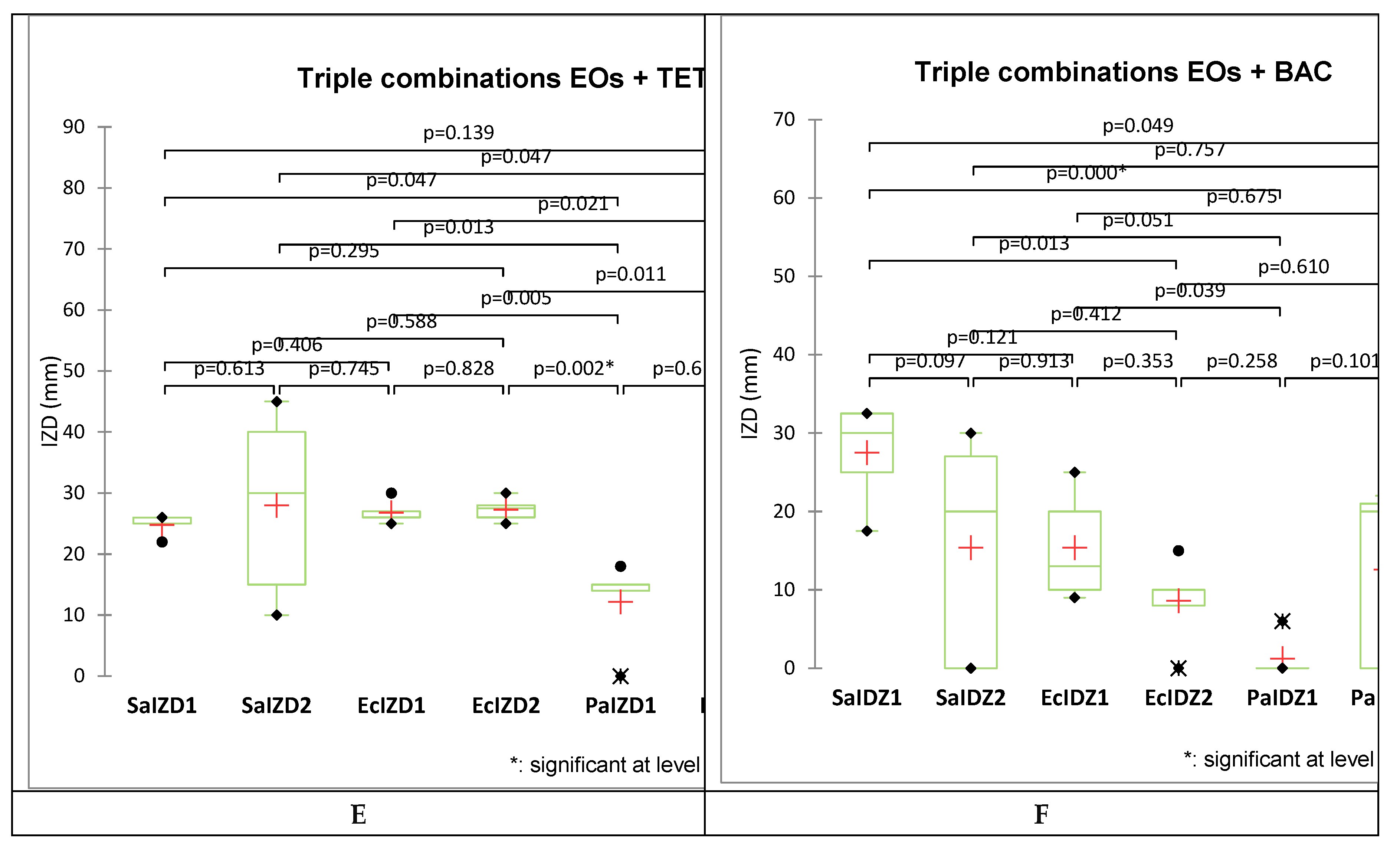
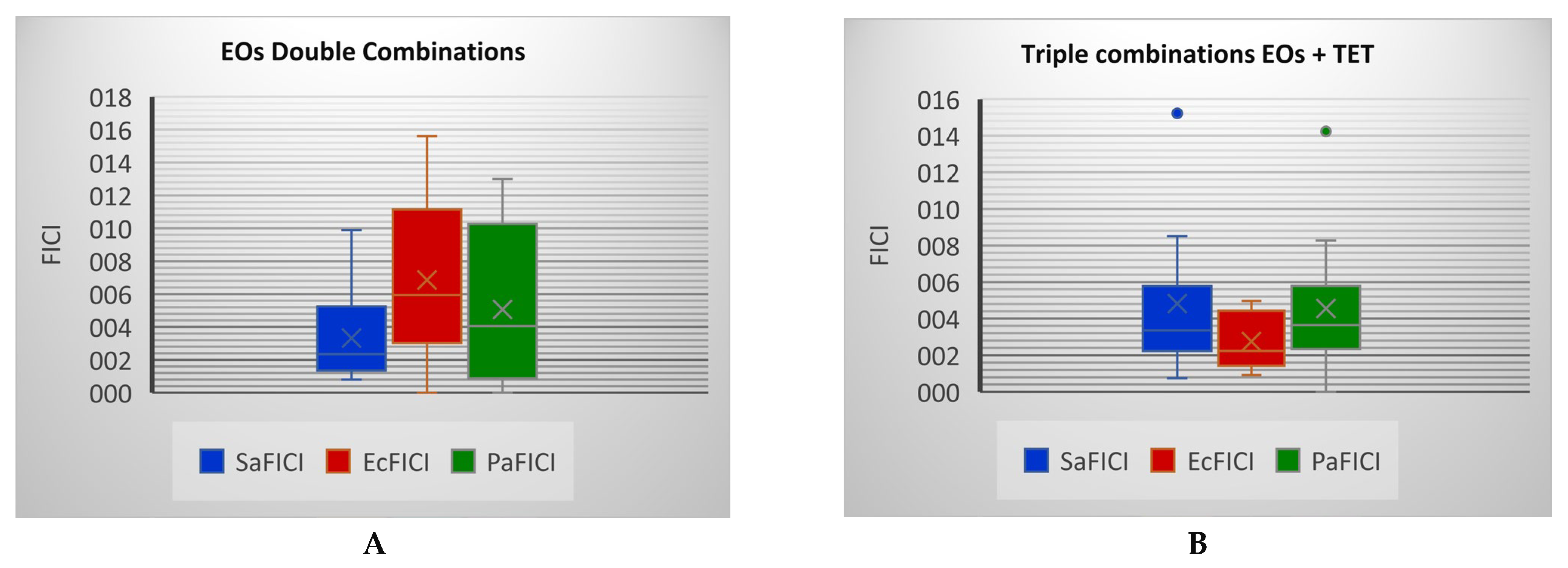
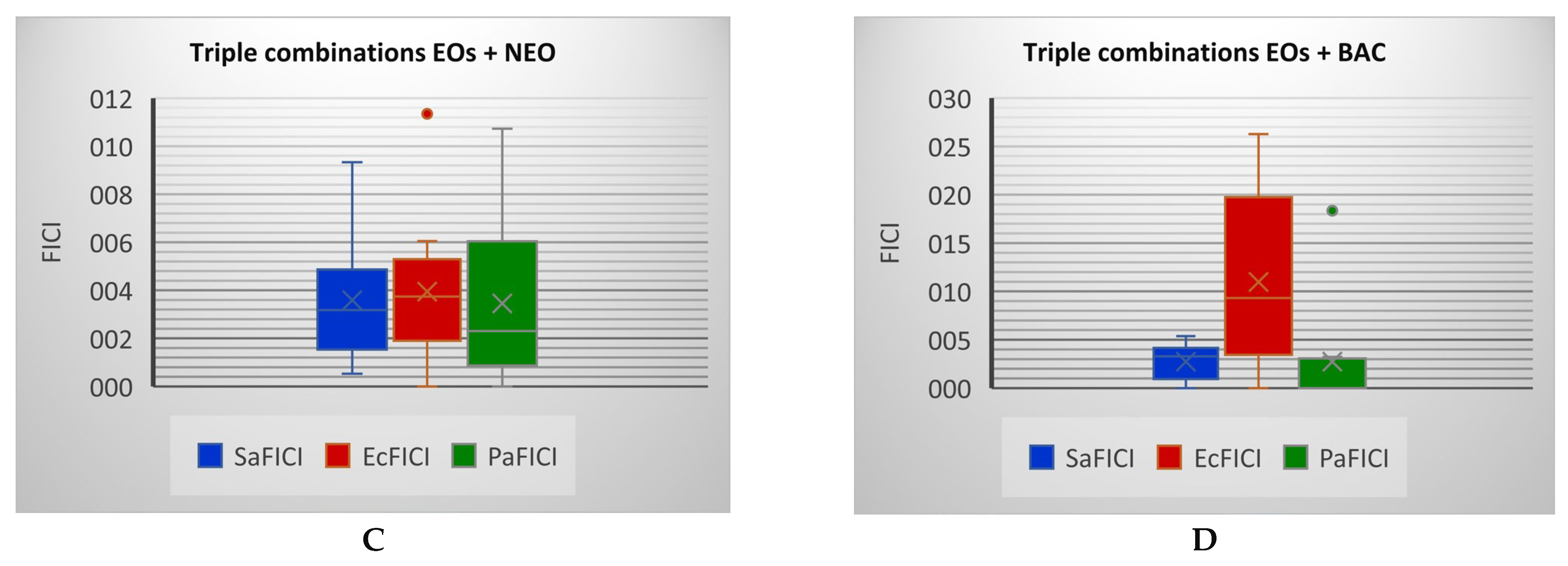
2.2. Antibacterial Activity
| Technique | Cellulose disc technique | Cylinder technique | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | S. aureus | |||||||
| IZD (mm) |
MIC (µg/mL) |
FICI | Obs | IZD (mm) |
MIC (µg/mL) |
FICI | Obs | |
| LEO | 40 | 2.1 | - | - | 40 | 21.2 | - | - |
| CEO | 27 | 4.7 | - | - | 25 | 54.3 | - | - |
| OEO | 40 | 2.1 | - | - | 40 | 21.2 | - | - |
| EEO | 20 | 8.5 | - | - | 25 | 54.3 | - | - |
| PEO | 25 | 5.4 | - | - | 15 | 150.9 | - | - |
| OEO+CEO | 30 | 3.8 | 2.6 | I | 15 | 150.9 | 9.9 | Ant. |
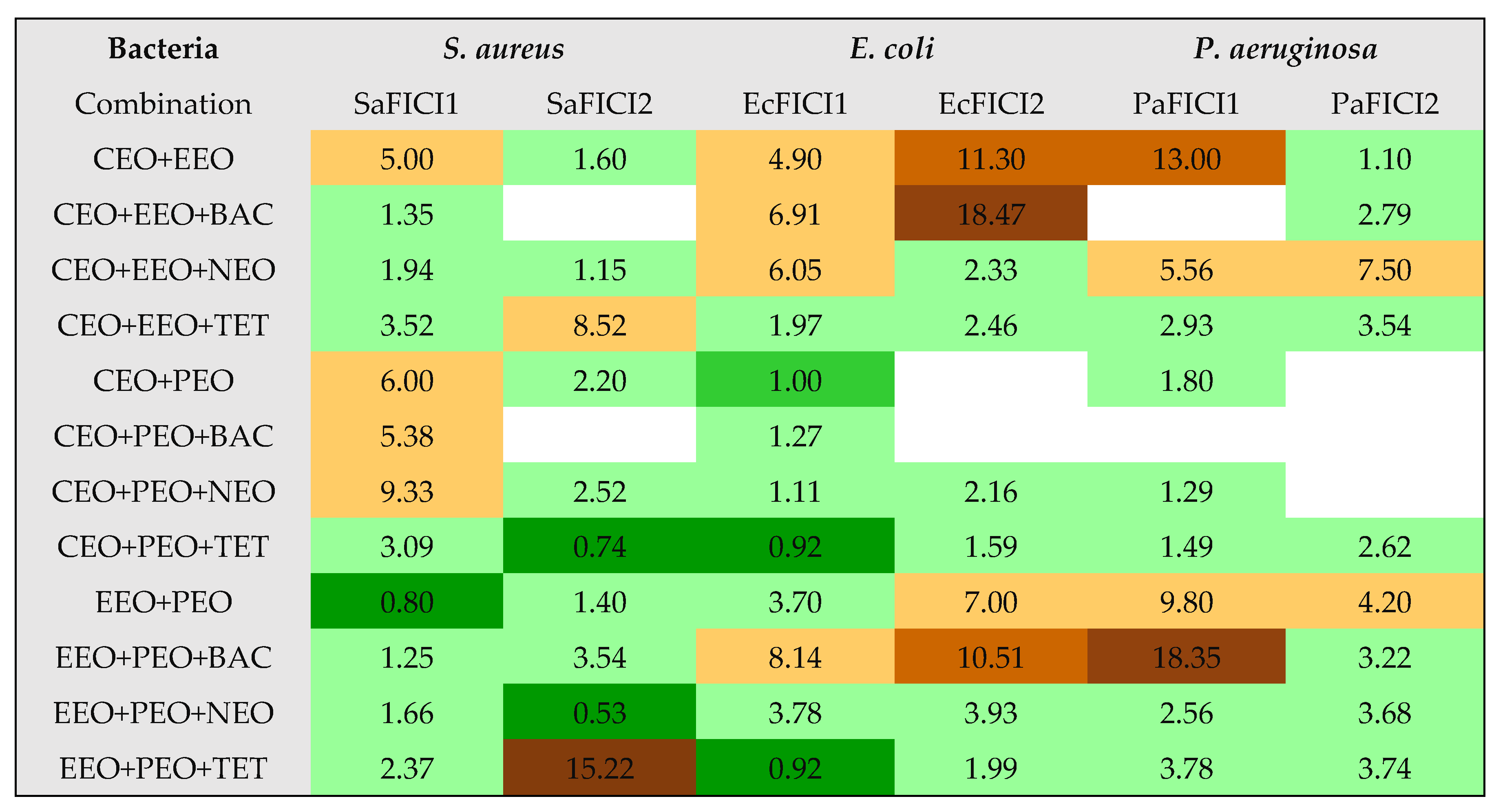
| CEO+EEO | 15 | 15.1 | 5.0 | Ant. | 27.5 | 44.9 | 1.6 | I |
| CEO+PEO | 15 | 15.1 | 6.0 | Ant. | 20 | 84.9 | 2.2 | I |
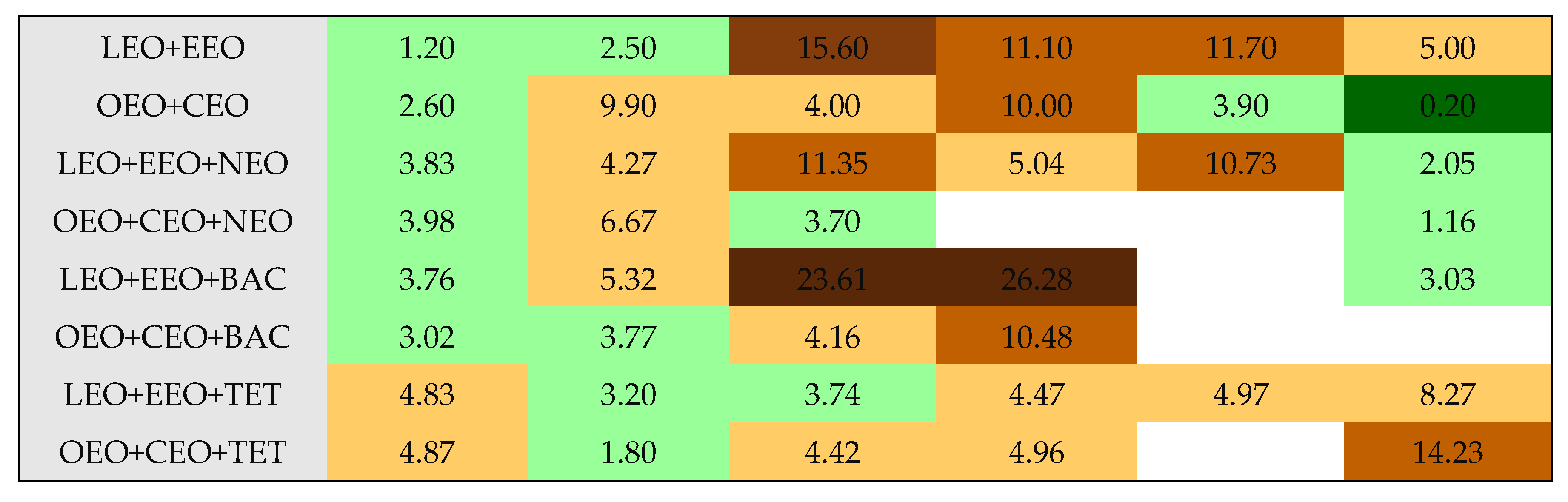
| LEO+EEO | 40 | 2.1 | 1.2 | I | 30 | 37.7 | 2.5 | I. |
| EEO+PEO | 35 | 2.8 | 0.8 | P.S. | 25 | 54.3 | 1.4 | I |
| NEO | 7 | 86.7 | - | - | 20 | 106.2 | - | - |
| OEO+CEO+NEO | 25 | 5.7 | 3.98 | I | 20 | 89.2 | 6.67 |
Ant. |
| CEO+EEO+NEO | 25 | 5.7 | 1.94 | I | 37.5 | 25.4 | 1.15 | I |
| CEO+PEO+NEO | 12.5 | 22.8 | 9.33 | Ant. | 22 | 73.7 | 2.52 | I |
| LEO+EEO+NEO | 23.5 | 6.5 | 3.83 | I | 25 | 57.1 | 4.27 | Ant. |
| EEO+PEO+NEO | 26 | 5.3 | 1.66 | I | 15 | 15.9 | 0.53 | P.S. |
| TET | 25 | 6.8 | - | - | 29 | 50.5 | - | - |
| OEO+CEO+TET | 25 | 5.7 | 4.87 | Ant. | 40 | 21.2 | 1.8 | I |
| CEO+EEO+TET | 22 | 7.4 | 3.52 | I | 15 | 150.9 | 8.52 |
Ant. |
| CEO+PEO+TET | 25 | 5.7 | 3.09 | I | 45 | 16.8 | 0.74 | P.S. |
| LEO+EEO+TET | 26 | 5.3 | 4.83 | Ant. | 30 | 37.7 | 3.20 | I |
| EEO+PEO+TET | 26 | 5.3 | 2.37 | I | 10 | 339.7 | 15.22 | Ant. |
| BAC | 17 | 14.7 | - | - | 25 | 67.9 | - | - |
| OEO+CEO+BAC | 30 | 4.0 | 3.02 | I | 30 | 47.2 | 3.77 | I |
| CEO+EEO+BAC | 32.5 | 3.4 | 1.35 | I | 0 | - | - | - |
| CEO+PEO+BAC | 17.5 | 11.6 | 5.38 | Ant. | 0 | - | - | - |
| LEO+EEO+BAC | 25 | 5.7 | 3.76 | I | 27 | 48.9 | 5.32 | Ant. |
| EEO+PEO+BAC | 32.5 | 3.4 | 1.25 | I | 20 | 89.2 | 3.54 | I |
| Technique | Cellulose disc technique | Cylinder technique | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | E. coli | |||||||
| IZD (mm) |
MIC (µg/mL) |
FICI | Obs | IZD (mm) |
MIC (µg/mL) |
FICI | Obs | |
| LEO | 40 | 2.1 | - | - | 40 | 21.2 | - | - |
| CEO | 22 | 7.0 | - | - | 15 | 150.9 | - | - |
| OEO | 45 | 1.7 | - | - | 45 | 16.8 | - | - |
| EEO | 25 | 5.4 | - | - | 30 | 37.7 | - | - |
| PEO | 0 | - | - | - | 10 | 339.7 | - | - |
| OEO+CEO | 25 | 5.4 | 4.0 | I | 15 | 150.9 | 10.0 | Ant |
| CEO+EEO | 15 | 15.1 | 4.9 | Ant | 10 | 339.7 | 11.3 | Ant |
| CEO+PEO | 22 | 7.0 | 1.0 | Add. | 0 | - | - | - |
| LEO+EEO | 12 | 23.6 | 15.6 | Ant | 15 | 150.9 | 11.1 | Ant |
| EEO+PEO | 13 | 20.1 | 3.7 | I | 12 | 235.9 | 7.0 | Ant |
| NEO | 15 | 18.9 | - | 25 | 67.9 | - | - | |
| OEO+CEO+NEO | 30 | 4.0 | 3.7 | I | 0 | - | - | - |
| CEO+EEO+NEO | 15 | 15.9 | 6.05 | Ant | 27 | 48.9 | 2.33 | I |
| CEO+PEO+NEO | 25 | 5.7 | 1.11 | I | 20 | 89.2 | 2.16 | I |
| LEO+EEO+NEO | 15 | 15.9 | 11.35 | Ant | 25 | 57.1 | 5.04 | Ant |
| EEO+PEO+NEO | 15 | 15.9 | 3.78 | I | 20 | 89.2 | 3.93 | I |
| TET | 14 | 21.7 | - | - | 30 | 47.2 | - | - |
| OEO+CEO+TET | 25 | 5.7 | 4.42 | Ant | 25 |
57.1 | 4.96 | Ant |
| CEO+EEO+TET | 26 | 5.3 | 1.97 | I | 28 | 45.5 | 2.46 | I |
| CEO+PEO+TET | 27 | 4.9 | 0.92 | P.S. | 26 | 52.8 | 1.59 | I |
| LEO+EEO+TET | 26 | 5.3 | 3.74 | I | 27.5 | 47.2 | 4.47 | Ant |
| EEO+PEO+TET | 30 | 4.0 | 0.92 | P.S. | 30 | 39.6 | 1.99 | I |
| BAC | 0 | - | - | - | 0 | - | - | - |
| OEO+CEO+BAC | 25 | 5.7 | 4.16 | Ant | 15 | 158.5 | 10.48 | Ant |
| CEO+EEO+BAC | 13 | 21.1 | 6.91 | Ant | 8 | 557.4 | 18.47 | Ant |
| CEO+PEO+BAC | 20 | 8.9 | 1.27 | I | 0 | - | - | - |
| LEO+EEO+BAC | 10 | 35.7 | 23.61 | Ant | 10 | 356.7 | 26.28 | Ant |
| EEO+PEO+BAC | 9 | 44.0 | 8.14 | Ant | 10 | 356.7 | 10.51 | Ant |
| Technique | Cellulose disc technique | Cylinder technique | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | P. aeruginosa | |||||||
| IZD (mm) |
MIC (µg/mL) |
FICI | Obs | IZD (mm) |
MIC (µg/mL) |
FICI | Obs | |
| LEO | 11 | 28.1 | - | - | 7 | 693.2 | - | - |
| CEO | 10 | 33.9 | - | - | 8 | 530.9 | - | - |
| OEO | 11 | 28.1 | - | - | 10 | 339.7 | - | - |
| EEO | 25 | 5.4 | - | - | 35 | 27.7 | - | - |
| PEO | 0 | - | - | - | 0 | - | - | - |
| OEO+CEO | 7.5 | 60.6 | 3.9 | I | 30 | 37.7 | 0.2 | S |
| CEO+EEO | 7.5 | 60.6 | 13.0 | Ant | 35 | 27.7 | 1.1 | I |
| CEO+PEO | 7.5 | 60.6 | 1.8 | I | 0 | - | - | - |
| LEO+EEO | 8 | 53.0 | 11.7 | Ant | 16 | 132.7 | 5.0 | Ant |
| EEO+PEO | 8 | 53.0 | 9.8 | Ant | 17 | 117.5 | 4.2 | Ant |
| NEO | 10 | 42.4 | - | - | 20 | 106.2 | - | - |
| OEO+CEO+NEO | 0 | - | - | - | 22 | 73.7 | 1.16 | I |
| CEO+EEO+NEO | 12.5 | 22.8 | 5.56 | Ant | 15 | 158.5 | 7.5 | Ant |
| CEO+PEO+NEO | 15 | 15.9 | 1.29 | I | 0 | - | - | - |
| LEO+EEO+NEO | 9 | 44.0 | 10.73 | Ant | 14 | 182.0 | 2.05 | I |
| EEO+PEO+NEO | 17 | 12.3 | 2.56 | I | 21 | 80.9 | 3.68 | I |
| TET | 15 | 18.9 | - | - | 25 | 67.9 | - | - |
| OEO+CEO+TET | 0 | - | - | - | 7 | 727.9 | 14.23 | Ant |
| CEO+EEO+TET | 18 | 11.0 | 2.93 | I | 23 | 67.4 | 3.54 | I |
| CEO+PEO+TET | 14 | 18.2 | 1.49 | I | 15 | 158.5 | 2.62 | I |
| LEO+EEO+TET | 14 | 18.2 | 4.97 | Ant | 15 | 158.5 | 8.27 | Ant |
| EEO+PEO+TET | 15 | 15.9 | 3.78 | I | 22 | 73.7 | 3.74 | I |
| BAC | 0 | - | - | - | 0 | - | - | - |
| OEO+CEO+BAC | 0 | - | - | - | 0 | - | - | - |
| CEO+EEO+BAC | 0 | - | - | - | 22 | 73.7 | 2.79 | I |
| CEO+PEO+BAC | 0 | - | - | - | 0 | - | - | - |
| LEO+EEO+BAC | 0 | - | - | - | 21 | 80.9 | 3.03 | I |
| EEO+PEO+BAC | 6 | 99.1 | 18.35 | Ant | 20 | 89.2 | 3.22 | I |
2.3. Data Analysis
4. Materials and Methods
4.1. Materials and Equipment
4.2. GC-MS Analysis
4.3. In Vitro Evaluation of Antibacterial Activity
4.3.1. Bacterial Inoculum
4.3.2. Sample Solutions
4.3.3. Technique
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T. Bin; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Manion, C.R.; Widder, R.M. Essentials of Essential Oils. American Journal of Health-System Pharmacy 2017, 74, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential - A Review. Curr Drug Metab 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Yeddes, W.; Ouerghemmi, I.; Hammami, M.; Gadhoumi, H.; Affes, T.G.; Mohamed, S.N.; Aidi-Wannes, W.; Witrowa-Rajchert, D.; Saidani-Tounsi, M.; Nowacka, M. Optimizing the Method of Rosemary Essential Oils Extraction by Using Response Surface Methodology (RSM)-Characterization and Toxicological Assessment. Sustainability (Switzerland) 2022, 14, 3927. [Google Scholar] [CrossRef]
- Khodaei, N.; Nguyen, M.M.; Mdimagh, A.; Bayen, S.; Karboune, S. Compositional Diversity and Antioxidant Properties of Essential Oils: Predictive Models. LWT 2021, 138. [Google Scholar] [CrossRef]
- Piatkowska, E.; Rusiecka-Ziółkowska, J. Influence of Essential Oils on Infectious Agents. Advances in Clinical and Experimental Medicine 2016, 25. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral, and Cytotoxic Properties - An Overview. Forsch Komplementarmed 2009, 16. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- LaLonde, T.; Bowser, T.; Jadeja, R. Essential Oils as Antimicrobials. Madridge Journal of Food Technology 2019, 4, 163–169. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol J 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Upadhyay, R. Essential Oils: Antimicrobial, Antihelminthic, Antiviral, Anticancer and Antiinsect Properties. J Appl Biosci 2010, 36, 1–22. [Google Scholar]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Facchiano, S.; Boscaino, F.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Towards Green Strategies of Food Security: Antibacterial Synergy of Essential Oils from Thymus Vulgaris and Syzygium Aromaticum to Inhibit Escherichia Coli and Staphylococcus Aureus Pathogenic Food Isolates. Microorganisms 2022, 10, 2446. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn Rev 2017, 11. [Google Scholar]
- Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds: Current Strategies and New Alternatives; 2014; Vol. 9783642404443.
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit Rev Microbiol 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline Antibiotics and Resistance Mechanisms. Biol Chem 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Deb, J.K. Molecular Understanding of Aminoglycoside Action and Resistance. Appl Microbiol Biotechnol 2006, 70, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamada, K.; Nagao, M.; Aoki, E.; Matsumoto, M.; Hirayama, T.; Yamamoto, H.; Hiramatsu, R.; Ichiyama, S.; Iinuma, Y. Antimicrobial Ointments and Methicillin-Resistant Staphylococcus Aureus USA300. Emerg Infect Dis 2011, 17, 1917–1920. [Google Scholar] [CrossRef]
- Vorobets, N.M.; Yavorska, H. V Modifications of Agar Diffusion Method to Determination of the Antimicrobial Effect of the Herbal Medicinal Products. Ukraïns’kij bìofarmacevtičnij žurnal 2016, 2, 80–84. [Google Scholar] [CrossRef]
- Boz, I.; Gille, E.; Necula, R.; Dunca, S.; Zamfirache, M.M. Chemical Composition and Antibacterial Activity of Essential Oils from Five Populations of Thymus Pulegioides L. Cellulose Chemistry and Technology 2015, 49, 169–174. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J Food Drug Anal 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Dias, C.; Vasconcelos, M.C.; Rosa, E.; Saavedra, M.J. Antibacterial Activity and Synergistic Effects between Eucalyptus Globulus Leaf Residues (Essential Oils and Extracts) and Antibiotics against Several Isolates of Respiratory Tract Infections (Pseudomonas Aeruginosa). Ind Crops Prod 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and Antiplasmid Activities of Essential Oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies – Methods and Approaches to Study the Interaction between Natural Products. Planta Med 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-E.; Kim, H.-Y.; Cha, J.-D. Synergistic Effect between Clove Oil and Its Major Compounds and Antibiotics against Oral Bacteria. Arch Oral Biol 2011, 56, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and Antibiofilm Effects of Different Samples of Five Commercially Available Essential Oils. 2023, 12, 1191.
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol Author Information. American Society For Microbiology 2012, 1–13. [Google Scholar]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea Barbata (L.) Weber Ex F.H. Wigg from C ă Limani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea Barbata (L.) Weber Ex F. H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ivan, I.M.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical Profile, Antioxidant and Cytotoxic Potential of Capsicum Annuum (L.) Dry Hydro-Ethanolic Extract. Pharmaceutics 2024, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Caraiane, A.; Botnarciuc, M. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea Barbata (L.) Weber Ex F. H. Wigg from Călimani Mountains, Romania. Pharmaceuticals 2022, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef] [PubMed]
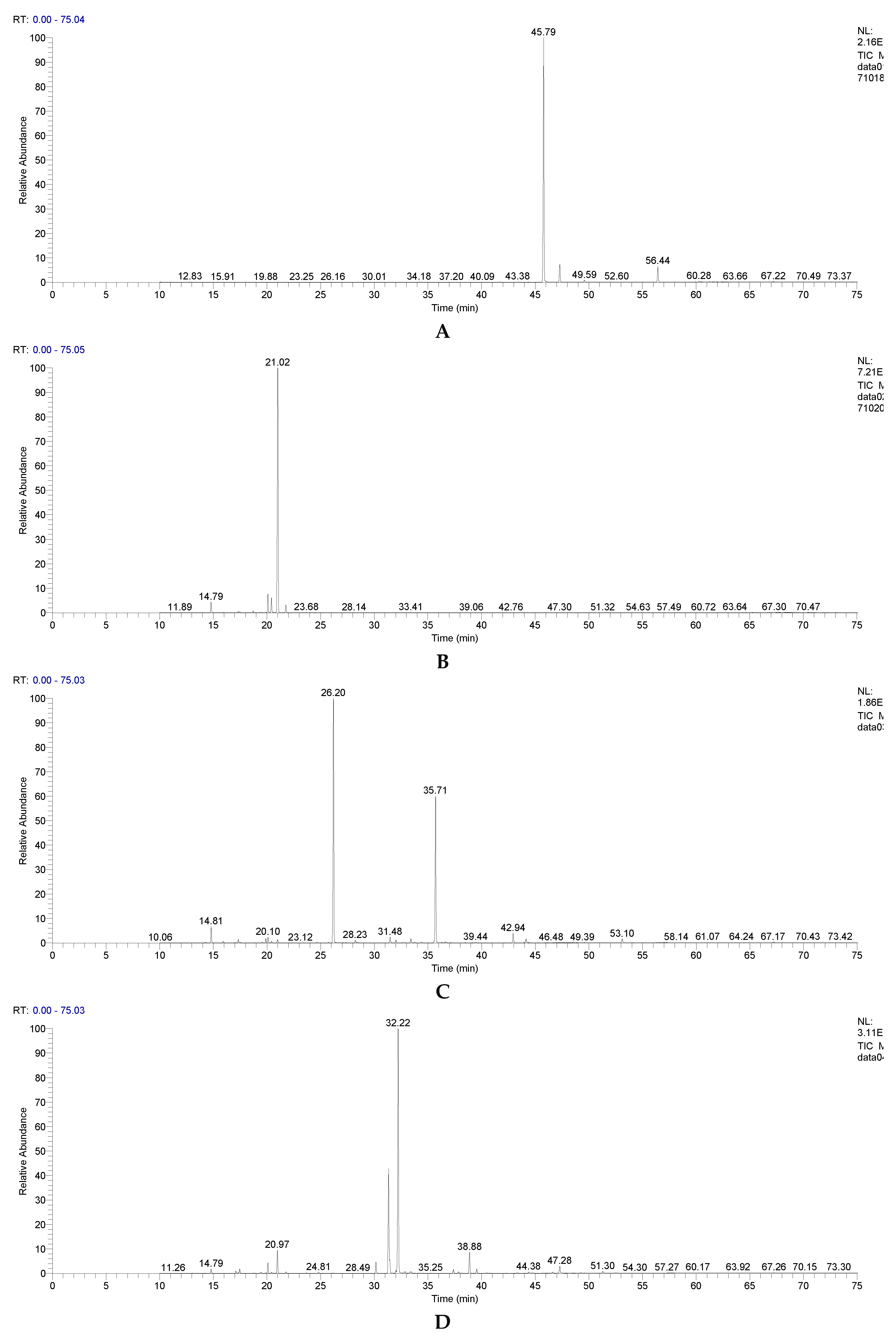
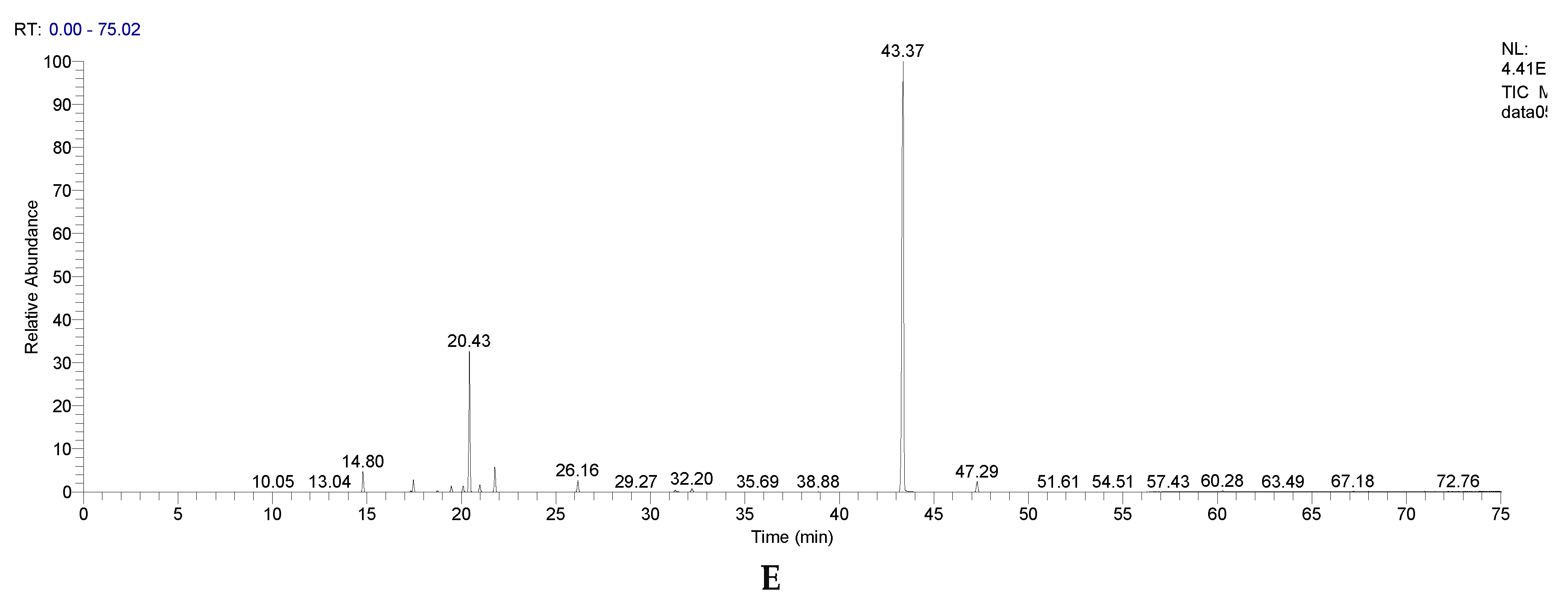
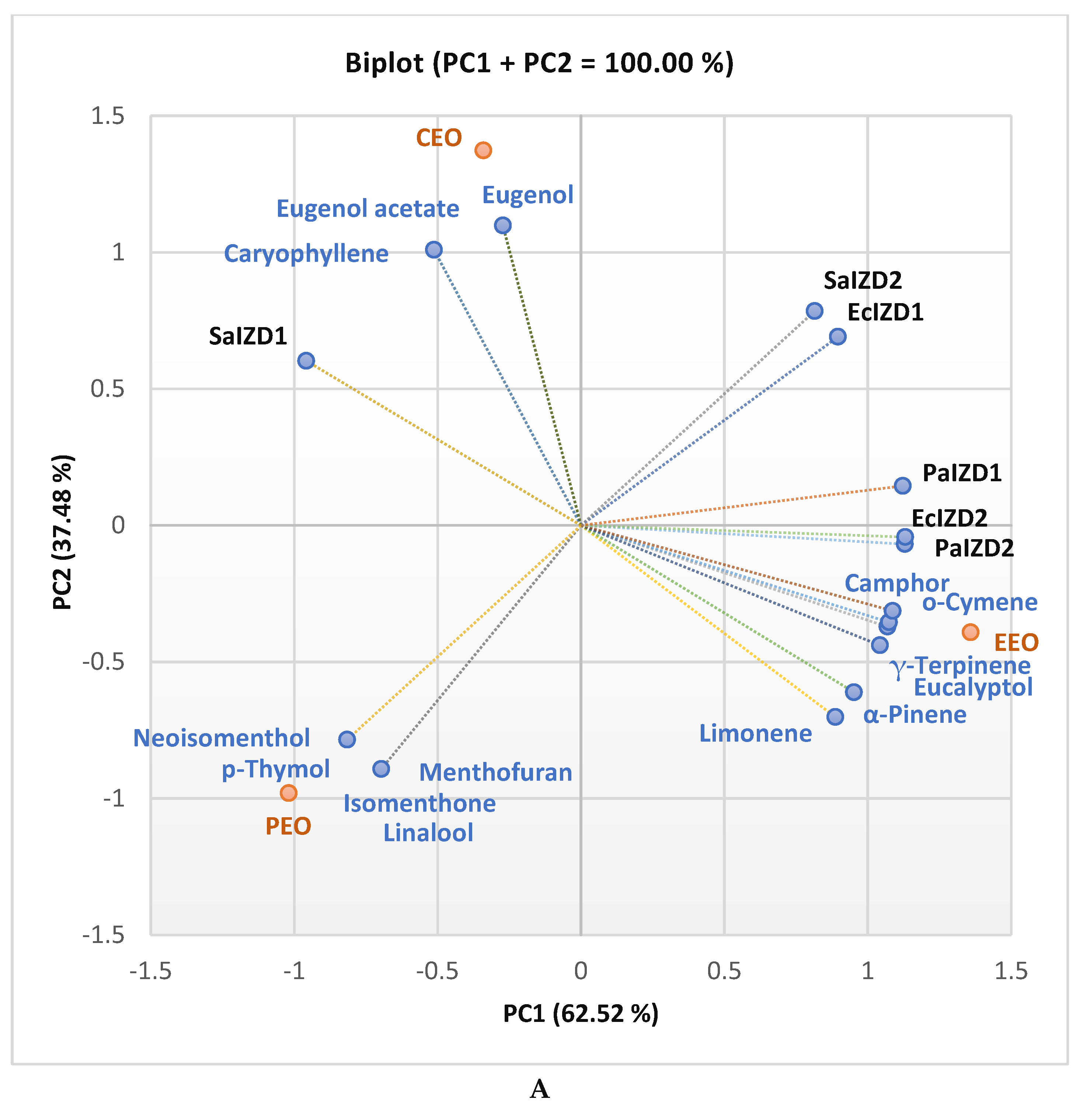
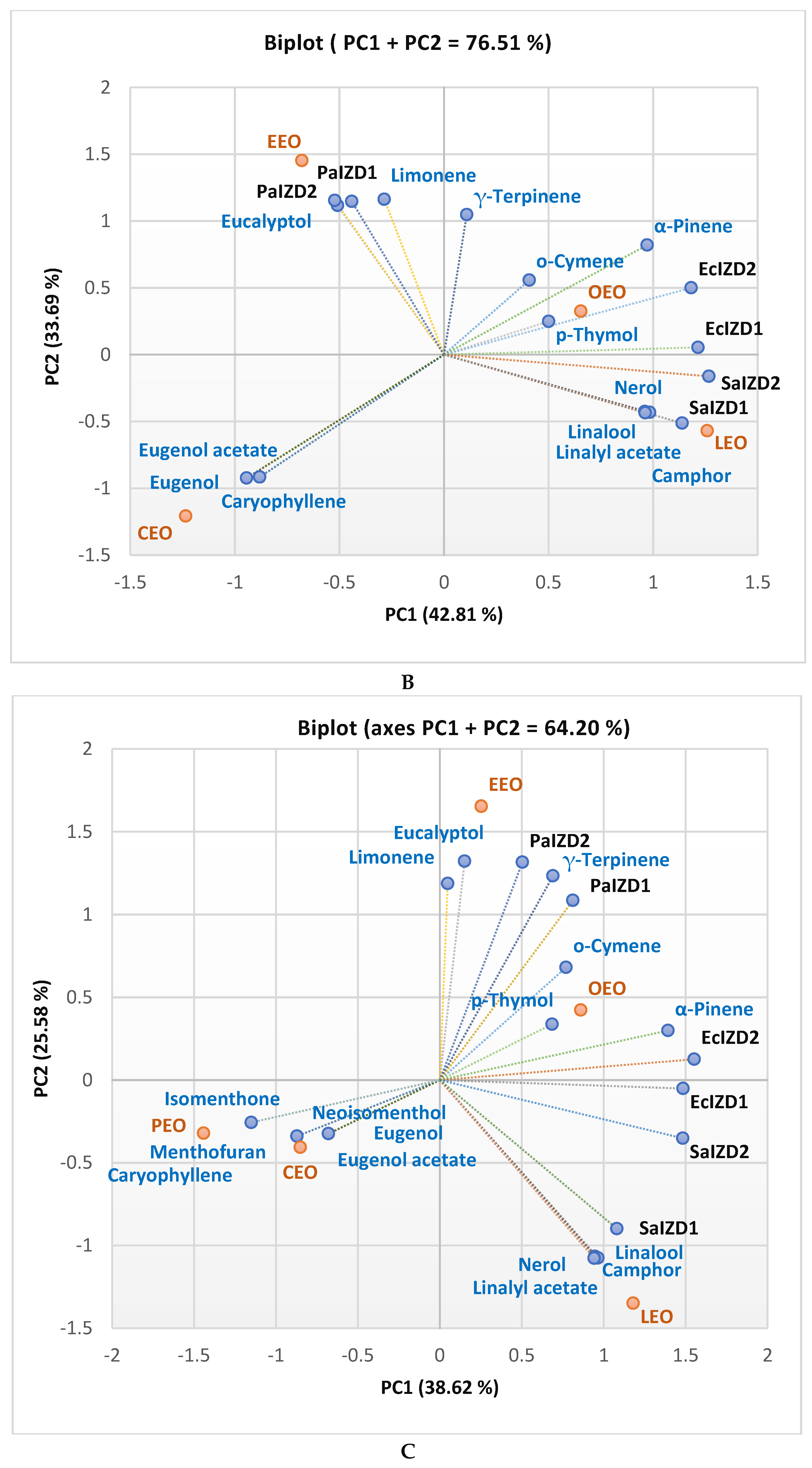
| RT [min] | Compound name | CEO | EEO | LEO | PEO | OEO |
|---|---|---|---|---|---|---|
| 45.79 | Eugenol | 86.2272 | - | - | - | - |
| 47.29 | Caryophyllene | 6.8733 | - | - | 1.6170 | 1.4581 |
| 56.44 | Eugenol acetate | 5.7515 | - | - | - | - |
| 21.00 | Eucalyptol | - | 83.7478 | 0.7360 | 5.0452 | 0.8904 |
| 21.77 | γ-Terpinene | - | 2.2315 | 0.0000 | 0.2864 | 2.9827 |
| 31.34 | Isomenthone | - | - | - | 26.5289 | - |
| 26.16 | Linalool | - | 0.0102 | 52.9348 | 0.0670 | 1.4072 |
| 31.48 | Camphor | - | 0.0138 | 1.3463 | - | - |
| 32.23 | Neoisomenthol | - | - | - | 55.0951 | - |
| 43.29 | p-Thymol | - | - | - | 0.0896 | 72.0862 |
| 35.69 | Linalyl acetate | - | - | 32.3146 | - | 0.0152 |
| 42.95 | Nerol | - | - | 2.1132 | - | - |
| 20.10 | Limonene | - | 5.4506 | 1.1219 | 2.0857 | 0.6877 |
| 20.42 | o-Cymene | - | 4.1323 | 0.2470 | 0.1803 | 16.2692 |
| 14.79 | α-Pinene | - | 2.8165 | 3.1612 | 0.8279 | 2.3971 |
| 17.46 | β-Pinene | - | 0.2631 | 0.0478 | 0.8802 | 1.3942 |
| 30.16 | Menthofuran | - | - | - | 2.4656 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





