Submitted:
23 April 2024
Posted:
25 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Possible Mechanisms
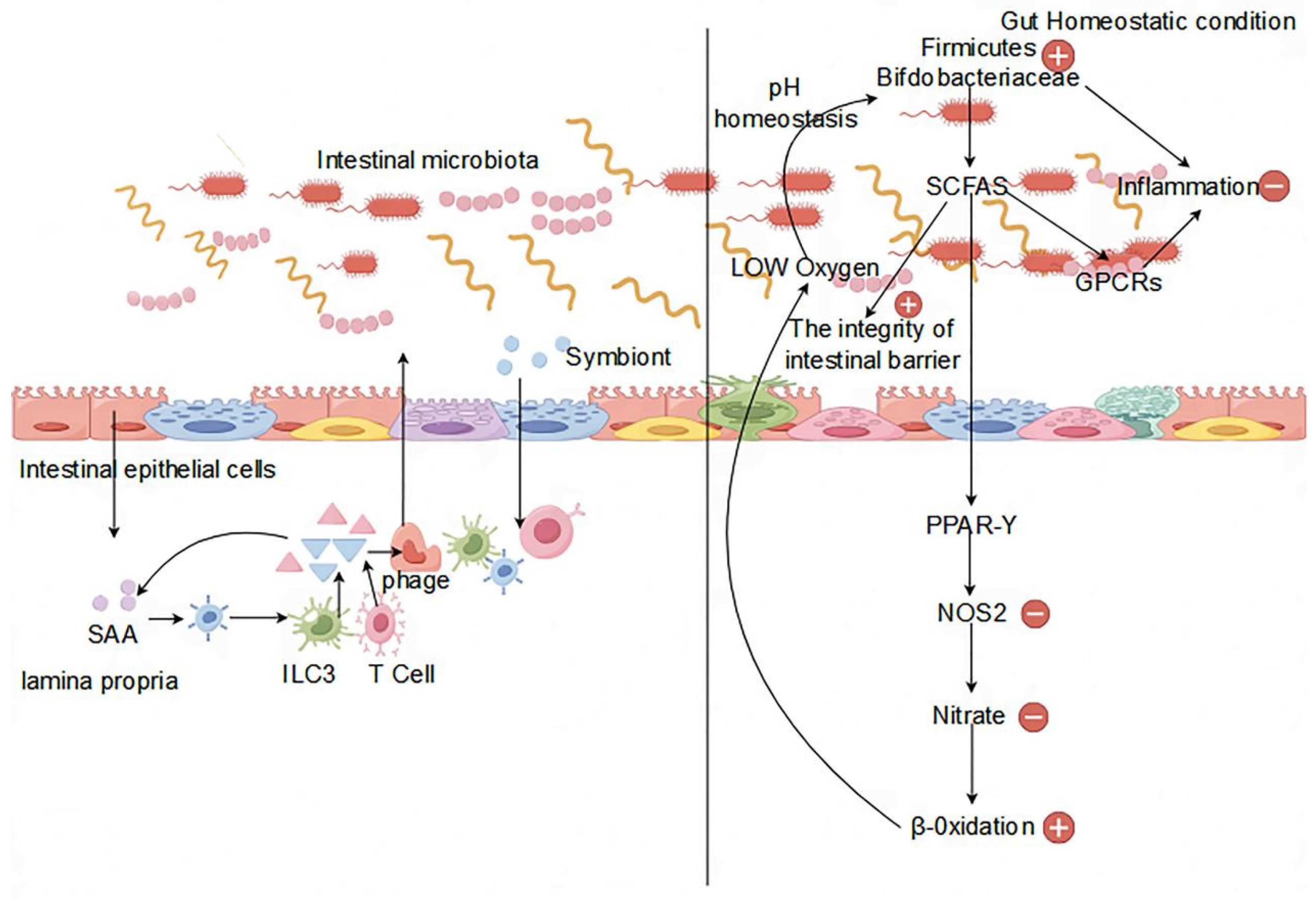
2.1. Regulation of the Immune System
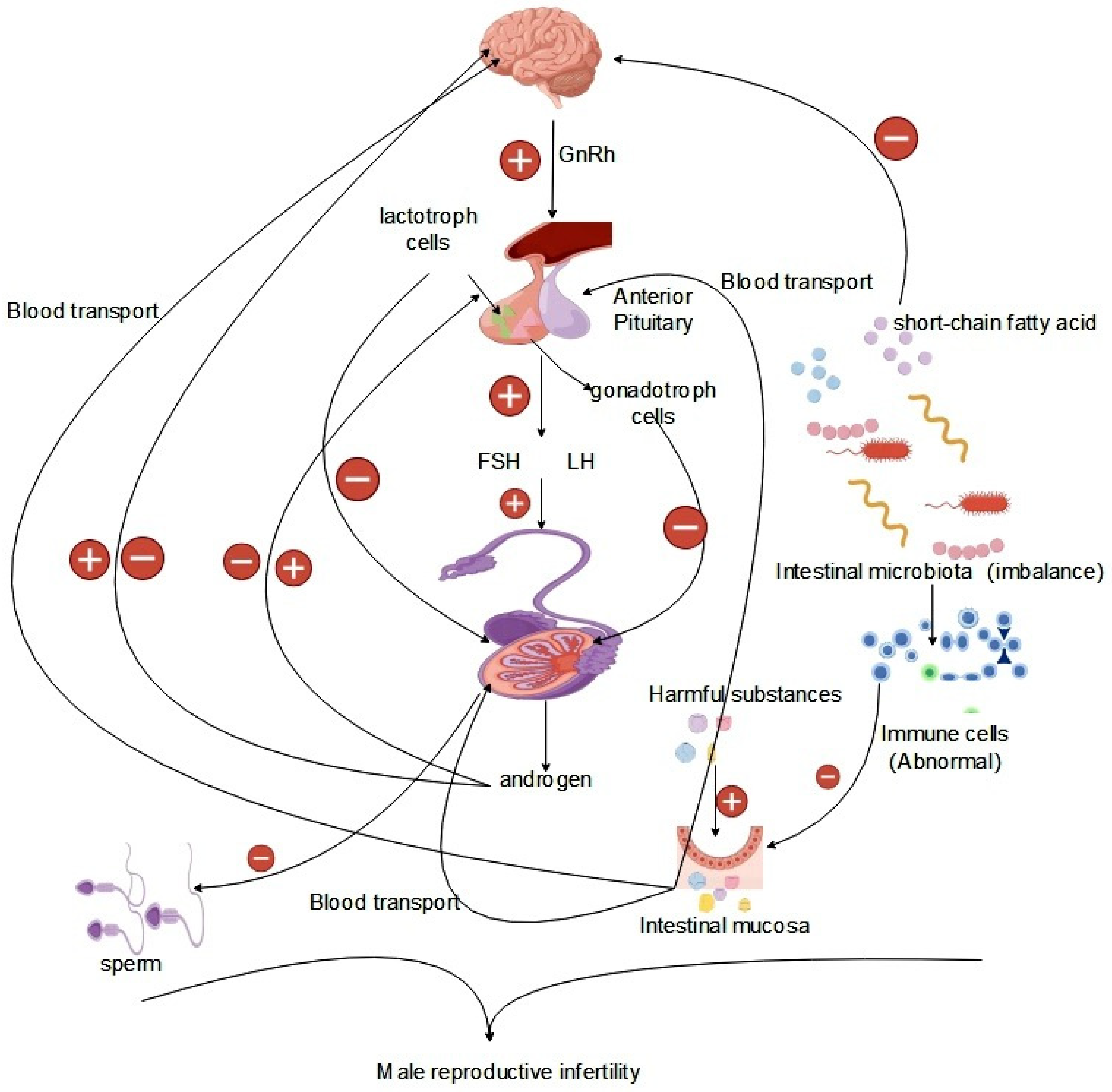
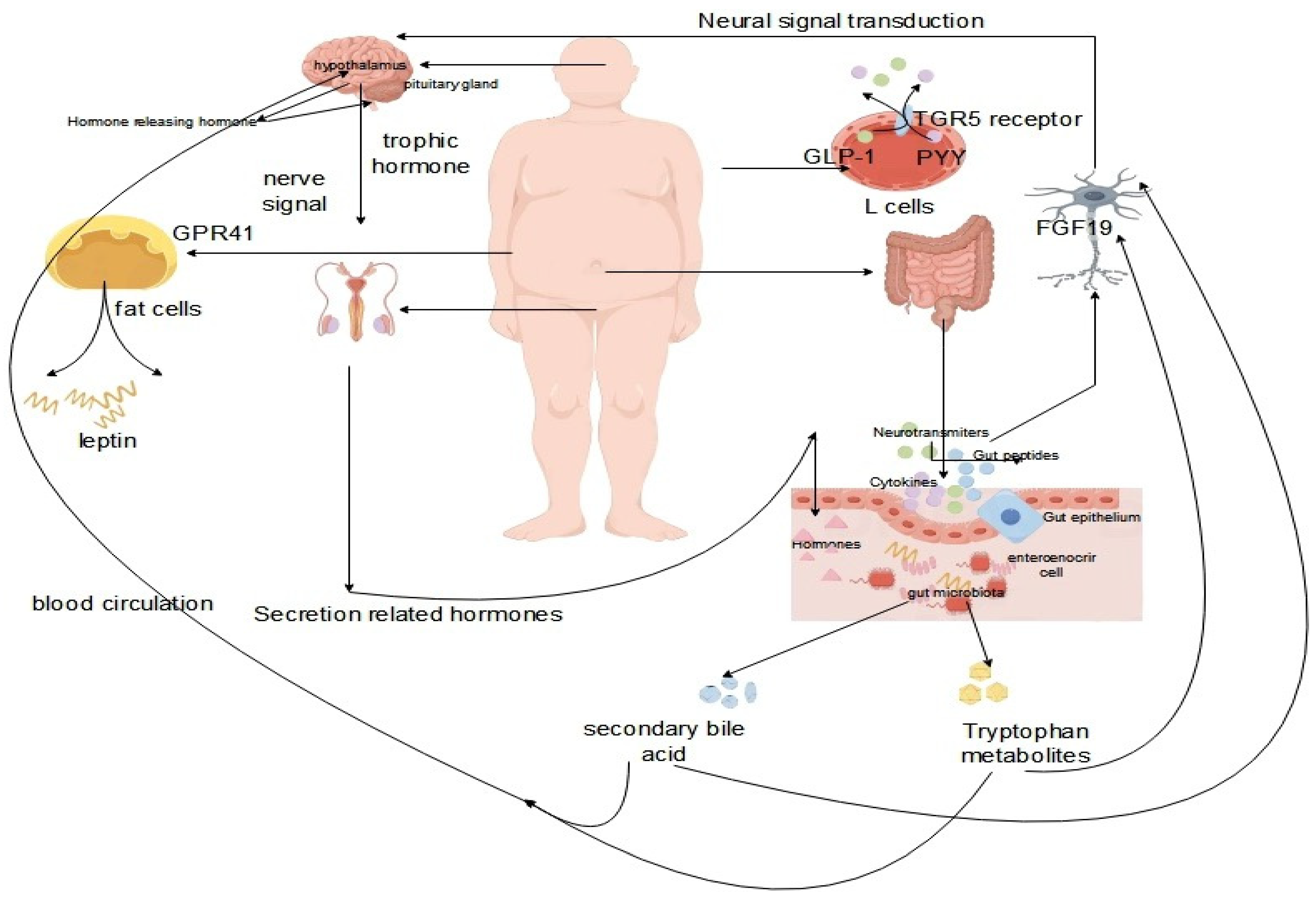
2.2. Effects of the Endocrine System
2.3. Interaction of the Gut-Brain-Reproductive Axis
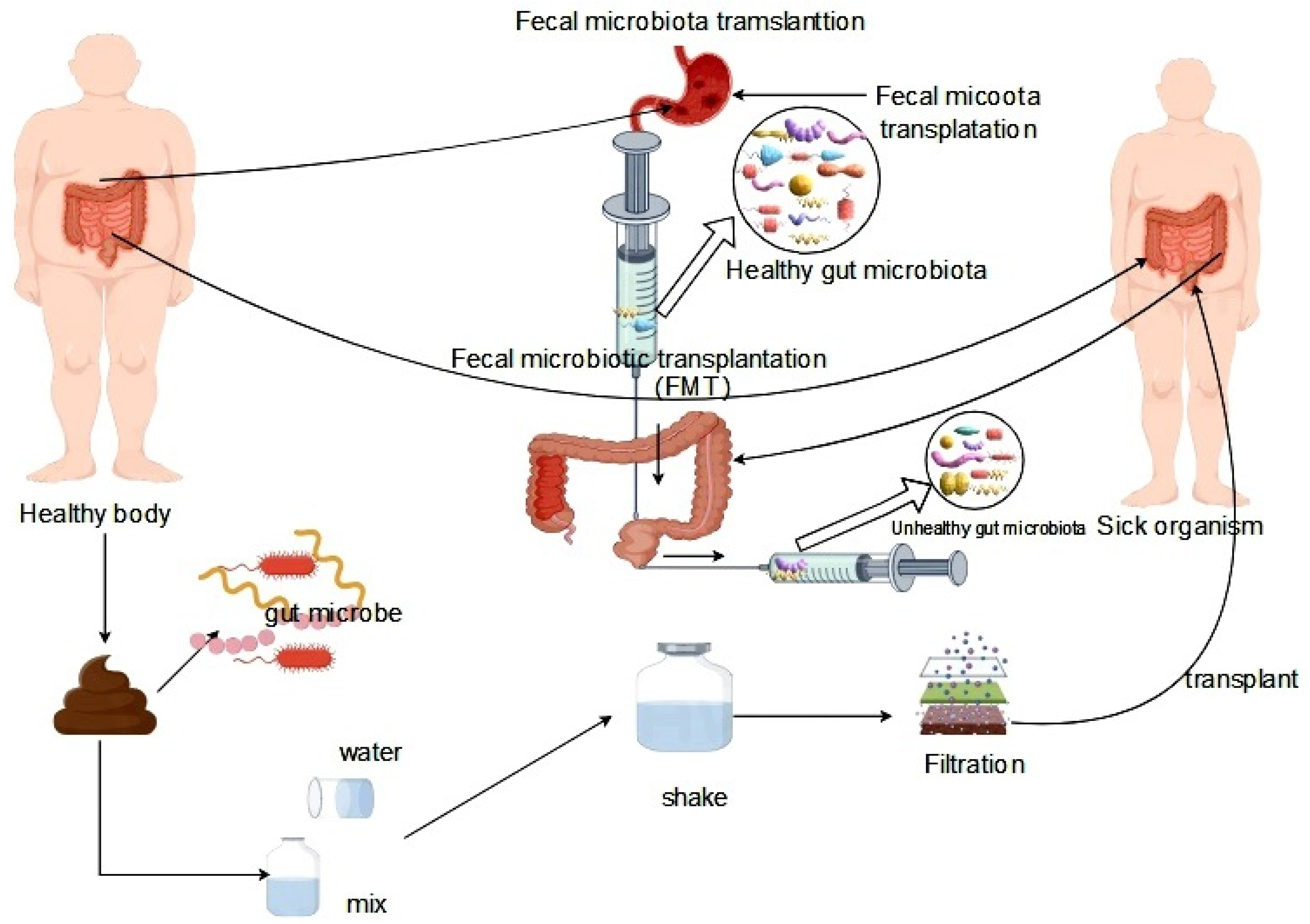
3. Intervention and Treatment Strategies
4. Challenges and Future Research Directions
5. Conclusions
References
- Jabeen, Z.; Bukhari, S.A.; Malik, S.A.; Hussain, G.; Kamal, S. Improved Gut Microbiota Escalates Muscle Function Rehabilitation and Ameliorates Oxidative Stress Following Mechanically Induced Peripheral Nerve Injury in Mice. The Pakistan Veterinary Journal 2023, 43, 707–713. [Google Scholar]
- de Theije, C.G.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. BRAIN BEHAV IMMUN 2014, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A 2016, 113, E7554–E7563. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. IMMUNITY 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Rossi, V.; Massini, G.; Regalbuto, C.; Hruby, C.; Panelli, S.; Bandi, C.; Zuccotti, G. Precocious puberty and microbiota: The role of the sex hormone-gut microbiome axis. Front Endocrinol (Lausanne) 2022, 13, 1000919. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Greenlee, A.R.; Taub, C.J.; Braun, R.E. Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. BIOL REPROD 2011, 85, 254–260. [Google Scholar] [CrossRef]
- Plant, T.M. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J ENDOCRINOL 2015, 226, T41–T54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, W.; Shang, H.; Wei, H.; Deng, C. The Interplay between Androgen and Gut Microbiota: Is There a Microbiota-Gut-Testis Axis. REPROD SCI 2022, 29, 1674–1684. [Google Scholar] [CrossRef]
- Li, Y.; Mehmood, K.; Chang, Y.F.; Guo, R.; Shang, P.; Zhang, H. Antibiotic resistance genes in Bacillus cereus isolated from wild Pere David’s deer (Elaphurus davidianus). J Infect 2021, 83, 709–737. [Google Scholar] [CrossRef]
- Reid, G. Probiotic Lactobacilli for urogenital health in women. J CLIN GASTROENTEROL 2008, 42 Pt 2 Suppl 3, S234–S236. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. FRONT MICROBIOL 2016, 7, 925. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.J. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Yu, J.; Yu, J.; Hu, Z. Autophagy and the Immune Response. ADV EXP MED BIOL 2019, 1206, 595–634. [Google Scholar]
- Eberl, G.; Pradeu, T. Towards a General Theory of Immunity? TRENDS IMMUNOL 2018, 39, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Kanneganti, T.D. The innate immune system and cell death in autoinflammatory and autoimmune disease. CURR OPIN IMMUNOL 2020, 67, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Li, Y.; Zhao, H.; He, C.; Wang, Z.; Zhao, H. Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis. Front Cell Infect Microbiol 2021, 11, 736204. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Mohr, A.E.; Crawford, M.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: considering structural variation. FEBS LETT 2022, 596, 849–875. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. IMMUNOL REV 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, W.; Xue, S.; Han, D. Testicular defense systems: immune privilege and innate immunity. CELL MOL IMMUNOL 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Wang, M.; Fijak, M.; Hossain, H.; Markmann, M.; Nusing, R.M.; Lochnit, G.; Hartmann, M.F.; Wudy, S.A.; Zhang, L.; Gu, H.; Konrad, L.; Chakraborty, T.; Meinhardt, A.; Bhushan, S. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J IMMUNOL 2017, 198, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Ponte, R.; Dupuy, F.P.; Brimo, F.; Mehraj, V.; Brassard, P.; Belanger, M.; Yurchenko, E.; Jenabian, M.A.; Bernard, N.F.; Routy, J.P. Characterization of myeloid cell populations in human testes collected after sex reassignment surgery. J REPROD IMMUNOL 2018, 125, 16–24. [Google Scholar] [CrossRef]
- Wang, J.; Wreford, N.G.; Lan, H.Y.; Atkins, R.; Hedger, M.P. Leukocyte populations of the adult rat testis following removal of the Leydig cells by treatment with ethane dimethane sulfonate and subcutaneous testosterone implants. BIOL REPROD 1994, 51, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: a new regulatory model. J REPROD IMMUNOL 2013, 97, 147–158. [Google Scholar] [CrossRef]
- Yee, J.B.; Hutson, J.C. Effects of testicular macrophage-conditioned medium on Leydig cells in culture. ENDOCRINOLOGY 1985, 116, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D.; Lukyanenko, Y.O.; Jia, Z.H.; Quideau, S.; Howald, W.N.; Pratum, T.K.; West, R.R.; Hutson, J.C. Identification of the lipophilic factor produced by macrophages that stimulates steroidogenesis. ENDOCRINOLOGY 2000, 141, 953–958. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.; Smith, L.B. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab 2015, 29, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Toocheck, C.; Clister, T.; Shupe, J.; Crum, C.; Ravindranathan, P.; Lee, T.K.; Ahn, J.M.; Raj, G.V.; Sukhwani, M.; Orwig, K.E.; Walker, W.H. Mouse Spermatogenesis Requires Classical and Nonclassical Testosterone Signaling. BIOL REPROD 2016, 94, 11. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. CELL REP 2015, 12, 1107–1119. [Google Scholar] [CrossRef]
- Walecki, M.; Eisel, F.; Klug, J.; Baal, N.; Paradowska-Dogan, A.; Wahle, E.; Hackstein, H.; Meinhardt, A.; Fijak, M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. MOL BIOL CELL 2015, 26, 2845–2857. [Google Scholar] [CrossRef]
- Wang, P.; Duan, Y.G. The role of dendritic cells in male reproductive tract. AM J REPROD IMMUNOL 2016, 76, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. IMMUNOL REV 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; Hoeksema, M.A.; de Vos, A.F.; de Winther, M.P. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. CELL REP 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Involvement of intratesticular IL-1 system in the regulation of Sertoli cell functions. MOL CELL ENDOCRINOL 2002, 187, 125–132. [Google Scholar] [CrossRef]
- Bhushan, S.; Meinhardt, A. The macrophages in testis function. J REPROD IMMUNOL 2017, 119, 107–112. [Google Scholar] [CrossRef]
- Murakami, G.; Tanabe, N.; Ishii, H.T.; Ogiue-Ikeda, M.; Tsurugizawa, T.; Mukai, H.; Hojo, Y.; Takata, N.; Furukawa, A.; Kimoto, T.; Kawato, S. Role of cytochrome p450 in synaptocrinology: endogenous estrogen synthesis in the brain hippocampus. DRUG METAB REV 2006, 38, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Pathophysiology of the Effects of Alcohol Abuse on the Endocrine System. Alcohol Res 2017, 38, 255–276. [Google Scholar]
- Ellsworth, B.S.; Stallings, C.E. Molecular Mechanisms Governing Embryonic Differentiation of Pituitary Somatotropes. Trends Endocrinol Metab 2018, 29, 510–523. [Google Scholar] [CrossRef]
- Estermann, M.A.; Major, A.T.; Smith, C.A. Gonadal Sex Differentiation: Supporting Versus Steroidogenic Cell Lineage Specification in Mammals and Birds. Front Cell Dev Biol 2020, 8, 616387. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Karkada, I.R.; Chinni, S.V. Endocrinopathies and Male Infertility. Life (Basel) 2021, 12. [Google Scholar] [CrossRef]
- Wu, N.; Mah, C.; Koentgen, S.; Zhang, L.; Grimm, M.C.; El-Omar, E.; Hold, G.L. Inflammatory bowel disease and the gut microbiota. P NUTR SOC 2021, 80, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Liu, Y.; Yi, J.; Li, Y.; Li, Q.; Li, Y.; Shang, P.; Guo, J.; Hu, L.; Pan, J.; Li, Y.; Chang, Y.F.; Tang, Z.; Zhang, H. Gut microbiota disturbance exaggerates battery wastewater-induced hepatotoxicity through a gut-liver axis. SCI TOTAL ENVIRON 2022, 809, 152188. [Google Scholar] [CrossRef] [PubMed]
- Ferasyi, T.R.; Barrett, P.H.; Blache, D.; Martin, G.B. Modeling the Male Reproductive Endocrine Axis: Potential Role for a Delay Mechanism in the Inhibitory Action of Gonadal Steroids on GnRH Pulse Frequency. ENDOCRINOLOGY 2016, 157, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Kaprara, A.; Huhtaniemi, I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. METABOLISM 2018, 86, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S.; Elliott, J.A. Opioid-induced androgen deficiency (OPIAD). PAIN PHYSICIAN 2012, 15, S145–S156. [Google Scholar]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. SEMIN CELL DEV BIOL 2014, 30, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Cobellis, G. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front Endocrinol (Lausanne) 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chang, Y.F.; Liu, J. Editorial: Regulation of Mitochondrial Function on Animal Diseases. Front Vet Sci 2022, 9, 943860. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. NAT REV UROL 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Dolci, S. Paracrine mechanisms involved in the control of early stages of Mammalian spermatogenesis. Front Endocrinol (Lausanne) 2013, 4, 181. [Google Scholar] [CrossRef]
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. HISTOL HISTOPATHOL 2016, 31, 499–513. [Google Scholar]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, J.; Sa, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; Socorro, S. Influence of 5alpha-dihydrotestosterone and 17beta-estradiol on human Sertoli cells metabolism. Int J Androl 2011, 34, e612–e620. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: formation, function, and regulation. BIOL REPROD 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Weckman, A.; Di Ieva, A.; Rotondo, F.; Syro, L.V.; Ortiz, L.D.; Kovacs, K.; Cusimano, M.D. Autophagy in the endocrine glands. J MOL ENDOCRINOL 2014, 52, R151–R163. [Google Scholar] [CrossRef] [PubMed]
- Organski, A.C.; Jorgensen, J.S.; Cross, T.L. Involving the life inside: The complex interplay between reproductive axis hormones and gut microbiota. Current Opinion in Endocrine and Metabolic Research 2021, 20, 100284. [Google Scholar] [CrossRef]
- Ullah, R.; Naz, R.; Batool, A.; Wazir, M.; Rahman, T.U.; Nabi, G.; Wahab, F.; Fu, J.; Shahab, M. RF9 Rescues Cortisol-Induced Repression of Testosterone Levels in Adult Male Macaques. FRONT PHYSIOL 2021, 12, 630796. [Google Scholar] [CrossRef] [PubMed]
- Dehdari, E.N.; Sadeghi, A.; Ala, M.; Ebrahimi, F.; Pakbaz, S.; Azarpira, N. Protective effects of melatonin against oxidative stress induced by metabolic disorders in the male reproductive system: a systematic review and meta-analysis of rodent models. Front Endocrinol (Lausanne) 2023, 14, 1202560. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.; Devereaux, B.; Dornhorst, A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol 2019, 34, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. FERTIL STERIL 2017, 107, 848–859. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Marsiglia, R.; Marangelo, C.; Vernocchi, P.; Scanu, M.; Pane, S.; Russo, A.; Guanziroli, E.; Del, C.F.; Valeriani, M.; Molteni, F.; Putignani, L. Gut Microbiota Ecological and Functional Modulation in Post-Stroke Recovery Patients: An Italian Study. Microorganisms 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. DIABETES 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. CELL 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Jo, Y.H.; Li, X.; Schwartz, G.J.; Zhang, Y.; Dun, N.J.; Lyu, R.M.; Blouet, C.; Chang, J.K.; Chua, S.J. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. MOL METAB 2014, 3, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Lee, S.; Ma, L.; Zhang, D.; Schlessinger, J.; Shulman, G.I. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. NAT COMMUN 2015, 6, 6980. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, M.; Balsari, A.; Rossini, A.; Selleri, S.; Calcaterra, C.; Gariboldi, S.; Zanobbio, L.; Arnaboldi, F.; Shirai, Y.F.; Serrao, G.; Rumio, C. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J IMMUNOL 2007, 178, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. BRAIN BEHAV IMMUN 2005, 19, 334–344. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. NAT REV NEUROSCI 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. CELL HOST MICROBE 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. ANNU REV IMMUNOL 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Zinselmeyer, B.H.; Corps, K.N.; McGavern, D.B. In vivo dynamics of innate immune sentinels in the CNS. Intravital 2012, 1, 95–106. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J NEUROENDOCRINOL 2018, 30, e12590. [Google Scholar] [CrossRef]
- Takahashi, A.; Kanda, S.; Abe, T.; Oka, Y. Evolution of the Hypothalamic-Pituitary-Gonadal Axis Regulation in Vertebrates Revealed by Knockout Medaka. ENDOCRINOLOGY 2016, 157, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J ENDOCRINOL 2015, 226, T41–T54. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Leone, M.; Boecker, H.; Sprenger, T.; Juergens, T.; Bussone, G.; Tolle, T.R. Hypothalamic deep brain stimulation in positron emission tomography. J NEUROSCI 2006, 26, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol 2009, 161, 20–29. [Google Scholar] [CrossRef]
- Nozaki, M. Hypothalamic-pituitary-gonadal endocrine system in the hagfish. Front Endocrinol (Lausanne) 2013, 4, 200. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Yu, Y.X.; Cao, S.Y.; Bai, X. Epigenetics of inflammation in hypothalamus pituitary gonadal and neuroendocrine disorders. SEMIN CELL DEV BIOL 2024, 154, 340–345. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, S.; Liu, K.; Li, Y.; Mehmood, K.; Nazar, M.; Hu, L.; Pan, J.; Tang, Z.; Liao, J.; Zhang, H. miR-181b-1-3p affects the proliferation and differentiation of chondrocytes in TD broilers through the WIF1/Wnt/beta-catenin pathway. Pestic Biochem Physiol 2023, 197, 105649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yi, J.; Li, Y.; Hussain, R.; Zhu, S.; Li, Y.; Ouyang, Z.; Mehmood, K.; Hu, L.; Pan, J.; Tang, Z.; Li, Y.; Zhang, H. Residue of thiram in food, suppresses immune system stress signals and disturbs sphingolipid metabolism in chickens. Vet Immunol Immunopathol 2022, 247, 110415. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Li, Y.; Yi, J.; Yang, B.; Li, Y.; Ouyang, Z.; Liu, B.; Shang, P.; Mehmood, K.; Abbas, R.Z.; Ahmed, S.; Chang, Y.F.; Guo, J.; Pan, J.; Hu, L.; Tang, Z.; Li, Y.; Zhang, H. The potential risks of herbicide butachlor to immunotoxicity via induction of autophagy and apoptosis in the spleen. CHEMOSPHERE 2022, 286, 131683. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Li, Y.; Tang, Z.; Li, A.; Zhang, H. Molecular mechanism of thiram-induced abnormal chondrocyte proliferation via lncRNA MSTRG.74.1-BNIP3 axis. PESTIC BIOCHEM PHYS 2024, 201, 105847. [Google Scholar] [CrossRef]
- Wu, S.; Liu, K.; Huang, X.; Sun, Q.; Wu, X.; Mehmood, K.; Li, Y.; Zhang, H. Molecular mechanism of miR-203a targeting Runx2 to regulate thiram induced-chondrocyte development. PESTIC BIOCHEM PHYS 2024, 200, 105817. [Google Scholar] [CrossRef]
- Sindi, R.A.; Alam, S.; Rizwan, M.; Ullah, M.I.; Ijaz, N.; Iqbal, Z.; Muzafar, R.; Akram, R.; Nazar, M.W.; Hussain, R. Investigations of Hemato-Biochemical, Histopathological, Oxidative Stress and Reproductive Effects of Thiram in Albino Rats. The Pakistan Veterinary Journal 2023, 43, 255–261. [Google Scholar]
- Gyawali, I.; Zhou, G.; Xu, G.; Li, G.; Wang, Y.; Zeng, Y.; Li, J.; Zhou, J.; Zhu, C.; Shu, G.; Jiang, Q. Supplementation of microencapsulated probiotics modulates gut health and intestinal microbiota. FOOD SCI NUTR 2023, 11, 4547–4561. [Google Scholar] [CrossRef] [PubMed]
- Galanis, A. Shaping the Future of Probiotics: Novel Methodologies, Applications, and Mechanisms of Action. Microorganisms 2023, 12. [Google Scholar] [CrossRef]
- Gul, S.T.; Alsayeqh, A.F. Probiotics as an Alternative Approach to Antibiotics for Safe Poultry Meat Production. The Pakistan Veterinary Journal 2022, 43, 285–291. [Google Scholar]
- Hu, S.; Wang, L.; Jiang, Z. Dietary Additive Probiotics Modulation of the Intestinal Microbiota. Protein Pept Lett 2017, 24, 382–387. [Google Scholar] [CrossRef]
- Chibbar, R.; Dieleman, L.A. Probiotics in the Management of Ulcerative Colitis. J CLIN GASTROENTEROL 2015, 49 Suppl 1, S50–S55. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. ANNU REV MED 2019, 70, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gou, X.; Ding, Y.; Liu, J.; Wang, Y.; Wang, Y.; Zhang, J.; Du, L.; Peng, W.; Fan, G. The interplay between herbal medicines and gut microbiota in metabolic diseases. FRONT PHARMACOL 2023, 14, 1105405. [Google Scholar] [CrossRef] [PubMed]
| Classification | Composition | Function |
|---|---|---|
| Bacteria | Probiotics | Maintain intestinal pH balance, inhibit pathogen growth and reproduction, and promote digestion and absorption. |
| Lactobacilli | Reduce intestinal pH value, inhibit the growth of harmful bacteria. | |
| Thick-walled bacteria | Influence the degradation and absorption of intestinal food. | |
| morphogenic bacteria | Plays an important role in the carbohydrate metabolism process in the intestinal tract. | |
| Enterococci | Regulate intestinal health and the immune system. | |
| Actinomycetes | Synthesize antibiotics. | |
| Streptococcus faecalis | Short-chain fatty acids and other metabolites that contribute to maintaining homeostasis in the intestinal environment. | |
| Fungi | Yeasts | Assist in the intestinal digestive process. |
| Viruses | Poliovirus | causes intestinal infections, leading to an immune response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





