Submitted:
22 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
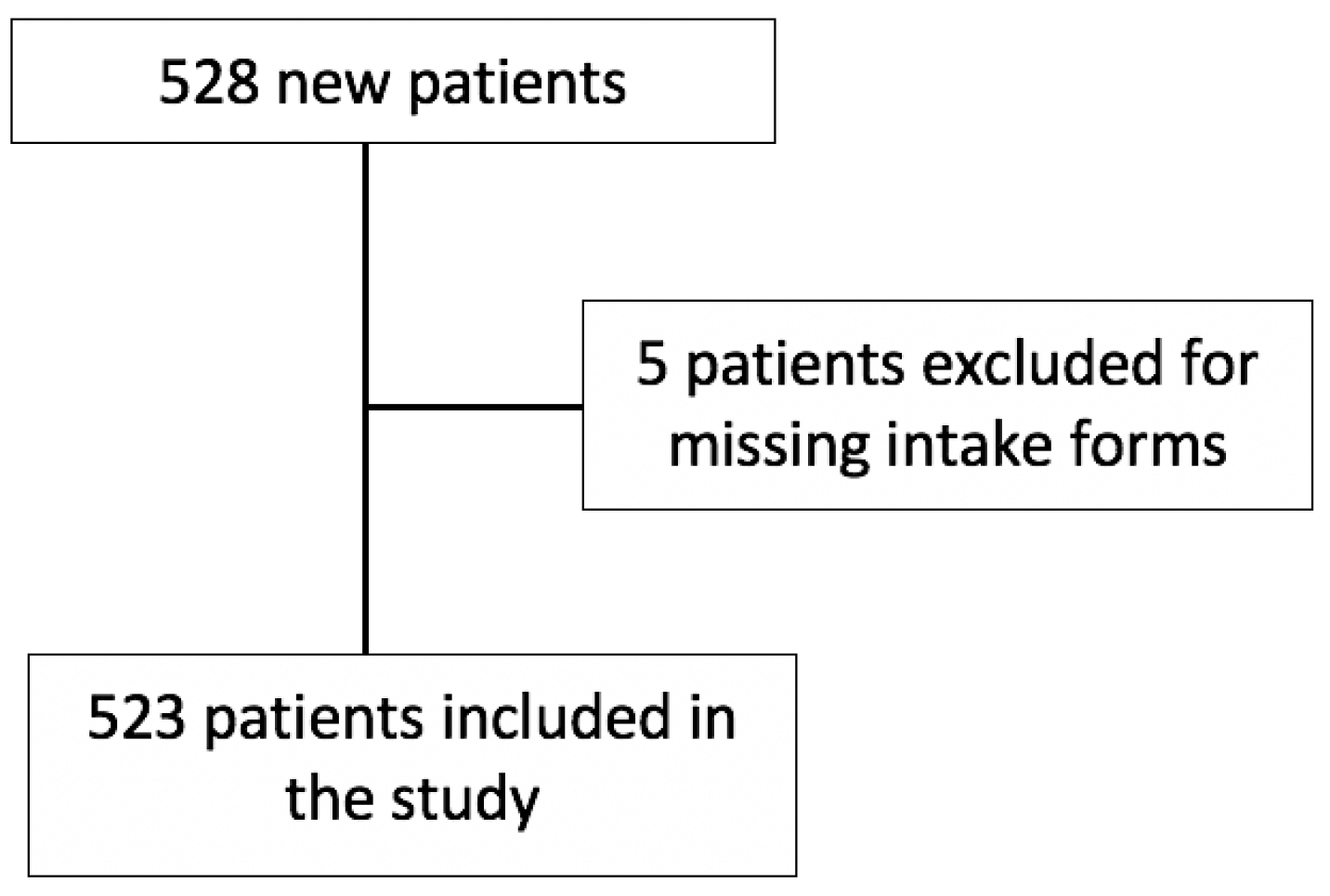
2.1. Study Design
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, N., Kucharczyk, K. M., Estes, J. L., Gerber, R. S., Lekovich, J. P., Elias, R. T., & Spandorfer, S. D. (2015). Human papillomavirus infection, infertility, and assisted reproductive outcomes. Journal of pathogens, 2015. [CrossRef]
- Centers for Disease Control and Prevention. (2022). Genital HPV Infection–Basic Fact Sheet.
- Moody, C. A., & Laimins, L. A. (2010). Human papillomavirus oncoproteins: Pathways to transformation. Nature Reviews Cancer, 10(8), 550-560. [CrossRef]
- Crosbie, E. J., Einstein, M. H., Franceschi, S., & Kitchener, H. C. (2013). Human papillomavirus and cervical cancer. The Lancet, 382(9895), 889-899. [CrossRef]
- Centers for Disease Control and Prevention (CDC. (2012). Human papillomavirus-associated cancers-United States, 2004-2008. MMWR: Morbidity & Mortality Weekly Report, 61(15).
- Harper, D.M. and DeMars, L.R., 2017. HPV vaccines–a review of the first decade. Gynecologic oncology, 146(1), pp.196-204. [CrossRef]
- Pingali, C. (2023). Vaccination coverage among adolescents aged 13–17 years—National immunization survey–teen, United States, 2022. MMWR. Morbidity and Mortality Weekly Report, 72. [CrossRef]
- Dilley, S., Miller, K. M., & Huh, W. K. (2020). Human papillomavirus vaccination: Ongoing challenges and future directions. Gynecologic oncology, 156(2), 498-502. [CrossRef]
- World Health Organization. (2020). Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization.
- Bruni, L., Saura-Lázaro, A., Montoliu, A., Brotons, M., Alemany, L., Diallo, M. S., ... & Bloem, P. (2021). HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Preventive medicine, 144, 106399. [CrossRef]
- Escoffery, C., Petagna, C., Agnone, C., Perez, S., Saber, L. B., Ryan, G., ... & Fernandez, M. E. (2023). A systematic review of interventions to promote HPV vaccination globally. BMC Public Health, 23(1), 1262. [CrossRef]
- Dykens, J. A., Peterson, C. E., Holt, H. K., & Harper, D. M. (2023). Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Frontiers in Public Health, 11, 1067299. [CrossRef]
- Isaguliants, M., Krasnyak, S., Smirnova, O., Colonna, V., Apolikhin, O., & Buonaguro, F. M. (2021). Genetic instability and anti-HPV immune response as drivers of infertility associated with HPV infection. Infectious Agents and Cancer, 16(1), 1-18. [CrossRef]
- Souho, T., Benlemlih, M., & Bennani, B. (2015). Human papillomavirus infection and fertility alteration: A systematic review. PLoS ONE, 10(5), e0126936. [CrossRef]
- Zacharis, K., Messini, C. I., Anifandis, G., Koukoulis, G., Satra, M., & Daponte, A. (2018). Human papilloma virus (HPV) and fertilization: A mini review. Medicina, 54(4), 50. [CrossRef]
- Farsimadan, M., & Motamedifar, M. (2021). The effects of human immunodeficiency virus, human papillomavirus, herpes simplex virus-1 and-2, human herpesvirus-6 and-8, cytomegalovirus, and hepatitis B and C virus on female fertility and pregnancy. British Journal of Biomedical Science, 78(1), 1-11. [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. (2018). Current recommendations for vaccines for female infertility patients: A committee opinion. Fertility and Sterility, 110(5), 838-841. [CrossRef]
- Garolla, A., De Toni, L., Bottacin, A., Valente, U., De Rocco Ponce, M., Di Nisio, A., & Foresta, C. (2018). Human Papillomavirus Prophylactic Vaccination improves reproductive outcome in infertile patients with HPV semen infection: A retrospective study. Scientific reports, 8(1), 1-9. [CrossRef]
- Gomez, L. M., Ma, Y., Ho, C., McGrath, C. M., Nelson, D. B., & Parry, S. (2008). Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Human reproduction, 23(3), 709-715. [CrossRef]
- Huang, Q. T., Zhong, M., Gao, Y. F., Huang, L. P., Huang, Q., Wang, W., ... & Yu, Y. H. (2014). Can HPV vaccine have other health benefits more than cancer prevention? A systematic review of association between cervical HPV infection and preterm birth. Journal of Clinical Virology, 61(3), 321-328. [CrossRef]
- Bonde, U., Joergensen, J. S., Mogensen, O., & Lamont, R. F. (2014). The potential role of HPV vaccination in the prevention of infectious complications of pregnancy. Expert Review of Vaccines, 13(11), 1307-1316. [CrossRef]
- Yuill, S., Egger, S., Smith, M., Velentzis, L., Wrede, C. D., Bateson, D., & Canfell, K. (2020). Has human papillomavirus (HPV) vaccination prevented adverse pregnancy outcomes? Population-level analysis after 8 years of a national HPV vaccination program in Australia. The Journal of infectious diseases, 222(3), 499-508. [CrossRef]
- Schmuhl, Nicholas B.; et al. "No association between HPV vaccination and infertility in US females 18–33 years old." Vaccine 38.24 (2020): 4038-4043. [CrossRef]
- McInerney, K. A., Hatch, E. E., Wesselink, A. K., Mikkelsen, E. M., Rothman, K. J., Perkins, R. B., & Wise, L. A. (2017). The effect of vaccination against human papillomavirus on fecundability. Paediatric and perinatal epidemiology, 31(6), 531-536. [CrossRef]
- Ciavattini, A., Marconi, C., Giannella, L., Delli Carpini, G., Sopracordevole, F., & Di Giuseppe, J. (2021). The Impact of 9-Valent HPV Vaccination on Couple Infertility Prevention: A Comprehensive Review. Frontiers in Medicine, 1332. [CrossRef]
- Estevez, S. L., Tarrash, M., Brownridge, S. R., Goldman, R. H., & Mullin, C. (2021). HPV Vaccination rate amongst women presenting for fertility care. Fertility and Sterility, 116(3), e404. [CrossRef]
- Lichter, K., Krause, D., Xu, J., Tsai, S. H. L., Hage, C., Weston, E., ... & Levinson, K. (2020). Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: A systematic review and meta-analysis. Obstetrics & Gynecology, 135(5), 1070-1083. [CrossRef]
- Di Donato, V., Caruso, G., Petrillo, M., Kontopantelis, E., Palaia, I., Perniola, G., ... & Bogani, G. (2021). Adjuvant HPV vaccination to prevent recurrent cervical dysplasia after surgical treatment: A meta-analysis. Vaccines, 9(5), 410. [CrossRef]
- Bruni, L., Albero, G., Rowley, J., Alemany, L., Arbyn, M., Giuliano, A. R., ... & Taylor, M. (2023). Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. The Lancet Global Health, 11(9), e1345-e1362. [CrossRef]
- Giuliano, A. R., Anic, G., & Nyitray, A. G. (2010). Epidemiology and pathology of HPV disease in males. Gynecologic oncology, 117(2), S15-S19. [CrossRef]
- Lyu, Z., Feng, X., Li, N., Zhao, W., Wei, L., Chen, Y., ... & Dai, M. (2017). Human papillomavirus in semen and the risk for male infertility: A systematic review and meta-analysis. BMC infectious diseases, 17(1), 1-9. [CrossRef]
- Xiong, Y. Q., Chen, Y. X., Cheng, M. J., He, W. Q., & Chen, Q. (2018). The risk of human papillomavirus infection for male fertility abnormality: A meta-analysis. Asian journal of andrology, 20(5), 493. [CrossRef]
- Moghimi, M., Zabihi-Mahmoodabadi, S., Kheirkhah-Vakilabad, A., & Kargar, Z. (2019). Significant correlation between high-risk HPV DNA in semen and impairment of sperm quality in infertile men. International Journal of Fertility & Sterility, 12(4), 306. [CrossRef]
- Garolla, A., Engl, B., Pizzol, D., Ghezzi, M., Bertoldo, A., Bottacin, A., ... & Foresta, C. (2016). Spontaneous fertility and in vitro fertilization outcome: New evidence of human papillomavirus sperm infection. Fertility and Sterility, 105(1), 65-72. [CrossRef]
- Garolla, A., Pizzol, D., Bertoldo, A., Menegazzo, M., Barzon, L., & Foresta, C. (2013). Sperm viral infection and male infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. Journal of reproductive immunology, 100(1), 20-29. [CrossRef]
- Yang, Y., Jia, C. W., Ma, Y. M., Zhou, L. Y., & Wang, S. Y. (2013). Correlation between HPV sperm infection and male infertility. Asian journal of andrology, 15(4), 529. [CrossRef]
- Foresta, C., Noventa, M., De Toni, L., Gizzo, S., & Garolla, A. (2015). HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology, 3(2), 163-173. [CrossRef]
- Foresta, C., Pizzol, D., Moretti, A., Barzon, L., Palù, G., & Garolla, A. (2010). Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertility and Sterility, 94(5), 1723-1727. [CrossRef]
- Weinberg, M., Nahshon, C. S. S., Feferkorn, I., & Bornstein, J. (2020). Evaluation of human papilloma virus in semen as a risk factor for low sperm quality and poor in vitro fertilization outcomes: A systematic review and meta-analysis. Fertility and sterility, 113(5), 955-969. [CrossRef]
- Bezold, G., Politch, J. A., Kiviat, N. B., Kuypers, J. M., Wolff, H., & Anderson, D. J. (2007). Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertility and sterility, 87(5), 1087-1097. [CrossRef]
- Kaspersen, M. D., Larsen, P. B., Ingerslev, H. J., Fedder, J., Petersen, G. B., Bonde, J., & Höllsberg, P. (2011). Identification of multiple HPV types on spermatozoa from human sperm donors. PLoS ONE, 6(3), e18095. [CrossRef]
- Jeršovienė, V., Gudlevičienė, Ž., Rimienė, J., & Butkauskas, D. (2019). Human papillomavirus and infertility. Medicina, 55(7), 377. [CrossRef]
- Damke, E., Kurscheidt, F. A., Balani, V. A., Takeda, K. I., Irie, M. M., Gimenes, F., & Consolaro, M. E. (2017). Male partners of infertile couples with seminal infections of human papillomavirus have impaired fertility parameters. BioMed Research International, 2017. [CrossRef]
- Martorell, M., Gil-Salom, M., Pérez-Vallés, A., Garcia, J. A., Rausell, N., & Senpere, A. (2005). Presence of human papillomavirus DNA in testicular biopsies from nonobstructive azoospermic men. Archives of Pathology & Laboratory Medicine, 129(9), 1132-1136. [CrossRef]
- Foresta, C., Pizzol, D., Bertoldo, A., Menegazzo, M., Barzon, L., & Garolla, A. (2011). Semen washing procedures do not eliminate human papilloma virus sperm infection in infertile patients. Fertility and Sterility, 96(5), 1077-1082. [CrossRef]
- Garolla, A., Pizzol, D., & Foresta, C. (2011). The role of human papillomavirus on sperm function. Current Opinion in Obstetrics and Gynecology, 23(4), 232-237. [CrossRef]
- Garolla, A., Engl, B., Pizzol, D., Ghezzi, M., Bertoldo, A., Bottacin, A., ... & Foresta, C. (2016). Spontaneous fertility and in vitro fertilization outcome: New evidence of human papillomavirus sperm infection. Fertility and sterility, 105(1), 65-72. [CrossRef]
- Foresta, C., Garolla, A., Parisi, S., Ghezzi, M., Bertoldo, A., Di Nisio, A., & De Toni, L. (2015). HPV prophylactic vaccination in males improves the clearance of semen infection. EBioMedicine, 2(10), 1487-1493. [CrossRef]
- Chido-Amajuoyi, O. G., Domgue, J. F., Obi-Jeff, C., Schmeler, K., & Shete, S. (2019). A call for the introduction of gender-neutral HPV vaccination to national immunisation programmes in Africa. The Lancet Global Health, 7(1), e20-e21. [CrossRef]
- Linertová, R., Guirado-Fuentes, C., Mar-Medina, J., & Teljeur, C. (2022). Cost-effectiveness and epidemiological impact of gender-neutral HPV vaccination in Spain. Human Vaccines & Immunotherapeutics, 18(6), 2127983. [CrossRef]
- Lechner, M., Jones, O. S., Breeze, C. E., & Gilson, R. (2019). Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. The Lancet Infectious Diseases, 19(2), 131-132. [CrossRef]
- Simons, J. J., Vida, N., Westra, T. A., & Postma, M. J. (2020). Cost-effectiveness analysis of a gender-neutral human papillomavirus vaccination program in the Netherlands. Vaccine, 38(30), 4687-4694. [CrossRef]
- Palmer, C., Tobe, K., Negishi, Y., You, X., Chen, Y. T., & Abe, M. (2023). Health impact and cost effectiveness of implementing gender-neutral HPV vaccination in Japan. Journal of Medical Economics, 26(1), 1546-1554. [CrossRef]
- Simoens, S., Bento-Abreu, A., Merckx, B., Joubert, S., Vermeersch, S., Pavelyev, A., ... & Morais, E. (2021). Health impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent human papillomavirus vaccine in Belgium. Frontiers in Pharmacology, 12, 628434. [CrossRef]
- Wähner, C., Hübner, J., Meisel, D., Schelling, J., Zingel, R., Mihm, S., ... & Reuschenbach, M. (2023). Uptake of HPV vaccination among boys after the introduction of gender-neutral HPV vaccination in Germany before and during the COVID-19 pandemic. Infection, 1-12. [CrossRef]
- Cheung, T. H., Cheng, S. S. Y., Hsu, D., Wing-Lei Wong, Q., Pavelyev, A., Sukarom, I., & Saxena, K. (2023). Health impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent HPV vaccine in Hong Kong. Human Vaccines & Immunotherapeutics, 2184605. [CrossRef]
- Thanasas, I., Lavranos, G., Gkogkou, P., & Paraskevis, D. (2020). Understanding of young adolescents about HPV infection: How health education can improve vaccination rate. Journal of Cancer Education, 35, 850-859. [CrossRef]
| Type of HPV vaccine | HPV strains covered |
|---|---|
| Quadrivalent HPV vaccine | 6, 11, 16, 18 |
| 9-valent HPV vaccine | 6, 11, 16, 18, 31, 33, 45, 52, 58 |
|
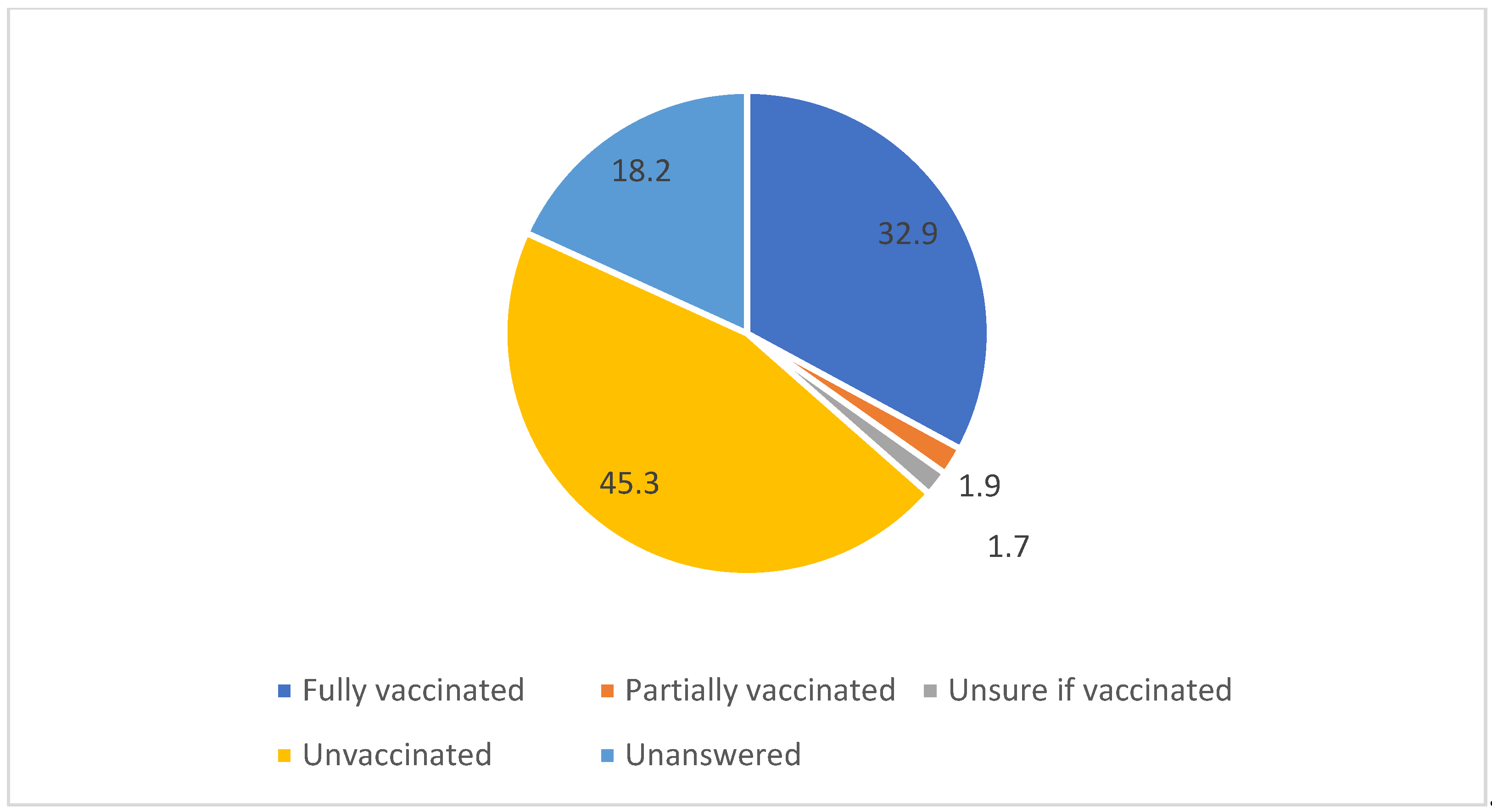
Vaccinated n=172 (32.9%) |
Unvaccinated n=237 (45.3%) |
p-value | |
|---|---|---|---|
|
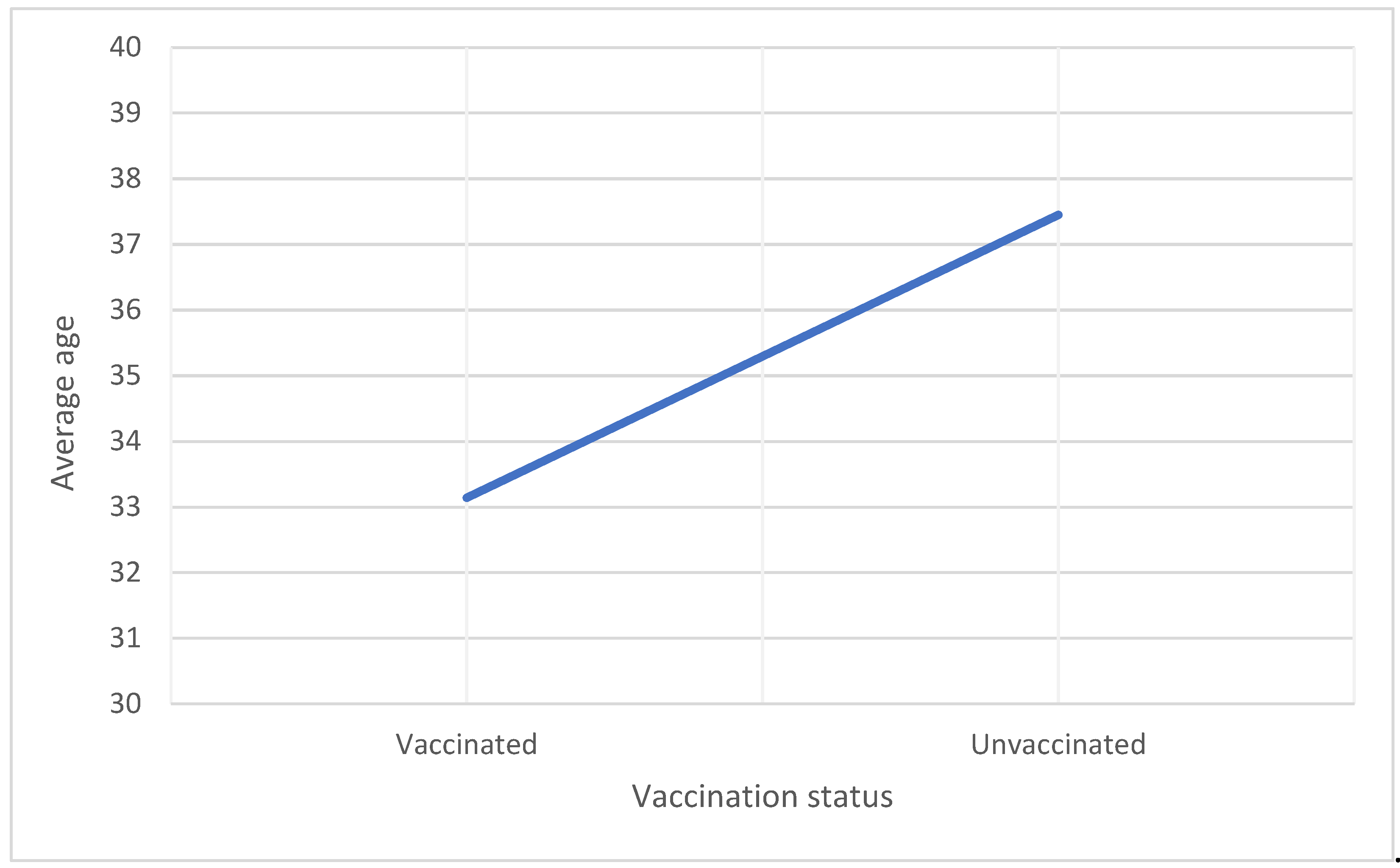
Average age (years) |
33.14 ± 4.65 |
37.45 ± 5.54 |
p=<0.001 |
|
History of abnormal Pap smear (n) |
49 (28.5%) |
53 (22.4%) | p=0.098 |
|
Relationship Status Married (n) |
115 (66.9%) |
159 (67.1%) |
p=0.961 |
| Gravidity |
0.65 |
1.11 |
p=0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





