Submitted:
23 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: biodiversity and infestation patterns. Diversity 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Totti, C.; Romagnoli, T.; De Stefano, M.; Camillo, D.C.G.; Bavestrello, G. The diversity of epizoic Diatoms. In All Flesh is Grass. Cellular origin, life in extreme habitats and astrobiology; Dubinsky, Z., Seckbach, J., Eds.; Springer: Dordrecht, Netherlands, 2010; Volume 16. [Google Scholar] [CrossRef]
- Tiffany, M.A. Epizoic and Epiphytic Diatoms. In The Diatom World; Seckbach, J., Kociolek, J.P., Eds.; Springer: New York, USA, 2011; pp. 197–209. [Google Scholar] [CrossRef]
- Wujek, D.E. Epizoic Diatoms on the cerci of Ephemenoptera (Caenidae) Naiads. Great Lakes Entomol. 2013, 46. [Google Scholar] [CrossRef]
- Cribb, A.B. Algae on the Hawks-Bill turtle. Qsl. Nat. 1969, 19, 108–109. [Google Scholar]
- Croll, D.A.; Holmes, R-W. A note on the occurrence of diatoms on the feathers of diving seabirds. Auk 1982, 99, 765–766. [Google Scholar] [CrossRef]
- Denys, L. Morphology and taxonomy of epizoic diatoms (Epiphalaina and Tursiocola) on a sperm whale (Physeter macrocephalus) stranded on the coast of Belgium. Diatom Res. 1997, 12, 1–18. [Google Scholar] [CrossRef]
- Hart, T.J. On the diatoms of the skin film of whales, and their possible bearing on problems of whale movements. Discov. Rep. 1935, 10, 247–282. [Google Scholar]
- Holmes, R.W. The Morphology of diatoms epizoic on Cetaceans and their transfer from Cocconeis to two new genera, Bennettella and Epipellis. Br. Phycol. J. 1985, 20, 43–57. [Google Scholar] [CrossRef]
- Becker, K. . Epibionts on carapaces of some Malacostracans from the Gulf of Thailand. J. Crustacean Biol. 1996, 16, 92–104. [Google Scholar] [CrossRef]
- Gaiser, E.E.; Bachmann, R.W. The ecology and taxonomy of epizoic diatoms on Cladocera. Limnol. Oceanogr. 1993, 38, 628–637. [Google Scholar] [CrossRef]
- Gibson, R.A. Pseudohimantidium pacificum, an epizoic diatom new to the Florida current (western North Atlantic Ocean). J. Phyc. 1978, 14, 371–373. [Google Scholar] [CrossRef]
- Prasad, A.K.S.K.; Livingston, R.J.; Ray, G.L. The marine epizoic diatom Falcula hyaline from Chactawatchee Bay, the northeastern Gulf of Mexico: frustule morphology and ecology. Diatom Res. 1989, 4, 119–129. [Google Scholar] [CrossRef]
- Russell, D.J.; Norris, R.E. Ecology and taxonomy of an epizoic diatom. Pac. Sci. 1971, 25, 357–367. [Google Scholar]
- Key, M.M.; Winston, J.E.; Volpe, J.W.; Jeffries, W.B.; Voris, H.K. Briozoan fouling of the blue crab Callinectes sapidus at Beaufort, North Caroline. Bull. Mar. Sci. 1999, 64, 513–533. [Google Scholar]
- Sánchez-Vargas, D.P.; Hendrickx, M.E. Utilization of algae and sponges by tropical decorating crabs (Majidae) in the Southeastern Gulf of California. Rev. Biol. Trop. 1987, 35, 161–164. [Google Scholar]
- Madkour, F.F.; Sallam, W.S.; Wicksten, M.K. Epibiota of the spider crab Schizophrys dahlak (Brachyura: Majidae) from the Suez Canal with special reference to epizoic diatoms. Mar. Biodivers. Rec. 2012, 5, e64. [Google Scholar] [CrossRef]
- Patil, J. S, Anil, A.C. Epibiotic community of the horseshoe crab Tachypleus gigas. Mar Biol. 2000, 136, 699–713. [Google Scholar] [CrossRef]
- Escamilla-Montes, R.; De la Cruz-Agüero, G.; Villalejo-Fuerte, M.T.; Duarte-Plata, G. Fecundidad de Callinectes arcuatus (Ordway, 1863) y C. bellicosus (Stimpson, 1859) (Decapoda: Brachyura: Portunidae) en la Ensenada de La Paz, Golfo de California, México. Universidad y Ciencia 2013, 29, 53–61. [Google Scholar]
- Hendrickx, M.E. Cangrejos. In Guía FAO para la identificación de especies para los fines de la pesca. Pacífico centro-oriental. Volumen I. Plantas e invertebrados; Fischer, W., Krupp, F., Schneider, W., Sommer, C., Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italia, 1995; Volume 1, pp. 565–636. [Google Scholar]
- Hernández, L.; Arreola-Lizárraga, J.A. Estructura de tallas y crecimiento de los cangrejos Callinectes arcuatus y C. bellicosus (Decapoda: Portunidae) en la laguna costera Las Guásimas, México. Rev. Biol. Trop. 2007, 55, 225–233. [Google Scholar]
- Hines, A.H.; Lipcius, R.N.; Haddon, A.M. Population dynamics and habitat partitioning by size, sex and molt stage of blue crab Callinectes sapidus in a subestuary of central Chesapeake Bay. Mar. Ecol. 1987, 36, 55–64. [Google Scholar] [CrossRef]
- McLay, C.L. Dispersal and use of sponges and ascidian camouflage by Cryptodromia hilgendorfi (Brachyura: Decapoda). Mar. Biol. 1983, 76, 17–32. [Google Scholar] [CrossRef]
- Sato, M.; Wada, K. Resource utilization for decorating in three intertidal Majidae crabs (Brachyura: Majidae). Mar. Biol. 2000, 137, 705–714. [Google Scholar] [CrossRef]
- López-Fuerte, F.O.; Siqueiros Beltrones, D.A. A checklist of marine benthic diatoms (Bacillariophyta) from Mexico. Phytotaxa 2016, 283, 201–258. [Google Scholar] [CrossRef]
- Cleve-Euler, A. Die Diatomeen von Schweden und Finnland. Part V. (Schluss.). K. Sven. Vetensk. Akad. Handl. Ser. 4 1952, 3, 1–153. [Google Scholar]
- Cleve-Euler, A. Die Diatomeen von Schweden un Finnland. I–V. K. Sven. Vetensk. Akad. Handl. Ser. 4 1953, 4, 1–158. [Google Scholar]
- Cleve-Euler, A. Die Diatomeen von Schweden un Finnland. I–V. K. Sven. Vetensk. Akad. Handl. Ser. 4 1953, 4, 1–255. 4.
- Cleve-Euler, A. Die Diatomeen von Schweden und Finnland. Part IV. Biraphideae 2. K. Sven. Vetensk. Akad. Handl. Ser. 4 1955, 5(4), 1-232, figs 971-1306.
- Foged, N. Some littoral diatoms from the coasts of Tanzania. Biblioth. Phycol. 1975, 16, 1–127. [Google Scholar]
- Foged, N. Diatoms in Eastern Australia. Biblioth. Phycol. 1978, 41, 1–243. [Google Scholar]
- Foged, N. Freshwater and littoral diatoms from Cuba. Biblioth. Diatom. 1984, 5, 1–242. [Google Scholar]
- Hustedt, F. Die kieselalgen Deutschlands, Osterreichs and der Schweis, Bacillariophyta (Diatomae) Heft 10. In Die Süsswasser-Flora Mitteleuropas; Pascher, A., Ed.; Gustav Fischer: Jena, Germany, 1930; p. 466. [Google Scholar]
- Hustedt, F. Die kieselalgen Deutschlands, Osterreichs and der Schweis. In Kryptogammen-Flora; VII Band, II Teil, Rabenhorts, L., Eds.; Koeltz Scientific Book: Leipzig, Germany, 1959; p. 845. [Google Scholar]
- Hustedt, F. Die kieselalgen Deutschlands, Osterreichs and der Schweis. In Kryptogammen-Flora; VII Band, III Teil, Rabenhorst, L., Eds.; Koeltz Scientific Book: Leipzig, Germany, 1966; p. 816. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Suesswasserflora von Mitteleuropa 2 (3). Gustav Fisher: Jena, Germany, 1991, 576 pp.
- Lange-Bertalot, H.; Krammer, K. Bacillariaceae, Epithemiaceae, Surirellaceae. Neue und wenig bekannte Taxa, neue Kombinationen und Synonyme sowie Bemerkungen und Ergänzungen zu den Naviculaceae. Biblioth. Diatom. 1987, 15, 1–289, 62 pls. [Google Scholar]
- Lange-Bertalot, H.; Kulbs, K.; Lauser, T.; Norpel-Schempp, M.; Willmann, M. Diatom taxa introduced by Georg Krasske: Documentation and revision. In Iconographia Diatomologica; Lange-Bertalot, H., Ed., Koeltz Scientific Books: Konigstein, Germany, 1996; Volume 3, 358p. [Google Scholar]
- Lobban, C.S.; Schefter, M.; Jordan, R.W.; Arai, Y.U.; Sasaki, A.S.; Theriot, E.C.; Ashworth, M.A.; Ruck, E.C.; Pennesi, C.H. Coral-reef diatoms (Bacillariophyta) from Guam: New records and preliminary checklist, with emphasis on epiphytic species from farmer-fish territories. Micronesica 2012, 43, 237–479. [Google Scholar]
- Loir, M.; Novarino, G. Marine Mastogloia Thwaites ex W. Sm. and Stigmaphora Wallich Species from the French Lesser Antille; Diatom Monographs, Koeltz Scientific Books: Königstein, Germany, 2013; Volume 16, p. 133. [Google Scholar]
- Moreno, J.L.; Licea, S.; Santoyo, H. Diatomeas del Golfo de California; Universidad Autónoma de Baja California Sur-SEP-FOMESPROMARCO: Tijuana, Mexico, 1996; p. 273. [Google Scholar]
- Peragallo, H.; Peragallo, M. Diatomées Marines de France et des Districts Maritimes Voisins; Tempère, J., Ed.; Micrographe-Editeur: À Grez-sur-Loing, France, 1908. [Google Scholar]
- Schmidt, A.; Schmidt, M.V.; Fricke, F.V.; Heiden, H.; Muller, O.; Hustedt, F. Atlas der Diatomaceenkunde; Heft 1–120, Tafeln 1–460; Reisland: Leipzig, Germany, 1959. [Google Scholar]
- Stidolph, S.R.; Sterrenburg, F.A.S.; Smith, K.E.L.; Kraberg, A.; Stuart, R. Stidolph Diatom Atlas. U.S. Geological Survey Open-File Report 2012-1163. Available online: http://pubs.usgs.gov/of/2012/1163/ (accessed on 1 December 2023).
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts. In Iconographia Diatomologica; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Königstein, Germany, 2000; pp. 1–925. [Google Scholar]
- Kingston, J.C. Araphid and monoraphid diatoms. In Freshwater Algae of North America, Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, California, USA, 2003; pp. 595–636. [Google Scholar]
- Kociolek, J.P.; Spaulding, S.A. Symmetrical naviculoid diatoms. In Freshwater Algae of North America, Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, California, USA, 2003; pp. 637–654. [Google Scholar]
- Medlin, L.K.; Kaczmarska, I. Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision Phycologia, 2004, 43, 245–270. [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. Algaebase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland. National University of Ireland: Galway. Available online: http://www.algaebase.org (accessed on 1 December 2023).
- WoRMS Editorial Board. World Register ofMarine Species. Available online: http://www.marinespecies.org (accessed on 1 December 2023).
- Siqueiros Beltrones, D.A. Diatomeas bentónicas asociadas a trombolitos vivos registrados por primera vez en México. CICIMAR Oceán. 2006, 21, 113–143. [Google Scholar]
- Siqueiros Beltrones, D.A.; Morzaria Luna, H. New records of marine benthic diatom species for the north-western Mexican region. Oceánides 1999, 14, 89–95. [Google Scholar]
- Siqueiros Beltrones, D.A.; Sánchez-Castrejón, E. Structure of benthic diatom assemblages from a mangrove environment in a Mexican Subtropical Lagoon. Biotropica 1999, 31, 48–70. [Google Scholar]
- López-Fuerte, F.O.; Siqueiros Beltrones, D.A.; Navarro, J.N. Benthic Diatoms Associated with Mangrove Environments in the Northwest Region of Mexico; CONABIO-UABCS-IPN: La Paz, Mexico, 2010; p. 217. [Google Scholar]
- López-Fuerte, F.; Siqueiros-Beltrones, D.; Jakes-Cota, U.; Tripp-Valdéz, A. New Records of Marine Diatoms for the American Continent Found on Stone Scorpionfish Scorpaena mystes. Open J. Mar. Sci. 2019, 9, 98–112. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; López-Fuerte, F.O.; Martínez, Y.J.; Altamirano-Cerecedo, M.C. A first estimate of species diversity for benthic diatom assemblages from the Revillagigedo Archipelago, Mexico. Diversity 2021, 13, 458. [Google Scholar] [CrossRef]




|
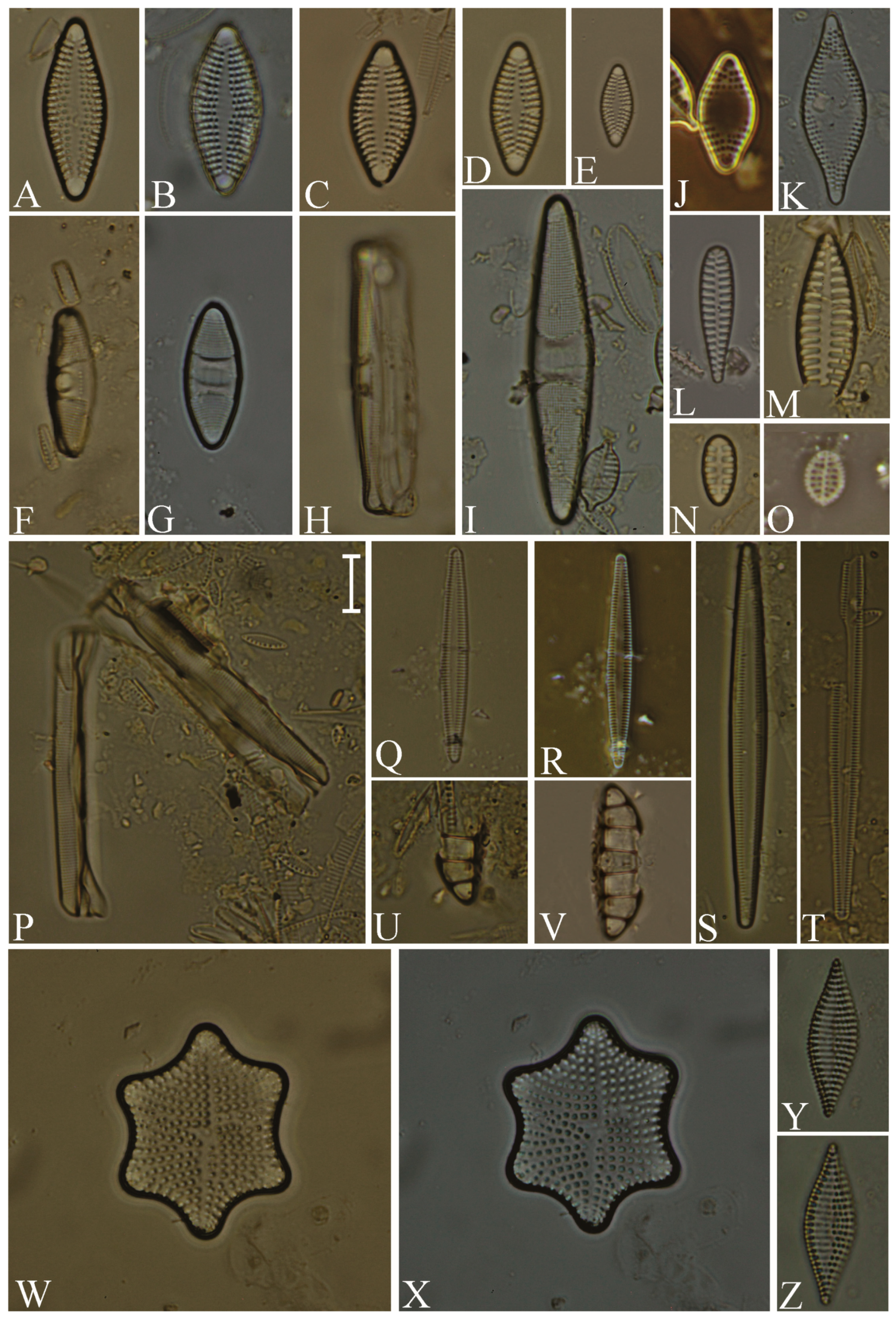
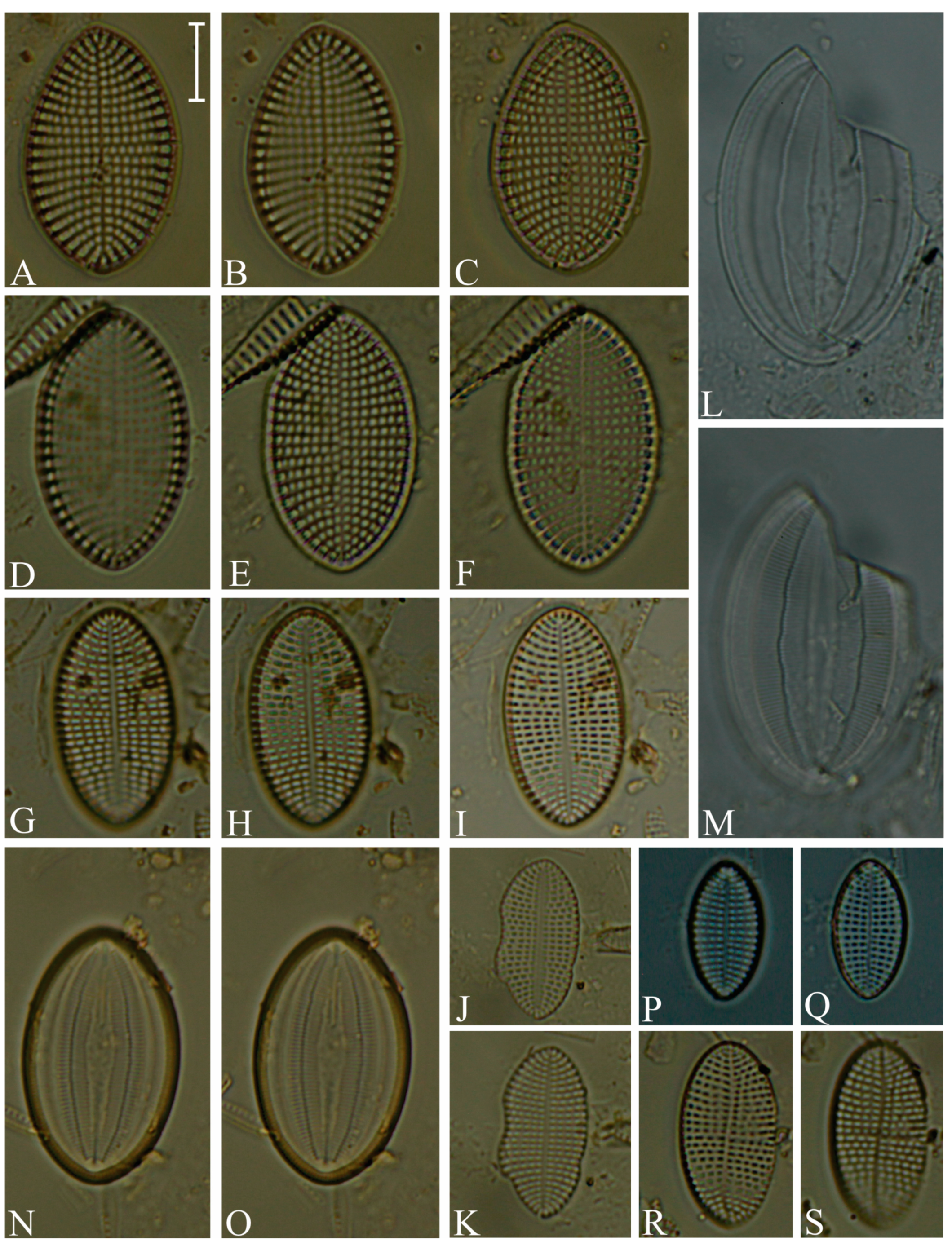
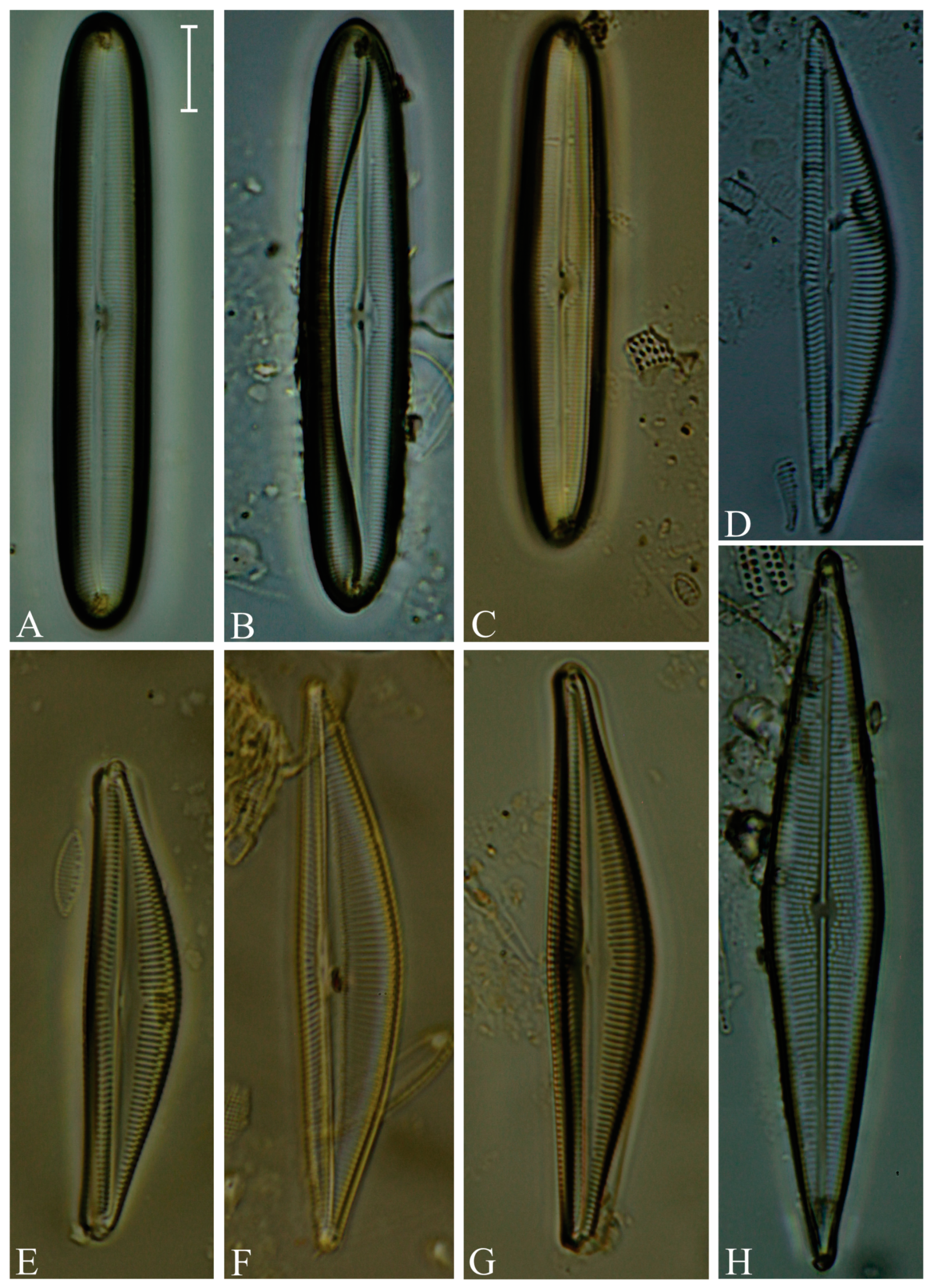
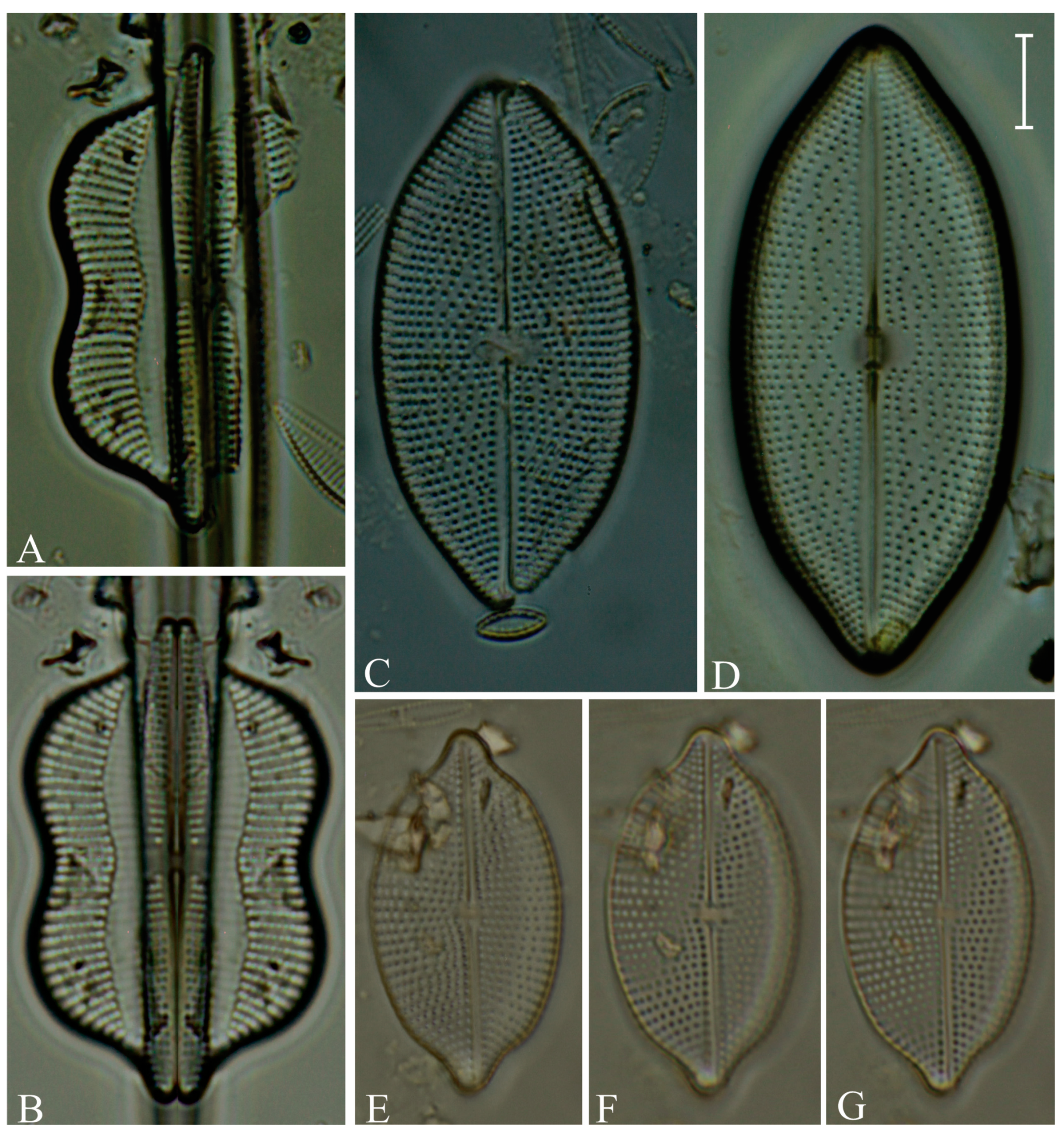
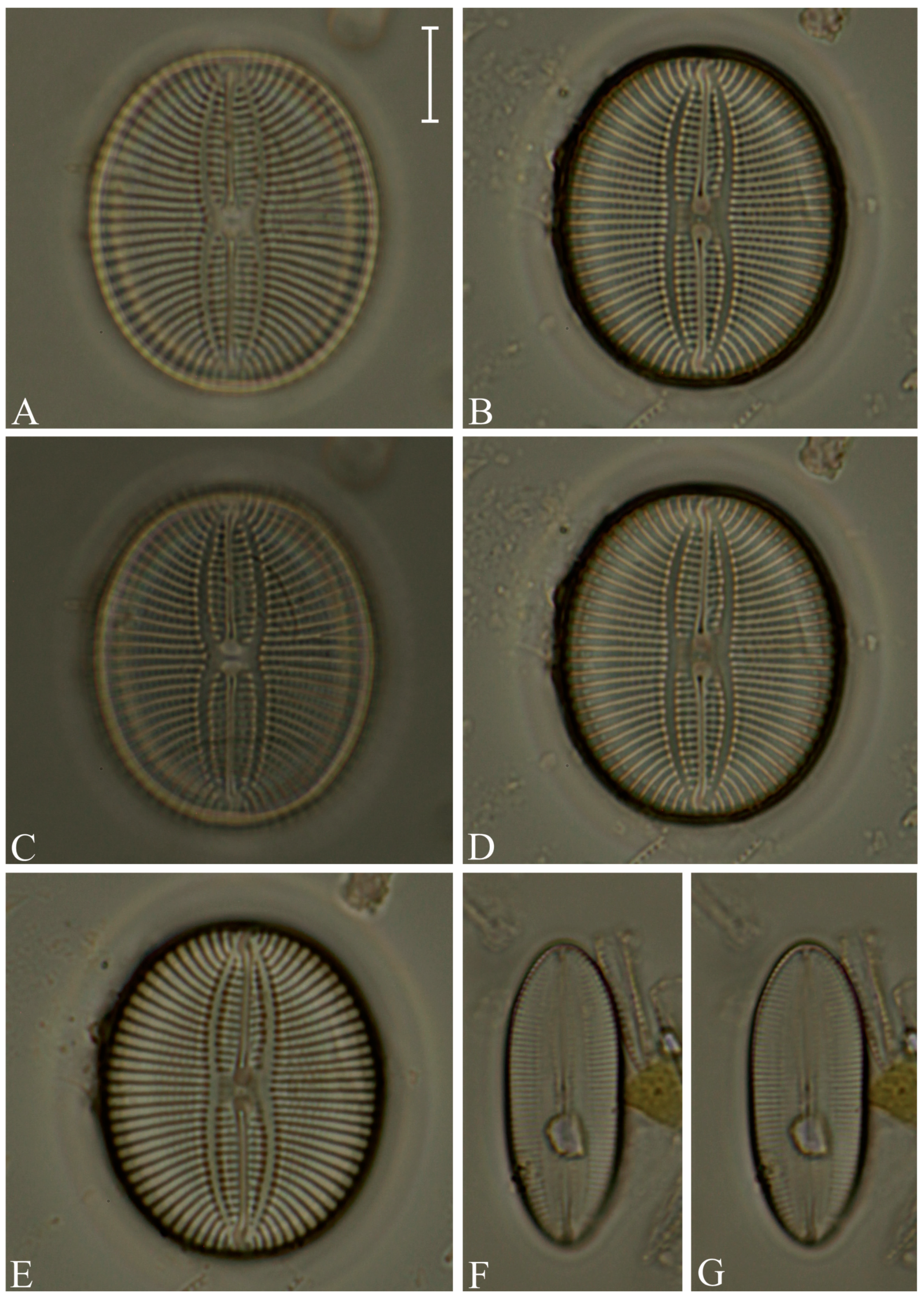
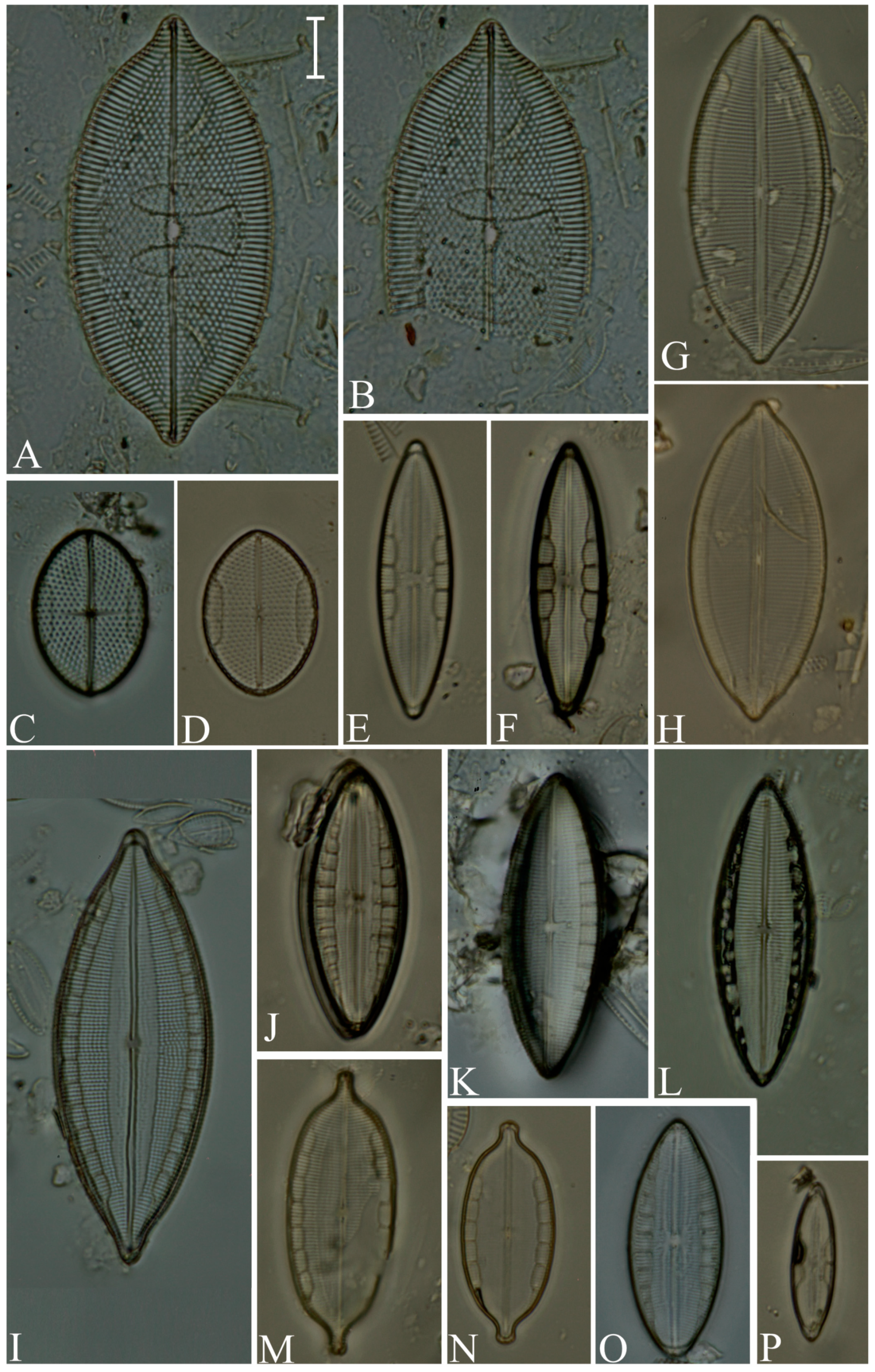
Division Bacillariophyta Silva Subdivision Bacillariophytina Medlin & Kaczmarska Class Coscinodiscophyceae Round et Crawford in Round et al. emend. Medlin & Kaczmarska Order Coscinodiscales Round & Crawford in Round et al. Family Heliopeltaceae Smith Genus Actinoptychus Ehrenberg Actinoptychus senarius (Ehrenberg) Ehrenberg (Figure 3E,F; SG) Order Melosirales Crawford in Round et al. Family Melosiraceae Kützing emend. Crawford in Round et al. Genus Melosira Agardh Melosira distans var. lyrata (Ehrenberg) Müller (Figure 3G) Melosira nummuloides Agardh (Figure 3J) Melosira westii var. quadrata Jurilj (Figure 3H,I; NR) Order Paraliales Crawford Family Paraliaceae Crawford Genus Paralia Heiberg Paralia sulcata (Ehrenberg) Cleve (Figure 3K,L; SG) Class Mediophyceae (Jousé & Proshkina-Lavrenko) Medlin & Kaczmarska Order Biddulphiales Krieger Family Biddulphiaceae Kützing Genus Biddulphia Gray Biddulphia californica (Schmidt) Wolle (Figure 3A,B; SG; NR) Order Anaulales Round & Crawford Family Anaulaceae (Schütt) Lemmermann Genus Eunotogramma Weisse Eunotogramma litorale Amspoker (Figure 4U,V; SG Order Eupodiscales Nikolaev & Harwood Family Odontellaceae Sims, Williams & Ashworth Genus Odontella Agardh Odontella aurita (Lyngbye) Agardh (Figure 3D; SG) Class Bacillariophyceae Haeckel emend. Medlin & Kaczmarska Order Achnanthales Silva Family Achnanthaceae Kützing Genus Achnanthes Bory Achnanthes angustata Bory (Figure 5P) Achnanthes pseudogroenlandica Hendey (Figure 5E–O) Achnanthes yaquinensis McIntire & Reimer (Figure 5A–D) Achnanthes pyrenaica Hustedt (Figure 5Z) Genus Planothidium Round & Bukhtiyarova Planothidium delicatulum (Kützing) Round & Bukhtiyarova (Figure 5T–Y) Planothidium hauckianum (Grunow) Bukhtiyarova (Figure 5Q–S) Family Cocconeidaceae Kützing Genus Cocconeis Ehrenberg Cocconeis neodiminuta Krammer (Figure 6P,Q) Cocconeis lineata Ehrenberg (Figure 6G–K) Cocconeis pseudodiruptoides Foged (Figure 6R,S) Cocconeis pseudomarginata Gregory (Figure 6L,O) Cocconeis scutellum Ehrenberg (Figure 6A–F) Order Bacillariales Hendey emend. Mann in Round et al. Family Bacillariaceae Ehrenberg Genus Homoeocladia Agardh, nom. rejic. Homoeocladia distans (Gregory) Kuntze (Figure 17D,E) Genus Nitzschia Hassall Nitzschia carnicobarica Desikachary & Prema (Figure 17O–R) Nitzschia frustulum (Kützing) Grunow (Figure 17T–W) Nitzschia fusoides Ehrlich (Figure 4Y,Z) Nitzschia ligowskii Witkowski, Lange-Bertalot, Kociolek & Brzezinska (Figure 17M,N; NR) Nitzschia persuadens Cholnoky (Figure 17K,L) Nitzschia longissima (Brébisson ex Kützing) Grunow (Figure 17Y,Z) Nitzschia scalpelliformis Grunow (Figure 17S) Nitzschia sigma (Kützing) Smith (Figure 17B,C) Nitzschia subconstricta Desikachary & Prema (Figure 17I,J) Nitzschia vidovichii (Grunow) Grunow (Figure 17A) Genus Psammodictyon Mann Psammodictyon constrictum (Gregory) Mann (Figure 16D,E) Psammodictyon panduriforme (Gregory) Mann (Figure 16A–C) Genus Tryblionella Smith Tryblionella coarctata (Grunow) Mann (Figure 16F–L) Tryblionella hungarica (Grunow) Frenguelli (Figure 16O,P) Tryblionella hyalina (Amossé) Ohtsuka (Figure 16M) Tryblionella pararostrata (Lange-Bertalot) Clavero & Hernández-Mariné (Figure 16N; NR) Order Cymbellales Mann in Round et al Family Cymbellaceae Greville Genus Navicymbula Krammer Navicymbula pusilla var. lata Krammer (Figure 14L; SG) Family Rhoicospheniaceae Chen & Zhu Genus Gomphoseptatum Medlin Gomphoseptatum aestuarii (Cleve) Medlin (Figure 17X; SG) Order Lyrellales Mann Family Lyrellaceae Mann Genus Lyrella Karayeva Lyrella exsul (Schmidt) Mann (Figure 9A,B; SG) Genus Petroneis Stickle & Mann Petroneis besarensis (Giffin) Witkowski, Lange-Bertalot & Witkowski (Figure 9E–G; NR) Petroneis granulata Mann, nom. illeg. (Figure 9C,D) Order Mastogloiales Mann Family Mastogloiaceae Mereschkowsky Genus Mastogloia Thwaites ex Smith Mastogloia acutiuscula var. elliptica Hustedt (Figure 12M,N) Mastogloia angulata Lewis (Figure 12A,B) Mastogloia apiculata Smith (Figure 12G,H) Mastogloia binotata (Grunow) Cleve (Figure 12C,D) Mastogloia exigua Lewis (Figure 12E,F) Mastogloia gieskesii Cholnoky (Figure 12P) Mastogloia braunii Grunow (Figure 12J,O) Mastogloia robusta Hustedt (Figure 12K,L) Mastogloia pisciculus Cleve (Figure 12I) Order Naviculales Bessey emend. Mann in Round et al. Family Diploneidaceae Mann in Round et al. Genus Diploneis (Ehrenberg) Cleve Diploneis coffeiformis (Schmidt) Cleve (Figure 8I) Diploneis gruendleri (Schmidt) Cleve (Figure 8A,F) Diploneis gravelleana Hagelstein (Figure 8B–E) Diploneis incurvata (Gregory) Cleve (Figure 8J) Diploneis nitescens (Gregory) Cleve (Figure 8P) Diploneis obliqua (Brun) Hustedt (Figure 8G,H) Diploneis smithii (Brébisson) Cleve (Figure 8K–O) Family Naviculaceae Kützing emend. Mann in Round et al. Genus Navicula Bory Navicula abunda Hustedt (Figure 13G) Navicula cancellata Donkin (Figure 13A,B) Navicula cincta Pantocsek, nom. illeg. (Figure 13F) Navicula longa var. irregularis Hustedt (Figure 13C) Navicula normaloides Cholnoky (Figure 13E) Navicula pennata Schmidt (Figure 13D) Navicula platyventris Meister (Figure 13H–K) Genus Trachyneis Cleve Trachyneis aspera (Ehrenberg) Cleve (Figure 15D,E) Trachyneis velata (Schmidt) Cleve (Figure 15B,C) Family Naviculaceae Kützing Genus Seminavis Mann Seminavis robusta Danielidis & Mann (Figure 7D–G; SG) Family Plagiotropidaceae Mann Genus Plagiotropis Pfitzer nom. illeg. Plagiotropis pusilla (Gregory) Kuntze (Figure 15A; SG) Family Pleurosigmataceae Mereschkowsky Genus Gyrosigma Hassall Gyrosigma balticum (Ehrenberg) Rabenhorst (Figure 11A) Gyrosigma eximium (Thwaites) Boyer (Figure 11F–H) Gyrosigma peisonis (Grunow) Hustedt (Figure 11D) Gyrosigma variistriatum Hagelstein (Figure 11B) Genus Pleurosigma Smith nom. et typ. cons Pleurosigma diversestriatum Meister (Figure 11E) Pleurosigma salinarum (Grunow) Grunow (Figure 11C) Family Berkeleyaceae Mann Genus Parlibellus Cox Parlibellus rhombicula (Hustedt) Witkowski, Lange-Bertalot & Metzeltin (Figure 7H; SG) Family Brachysiraceae Mann in Round et al. Genus Brachysira Kützing Brachysira cf. estoniarum Witkowski, Lange-Bertalot & Metzeltin (Figure 13N,O; SG) Family Diadesmidaceae Mann in Round et al. Genus Caloneis Cleve Caloneis linearis (Cleve) Boyer (Figure 7A–C; SG) Family Scoliotropidaceae Mereschkowsky Genus Biremis Mann & Cox Biremis ridicula (Giffen) Mann (Figure 13L,M; SG) Order Rhopalodiales Mann in Round et al. Family Rhopalodiaceae (Karsten) Topachevskyj & Oksiyuk Genus Rhopalodia Müller Rhopalodia acuminata Krammer (Figure 18B) Rhopalodia gibberula (Ehrenberg) Müller (Figure 18A) Rhopalodia musculus (Kützing) Müller (Figure 18C) Order Surirellales Mann in Round et al. Family Entomoneidaceae Reimer Genus Entomoneis Ehrenberg Entomoneis paludosa (Smith) Reimer (Figure 15F,G; SG) Family Surirellaceae Kützing Genus Campylodiscus Ehrenberg ex Kützing Campylodiscus subangularis Cleve & Möller (Figure 18L–O, S; NR) Genus Coronia (Ehrenberg ex Grunow) Ehrenberg Coronia ambigua (Greville) Ruck & Guiry (Figure 18D–K; SG) Order Thalassiophysales Mann in Round et al. Family Catenulaceae Mereschkowsky Genus Amphora Ehrenberg ex Kützing Amphora cingulata Cleve (Figure 14G) Amphora holsaticoides Nagumo & Kobayasi (Figure 14F) Amphora marina Smith (Figure 14D,E) Amphora proteus var. contigua Cleve (Figure 14C) Amphora proteus var. proteus Gregory (Figure 14A,B) Genus Halamphora (Cleve) Levkov Halamphora acutiuscula (Kützing) Levkov (Figure 14K) Halamphora coffeiformis (Agardh) Mereschkowsky (Figure 14I,J) Halamphora holsatica (Hustedt) Levkov (Figure 14H) Family Sellaphoraceae Mereschkowsky Genus Fallacia Stickle & Mann Fallacia litoricola (Hustedt) Mann (Figure 10F,G) Fallacia nummularia (Greville) Mann (Figure 10A–E) Order Fragilariales Silva emend. Round in Round et al. Family Staurosiraceae Medlin Genus Opephora Petit Opephora mutabilis Sabbe & Wyverman, nom. inval. (Figure 4L) Opephora pacifica (Grunow) Petit (Figure 4M) Genus Staurosirella Wiliams & Round Staurosirella martyi (Héribaud) Morales & Manoylov (Figure 4N; SG) Order Licmophorales Round in Round et al. Family Ulnariaceae Cox Genus Tabularia Williams & Round Tabularia fasciculata (Agardh) Williams & Round (Figure 4Q–S) Tabularia tabulata (Agardh) Snoeijs (Figure 4T) Order Rhabdonematales Round & Crawford Family Grammatophoraceae Lobban & Ashworth Genus Grammatophora Ehrenberg Grammatophora oceanica Ehrenberg (Figure 4P; SG) Order Plagiogrammales Cox Family Plagiogrammaceae De Toni Genus Dimeregramma Ralfs Dimeregramma maculatum (Cleve) Frenguelli (Figure 4J,K; SG) Genus Plagiogramma Greville Plagiogramma minus (Gregory) Li, Ashworth & Witkowski (Figure 4A–E) Plagiogramma tenuistriatum Cleve (Figure 4F–I) Order Rhaphoneidales Round Family Rhaphoneidaceae Forti Genus Delphineis Ehrenberg Delphineis minutissima (Hustedt) Simonsen (Figure 4O; SG) Genus Rhaphoneis Ehrenberg Rhaphoneis castracanei Grunow (Figure 4W,X; SG) Class Bacillariophyceae incertae sedis Order Bacillariophyceae ordo incertae sedis Family Bacillariophyceae familia incertae sedis Genus Ralfsiella Sims, Williams & Ashworth Ralfsiella smithii (Ralfs) Sims, Williams & Ashworth (Figure 3C; SG) Conflicts of Interest: The authors declare no conflicts of interest. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





