1. Introduction
Coffee is cultivated in 14 areas of Mexico, generating 1 million 25 thousand tons of cherry coffee: four areas—Chiapas, Veracruz, Puebla and Oaxaca—account for 90% of the country’s coffee production. The value of coffee production in 2022 reached 6,535 million pesos. In 2021, Mexico ranked 12th in coffee production globally, while exports amounted to 428 million dollars, the main commercial destination being the United States of America. In Veracruz, coffee crops cover 139 thousand hectares, with high-quality varieties of Coffea arabica, such as typica, bourbon, mundo novo, caturra and garnica, being the most cultivated. The countries that stand out in terms of the production of Arabica coffee are Brazil, Colombia, Ethiopia and Honduras, which together contribute 71.9% of the global arabica coffee volume; however, for Robusta coffee, Vietnam, Brazil and Indonesia account for 77.6% of the global production. The beneficiaries of the world coffee trade today are large corporations despite the cultivation and production of coffee being carried out by small producers [
1]. Data from Fairtrade International [
2] indicate that approximately 125 million people globally depend on coffee cultivation for their livelihood and that for most of those people, coffee cultivation does not provide a reliable livelihood.
Coffee plantations are cultivated at elevations ranging from 300 to almost 2,000 meters above sea level and in areas with diverse climates, soils, and vegetation; however, coffee plantations develop better between 600 and 1,200 meters above sea level and are found mainly in hilly and mountainous areas on the slopes facing the Gulf of Mexico and those facing the Pacific. The coffee growing areas coincide with very rich and diverse regions; coffee cultivation areas play a very important role in the dynamics of the basins and in the conservation of soils since they help prevent the loss of soil on the slopes [
3]. The structure of shaded coffee plantations is like that of natural ecosystems; thus, such plantations function as refuges for innumerable species of plants and animals and as sources for germplasms of saprobic and mycorrhizal fungi [
4,
5,
6,
7,
8,
9,
10].
Despite the coffee production areas in the state of Veracruz being extensive, they face various problems; for example, there are issues related to the soil and its composition, as the soil is acidic and deficient in nutrients. To address these issues, producers resort to the application of fertilizers; however, the excessive use of chemical fertilizers creates other problems, such as eutrophication, soil degradation and subsequently the loss of soil biodiversity [
11]. In the soil, interactions occur between microorganisms, including mycorrhizae, which are symbiotic associations established between fungi and plant roots [
12].
Arbuscular mycorrhizal fungi (AMF) have been shown to be a sustainable alternative for optimizing resources since they offer multiple benefits, such as a high tolerance to abiotic stressors [
13], the ability to contribute to bioremediation following contamination by heavy metals [
14], and bioprotection [
15], as well as the ability to improve soil structure and aggregation properties [
16]. Furthermore, AMF provide plants with nutrition [
17]. Sustainability has become a requirement of coffee production among the largest consumer groups, such as Europeans, and is necessary for marketing. To fulfil this requirement, alternative systems have been developed; these systems are referred to as sustainable, organic, and special systems, because they aim to conserve biodiversity.
Among the approaches for producing, marketing and consuming coffee, organic coffee is considered a holistic production management system that promotes and improves the health of agroecosystems, and particularly the health, biological cycles, and activity of soil. These achievements are reached through practices that avoid the use of chemical products as well as genetically modified organisms, sewage, sweeteners, and synthetic preservatives [
18]. The application of AMF as bioinoculants to crops can minimize the use of chemical fertilizers in agricultural practices and guarantee agricultural sustainability by improving symbiotic associations with plants. However, given their biotrophic nature, these bacteria cannot be propagated in artificial culture media in the laboratory. This has complicated the development of large-scale production methods, limiting their commercial exploitation. Hence, the use of host trap plants with favorable sporulation and mycorrhizal colonization qualities is highly relevant for the propagation of native AMF [
19]. Another important factor is the use of native or local AMF strains, which provide better results without the introduction of allochthonous organisms that compete with or lead to an imbalance of native organisms and are local resources typical of the coffee growing areas.
2. Materials and Methods
Study sites. The study sites were established in three locations in the center of the state of Veracruz, Mexico. Five shade-grown coffee farms that used conventional or traditional management of
Coffea arabica var. costa rica were selected (
Figure 1). The characteristics of each of the sites are shown in
Table 1.
Sampling. Soil sampling was carried out on five coffee farms. On each farm, five sampling points were established, each separated by a distance of 50 m to ensure that they were independent. At each point, a coffee plant was considered the center, from which two axes measuring 1 m were defined—one north‒south and another east‒west. At the end of each axis, a 250 g soil sample was taken at a depth of 0-15 cm. The soil was dried at room temperature, after which physicochemical analysis was performed.
Propagation test in Sorghum (Sorghum vulgare) trap plants. Soil from the coffee farms was used for the AMF propagation test in the trap plants, and five pots were prepared with soil from each of the farms and sterile sand at a 50:50 ratio. S. vulgare was used as the trap plant, and individuals were planted in 5 kg pots that were kept in a greenhouse for 120 days. Irrigation was carried out every third day with Hewitt nutrient solution without phosphorus. At the end of the experiment, the irrigation was suspended, the aerial biomass of the plants was measured, the spore morphotypes were isolated and counted, and the percentage of mycorrhization was quantified.
Isolation and counting of spore morphotypes of arbuscular mycorrhizal fungi. The AMF spores were separated by wet sieving and decantation [
20]. A total of 50 g of soil was placed in a flask with 250 mL of water, and the sample was vigorously shaken for 10 minutes. Subsequently, the flask was left to rest for 10-15 minutes. The supernatant was passed through a series of Tyler sieves with 750, 250, 150 and 50 µm apertures. The supernatant from the last sieve was placed in 50 mL Falcon tubes, which were subsequently centrifuged at 2000 rpm for 5 minutes (Thermo Ice Centra CL2 Centrifuge). Once the samples were centrifuged, they were decanted, and a 70% sucrose solution was added. After vigorously shaking the sample, it was centrifuged again at 2500 rpm for 1 minute. The supernatant was passed through a 50 µm sieve, washed under running water and placed in plastic bottles. The samples were subsequently placed in Petri dishes and observed under a stereoscopic microscope (Carl Zeiss). The spores were grouped according to their morphological characteristics, such as size, color, and presence of supporting hyphae. The abundance of the spores was quantified, and the spores were mounted in permanent preparations in polyvinyl alcohol with or without Melzer solution for observation under a compound microscope (Carl Zeiss).
Thinning and staining of the roots of trap plants (
S. vulgare). The roots were washed under running water, cut into small pieces, and placed in test tubes. The roots were stained following the thinning and staining technique [
21], in which 10% KOH was added until the roots were completely covered, after which they were left to rest for 30 seconds and then placed in a water bath for 15 minutes. The roots were then washed with running water to remove the KOH. Next, 10% HCL was added, and the mixture was left for 3 minutes. The mixture was washed again with running water until the remaining HCL was removed. Then, 0.05% trypan blue was added, and the roots were placed in a water bath for 10 min. Finally, the stained roots were placed in lactic acid for observation.
Measurement of the percentage of mycorrhization. To determine the percentage of root colonization, the technique described by Giovannetti and Mosse [
22] was used. The stained roots were placed in a square Petri dish (1x1 cm) filled with lactic acid, and the fungal structures in the roots were observed under a compound microscope. The quantification of the percentage of mycorrhization was carried out according to the methods described by McGonigle et al. [
23]. A simple percentage count of the stained roots was carried out; each time a longitudinal portion of a root was observed with hyphae, vesicles or arbuscules that also touched the axis of the reticule that crossed the root, the result was considered positive; otherwise, the result was considered negative. The counts are expressed as percentages and were calculated as the difference between the number of positive intersections and the total number of intersections (set at 100).
Physicochemical analysis of soils. Physicochemical analyses of the soil from the coffee farms were carried out in accordance with NOM 021-RECNAT-2000 [
24]. The organic matter (OM) and the organic carbon (CO) were quantified by the modified Walkley-Black method [
25], the pH was measured by the electrometric method, the cation exchange capacity (CEC) was determined with 1 N ammonium acetate (pH 7.0), the total nitrogen was determined by micro-Kjeldahl [
26], the available phosphorus (P) was measured by the Bray Kurtz1 method [
27], and the retained phosphorus was quantified by the Blakemore method [
28]. The analyses were carried out in the Soil, Plant and Water Analysis Laboratory of the Institute of Ecology, AC (
Table 2).
Statistical analysis. Spore abundance was expressed as the total number of spores found in 100 g of soil. Species-abundance distributions were plotted (Whittaker plots) to elucidate AMF dominance patterns in each one of samples. Distributions were obtained by plotting the abundance of AMF (from most abundant to least abundant species). To identify differences in spore abundance between the initial count in the coffee-growing soil and the final count after propagation in the sorghum trap plants, we conducted one-way analysis of variance was performed after determining whether the data met the assumption of a normal distribution and homogeneity of variance using the Kolmogorov‒Smirnov test and Bartlett test, respectively).
Percentage of root colonization were compared between samples using one-way ANOVAs (with 5 replicates). When the effects of factors were significant in the ANOVAs, a Tukey’s HSD post hoc test was run to test for pair-wise mean differences at P = 0.05. These analyses were conducted using Statistica 12.0 software. To estimate the relationships between the physicochemical variables of the soil and abundance of spores, Pearson’s correlation analysis was performed with a significance level of p ≤0.05.
3. Results
Abundance of arbuscular mycorrhizal fungal spores
The abundance of spores increased significantly (p <0.05) between the initial count in the coffee-growing soil and the final count after propagation in the sorghum trap plants. This was observed for all the sites analyzed. On the San Isidro farm, the number of spores increased from 136 to 918; on the Los bambus farm, the number of spores increased from 89 to 867; on the La Barranca farm, the number of spores increased from 130 to 801; on the Tuzamapan farm, the number of spores increased from 195 to 990; and on the San Marcos farm, the number of spores increased to 809. The greatest increase was detected on the Los bambus farm (9.7 times), and the smallest increase was detected on the San Marcos farm (3.87 times) (
Table 3).
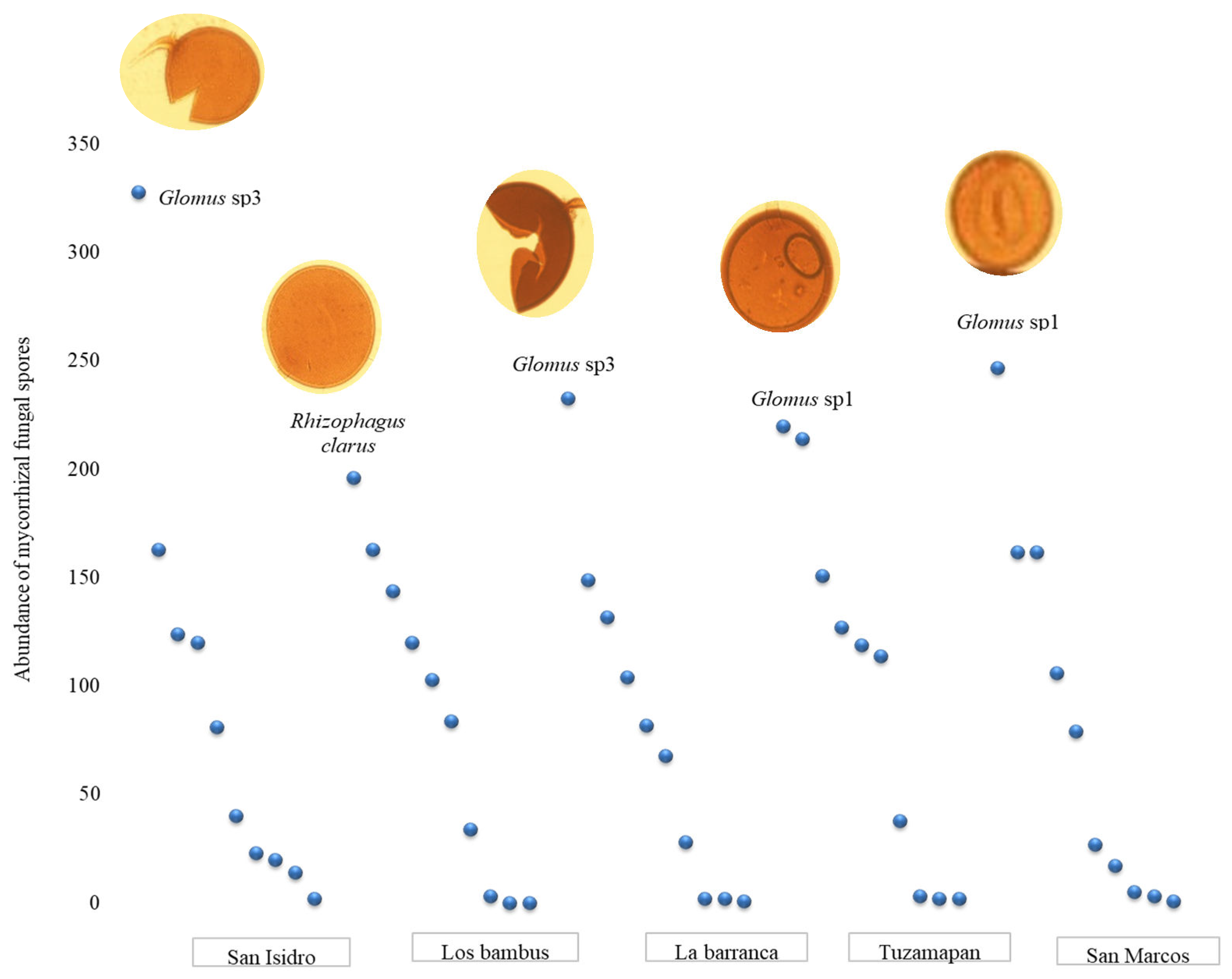
The results demonstrate the presence of morphotypes of the Glomus and Acaulospora genera in all the coffee plantations and a large increase in these genera through propagation in trap plants of S. vulgare. It is important to highlight that the abundances of the morphotypes belonging to the genus Glomus (Glomus sp3 and Glomus sp1) increased more than the abundances of the morphotypes of the genus Acaulospora. Within the genus Acaulospora, the number of A. scrobiculata spores increased on the trap plants. The genus Gigaspora was represented by a single morphotype (Gigaspora sp1), and the number of spores from this morphotype did not increase in the S. vulgare trap plants. This result could be due to the low number of spores detected at the beginning of the experiment or poor compatibility with the trap plant.
Dominance of arbuscular mycorrhizal fungal morphotypes in trap plants
The dominance of the morphospecies is illustrated by Whittaker plots (
Figure 2). A In total, 10 spore morphotypes were observed in the soil from the coffee plantations in both the initial and final samples. During the initial sampling, the
Glomus sp3 morphotype was dominant on the San Isidro farm, the Los bambus farm and the La barranca farm. The
Glomus sp1 morphotype was dominant on the Tuzamapan and San Marcos farms; however, after propagation in the trap plants,
Glomus sp3 and
Glomus sp1 were dominant. At the Los bambus farm,
Rhizophagus clarus was the dominant morphotype.
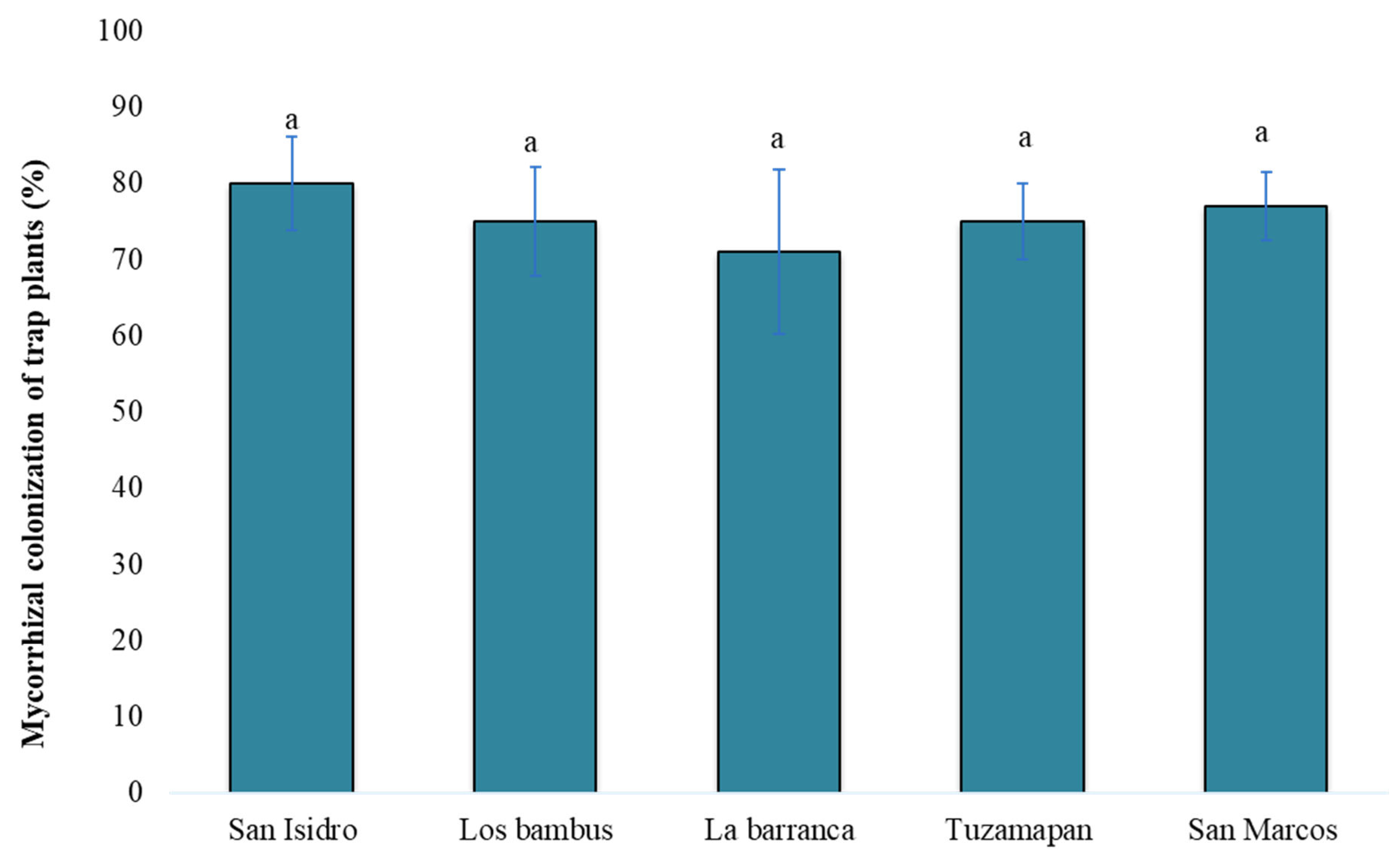
Mycorrhizal colonization of trap plants (S. vulgare)
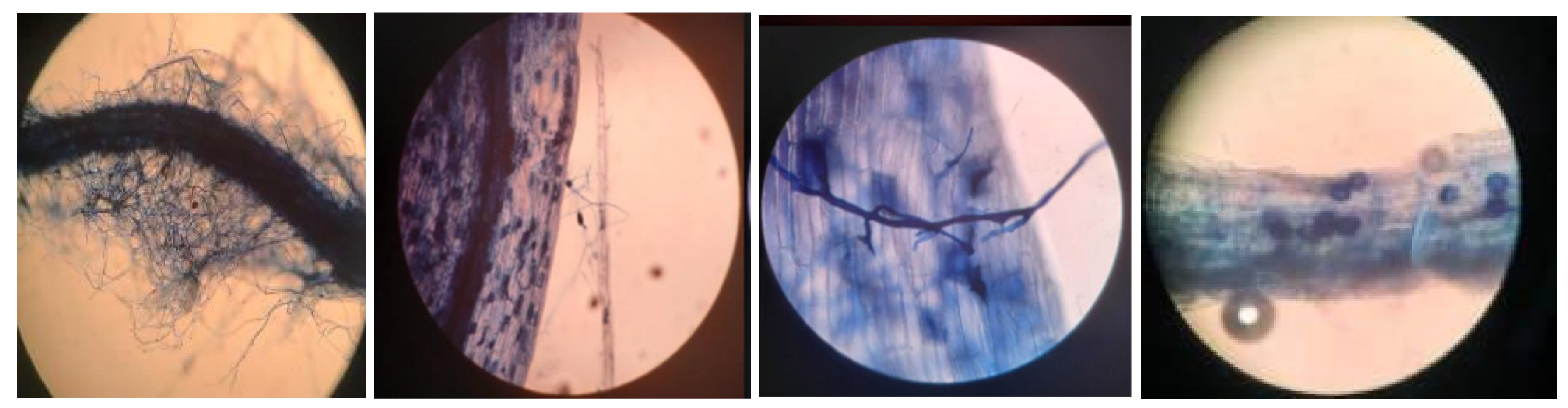
Characteristic structures of AMF (
Figure 3), such as hyphae, vesicles, arbuscules and spores, were observed within the roots of the trap plants (
S. vulgare) 120 days after sowing. The percentages of mycorrhizal colonization did not significantly differ among the trap plants inoculated with soil from different coffee farms (p> 0.05). The mycorrhization percentages ranged from 71-80% (
Figure 4). However, the differences between the values were not significant, and the highest colonization rate was observed on the San Isidro farm (80%). Kormanic and McGraw [
29] defined 5 degrees of mycorrhizal colonization: null (0%), low (1-25%), moderate (26-50%), high (51-75%) and very high (76-100%). According to these categories, the extent of mycorrhizal colonization was classified as very high.
4. Discussion
The
Glomus sp1 and
Glomus sp3 morphotypes were dominant in the coffee plantations analyzed in this study. Arias et al. [
6] indicated that the genera
Glomus and
Acaulospora were dominant in coffee plantations in Veracruz under different management practices; in particular,
Glomus sp. and
G. clarum were the dominant morphospecies at the regional level. This work highlights the greater abundance of the
Gigaspora morphotype on San Isidro Farm. Compost is applied exclusively on this farm, resulting in a higher organic matter content; thus, it is important to highlight that various agroecological practices, such as the use of compost, can favor the presence and abundance of spores. Based on a study of coffee plantations in Chiapas, Bertolini et al. [
30] report that there may be specific AMF consortia associated with different levels of P and soil acidity. In addition, they noted that various species of
Acaulospora and
Glomus could be common in the environmental conditions in which coffee is grown. They even suggested that functional compatibility studies be carried out before the application of biofertilizers.
The physicochemical characteristics of the soil determine the distribution of these microorganisms, and additional studies of different types of vegetation can help improve the understanding of the ecological preferences of AMF species. Species belonging to the genus
Glomus adapt to almost any edaphoclimatic condition; likewise,
Acaulospora morrowiae and
Acaulospora scrobiculata have been reported to exist at a wide range of pH values (between 3.8 and 8.0) and to adapt to various levels of fertility [
31].
In the present study, the soil pH (4.09) and clay content (29.8) at the San Isidro farm favored high abundances of
Acaulospora sp1,
Glomus sp2 and
Gigaspora morphospecies. Peña-Venegas et al. [
32] argue that the pH affects the number of spores in the soil because a slight increase in pH changes the level of aluminum saturation, causing a decrease in this parameter; therefore, in clays, for example, an increase in pH improves the cation exchange capacity of the soil, favoring higher population densities of microorganisms.
The genera
Scutellospora and
Entrophospora are highly diverse in the tropics [
31]; however, in altered agroecosystems, the genera
Scutellospora and
Gigaspora are less common [
33]. Arias et al. [
6] concluded that various factors related to management practices, such as weed control, soil conditions and chemical fertilization, interfere with the abundance and composition of AMF in coffee plantations.
In coffee-growing soils in the study area, the use of
S. vulgare plants is recommended for promoting the spread of AMF spores (801-918 in 50 g of soil) and supporting high rates of mycorrhization (71-80%). This high colonization rate could be due to the high level of compatibility with the trap plant used. In a study by Chaiyasen et al. [
34] in soil from coffee plantations in Vietnam
, Zea mays was selected among different herbaceous plants (
Zea mays, Plantago spp.,
Oryza sativa,
Bidens pilosa and
Pensacola bahia) due to its ability to support a larger increase in AMF spores (3690 spores/100 cm3 and 65% mycorrhizal colonization). The International Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM [
35], sorghum plants are routinely used in germplasm banks, and these plants are considered excellent hosts for a wide range of AMF under greenhouse conditions; however, these plants may not behave as optimally in all geographic regions because each host plant has particularities or characteristics that can affect or limit the spread of AMF spores in a trap crop [
36]. Thus, it is important to choose the species with the best performance when carrying out this type of work.
There are different methods for propagating AMF, such as monosporic culturing [
37,
38], solid substrate culturing [
39,
40], aeroponic culturing [
41] and hydroponic culturing [
42]. However, storing propagated AMF spores requires technical skills and specialized knowledge, making this task difficult for farmers. One of the common practices for the propagation of spores is the trap culture method. In the production of inoculum at a commercial level, host plants such as
Allium cepa, Cenchrus ciliaris, Panicum maxim, Paspalum notatum, Sorghum halepense, Trifolium subterraneum and
Zea mays, which require 3-4 months for development, are used [
43,
44]; however, it is recommended that evaluations be carried out with other plants that could be compatible. Substrates and host plants influence the propagation of AMF species or AMF consortia, so it is extremely important that these factors be considered for successful propagation. Likewise, the dominant species should be considered since the effectiveness of propagation varies depending on the plant-AMF interaction.
The application of AMF requires careful selection of viable species that are effective for propagation since one of the problems in the use of commercial mycorrhizae is that they are delivered in inoculum in low concentrations, and their composition is generally not revealed by the manufacturer, reducing their effectiveness in the field [
45]. Thus, it is necessary to expand what is known about the multiplication and characterization of viable AMF spores to produce AMF inoculum within a specific host plant to improve plant growth and guarantee sustainable agricultural practices [
46]. It is important to note that both a host plant and a consortium of host plants can benefit the propagation of AMF spores, as demonstrated by Yao et al. [
47], who found that cocultures of certain host plant species were more effective than monocultures for propagating AMF spores. Recently, a coculture of corn and sorghum was shown to have a greater capacity as a host consortium of trap crops than individual trap plants [
48]. Trejo and Bañuelos [
49] recommend the rotation of host plants after every four cycles of trap cultivation to maintain the original diversity of AMF. The production of native AMF inoculum provides several benefits, thus enabling the production of viable and low-cost inoculum; additionally, the use of native AMF inoculum is environmentally friendly and can facilitate the characterization of the propagated AMF inoculum.
5. Conclusions
The propagation of AMF spores from soils in coffee-growing areas using S. vulgare as a trap plant promoted efficient spore growth and high rates of mycorrhizal colonization. This host plant was able to maintain the AMF community present in the soils. However, it is suggested that experiments with plant consortia be performed to increase the efficiency of AMF propagation. In general, the Glomus sp1 and Glomus sp3 morphotypes are considered the dominant species of coffee plantations and could be used in the formulation of biofertilizers. This technique of propagating native AMF of coffee plantations by using trap plants offers an alternative to sustainable coffee production and the possibility of obtaining an additional coffee product for consumption or sale.
Author Contributions
Conceptualización, *Rosa María Arias; Adquisición de financiación, Laura Ruelas Monjardín; Metodología, *Rosa María Arias, Yadeneyro de la Cruz Elizondo, Laura Ruelas Monjardín y Yamel Perea-Rojas; Administración de proyecto, Laura Ruelas Monjardín; Supervisión, *Rosa María Arias y Yadeneyro de la Cruz Elizondo; Validación, *Rosa María Arias y Yadeneyro de la Cruz Elizondo; Escritura – borrador original, *Rosa María Arias, Yadeneyro de la Cruz Elizondo y Yamel Perea-Rojas; Escritura – revisión y edición, *Rosa María Arias y Yamel Perea-Rojas.
Funding
Please add: “This research was funded COVEICYDET for financing the CLAVE CP1111 2112/2023 project: “Boosting sustainable coffee growing through technological innovations for the use of water and soil in Jilotepec, Veracruz”.
Institutional Review Board Statement
“Not applicable
Informed Consent Statement
Not applicable.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors thank Elizabeth Abigail López Martínez for her help in processing the samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SIAP (Servicio de Información agroalimentaria y pesquera), Panorama Agroalimentario. Available online: https://drive.google.com/file/d/1FWHntHMgjw_uOse_MsOF9jZQDAm_FOD9/view (accessed on 10 december 2023).
- Fairetrade international. Available online: https://www.fairtrade.net/ (accessed on 1 april 2023).
- Moguel, P.; Toledo, V.M. Biodiversity conservation in traditional coffee systems of Mexico. Biol. Conserv. 1999, 13, 11–21. [Google Scholar] [CrossRef]
- Heredia, G.; Arias, R.A. Hongos saprobios y endomicorrizógenos en suelos cafetaleros. In Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación, Manson, R.H.; Hernández-Ortiz, V.; Gallina, S.; Mehltreter, K.,Eds.; Instituto de Ecología A.C. (INECOL) e Instituto Nacional de Ecología (INE-SEMARNAT), México, 2008, pp 193-212.
- Trejo, D.; Ferrera-Cerrato, R.; García, R.; Varela, L.; Lara, L.; Alarcón, A. Efectividad de siete consorcios nativos de hongos micorrízicos arbusculares en plantas de café en condiciones de invernadero y campo. Rev. Chil. de Hist. Nat. 2011, 84, 23–31. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia, G.; Sosa, V.; Fuentes-Ramírez, L.E. Diversity and abundance of arbuscular mycorrhizal fungi spores under different coffee production systems and in a tropical montane cloud forest patch in Veracruz, Mexico. Agroforest Syst. 2012, 85, 179–193. [Google Scholar] [CrossRef]
- Pérez-Luna, Y.; Alvarez-Solís, D.; Hernández-Cuevas, L.; Sánchez-Roque, Y. Acaulospora excavata (Glomeromycota) in agricultural soils of Chiapas, México. Int. j. adv. agric. res. 2016, 4, 6–9. [Google Scholar]
- Posada, R.H.; Sánchez, M.; Heredia-Abarca, G.; Sieverding, E. Effects of soil physical and chemical parameters, and farm management practices on arbuscular mycorrhizal fungi communities and diversities in coffee plantations in Colombia and Mexico. Agrofor. Syst. 2018, 92, 555–574. [Google Scholar] [CrossRef]
- Herrera-Monroy, S.; Castro-Brindis, R.; Pérez-Moreno, J.; Valdés-Velarde, E. Endomycorrhizal diversity in coffee plants (Coffea arabica L.) infected with rust (Hemileia vastatrix). Nova Scientia 2019, 11, 102–123. [Google Scholar] [CrossRef]
- Bertolini, V.; Montaño, N.M.; Salazar, B.L.; Chimal, E.; Varela, L. Diversidad de hongos micorrizógenos arbusculares en plantaciones de café (Coffea arabica) del volcán Tacaná, Chiapas, México. Act. Bot. Mex. 2020, 127, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. La contaminación del suelo: una realidad oculta; FAO Italia, Roma, 2019,pp. 144.
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; et al. Una clasificación filogenética a nivel de filo de hongos zigomicetos basada en datos a escala genómica. Micología 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Pérez-Moncada, U.A.; Gómez, M.R.; Serralde-Ordoñez, D.P.; Peñaranda-Rolón, A.M.; Wilches-Ortiz, W.A.; Ramírez, L.; Rengifo-Estrada, G.A. Hongos formadores de micorrizas arbusculares (HFMA) como estrategia para reducir la absorción de cadmio en plantas de cacao (Theobroma cacao). Terra Latinoamericana 2019, 37, 121–130. [Google Scholar] [CrossRef]
- Mohamed, I.; Eid, K.E.; Abbas, M.H.H.; Salem, A.A.; Ahmed, N.; Ali, M.; Shah, G.M.; Fang, C. Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Saf. 2019, 171, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.V.; Pedroso, D.D.F.; Curi, N.; Carneiro, M.A.C. Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates? Cienc. e Agrotecnologia 2019, 43, e003519. [Google Scholar] [CrossRef]
- Muñoz, M.G. Análisis de expresión de genes de respuesta al estrés hídrico en plantas de Sorghum bicolor (L) Moench en presencia y ausencia de asociaciones con hongos micorrízicos, PhD dissertation, Universidad Autónoma de Aguascalientes, Aguascalientes, México, 2018.
- Gómez-Cruz, M.A.; Schwentesius, R.R.; Meraz, A.M. del R.; Lobato, G.A.; Gómez, T. Agricultura, apicultura y ganadería orgánicas de México-2005-Situación–retos–tendencias, 1st ed.; PIAI- CIESTAAM: Texcoco, México, pp. 1–65.
- Oehl, F.; Sieverding, E.; Ineichen, K.; Maeder, P.; Wiemken, A.; Boller, T. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 2009; 134, 257–268. [Google Scholar] [CrossRef]
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. mycol. soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 489–500. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- NORMA Oficial Mexicana NOM-021-SSA1-2021, Salud ambiental. Criterio para evaluar la calidad del aire ambiente, con respecto al monóxido de carbono (CO). Valores normados para la concentración de monóxido de carbono (CO) en el aire ambiente, como medida de protección a la salud de la población. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5634084&fecha=29/10/2021#gsc.tab=0 (accessed on 26 april 2023).
- Bahadori, M.; Tofighi, H. A modified Walkley-Black method based on spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 2016, 47, 213–220. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Bray, R.; Kurtz, L. Determination of total, organic and available forms of phosphorus in soil. Soil Sci. 1945; 59, 39–45. [Google Scholar] [CrossRef]
- Metson, A.J.; Blakemore, L.C.; Rhoades, D.A. Methods for the Determination of Soil Organic Carbon: A Review, and Application to New Zealand Soils. N. Z. J. Sci. 1979, 22, 205–228. [Google Scholar]
- Kormanik, P.P.; McGraw, A.C. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In Methods and Principles of Mycorrhizal Research, American Phytopathological Society, Schenck, N., Ed.; St Paul 1982, pp. 37–45.
- Bertolini, V.; Montaño, N.M.; Chimal, E.; Varela, L.; Gómez, J.; Martínez, J.M. Abundancia y riqueza de hongos micorrizógenos arbusculares en cafetales de Soconusco, Chiapas, México. Rev. biol. Trop. 2018, 66, 91–105. [Google Scholar] [CrossRef]
- Sieverding, E.; Friedrichsen, J.; Suden, W. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; Sonderpublikation der GTZ: Eschborn, Germany, 1991. [Google Scholar]
- Peña-Venegas, C.P.; Cardona, G.I.; Arguelles, J.H.; Arcos, A.L. Micorrizas arbusculares del sur de la amazonia colombiana y su relación con algunos factores fisicoquímicos y biológicos del suelo. Acta Amaz. 2007, 37, 327–336. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Colozzi-Filho, A.; de Oliveira, E. Ocorrência de micorrizas vesicular-arbusculares em agro e ecossistemas do Estado de Minas Gerais. Pesqui. Agropecu. Bras. 1989, 24, 1499–1506. [Google Scholar]
- Chaiyasen, A.; Douds, D.D.; Gavinlertvatana, P.; Lumyong, S. Diversity of arbuscular mycorrhizal fungi in Tectona grandis Linn. f. plantations and their effects on growth of micropropagated plantlets. New For. 2017, 48, 547–562. [Google Scholar] [CrossRef]
- Invam (International culture collection of vesicular arbuscular mycorrhizal fungi). Available online: http://invam.caf.wvu.edu/ (accessed on 10 december 2023).
- Tenzin, U.W.; Noirungsee, N.; Runsaeng, P.; Noppradit, P.; Klinnawee, L. Dry-season soil and co-cultivated host plants enhanced propagation of arbuscular mycorrhizal fungal spores from sand dune vegetation in trap culture. J. Fungi 2022, 8, 1061. [Google Scholar] [CrossRef]
- Fracchia, S.; Menendez, A.; Godeas, A.; Ocampo, J.A. A method to obtain monosporic cultures of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2001, 33, 1283–1285. [Google Scholar] [CrossRef]
- Selvakumar, G.; Krishnamoorthy, R.; Kim, K.; Sa, T. Propagation technique of arbuscular mycorrhizal fungi isolated from coastal reclamation land. Eur. J. Soil. Biol. 2016, 74, 39–44. [Google Scholar] [CrossRef]
- Millner, P.D.; Kitt, D.G. (1992). The Beltsville method for soilless production of vesicular-arbuscular mycorrhizal fungi. Mycorrhiza 1992, 2, 9–15. [Google Scholar] [CrossRef]
- Douds, Jr.D.D.; Nagahashi, G.; Hepperly, P.R. On-farm production of inoculum of indigenous arbuscular mycorrhizal fungi and assessment of diluents of compost for inoculum production. Bioresour. 2010, 101, 2326–2330. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, A.G.; Kuek, C. Improved aeroponic culture of inocula of arbuscular mycorrhizal fungi. Mycorrhiza 2000, 9, 337–339. [Google Scholar] [CrossRef]
- Tajini, F.; Suriyakup, P.; Vailhe, H.; Jansa, J.; Drevon, J.J. Assess suitability of hydroaeroponic culture to establish tripartite symbiosis between different AMF species, beans, and rhizobia. BMC Plant Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Chellappan, P.; Jeong, Y.J.; Kim, H. Occurrence and quantification of vesicular-arbuscular mycorrhizal (VAM) fungi in industrial polluted soils. J. Microbiol. Biotechnol 2005, 15, 147–154. [Google Scholar]
- Tahat, M.; Kamaruzaman, S.; Radziah, O.; Kadir, J.; Masdek, H. Plant Host Selectivity for Multiplication of Glomus mosseae Spore. Int. J. Bot. 2008, 4, 466–470. [Google Scholar]
- Douds, Jr.D.D.; Nagahashi, G.; Pfeffer, P.E.; Kayser, W.M.; Reider, C. On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Can. J. Plant Sci. 2005, 85, 15–21. [Google Scholar] [CrossRef]
- Mukhongo, R.W.; Tumuhairwe, J.B.; Ebanyat, P.; AbdelGadir, A.H.; Thuita, M.; Masso, C. Production and use of arbuscular mycorrhizal fungi inoculum in sub-Saharan Africa: challenges and ways of improving. Int. J. Soil Sci. 2016; 11, 108–122. [Google Scholar] [CrossRef]
- Yao, Q.; Gao, J.L.; Zhu, H.H.; Long, L.K.; Xing, Q.X.; Chen, J.Z. Evaluation of the potential of trap plants to detect arbuscular mycorrhizal fungi using polymerase chain reaction-denaturing gradient gel electrophoresis analysis. Soil Sci. Plant Nutr 2010, 56, 205–211. [Google Scholar] [CrossRef]
- Vieira, L.C.; Silva, D.K.A.D.; Escobar, I.E.C.; Silva, J.M.D.; Moura, I.A.D.; Oehl, F.; Silva, G.A.D. Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants 2020, 9, 52. [Google Scholar] [CrossRef]
- Trejo-Aguilar, D.; Bañuelos, J. Isolation and Culture of Arbuscular Mycorrhizal Fungi from Field Samples. Methods mol. biol. 2020, 2146, 1–18. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).