1. Introduction
In recent years, growing literature has centered its interest on the role of the mechanisms related to aging and sex-related factors and able in modulating and impairing immune system [
1,
2,
3,
4,
5,
6,
7,
8]. This last is composed, as well recognized, by two components, the innate and clonotypic compartments, which protect the organisms by external and internal pathogens and interact with other compartments of the same organism, such as the microbiota and central nervous system (CNS) [
4,
5,
6,
7,
8]. Aging, as physiological condition, impairs cognitive and physical functions, such as the activities of immune system. This last shows, indeed, with the advancing age a progressive decline, called “immunosenescence”, accompanied by a concomitant chronic and low-grade of inflammation, called “inflamm-aging” [
2,
3]. This also includes an increased production and release of pro-inflammatory mediators, such as Interleukin-1 (IL-1), IL-6, and Tumor Necrosis Factor alpha (TNF-α), that constitute the so-called phenotype associated with the senescence, [
9] at local and systemic level. Such mediators attempt to contrast and counteract the continuous antigenic load due to various forms of stresses, such as physical (i.e., UV radiation and heat), chemical (i.e., reactive oxygen species - ROS), and biological ones (i.e., viruses and bacteria) [
9] .This immune aging condition has been also demonstrated to induce a “fatigue” of immune system [
10], which contribute to onset of age-related disorders, such as neurodegenerative disorders [
11], cancer [
12,
13,
14], cardiovascular disorders [
12,
13,
14], and Covid-19 [
15], as well as autoimmune diseases [
2], resulting in organ failure and subsequently in death [
16].
Here we review the current literature on the biological effects of age- and sex- related factors on clinical, neuropsychological, and biologic features of a typical autoimmune brain disease, the Myasthenia gravis (MG). For a clear understanding, we report of follows some concepts on the close relationship between autoimmunity and aging.
The Close Relationship between Autoimmunity and Aging
With the term autoimmunity defines a process, in which the immune system responds to self-antigens with the production of autoantibodies (Auto-Ab), that, in turn, affect target tissues by causing dysfunctions or disruption; in this process, a genetic predisposition, especially in Human Leucocyte Antigen (HLA) genes, has been demonstrated to be implicated [
17]. Moreover, autoimmunity is characterized by immune system dysfunctions, and evocated by the action of hormonal factors (i.e., estrogens) and environmental factors. Accordingly, sex/gender and age are associated with the distinctive differences in clinical presentation, onset, progression, and outcome of several autoimmune disorders, such as MG [
18].
2. MG: Its Features
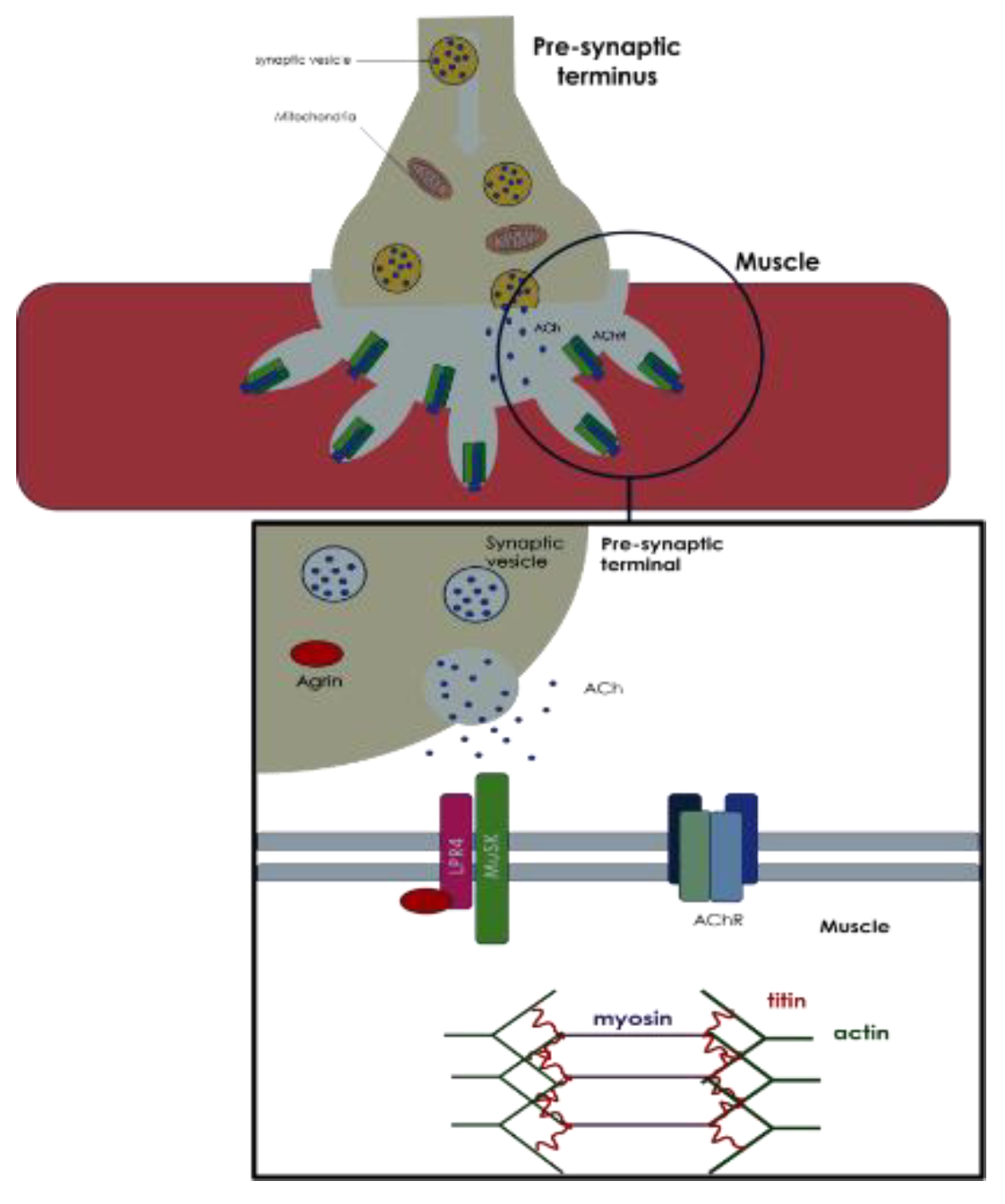
MG is a chronic brain autoimmune disease, characterized by the presence of specific autoantibodies, which impact the components of neuromuscular junction (NMJ) (
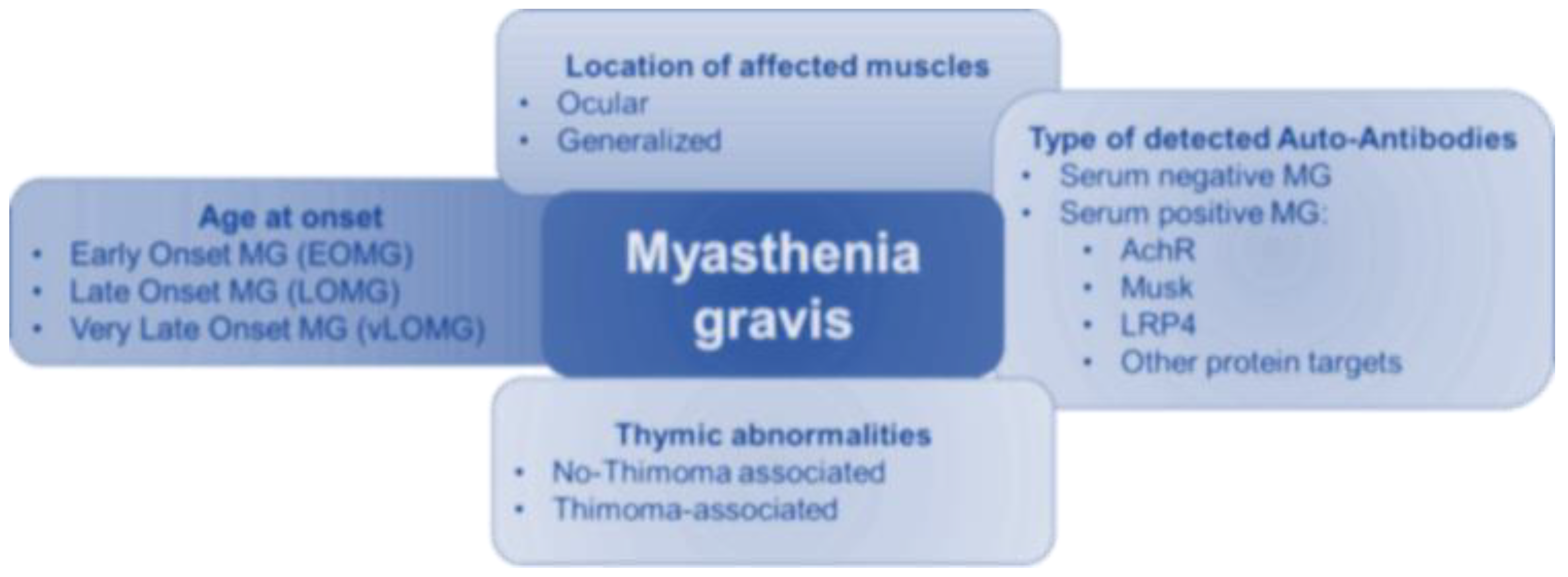
Figure 1). As a result, MG patients show a fluctuating muscle weakness, which could be restricted to local districts (i.e., extraocular muscles) or generalized. MG also shows a clinical and immunopathological complexity, which has required a system of classification to facilitate its management. During the years, many systems of MG classification have been, however, suggested, by considering the onset’s age, the location of affected muscles, the type of detected autoantibodies, and thymic abnormalities (
Figure 2) [
19]. According to onset ‘s age, it is possible to identify two MG forms: I) an early-onset MG (EOMG), a clinical form, which occurs before 40 years old, and consequently considered as a disease of young women (<40 years old), having some common aspects with other autoimmune disorders, such as Multiple Sclerosis (MS) or Systemic Lupus Erythematosus Lupus (SLE) [
19] and II) a late-onset MG (LOMG), typically defined by most authors, as typical MG, which occurs after 50 years old. Some authors have also proposed a further subdivision of the LOMG into two subgroups: “not elderly LOMG”, with an age at onset between 50 and 64 years, and “elderly LOMG”, with an age at onset after 65 years (the so called elderly LOMG) [
19].
The correct individuation of the different MG subtypes [
19,
20], forms, according to various systems of classification, is important for the MG management thanks to precise biomarkers as well as to apply diverse therapeutic strategies to improve muscle strength, reduce fatigue, and improve the quality of life of affected patients. Among these, medications such as acetylcholinesterase inhibitors, immunosuppressive drugs, and plasmapheresis, and in some cases, thymectomy (surgery to remove the thymus) may be considered.
2.1. The Contribution of Age- and Sex-Related Factors in Etiopathogenesis of MG
Over the years, the contribution of age- and sex-related factors in etiopathogenesis of MG has remained widely debated, although no significant differences in incidence and severity of disease between male and female have been found. However, some studies have suggested that women can develop more likely certain subtypes of MG, such as the ocular form (which affects the muscles of the eyes), and men may likely have the generalized form (which affects multiple muscle groups) [
21,
22].
2.2. Clinical Presentation of MG According to Age and Gender
Principal MG symptoms include fatigue and a fluctuating fatigable weakness, related to the damage of striated muscles. The muscle weakness is related to an impairment of NMJ and can be generalized or localized, involving mainly the extraocular muscles and/or the levator palpebrae muscles. The fluctuant nature of the symptoms may vary in intensity from day to day, or even from hour to hour, and is due to an excessive contraction of affected muscles [
19,
20,
23,
24].
Based on age at onset, MG is classified into two subgroups, as above mentioned, the EOMG and LOMG, with a prevalence in women [
23,
24]. However, the number of diagnoses of LOMG in both sexes has recently been increasing [
23,
24]. Indeed, the natural history of the disease is influenced by age-related parameters: for example, treatment options in the elderly patients are often hampered by the numerous comorbidities and contraindications [
23,
24], and LOMG is often burdened by worse prognosis [
23,
24]. Thus, the prevalence of LOMG has increased in recent years [
25]. However, it is not clear if the increase reflects the biological phenomenon of ageing or is influenced by an increased awareness, the availability of new assays for detection of auto-Ab and a longer lifespan [
25].
Both in EOMG and LOMG, ocular onset is more frequent than the generalized onset [
23,
24,
25]. However, ocular MG is more frequent in LOMG than in EOMG [
25,
26]. Furthermore, although LOMG tends to be characterized exclusively by ocular symptoms, it is possible to identify two subgroups within the LOMG based on the prevalence of ocular symptoms. In fact, in non-elderly LOMG, a greater frequency of clinical subtypes with exclusively ocular symptoms than in elderly LOMG has been identified; however, a generalized clinical phenotype starting from an ocular onset occurs with more frequency in elderly LOMG [
25,
26] The percentage of patients presenting ocular symptoms and transforming into generalized MG is higher in LOMG in comparison to EOMG [
25,
26]. Cortés-Vicente has shown that the patients with elderly LOMG presented more life-threatening events at onset, such as bulbar symptoms or myasthenic crisis rapidly progressive to respiratory pump failure. However, they required fewer drugs and were less frequently drug-refractory, so that they achieve a good outcome with fewer immunosuppressants when diagnosed and treated properly. Myasthenic crisis in LOMG occurred in about 6-11% of the patients [
27].
3. Genetic Susceptibility for MG and Epigenetic Modifications
Genetic susceptibility for MG in relationship with age has been widely studied, with the identification of specific polymorphisms in some genes involved in autoimmunity, which correlate specifically with the LOMG or EOMG. Among the identified polymorphisms, TNFAIP3-interacting protein 1 (TNIP1), sphingolipid biosynthesis regulator 3, Gasdermin-B (GSDMB) and Tumor Necrosis Factor receptor-associated factor (TRAF3) are related exclusively to the EOMG, while Bcl-6 Interacting Corepressor, Cluster of differentiation 28 (CD28) and Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) increase specifically the risk of LOMG [
28,
29,
30,
31]. In fact, EOMG-related polymorphisms, such as TRAF3 and TNIP1, concern regulatory genes of toll-like receptors (TRL) signaling, involved in innate immunity. This agrees with the previous evidence that the aberrant expression of TRL-4 in the hyperplastic thymuses of patients with MG can induce autoreactive T-cell activation and recruitment [
28,
29,
30,
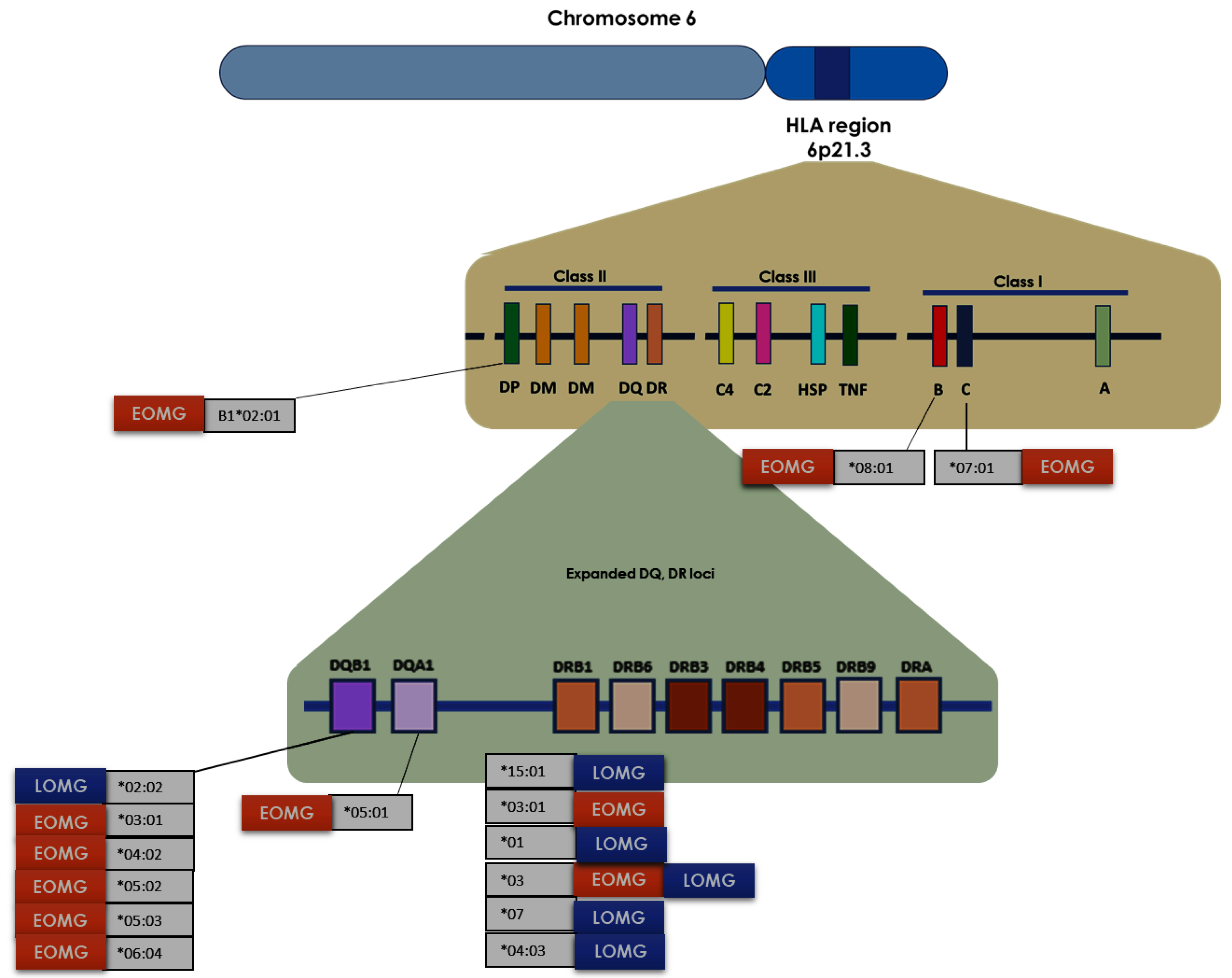
31]. These findings have allowed us to evidence that the pathways of cell-mediated immunity are more enriched in EOMG, while those of adaptive immunity are more involved in LOMG. Moreover, the genetic predisposition conferred by some polymorphisms of the Human Leucocyte Antigens (HLA) has been described to increase the risk of MG and some of these HLA polymorphisms are specifically correlated with age of onset (
Figure 3) [
32,
33,
34].
Most of the polymorphisms analyzed concern HLA class II molecules. The
Table 1 shows the main known associations between EOMG/LOMG and HLA variants [
34,
35]. Indeed, some of these polymorphisms may have a role more in women than men and vice versa. DRB1*04, DQB1*0302, and HLA-B*08 haplotypes are common in women with MG, especially with EOMG [
34,
35]. Otherwise, HLA-A*24:02 would seem to confer a phenotype-protective effect in women as shown by an analysis in a Spanish cohort [
36].
Recently, an epigenetic mechanism has been also proposed to explain the female preponderance in MG. Preferential X-chromosome inactivation (PXCI) is a phenomenon observed in certain genetic diseases, including MG, in which one of the two X chromosomes in females is preferentially inactivated. This means that only one of the two X chromosomes is active at any given time, while the other is essentially "turned off". Studies have shown that PXCI is present in some women with MG, with the affected X chromosome being the one carrying the genetic mutation associated with the disease. This means that the active X chromosome does not carry the genetic mutation, and therefore does not contribute to the development of the disease. This phenomenon has been observed in a small proportion of women with MG and has been found to be associated with a milder form of the disease, as well as better response to treatment [
37]
4. Clinical and Biological Heterogeneity in MG Associated with Typical Change in the Thymus Morphology According to Age and Sex
Clinical and biological heterogeneity in MG is characterized by a different change affecting the thymus, with a prevalence of thymic hyperplasia in cases of EOMG, especially in women, while the thymus goes more frequently to involution in LOMG cases [
38,
39,
40]. In seropositive EOMG, some thymic medullary alterations have been described, mainly characterized by the expansion of T lymph node areas, by the finding of ectopic lymph node-like germinal centers (GCs) in the context of expanded perivascular spaces, and by the presence of thymic epithelial cells (TECs) reported to be hyperplastic and arranged in a palisade, while normally they have a typical monolayer deposition. TECs appear to play an important role in the immunization process since they express AChR subunits on their surface and can act as antigen-presenting cells to the near T lymphocytes. It is not surprising that EOMG improves after thymectomy. Furthermore, in patients with EOMG the surrounding basement membranes near the perivascular spaces are focally disrupted and show fenestrations through which the TECs protrude. It is possible that this allows aberrant communication between TEC and GC [
40]. In LOMG, the thymic myoid cells that typically make up lymphoid follicles in anti-AChR positive patients with EOMG are infrequent and tend to decrease with advancing age with replacement by fat. However, in some cases of LOMG some follicular aggregates like those of EOMG were observed in the thymus with a prevalence in women. In these cases, thymectomy was clinically advantageous, except in cases of positivity to the anti-titin antibodies [
40].
5. Distinctive Biomarkers in Early and Late-Onset MG, According to Sex/Gender and Age Differences
As above described, MG has a well fluctuating nature and a high heterogeneity in the onset age, progression, and forms. These features require appropriate biomarkers for facilitating the differential management of various MG forms. Unfortunately, no biomarkers are currently available to identify disease activity.
Recently, many studies have been focused on the inflammatory circulating proteins to identify eventual profiles associated with the disease onset and progression. Moling and coworkers [
41] analyzed 92 different inflammatory proteins associated with inflammation within the clinical subgroups of EOMG and LOMG. Eleven proteins were significantly increased in the sera of patients with MG, and among them the matrix metalloproteinase (MMP)-10 had the highest serum levels. However, some increased biomarkers showed different levels between the EOMG and the LOMG, with no sex-mediated effect emerging since similar levels were found in males and females. Patients with EOMG had increased levels of MMP-10 and C-X-C motif ligand 1 (CXCL1) and reduced levels of brain-derived neurotrophic factor (BDNF) in comparison to patients with LOMG [
41]. A dysfunction of MMPs in MG is already known [
42], but there are no other studies that evaluate their difference based on the age of onset of the disease.
The different sera levels of BDNF in the comparison between EOMG and LOMG [
43] could be the expression of a compensatory mechanism. Indeed, several cells present in the thymus, including immune cells and thymic epithelial cells (TECs), express receptors for BDNF. This neurotrophic factor could therefore arise at the interface between the immune system and the nervous system. Moreover, it regulates the turnover of TECs [
42,
43] and is increased in patients thymomas compared to patients with thymic hyperplasia [
42,
43] It could be hypothesized that, since thymic involution occurs in patients with LOMG, the increase in BDNF expression is a reactive process or, as suggested by Molin and colleagues, this difference on BDNF levels could be due to nature of EOMG, characterized by with a greater inflammatory component, capable of reducing BDNF production [
42,
43].
Finally, some studies have detected specific profiles of circulating miRNAs in MG cases from diverse subclinical groups. In EOMG cases, miR-150-5p and miR-21-5p resulted the most elevated miRNAs, with lower levels observed upon treatment with immunosuppression and thymectomy. In LOMG, the miR-150-5p, miR-21-5p, and miR-30e-5p levels have been assessed elevated and decreased in accordance with the clinical response after immunosuppression [
44].
6. Typical MG Autoantibodies in Relationship to Age and Sex-Related Factors
As previously seen, one of the systems of classification for MG consists in subgrouping based on the presence /absence of autoantibodies against components of NMJ (see
Figure 1 and 2). The NMJ is the synapse between a motor neuron (i.e., pre-synaptic element) and a muscle fiber (i.e., post-synaptic element), allowing the transmission of electrical signals from the neuron to the muscle to induce muscle contraction. The motor neuron releases Acetylcholine (ACh), as neurotransmitter, which binds specific nicotinic receptors (AChR) on the muscle membranes, triggering a positive ion influx and, subsequently, the release of calcium ions from sarcoplasm. The increase of intracellular calcium ions results in binding of calcium ions to Troponin, a protein complex that modified the conformation of globular actin, that forms filaments. This conformational change leads the conformational change of Tropomyosin, with a consequent interaction between actin and myosin, resulting in a series of cross-bridging that allows the slide of the filaments, which in turn leads to a cycling shortening of muscle fibers, contributing to the muscle contraction. During the muscle contraction, Titin acts as molecular spring that is passively stretched during the initial stages of muscular lengthening and then helps to restore the resting length of the muscle; another property of this protein is to regulate the passive tension in the muscle. Moreover, some proteins are important in the regulation of NMJ. Among these, Muscle-specific kinase (MuSK) plays a role in the clustering and anchoring of AChRs and in the maintenance and function of NMJ. Anti-AChR antibodies can be found in about 85% of MG patients and antibodies against the muscle-specific kinase (MuSK) are detected in about 6% of the patients. An increasing frequency of positivity for antibodies anti-AChRs has been reported during aging, with about 93% in very-late-onset MG (≥ 65 years), while anti-MuSK antibodies were found to be uncommon after 65-70 years of age [
47]. About 10% of the patients remain seronegative to anti-AChRs and anti-MuSK antibodies [
48]. Interestingly, most seronegative patients with LOMG are women, while serum-negativity in EOMG is prevalent in men [
49]. In the cases of double serum-negativity for anti-AChRs and anti-MuSK, it is possible to find a positivity for other antibodies, including anti- LRP4 and anti-titin antibodies [
47]. Anti-titin antibodies could be expression of some immunological differences between EOMG and LOMG since they are present in more than half of patients with thymoma but also in a similar percentage in patients with LOMG without thymoma. The group of Szczudlik and coworkers have also detected that anti-titin antibodies can constitute appropriate biomarkers for non thymomatous LOMG and EOMG with thymoma [
50]. Moreover, although several studies have associated the positivity of anti-titin antibodies with a more unfavorable clinical course, other studies have not confirmed this association [
50].
7. Neuropsychology of MG Patients
The study of neuropsychological features in autoimmune disorders, and in MG, represents a core challenge of neuroscience research. In particular, the study of cognitive functions, but also of depressive status and quality of life, was widely analyzed.
Some authors evidenced the presence of cognitive impairment in MG, which may suggest a partial CNS damage due to autoimmunity [
51]. Among different mechanisms that can explain the presence of cognitive deficits and neuropsychiatric symptoms in MG patients, hypothetical pathogenic central effects of autoantibodies against AChR were suggested [
52]. The impaired cognitive domains found in patients with MG included language, visuospatial function, information processing, verbal immediate and delayed recall memory, visual immediate recall memory, and response fluency, while attention, executive function, and visual delayed recall memory were unimpaired [
53]. The presence of cognitive impairments could be linked to age of onset and to disease severity. Indeed, patients with EOMG and generalized MG obtained poorer results than healthy controls in multiple cognitive domains [
53]. However, other authors did not confirm these results and in the studies available to date, no significant differences emerged in the cognitive tests between EOMG and LOMG and between female and male patients [
53].
Furthermore, MG patients could present as co-morbidities mood disturbances, such as anxiety and depression. MG patients, due to the fatigue and fluctuating weakness, often develop behavioral patterns of excessive advanced planning, avoiding some social situations to preserve muscle strength and evade embarrassment. For example, bulbar dysfunctions, such as dysarthria, dysphagia, and dysphonia, could determine social anxiety due to an impaired verbal and non-verbal communication, caused by the compromised ability to speech, to communicate, especially for facial expression and swallowing abilities. Limiting social interactions is translated with lower satisfaction with life and with a poorer quality of life (QoL). Moreover, patients who needs to ventilator support had a poorer QoL prior to ventilation, because increased work of breathing and the emotional distress of anticipatory planning are used to circumvent social anxiety [
54]. MG patients may also present a concomitant depression, which often represent a key confounder in differential diagnosis. This is because MG patients with their facial weakness and eye ptosis gave the impression of apathy and depressive mood, and the fluctuating nature of myasthenic symptoms can be confused with depressive symptoms. It was shown that the nature of MG, social disadvantages, and adverse effects of corticosteroid therapies are related with the high presence of depressive symptoms in MG. Moreover, abnormalities in both innate and adaptative immune system seems to contribute to a higher susceptibility for depression [
55].
A study analyzed the effect of sex on QoL of MG patients; indeed, it was demonstrated that the QoL of female MG patients is less than male patients, and that the relationship between gender and QoL is modulated by the number of co-morbidities and the way by which they are treated. Additionally, factors, such as employment status, MG exacerbations, and an active lifestyle, are related to QoL: so, interventions able to cope with these factors are useful to improve quality of life of MG patients [
56].
Another important aspect is linked to sexual sphere because it was demonstrated that MG patients could be affected by sexual dysfunctions, which consist in a series of symptoms, including decreased libido, impaired sexual arousal, discomfort during intercourse, and decreased sexual satisfaction. Generally, sexual dysfunctions are related to abnormalities in the genitourinary, nervous, vascular, and endocrine systems. Wang et colleagues showed that MG patients, especially females, have a higher incidence of sexual dysfunctions in comparison to healthy people. Female MG patients manifests a decline in overall sexual function, such as sexual desire, arousal, lubrication, and orgasm, while male MG patients showed an overall decline in the patient’s ejaculation functions. All these dysfunctions are related to the state of anxiety and depression of each MG patient, and the age represents another risk factor able to worse the sexual dysfunctions [
57].
8. Conclusions
When comparing the available data, some differences emerge in patients with MG according to age and sex. Despite the lower clinical activity, LOMG tends to have a worse prognosis, with a higher rate of generalization and its course is often complicated by the presence of concomitant diseases. Moreover, ocular symptoms are more frequent in LOMG [
47], although there may be some difficulty in recognizing ocular symptoms in the elderly. In fact, it is important to underline that ocular symptoms are more difficult to detect in elderly patients, as diplopia may be masked by eye pathologies such as degenerative maculopathy or ptosis may be unrecognized due to decreased eyelid area during normal aging [
58]. Considering the neuropsychological aspects, few data are available, although some studies have highlighted the presence of cognitive decline in patients with MG [
53]. The possible presence of a difference in cognitive performances between patients with EOMG and LOMG needs to be investigated, since there is no evidence, although a study has shown that patients with EOMG obtain worse cognitive performance [
53].
MG is considered an autoimmune disease [
58] and autoimmune diseases are more common in young people. In recent years incidence of LOMG is increasing and MG is more frequent in elderly than in young people [
58]. According to some authors, the higher incidence of MG in elderly subjects could be linked to the action of environmental factors but to date no explanation of this link has been offered. Probably, a possible explanation of this apparent inconsistency could lie in the inflammaging, whereby the onset of autoimmune-based diseases in the elderly could be the consequence of the dysregulation of the immune system during inflammaging. A difference between EOMG and LOMG and between men and women is also reported in terms of antibodies. A frequent finding in most of the studies examined showed that patients with LOMG and non-thymoma MG are more frequently positive for anti-titin antibodies, while these are rarely found in young subjects. However, a higher incidence of anti-AChR antibody positivity during aging has been reported in recent years, especially in men and although LOMG was previously reported in a review by Aarli to be characterized by lower anti-AChR antibody titers than EOMG, one most recent study showed that patients with elderly LOMG are more often positive for anti-AChR antibodies than patients with EOMG and not elderly LOMG [
58].
In conclusion, EOMG and LOMG can be considered two distinct phenotypic expressions of the same disease, at the base of which different immunopathogenic mechanisms occur. Regarding the effects of sex-related factors in etiopathogenesis of MG, it was demonstrated that hormones, such as estrogen, progesterone, and testosterone, can modulate the immune system and influence the development of autoimmunity [
58]. Estrogen has been shown to increase the expression of acetylcholine receptors on the surface of muscle cells, which may make them more susceptible to attack by autoantibodies in MG [
59]. Estrogen can modulate the activity of T-helper cells and modulate the production of autoantibodies [
59]. However, it remains to be demonstrated whether a different gender-based mechanism underlies the loss of immune tolerance towards AChR and other antigens [
60].
Further studies are needed to improve understanding the impact of sex and aging-related factors to the disease, to allow for better clinical management.
Author Contributions
Conceptualization G.S, T.C. and C.R.B.; literature research and data curation, G.S, T.C.; figure preparation, T.C.; writing—original draft preparation, G.S., T.C., T.P., P.R. M.D. C.R.B.; writing—review and editing P.R., M.D., and C.R.B.; visualization, P.R., M.D., C.R.B.; supervision, P.R., M.D., G.S. and C.R.B.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
These authors declare no conflict of interest.
References
- Balistreri, C. R., Candore, G., Accardi, G., Bova, M., Buffa, S., Bulati, M., Forte, G. I., Listì, F., Martorana, A., Palmeri, M., Pellicanò, M., Vaccarino, L., Scola, L., Lio, D., & Colonna-Romano, G. (2012). Genetics of longevity. data from the studies on Sicilian centenarians. Immunity & ageing : I & A, 9(1), 8. [CrossRef]
- Santoro, A., Bientinesi, E., & Monti, D. (2021). Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity?. Ageing research reviews, 71, 101422. [CrossRef]
- Wang, Y., Dong, C., Han, Y., Gu, Z., & Sun, C. (2022). Immunosenescence, aging and successful aging. Frontiers in immunology, 13, 942796. [CrossRef]
- Hohman, L. S., & Osborne, L. C. (2022). A gut-centric view of aging: Do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence?. Aging cell, 21(9), e13700. [CrossRef]
- Bulut, O., Kilic, G., & Domínguez-Andrés, J. (2022). Immune Memory in Aging: A Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics. Clinical reviews in allergy & immunology, 63(3), 499–529. [CrossRef]
- Alrosan, A. Z., Heilat, G. B., Alrosan, K., Aleikish, A. A., Rabbaa, A. N., Shakhatreh, A. M., Alshalout, E. M., & Al Momany, E. M. A. (2024). Autonomic brain functioning and age-related health concerns. Current research in physiology, 7, 100123. [CrossRef]
- Balistreri, C. R., & Monastero, R. (2023). Neuroinflammation and Neurodegenerative Diseases: How Much Do We Still Not Know?. Brain sciences, 14(1), 19. [CrossRef]
- Schirò, G., Iacono, S., & Balistreri, C. R. (2023). The Role of Human Microbiota in Myasthenia Gravis: A Narrative Review. Neurology international, 15(1), 392–404. [CrossRef]
- Balistreri, C. R., Candore, G., Accardi, G., Colonna-Romano, G., & Lio, D. (2013). NF-κB pathway activators as potential ageing biomarkers: Targets for new therapeutic strategies. Immunity & ageing : I & A, 10(1), 24. [CrossRef]
- Jeejeebhoy K. N. (2012). Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Current opinion in clinical nutrition and metabolic care, 15(3), 213–219. [CrossRef]
- Bowirrat A. (2022). Immunosenescence and Aging: Neuroinflammation Is a Prominent Feature of Alzheimer's Disease and Is a Likely Contributor to Neurodegenerative Disease Pathogenesis. Journal of personalized medicine, 12(11), 1817. [CrossRef]
- Balistreri C. R. (2022). Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers. International journal of molecular sciences, 23(14), 7548. [CrossRef]
- Vaiserman, A., De Falco, E., Koliada, A., Maslova, O., & Balistreri, C. R. (2019). Anti-ageing gene therapy: Not so far away?. Ageing research reviews, 56, 100977. [CrossRef]
- Balistreri C. R. (2018). Anti-Inflamm-Ageing and/or Anti-Age-Related Disease Emerging Treatments: A Historical Alchemy or Revolutionary Effective Procedures?. Mediators of inflammation, 2018, 3705389. [CrossRef]
- Lio, D., Scola, L., Giarratana, R. M., Candore, G., Colonna-Romano, G., Caruso, C., & Balistreri, C. R. (2021). SARS CoV2 infection _The longevity study perspectives. Ageing research reviews, 67, 101299. [CrossRef]
- Fulop, T. (2020) ‘Immunosenescence is both functional / adaptive and dysfunctional / maladaptive’, pp. 521–536.
- Ray, D. and Yung, R. (2018) ‘Immune senescence, epigenetics and autoimmunity’, Clinical Immunology, 196, pp. 59–63. [CrossRef]
- Nussinovitch, U. and Shoenfeld, Y. (2012) ‘The role of gender and organ specific autoimmunity’, Autoimmunity Reviews. [CrossRef]
- Wiendl, H., Abicht, A., Chan, A., Della Marina, A., Hagenacker, T., Hekmat, K., Hoffmann, S., Hoffmann, H. S., Jander, S., Keller, C., Marx, A., Melms, A., Melzer, N., Müller-Felber, W., Pawlitzki, M., Rückert, J. C., Schneider-Gold, C., Schoser, B., Schreiner, B., Schroeter, M., … Meisel, A. (2023). Guideline for the management of myasthenic syndromes. Therapeutic advances in neurological disorders, 16, 17562864231213240. [CrossRef]
- García Estévez, D. A., & Pardo Fernández, J. (2023). Myasthenia gravis. Update on diagnosis and therapy. Miastenia gravis. Actualización diagnóstica y terapéutica. Medicina clinica, 161(3), 119–127. [CrossRef]
- Iyer, S. R., Shah, S. B., & Lovering, R. M. (2021). The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. International journal of molecular sciences, 22(15), 8058. [CrossRef]
- Bubuioc, A. M., Kudebayeva, A., Turuspekova, S., Lisnic, V., & Leone, M. A. (2021). The epidemiology of myasthenia gravis. Journal of medicine and life, 14(1), 7–16. [CrossRef]
- Mishra, A. K., & Varma, A. (2023). Myasthenia Gravis: A Systematic Review. Cureus, 15(12), e50017. [CrossRef]
- Zhu, Y., Wang, B., Hao, Y., & Zhu, R. (2023). Clinical features of myasthenia gravis with neurological and systemic autoimmune diseases. Frontiers in immunology, 14, 1223322. [CrossRef]
- Deymeer F. (2020). Myasthenia gravis: MuSK MG, late-onset MG and ocular MG. Acta myologica : Myopathies and cardiomyopathies : Official journal of the Mediterranean Society of Myology, 39(4), 345–352. [CrossRef]
- Fan, L., Ma, S., Yang, Y., Yan, Z., Li, J., & Li, Z. (2019). Clinical differences of early and late-onset myasthenia gravis in 985 patients. Neurological research, 41(1), 45–51. [CrossRef]
- Cortés-Vicente, E., Álvarez-Velasco, R., Segovia, S., Paradas, C., Casasnovas, C., Guerrero-Sola, A., Pardo, J., Ramos-Fransi, A., Sevilla, T., López de Munain, A., Gómez, M. T., Jericó, I., Gutiérrez-Gutiérrez, G., Pelayo-Negro, A. L., Martín, M. A., Mendoza, M. D., Morís, G., Rojas-Garcia, R., Díaz-Manera, J., Querol, L., … Illa, I. (2020). Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology, 94(11), e1171–e1180. [CrossRef]
- Renton, A. E., Pliner, H. A., Provenzano, C., Evoli, A., Ricciardi, R., Nalls, M. A., Marangi, G., Abramzon, Y., Arepalli, S., Chong, S., Hernandez, D. G., Johnson, J. O., Bartoccioni, E., Scuderi, F., Maestri, M., Gibbs, J. R., Errichiello, E., Chiò, A., Restagno, G., Sabatelli, M., … Traynor, B. J. (2015). A genome-wide association study of myasthenia gravis. JAMA neurology, 72(4), 396–404. [CrossRef]
- Li, J., Wang, F., Li, Z., Feng, J., Men, Y., Han, J., Xia, J., Zhang, C., Han, Y., Chen, T., Zhao, Y., Zhou, S., Da, Y., Chai, G., & Hao, J. (2024). Integrative multi-omics analysis identifies genetically supported druggable targets and immune cell specificity for myasthenia gravis. Journal of translational medicine, 22(1), 302. [CrossRef]
- Chia, R., Saez-Atienzar, S., Murphy, N., Chiò, A., Blauwendraat, C., International Myasthenia Gravis Genomics Consortium, Roda, R. H., Tienari, P. J., Kaminski, H. J., Ricciardi, R., Guida, M., De Rosa, A., Petrucci, L., Evoli, A., Provenzano, C., Drachman, D. B., & Traynor, B. J. (2022). Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: A genome-wide association study. Proceedings of the National Academy of Sciences of the United States of America, 119(5), e2108672119. [CrossRef]
- Zhu, Z., Chen, X., Wang, C., & Cheng, L. (2022). Novel genes/loci validate the small effect size of ERBB2 in patients with myasthenia gravis. Proceedings of the National Academy of Sciences of the United States of America, 119(36), e2207273119. [CrossRef]
- Spagni, G., Todi, L., Monte, G., Valentini, M., Di Sante, G., Damato, V., Marino, M., Evoli, A., Lantieri, F., & Provenzano, C. (2021). Human Leukocyte Antigen Class II associations in late-onset Myasthenia Gravis. Annals of clinical and translational neurology, 8(3), 656–665. [CrossRef]
- Hong, Y., Li, H. F., Romi, F., Skeie, G. O., & Gilhus, N. E. (2018). HLA and MuSK-positive myasthenia gravis: A systemic review and meta-analysis. Acta neurologica Scandinavica, 138(3), 219–226. [CrossRef]
- Panhuber, A., Lamorte, G., Bruno, V., Cetin, H., Bauer, W., Höftberger, R., Erber, A. C., Frommlet, F., & Koneczny, I. (2022). A systematic review and meta-analysis of HLA class II associations in patients with IgG4 autoimmunity. Scientific reports, 12(1), 9229. [CrossRef]
- Zhong, H., Zhao, C., & Luo, S. (2019). HLA in myasthenia gravis: From superficial correlation to underlying mechanism. Autoimmunity reviews, 18(9), 102349. [CrossRef]
- Salvado, M., Caro, J. L., Garcia, C., Rudilla, F., Zalba-Jadraque, L., Lopez, E., Sanjuan, E., Gamez, J., & Vidal-Taboada, J. M. (2022). HLA-DQB1*05:02, *05:03, and *03:01 alleles as risk factors for myasthenia gravis in a Spanish cohort. Neurological sciences : Official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 43(8), 5057–5065. [CrossRef]
- Nicolì, V., Tabano, S. M., Colapietro, P., Maestri, M., Ricciardi, R., Stoccoro, A., Fontana, L., Guida, M., Miozzo, M., Coppedè, F., & Migliore, L. (2022). Preferential X Chromosome Inactivation as a Mechanism to Explain Female Preponderance in Myasthenia Gravis. Genes, 13(4), 696. [CrossRef]
- Asmail, A., Kesler, A., Kolb, H., Drory, V. E., & Karni, A. (2019). A tri-modal distribution of age-of-onset in female patients with myasthenia gravis is associated with the gender-related clinical differences. The International journal of neuroscience, 129(4), 313–319. [CrossRef]
- Marx, A., Pfister, F., Schalke, B., Saruhan-Direskeneli, G., Melms, A., & Ströbel, P. (2013). The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmunity reviews, 12(9), 875–884. [CrossRef]
- Gradolatto, A., Nazzal, D., Foti, M., Bismuth, J., Truffault, F., Le Panse, R., & Berrih-Aknin, S. (2012). Defects of immunoregulatory mechanisms in myasthenia gravis: Role of IL-17. Annals of the New York Academy of Sciences, 1274, 40–47. [CrossRef]
- Molin, C.J., Westerberg, E. and Punga, A.R. (2016) ‘Profile of upregulated inflammatory proteins in sera of Myasthenia Gravis patients OPEN’. [CrossRef]
- Romi, F.R., Gilhus, N.E. and Luckman, S.P. (2008) ‘Serum matrix metalloproteinase-3 levels are elevated in myasthenia gravis’, Journal of Neuroimmunology, 195(1–2), 96–99. [CrossRef]
- Molin, C.J., Westerberg, E. and Punga, A.R. (2016) ‘Profile of upregulated inflammatory proteins in sera of Myasthenia Gravis patients OPEN’. [CrossRef]
- Berzi, A. et al. (2008) ‘BDNF and its receptors in human myasthenic thymus: Implications for cell fate in thymic pathology’, Journal of Neuroimmunology, 197(2), pp. 128–139. [CrossRef]
- Schirò, G., Iacono, S., Ragonese, P., Aridon, P., Salemi, G., & Balistreri, C. R. (2022). A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis?. Frontiers in neurology, 13, 917527. [CrossRef]
- Sabre, L., Evoli, A. and Punga, A.R. (2019) ‘Cognitive dysfunction in mice with passively induced MuSK antibody seropositive myasthenia gravis’, Journal of the neurological sciences, 399, 15–21. [CrossRef]
- Cortés-Vicente, E. et al. (2020a) ‘Clinical and therapeutic features of myasthenia gravis in adults based on age at onset’, Neurology, 94(11), e1171. [CrossRef]
- Lazaridis, K., & Tzartos, S. J. (2020). Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Frontiers in neurology, 11, 596981. [CrossRef]
- Živković, S.A., Clemens, P.R. and Lacomis, D. (2012) ‘Characteristics of late-onset myasthenia gravis’, Journal of Neurology, 259(10), 2167–2171. [CrossRef]
- Szczudlik, P., Szyluk, B., Lipowska, M., Ryniewicz, B., Kubiszewska, J., Dutkiewicz, M., Gilhus, N. E., & Kostera-Pruszczyk, A. (2014). Antititin antibody in early- and late-onset myasthenia gravis. Acta neurologica Scandinavica, 130(4), 229–233. [CrossRef]
- Vitturi, B. K., Kim, A. I. H., Mitre, L. P., Pellegrinelli, A., & Valerio, B. C. O. (2021). Social, professional and neuropsychiatric outcomes in patients with myasthenia gravis. Neurological sciences : Official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(1), 167–173. [CrossRef]
- Kaltsatou, A., Fotiou, D., Tsiptsios, D., & Orologas, A. (2015). Cognitive impairment as a central cholinergic deficit in patients with Myasthenia Gravis. BBA clinical, 3, 299–303. [CrossRef]
- Zhou, X., Cao, S., Hou, J., Gui, T., Zhu, F., & Xue, Q. (2023). Association between myasthenia gravis and cognitive disorders: A PRISMA-compliant meta-analysis. The International journal of neuroscience, 133(9), 987–998. [CrossRef]
- Law, C., Flaherty, C. V., & Bandyopadhyay, S. (2020). A Review of Psychiatric Comorbidity in Myasthenia Gravis. Cureus, 12(7), e9184. [CrossRef]
- Tarawneh, R., D'Angelo, G., Crimmins, D., Herries, E., Griest, T., Fagan, A. M., Zipfel, G. J., Ladenson, J. H., Morris, J. C., & Holtzman, D. M. (2016). Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA neurology, 73(5), 561–571. [CrossRef]
- Dong, D., Chong, M. K., Wu, Y., Kaminski, H., Cutter, G., Xu, X., Li, H., Zhao, C., Yin, J., Yu, S., & Zhu, J. (2020). Gender differences in quality of life among patients with myasthenia gravis in China. Health and quality of life outcomes, 18(1), 296. [CrossRef]
- Wang, J., Yan, C., Zhao, Z., Chen, H., Shi, Z., Du, Q., Zhang, Y., Qiu, Y., Lang, Y., Kong, L., Cai, L., & Zhou, H. (2021). Sexual dysfunction in patients with myasthenia gravis. Journal of neuroimmunology, 358, 577669. [CrossRef]
- Tannemaat, M. R., Huijbers, M. G., & Verschuuren, J. J. G. M. (2024). Myasthenia gravis-Pathophysiology, diagnosis, and treatment. Handbook of clinical neurology, 200, 283–305. [CrossRef]
- Crisafulli, S., Boccanegra, B., Carollo, M., Bottani, E., Mantuano, P., Trifirò, G., & De Luca, A. (2024). Myasthenia Gravis Treatment: From Old Drugs to Innovative Therapies with a Glimpse into the Future. CNS drugs, 38(1), 15–32. [CrossRef]
- Matic, A., Alfaidi, N., & Bril, V. (2023). An evaluation of rozanolixizumab-noli for the treatment of anti-AChR and anti-MuSK antibody-positive generalized myasthenia gravis. Expert opinion on biological therapy, 23(12), 1163–1171. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).