Introduction
The placenta represents a specialized intermediary structure facilitating the transfer of oxygen and essential nutrients from the maternal circulation to the developing fetus via the umbilical vein1-2. Its circulatory dynamics assume critical significance in various pathological contexts affecting either the fetus or the pregnant woman. Failure in placental vascular remodeling during the initial trimester precipitates placental insufficiency, significantly amplifying the likelihood of perinatal morbidity and mortality. Manifestations include diminished fetal growth velocity or restriction, premature delivery, fetal neurodevelopmental impairments, hypoxia, and instances of stillbirth. Moreover, such deficiencies also lead to the obstetric complications associated with pre-eclampsia3-7.
Consequently, ultrasound assessment of placental insufficiency has gained significance in prenatal care. Previous studies commonly utilized uterine artery Doppler and umbilical artery Doppler to evaluate placental impedance. Umbilical artery flow serves as an indicator of fetal side placental resistance, while uterine artery flow reflects maternal upstream blood flow resistance8-13.
Doppler ultrasound has emerged as a valuable tool for assessing placental function and perfusion. While traditional Doppler indices, such as umbilical artery (Um A) and uterine artery (Ut A) Doppler, provide valuable information, recent studies have highlighted the potential of a novel index: the placental pulsatility index (PPI). Gudmundsson et al. (year) introduced the PPI, a novel metric designed to concurrently assess the impedance on both the maternal and fetal sides of the placenta. This index offers a comprehensive evaluation compared to traditional Doppler techniques focused solely on umbilical artery (UA) and uterine artery (Ut A) impedance. Gudmundsson et al.‘s findings suggest that the inclusion of PPI enhances the predictive sensitivity for adverse pregnancy outcomes14. In a study by Todumrong et al., they investigated the association between PPI and adverse pregnancy outcomes in high-risk pregnancies during the second trimester. Their results indicate a significant correlation between elevated PPI values and adverse outcomes such as small for gestational age (SGA), fetal growth restriction (FGR), and adverse perinatal events15.
Fetal growth and development in utero are contingent upon two primary determinants: genetic predisposition and vascular perfusion. Among the prevailing causes of intrauterine growth restriction in perinatology is placental insufficiency, characterized by compromised blood flow. Decreased placental perfusion stemming from maternal factors can result in fetal hypoxia and inadequate nutrient delivery. Assessment of placental perfusion and function may be facilitated through evaluation of umbilical vein (UV) blood volume, indicative of oxygenation and nourishment reaching the fetus. Consequently, monitoring blood flow within the umbilical vein assumes pivotal significance in gauging placental-fetal circulatory dynamics1, 16-18.
In recent years, advancements in Doppler ultrasound technology and software have facilitated the measurement of umbilical venous blood flow (UVBF), enabling a more comprehensive assessment during prenatal Doppler ultrasound examinations17, 19-23. Despite these advancements, no prior investigation has explored the correlation between UVBF and the PPI in human fetuses. This study endeavors to elucidate the relationship between UVBF and PPI, aiming to establish a novel reference chart for UVBF based on PPI values. Given that PPI reflects combined placental resistance across maternal and fetal circulations, our research aims to contribute theoretical insights into predicting fetal intrauterine growth and development. Consequently, our findings could inform clinical practice by facilitating the prognostication and intensified monitoring of fetuses at risk of adverse pregnancy outcomes.
Materials and Methods
Our study utilized data obtained from 792 women with singleton pregnancies, spanning gestational ages from 21+1 to 40+6 weeks, collected between January 2022 and December 2022, for cross-sectional analyses. Approval for our research was obtained from the Medical Research Ethics Review Committee of Fujian Medical University affiliated Mindong Hospital Ningde (Issued: 2022083101K). All research procedures adhered to the guidelines established by our institutional medical research ethics committee.
With the exception of pregnancies conceived through assisted reproductive technology (ART), gestational age was determined based on the estimated date of confinement derived from fetal crown-rump length (CRL) measurements obtained during ultrasound examinations conducted between 11 and 14 weeks of gestation. Exclusion criteria encompassed prior occurrences of chromosomal abnormalities, fetal structural anomalies or congenital heart defects (CHD), and maternal complications. Consistent with the guidelines set forth by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) and the American Institute of Ultrasound in Medicine (AIUM), assessments included CRL, fetal biometry, umbilical artery Doppler pulsatility index (Um A PI), umbilical artery resistance index (Um A RI), uterine artery Doppler pulsatility index (Ut A PI), uterine artery resistance index (Ut A RI), time-averaged maximum velocity (TAMXV), and time-averaged intensity-weighted mean velocity (TAV) of the umbilical vein (UV)24-28. Employing the built-in computational capabilities of the ultrasound equipment, most Doppler index data were automatically calculated following waveform tracing, enhancing efficiency and accuracy in data processing.
Fetal examinations were conducted using a Voluson E10 ultrasound platform (General Electric Medical Systems, Milwaukee, WI, USA) equipped with C2-9-D multifrequency transabdominal transducers (XD Clear Wide Band Convex Probe). Adherence to fetal safety protocols was ensured by implementing procedures consistent with the principles of as low as reasonably achievable (ALARA). A single registered physician with over a decade of experience in obstetric ultrasound scanning performed all scans and fetal measurements. Intraobserver repeatability was assessed using reliability statistics to evaluate the consistency of repeated fetal biometry measurements conducted by the same examiner.
The appropriate dimensions of the color box, as well as optimal pulse repetition frequency (PRF) and gain settings devoid of noise and artifacts, were meticulously adjusted to facilitate accurate color flow mapping and Doppler index acquisition. This was undertaken to mitigate the risk of erroneously identifying absent or abnormal Doppler velocities and waveforms. In order to minimize potential inaccuracies, efforts were made to maintain the angle of insonation as close to 0 degrees as practicable, or to ensure stability within a range of less than 20 degrees. Doppler spectra were recorded during periods of fetal rest states. Exposure to Doppler ultrasound was strictly limited to durations not exceeding 5 minutes whenever feasible. All Doppler indices obtained were automatically computed by the ultrasound system’s software.
In conducting umbilical artery (Um A) flow measurements, umbilical artery Doppler waveforms were acquired via transabdominal ultrasound with the umbilical cord in a free loop configuration. Doppler indices assessed included the Systolic-to-Diastolic Ratio (S/D), Pulsatility Index (PI), and Resistance Index (RI) of the umbilical artery. For Uterine Artery (Ut A) flow measurements, uterine artery Doppler waveforms were obtained via transabdominal examination, positioning the probe medially in the parasagittal plane and longitudinally in the lower lateral quadrant of the abdomen. The site of the uterine artery, approximately 1 cm downstream from the crossing of the external iliac artery, was identified using color flow mapping, and the probe position was adjusted for optimal insonation angle based on the orientation of the uterine artery. Doppler indices assessed included the Systolic-to-Diastolic Ratio (S/D), Pulsatility Index (PI), and Resistance Index (RI) of the uterine artery. The equation of PPI [
14]: PPI=(Um A PI + mean of the left and right Ut A PI)/2
14. For the measurement of Umbilical Venous Blood Flow (UV), the site of assessment was located at the same location as the free loop of the umbilical vein. The UVBF (mL/min) calculated according to the formula: UVBF=Cross-section area of UV (mm
2) × mean velocity (mm/s) × 60
29
Statistical analysis was conducted utilizing the Statistical Package for the Social Sciences (SPSS, Ver. 25.0 for Windows; Chicago, IL, USA). Statistical significance was established with a threshold probability value of p<0.05. Descriptive statistics, including mean, standard deviation (SD), and percentiles, were computed for GA, fetal biometry parameters, estimated fetal body weight, as well as pulsatility indices (PIs) of the umbilical artery (Um A), uterine artery (Ut A), and umbilical vein (UV) flow.
Results
In our study, we enrolled a total of 492 pregnant women who met the inclusion criteria. The median maternal age was 30.0 years (range, 18–44 years), with a median gravidity of 2 (range, 1–9) and median parity of 1 (range, 0–4). Gestational age at ultrasound examination had a median of 26+2 weeks (range, 20
+0 – 40
+1 weeks). Descriptive obstetric characteristics and fetal hemodynamic parameters are presented in
Table 1. In our study, the incidence rates of preterm birth (PTB) and low birth weight (LBW) were 8.94% and 6.91%, respectively. Comparing the UVBF/PPI ratio between PTB and non-PTB groups, we found significant differences (146.20±89.11 vs. 187.79±123.70, p=0.034) via Mann-Whitney test, indicating lower ratios in the PTB group. Similarly, comparing UVBF/PPI ratio between LBW and non-LBW groups revealed significant differences (138.98±94.91 vs. 187.11±122.64, p=0.021), suggesting lower ratios in the LBW group. Notably, UVBF/PPI ratio was significantly higher in the group of healthy fetuses. These findings underscore the potential utility of UVBF/PPI ratio as a predictive marker for adverse pregnancy outcomes, particularly PTB and LBW.
The mean UVBF was 151.58±80.69 ml/min. Mean values for middle cerebral artery pulsatility index (PI) and resistance index (RI) were 1.93±0.43 and 0.83±0.08, respectively. Umbilical artery PI and RI were 1.05±0.23 and 0.65±0.09, while mean values for mean uterine artery PI and RI were 0.81±0.26 and 0.51±0.09, respectively. Additionally, the mean cerebroplacental ratio (CPR) and PPI were 1.92±0.58 and 0.93±0.20, respectively. The UVBF/PPI ratio averaged 180.45±1.19.
Table 2 presents the 10th, 50th, and 90th centiles of the UVBF/PPI ratio across different gestational ages.
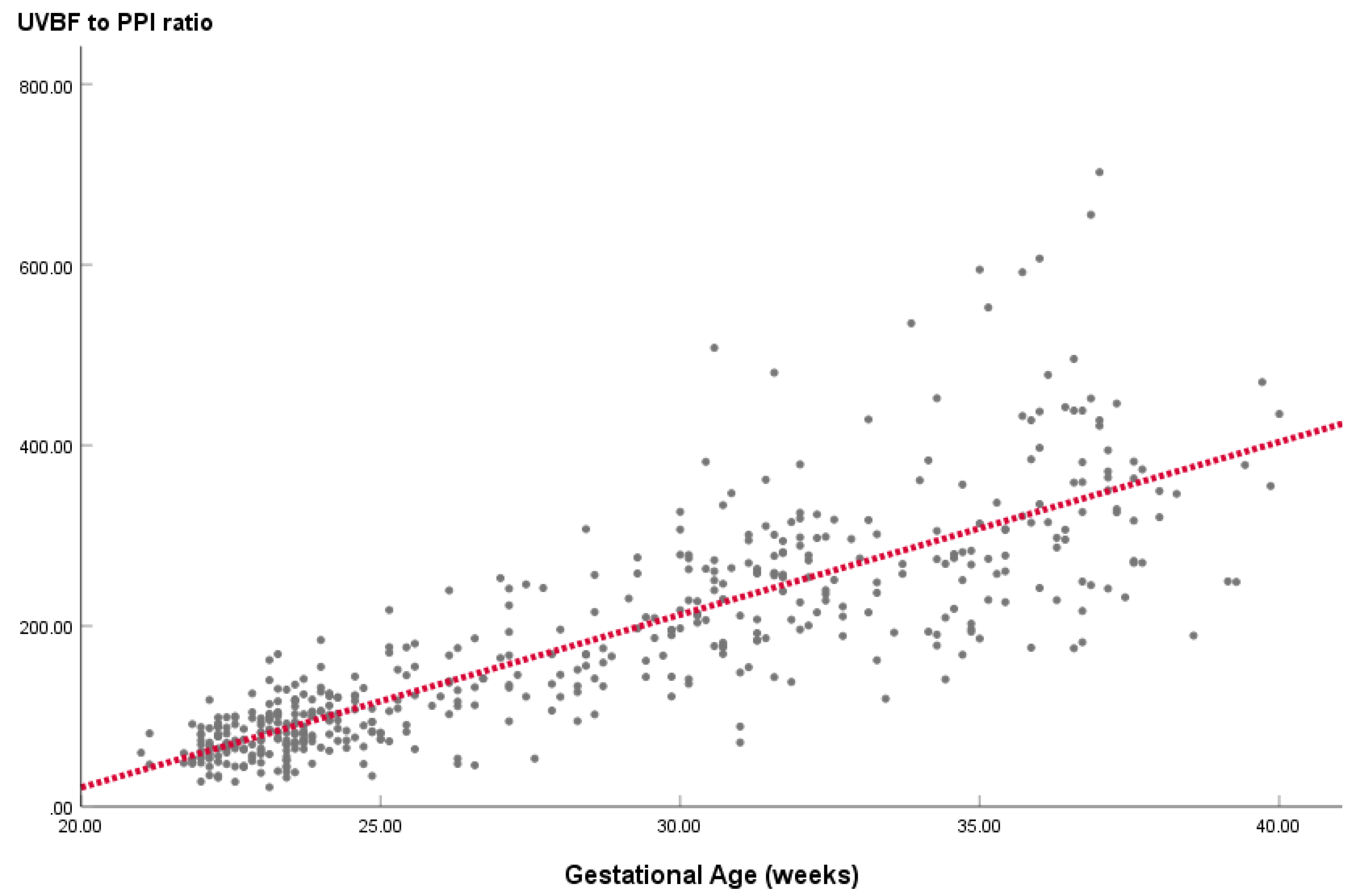
Figure 1 illustrates the linear regression equation line, indicating a linear relationship between UVBF/PPI ratio and gestational age. Regression analysis across all 492 fetuses revealed a quantifiable linear relationship expressed as: UVBF/PPI ratio = 19.138 x GA – 361.677 (R
2 = 0.695).
Our results revealed a significant negative correlation between UVBF and PPI (r = -0.55, p < 0.001), indicating that as PPI increased, UVBF decreased. Reference ranges for UVBF based on PPI values were established, with UVBF decreasing as PPI values increased. In addition, the correlation coefficients of UVBF/PPI ratio with GA as calculated by Pearson correlation were 0.833 (p=0.000). The correlation coefficients of UVBF/PPI ratio with CPR as calculated by Pearson correlation were 0.471 (p=0.000).
Discussion
This study offers a reference range for the ratio of umbilical vein blood flow (UVBF) to placental perfusion index (PPI) across gestational weeks 21 to 40, focusing on typical Chinese fetal development. Each participant contributed a single set of measurements obtained during routine prenatal ultrasound evaluations. It was observed that the UVBF to PPI ratio demonstrated a statistically significant increase with advancing gestational age in our study population. In our investigation, we observed a notable reduction in UVBF concurrent with an elevation in PPI levels. Our analysis of cross-sectional data revealed a negative relationship between UVBF and PPI. Furthermore, we derived reference ranges for UVBF to PPI ratio in normal Chinese fetuses based on gestational age, and formulated equations to determine the mean values of these parameters, serving as lower normal thresholds. A key advantage of our innovation lies in the construction of UVBF to PPI ratio charts incorporating gestational age due to as we found firmly the strong correlation between UVBF and PPI.
In a previous investigation, Wang et al. meticulously documented longitudinal data on 907 fetuses, providing valuable insights into the trajectory of umbilical vein (UV) blood flow across gestational ages. Their study unveiled a progressive increase in mean UV blood flow from 32.66 ml/min to 381.88 ml/min between the 22nd and 39th weeks of gestation. This longitudinal perspective offered a comprehensive understanding of UV blood flow dynamics throughout the latter stages of pregnancy22. Additionally, DeVore and Epstein conducted a noteworthy study involving 240 fetuses, spanning from the 20th to the 42nd week of gestation. Their observation of elevated UV blood flow across this extended gestational period further underscored the dynamic nature of fetal hemodynamics and the intricate interplay between placental function and fetal circulatory adaptations30. Furthermore, Barbieri et al. undertook a systematic review to synthesize existing data on UVBF in human fetuses. Their comprehensive review revealed a consistent augmentation in mean fetal UV blood flow with advancing gestational age, providing crucial context for interpreting UVBF measurements in clinical practice19. Building upon this foundation of knowledge, our current investigation delves into UVBF dynamics in a cohort of 492 healthy fetuses, spanning gestational ages from 21 to 40 weeks. Our findings not only confirm the trend of increasing UVBF with advancing gestation but also offer additional granularity by quantifying UVBF values within this specific index: PPI.
The present investigation examines the distribution of placental perfusion index (PPI) across trimesters. Our dataset is juxtaposed with the reference values established by Gudmundsson et al., derived from 53 low-risk pregnancies (comprising 248 observations) spanning from 20 to 40 weeks of gestation. Both studies demonstrate a decline in PPI with GA14. Assessment of the reliability of PPI across studies indicates substantial consistency, as evidenced by a Cronbach’s alpha coefficient of 0.861. Notably, our findings offer valuable insights into the normative UVBF patterns in healthy fetuses, contributing to the existing body of knowledge on prenatal hemodynamics. Comparing our results with the 50th percentile UVBF values reported in the comprehensive systematic review conducted by Barbieri et al.,19 we observed a remarkable concordance. Utilizing the Cronbach’s alpha coefficient as a measure of agreement, we obtained a high coefficient of 0.991. This robust level of agreement underscores the accuracy and consistency of our UVBF assessments, further reinforcing the reliability of our findings. This alignment not only corroborates the validity of our findings but also highlights the consistency of UVBF trends across diverse study populations.
Abnormal development of the vascular supply to the placenta is a known contributor to slow fetal growth. Throughout gestation, the umbilical vein plays a critical role in efficiently delivering essential nutrients and oxygen from the mother to the fetus, ensuring proper fetal development. However, fluctuations in the placental vascular situation can lead to changes in the flow volume of the umbilical vein, potentially serving as an indicator of fetal growth issues17. Specifically, the UVBF/PPI ratio serves as a valuable metric for assessing placental resistance and umbilical vein blood flow. A mild increase in placental resistance, accompanied by mild reductions in umbilical vein blood flow, is reflected in an elevated UVBF/PPI ratio. An abnormal UVBF/PPI ratio can manifest in various scenarios, including low normal range UVBF and upper normal range PPI, abnormal low UVBF with normal PPI, and abnormal low UVBF with high PPI.
Hamidi et al. reported the presence of abnormal absolute umbilical vein blood flow volume in fetuses exhibiting growth restriction17. Similarly, Stampalija et al. suggested that umbilical vein blood flow could serve as a marker for identifying fetuses with growth restriction16. Additionally, Gudmundsson et al. found that PPI enhances the detection of fetal growth restriction, particularly in normal pregnancies characterized by increased placental vascular impedance14. Similarly, our study observed a lower UVBF/PPI ratio in the low birth weight group (p=0.021). In our study, we observed a remarkable association between a lower UVBF/PPI ratio and a significantly higher preterm delivery rate (p=0.034). This finding underscores the potential role of placental vascular dynamics in the timing of delivery. Building upon this, Jaiman et al. elucidated the correlation between defects in placental villi and spontaneous preterm labor and delivery, independent of acute inflammatory lesions within the placenta31. Furthermore, Preston et al.‘s literature review adds depth to our understanding by highlighting placental insufficiency as a growing contributor to spontaneous preterm birth32. These findings collectively emphasize the intricate relationship between placental function, vascular dynamics, and preterm delivery. These findings underscore the potential utility of UVBF/PPI ratio as a predictive marker for adverse pregnancy outcomes, particularly PTB and LBW.
The quantification of umbilical vein blood flow (UVBF) plays a pivotal role in assessing the vital exchange of nutrients and oxygen between the placenta and the developing fetus. Understanding UVBF dynamics offers valuable insights into the intricate interplay of maternal and fetal physiology, particularly in the context of fetal adaptation to varying environmental conditions. Despite its evident clinical significance, UVBF assessment has historically received less attention compared to traditional measurements focusing on umbilical artery and uterine artery resistance.
Nevertheless, recent advancements have shed light on the pivotal role of UVBF in prenatal care. Contrary to the traditional emphasis on resistance measurements, attention is gradually shifting towards the qualitative evaluation of UV Doppler patterns. While the presence of pulsations in UV Doppler waveforms has often been associated with fetal hypoxia, this manifestation typically emerges as a late sign, limiting its utility as an early indicator of fetal distress. In contrast, diminished UVBF/PPI ratio may serve as an early marker of placental insufficiency, offering clinicians an opportunity for timely intervention and management.
Our findings indicate that the resistance values of middle cerebral artery flow did not exhibit a statistically significant correlation with UVBF/PPI ratio. However, we observed that the CPR, a prenatal measurement assessing blood flow to the fetal brain and placenta, showed a high correlation with the UVBF/PPI ratio (r=0.471, p=0.000). The CPR plays a crucial role in providing insights into the fetal circulatory system, demonstrating higher sensitivity than individual Doppler indices of MCA alone33. It serves as a valuable tool in obstetrics for evaluating fetal well-being and assessing the risk of adverse outcomes. Anomalies in placental perfusion can lead to alterations in fetal blood flow distribution, resulting in decreased CPR34. This underscores the significance of UVBF/PPI ratio in predicting perinatal outcomes and highlights its potential as a sensitive indicator of fetal health and placental function.
A refinement understanding of UVBF/PPI ratio patterns change in different GA is crucial for deciphering fetal hemodynamics and optimizing prenatal care strategies. By integrating UVBF combined PPI assessment into routine clinical practice, healthcare providers can enhance their ability to monitor fetal well-being and detect pathological deviations at earlier stages. Furthermore, ongoing research efforts aimed at elucidating the complex mechanisms underlying UVBF combined PPI regulation hold promise for further refining prenatal assessment techniques and improving pregnancy outcomes.
Strengths and Limitations
Our study’s robustness lies in its exclusive examination of prenatal hemodynamic shifts in low-risk pregnancies without complications. Previous literature predominantly emphasizes prenatal Doppler assessments during pregnancy. Our findings potentially contribute to a meticulous scrutiny of decision-making processes and the feasibility assessment of combined UVBF and PPI regulation throughout pregnancy.
This study is subject to several limitations. Firstly, inherent biases may be present due to its retrospective nature and lack of randomization. Secondly, the analysis relied on obstetric sonographic reports and clinical data within a restricted sample size. Thirdly, the study’s scope is confined to data obtained from a single local tertiary hospital, thus precluding generalization to other regional medical facilities. Lastly, the inclusion criteria encompass solely normal fetuses, thereby excluding cases of pregnancy complications, fetal anomalies, and genetic disorders.
Conclusion
In this study, our investigation sheds light on the association between UVBF to PPI ratio and gestational age in a substantial cohort of unaffected fetuses, offering valuable insights into the complex interplay of placental perfusion and fetal hemodynamics. These findings augment comprehension of fetal development and carry substantial clinical implications, furnishing clinicians with a dependable benchmark for evaluating fetal health and recognizing deviations from typical UVBF to PPI ratios suggestive of underlying pathology. Furthermore, our results emphasize the potential of UVBF/PPI ratio as a prognostic indicator for adverse pregnancy outcomes, in particular preterm birth, low birth weight, or potential fetal hypoxemia.
Acknowledgments
We acknowledge support from the Funding: Natural Science Foundation of Fujian (No. 2021J011449). Our work is supported by the Department of ultrasound and the Department of obstetrics and gynecology partnership Mindong Hospital Affiliated to Fujian Medical University, and funding from the Natural Science Foundation of Fujian funding scheme.
Conflicts of Interest
All authors declare that he does not have any competing interests.
References
- Basta, M.; Lipsett, B.J. , Anatomy, Abdomen and Pelvis: Umbilical Cord. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing: 2023.
- Gaccioli, F.; Lager, S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front Physiol 2016, 7, 40. [Google Scholar] [CrossRef]
- Gumina, D.L.; Su, E.J. Mechanistic insights into the development of severe fetal growth restriction. Clinical Science 2023, 137, 679–695. [Google Scholar] [CrossRef]
- Colson, A.; Sonveaux, P.; Debiève, F.; Sferruzzi-Perri, A.N. Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction. Human Reproduction Update 2020, 27, 531–569. [Google Scholar] [CrossRef]
- Ramirez Zegarra, R.; Dall’Asta, A.; Ghi, T. Mechanisms of Fetal Adaptation to Chronic Hypoxia following Placental Insufficiency: A Review. Fetal Diagn Ther 2022, 49, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol 2019, 221, 437–456. [Google Scholar] [CrossRef]
- Ghesquière, L.; Perbet, R.; Lacan, L.; Hamoud, Y.; Stichelbout, M.; Sharma, D.; Nguyen, S.; Storme, L.; Houfflin-Debarge, V.; De Jonckheere, J.; et al. Associations between fetal heart rate variability and umbilical cord occlusions-induced neural injury: An experimental study in a fetal sheep model. Acta Obstetricia et Gynecologica Scandinavica 2022, 101, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Parks, W.T.; Zeng, H.D.; Ravichandran, A.; Ashwal, E.; Windrim, R.C.; Hobson, S.R.; Melamed, N.; Kingdom, J.C. Diagnostic utility of serial circulating placental growth factor levels and uterine artery Doppler waveforms in diagnosing underlying placental diseases in pregnancies at high risk of placental dysfunction. Am J Obstet Gynecol 2022, 227, 618–e1. [Google Scholar] [CrossRef] [PubMed]
- Levytska, K.; Higgins, M.; Keating, S.; Melamed, N.; Walker, M.; Sebire, N.J.; Kingdom, J.C. Placental Pathology in Relation to Uterine Artery Doppler Findings in Pregnancies with Severe Intrauterine Growth Restriction and Abnormal Umbilical Artery Doppler Changes. Am J Perinatol 2017, 34, 451–457. [Google Scholar]
- Kingdom, J.C.; Audette, M.C.; Hobson, S.R.; Windrim, R.C.; Morgen, E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol 2018, 218, S803–S817. [Google Scholar] [CrossRef]
- Redline, R.W. Classification of placental lesions. Am J Obstet Gynecol 2015, 213 (Suppl. S4), S21–S28. [Google Scholar] [CrossRef]
- Rocha, A.S.; Andrade, A.R. A.; Moleiro, M.L.; Guedes-Martins, L. Doppler Ultrasound of the Umbilical Artery: Clinical Application. Rev Bras Ginecol Obstet 2022, 44, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Paranavitana, L.; Walker, M.; Chandran, A.R.; Milligan, N.; Shinar, S.; Whitehead, C.L.; Hobson, S.R.; Serghides, L.; Parks, W.T.; Baschat, A.A.; et al. Sex differences in uterine artery Doppler during gestation in pregnancies complicated by placental dysfunction. Biology of Sex Differences 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Flo, K.; Ghosh, G.; Wilsgaard, T.; Acharya, G. Placental pulsatility index: a new, more sensitive parameter for predicting adverse outcome in pregnancies suspected of fetal growth restriction. Acta Obstet Gynecol Scand 2017, 96, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Todumrong, N.; Charoenvidhya, D.B.U. Use of Placental Pulsatility Index in High Risk Pregnancy to Predict Fetal Growth Restriction. Thai J. Obstet. Gynaecol. 2022, 30, 8. [Google Scholar]
- Stampalija, T.; Monasta, L.; Barbieri, M.; Chiodo, A.; Quadrifoglio, M.; Fantasia, I.; Bello, L.L.; Barresi, V.; Ottaviani, C.; Di Martino, D.D.; et al. Late-term fetuses with reduced umbilical vein blood flow volume: An under-recognized population at increased risk of growth restriction. Eur J Obstet Gynecol Reprod Biol 2022, 272, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, O.P.; Driver, C.; Steller, J.G.; Peek, E.E.; Monasta, L.; Stampalija, T.; Gumina, D.L.; DeVore, G.R.; Hobbins, J.C.; Galan, H.L. Umbilical Venous Volume Flow in Late-Onset Fetal Growth Restriction. J Ultrasound Med 2023, 42, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Di Martino, D.D.; Ferrazzi, E.M.; Stampalija, T. Umbilical vein blood flow: State-of-the-art. J Clin Ultrasound 2023, 51, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Zamagni, G.; Fantasia, I.; Monasta, L.; Lo Bello, L.; Quadrifoglio, M.; Ricci, G.; Maso, G.; Piccoli, M.; Di Martino, D.D.; et al. Umbilical Vein Blood Flow in Uncomplicated Pregnancies: Systematic Review of Available Reference Charts and Comparison with a New Cohort. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, A.; Dickinson, J.E. Umbilical venous blood flow and its measurement in the human fetus. J Clin Ultrasound 2012, 40, 502–11. [Google Scholar] [CrossRef]
- Lees, C.; Visser, G.H. A.; Hecher, K. , Placental-Fetal Growth Restriction. Cambridge University Press: Cambridge, 2018.
- Wang, L.; Zhou, Q.; Zhou, C.; Wang, J.; Shi, C.; Long, B.; Hu, L.; Peng, Y.; Liu, Y.; Xu, G. Z-Score Reference Ranges for Umbilical Vein Diameter and Blood Flow Volume in Normal Fetuses. Journal of Ultrasound in Medicine 2022, 41, 907–916. [Google Scholar] [CrossRef]
- Opheim, G.L.; Moe Holme, A.; Blomhoff Holm, M.; Melbye Michelsen, T.; Muneer Zahid, S.; Paasche Roland, M.C.; Henriksen, T.; Haugen, G. The impact of umbilical vein blood flow and glucose concentration on blood flow distribution to the fetal liver and systemic organs in healthy pregnancies. The FASEB Journal 2020, 34, 12481–12491. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Fernandez, S.; Hernandez-Andrade, E.; Gratacos, E. Umbilical venous blood flow measurement: accuracy and reproducibility. Ultrasound Obstet Gynecol 2008, 32, 587–91. [Google Scholar] [CrossRef] [PubMed]
- Flo, K.; Wilsgaard, T.; Acharya, G. Longitudinal reference ranges for umbilical vein blood flow at a free loop of the umbilical cord. Ultrasound Obstet Gynecol 2010, 36, 567–72. [Google Scholar] [CrossRef]
- Bhide, A.; Acharya, G.; Baschat, A.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Ebbing, C.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; et al. ISUOG Practice Guidelines (updated): use of Doppler velocimetry in obstetrics. Ultrasound Obstet Gynecol 2021, 58, 331–339. [Google Scholar] [CrossRef]
- AIUM Practice Parameter for the Performance of Detailed Second- and Third-Trimester Diagnostic Obstetric Ultrasound Examinations. Journal of Ultrasound in Medicine 2019, 38, 3093–3100. [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.M.; Chalouhi, G.E.; Da Silva Costa, F.; Hernandez-Andrade, E.; Malinger, G.; Munoz, H.; Paladini, D.; et al. ISUOG Practice Guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2022, 59, 840–856. [Google Scholar] [CrossRef]
- Dev Maulik; Lees, C.C., Doppler Ultrasound in Obstetrics and Gynecology, 3 ed.; Springer: Cham, 2023; p XI, 649.
- DeVore, G.R.; Epstein, A. Computing Z-Score Equations for Clinical Use to Measure Fetal Umbilical Vein Size and Flow Using Six Independent Variables of Age and Size. J Ultrasound Med 2022, 41, 1949–1960. [Google Scholar] [CrossRef]
- Jaiman, S.; Romero, R.; Bhatti, G.; Jung, E.; Gotsch, F.; Suksai, M.; Gallo, D.M.; Chaiworapongsa, T.; Kadar, N. The role of the placenta in spontaneous preterm labor and delivery with intact membranes. J Perinat Med 2022, 50, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.; Hall, M.; Shennan, A.; Story, L. The role of placental insufficiency in spontaneous preterm birth: A literature review. Eur J Obstet Gynecol Reprod Biol 2024, 295, 136–142. [Google Scholar] [CrossRef]
- Mecke, L.; Ignatov, A.; Redlich, A. The importance of the cerebroplacental ratio for the prognosis of neonatal outcome in AGA fetuses. Arch Gynecol Obstet 2023, 307, 311–317. [Google Scholar] [CrossRef]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front Endocrinol (Lausanne) 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).