Submitted:
23 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Description and Distribution
3. Ecological Requirements and Production
4. Threats and Diseases
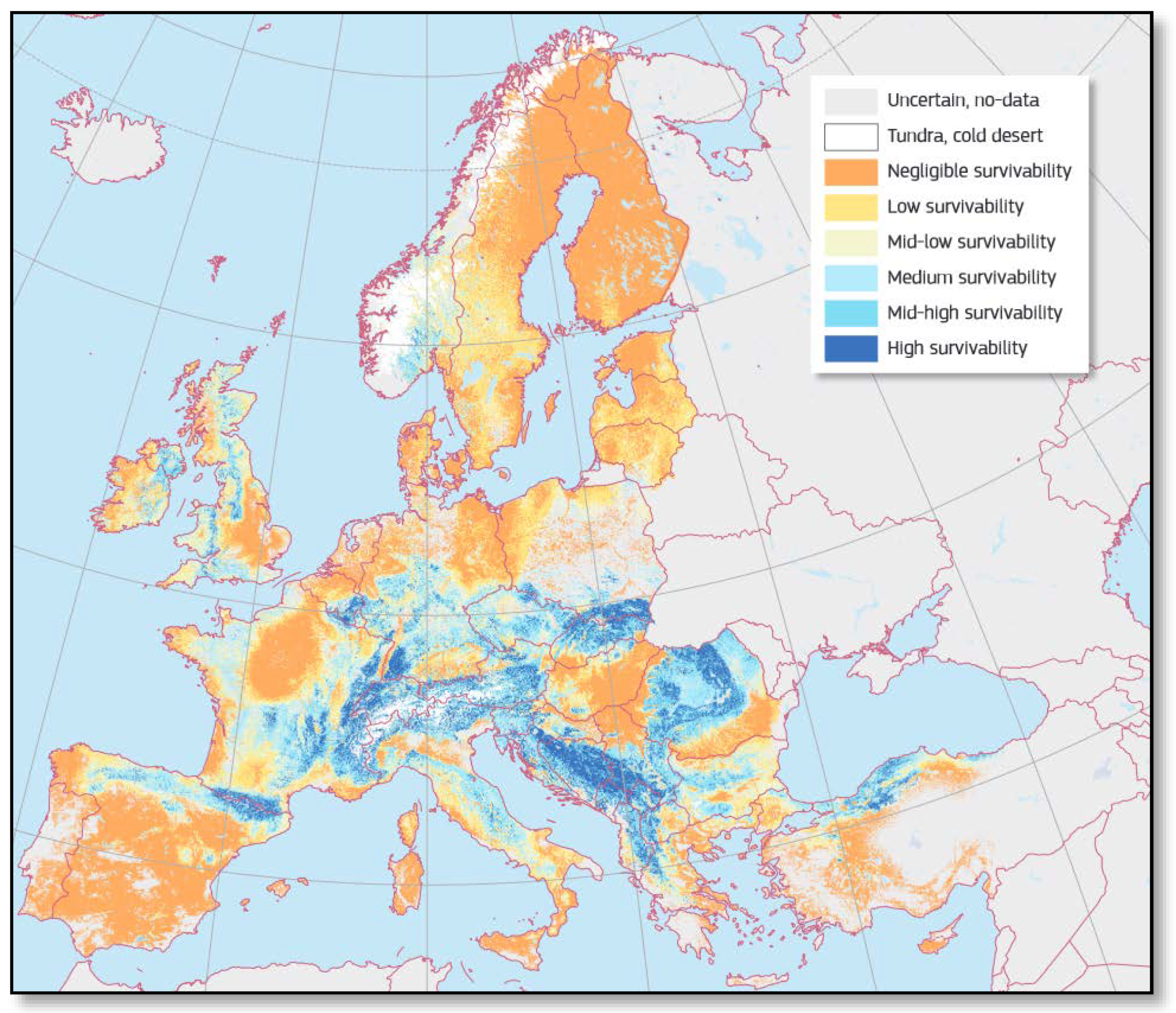
5. Impacts of Ongoing Climate Change on the Well-Being of Fir Trees
6. Seed Production and Nursery Management in the Context of Climate Change
7. Close-to-Nature Silvicultural Methods in the Context of Climate Change
8. Conclusions
Acknowledgments
References
- Prpic, B. Obicna jela (Abies alba Mill.) u Hrvatskoj; Akademija sumarskih znanosti: Zagreb, 2001. [Google Scholar]
- Gazol, A.; Camarero, J. J.; Gutiérrez, E.; Ionel, P.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Mauri, A.; de Rigo, D.; Caudullo, G. Abies alba in Europe: distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; p. E01493b+. [Google Scholar]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): a review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Bošeľa, M.; Lukac, M.; Castagneri, D.; Sedmák, R.; Biber, P.; Carrer, M.; Konôpka, B.; Nola, P.; Nagel, T.; Ionel, P.; et al. Contrasting effects of environmental change on the radial growth of co-occurring beech and fir trees across Europe. Sci. Total Environ. 2018, 615, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Dinca, L.; Marin, M.; Vlad, R.; Murariu, G.; Drasovean, R.; Cretu, R.; Georgescu, L.; Voichița, T.-G. Which are the best site and stand conditions for silver fir (Abies alba Mill.) located in the Carpathian Mountains? Diversity 2022, 14, 547. [Google Scholar] [CrossRef]
- Hofmeister, Š.; Svoboda, M.; Souček, J.; Vacek, S. Spatial pattern of Norway spruce and silver fir natural regeneration in uneven-aged mixed forests of northeastern Bohemia. J. For. Sci. 2008, 54, 92–101. [Google Scholar] [CrossRef]
- Vacek, S.; Vacek, Z.; Bulušek, D.; Bílek, L.; Schwarz, O.; Simon, J.; Štícha, V. The role of shelterwood cutting and protection against game browsing for the regeneration of silver fir. Austrian J. For. Sci. 2015, 132, 81–102. [Google Scholar]
- Mikulenka, P.; Prokůpková, A.; Vacek, Z.; Vacek, S.; Bulušek, D.; Simon, J.; Šimůnek, V.; Hájek, V. Effect of climate and air pollution on radial growth of mixed forests: Abies alba Mill. vs. Picea abies (L.) Karst. Cent. Eur. For. J. 2020, 66, 23–36. [Google Scholar]
- Šimůnek, V.; Prokůpková, A.; Vacek, Z.; Vacek, S.; Cukor, J.; Remeš, J.; Hájek, V.; D'Andrea, G.; Šálek, M.; Nola, P.; et al. Silver fir tree-ring fluctuations decrease from north to south latitude—total solar irradiance and NAO are indicated as the main influencing factors. For. Ecosyst. 2023, 10, 100150. [Google Scholar] [CrossRef]
- MZE. Zpráva o stavu lesa a lesního hospodářství České republiky v roce 2021, Ministerstvo zemědělství: Praha, 2022.
- Zlatník, A. Nástin lesnické typologie na biogeocenologickém základě a rozlišení československých lesů podle skupin lesních typů. Pěstění lesů III.; SZN: Praha, 1956; pp. 317–401. [Google Scholar]
- Málek, J. Problematik der Ökologie der Tanne (Abies alba Mill.) und ihres Sterbens in der ČSSR. Forstw. Cbl. 1981, 100, 170–174. [Google Scholar] [CrossRef]
- Málek, J. Problematika ekologie jedle bělokoré a jejího odumírání; Československá Akademie Věd Praha: Studie ČSAV: Praha, 1983; Vol. 11, p. 108. [Google Scholar]
- Klika, J. Lesní dřeviny; Československá matice lesnická: Písek, 1947; p. 394. [Google Scholar]
- Svoboda, P. Život lesa; Brázda: Praha, 1952; p. 894. [Google Scholar]
- Zlatník, A. Lesnická fytocenologie; SZN: Praha, 1976; p. 495. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers; Leiden & Boston: Brill, 2017; Vol.1 & 2, p. 1112. [Google Scholar]
- Pinto, P. E.; Gégout, J.-C.; Hervé, J.-C.; Dhôte, J.-F. Respective importance of ecological conditions and stand composition on Abies alba Mill. dominant height growth. For. Ecol. Manag. 2008, 255, 619–629. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Rebetez, M.; Conedera, M.; Augustin, S.; Brang, P.; Tinner, W. What is the potential of silver fir to thrive under warmer and drier climate? Eur. J. For. Res. 2019, 138, 547–560. [Google Scholar] [CrossRef]
- Kučeravá, B.; Dobrovolný, L.; Remeš, J. Responses of Abies alba seedlings to different site conditions in Picea abies plantations. Dendrobiology 2013, 69, 49–58. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J. J.; Colangelo, M.; de Luis, M.; Martinez del Castillo, E.; Serra-Maluquer, X. Summer drought and spring frost, but not their interaction, constrain European beech and silver fir growth in their southern distribution limits. Agric. For. Meteorol. 2019, 278, 107695. [Google Scholar] [CrossRef]
- Maxime, C.; Hendrik, D. Effects of climate on diameter growth of co-occurring Fagus sylvatica and Abies alba along an altitudinal gradient. Trees 2011, 25, 265–276. [Google Scholar] [CrossRef]
- Świercz, A.; Świątek, B.; Pietrzykowski, M. Changes in the concentrations of trace elements and supply of nutrients to silver fir (Abies alba Mill.) needles as a bioindicator of industrial pressure over the past 30 years in Świętokrzyski National Park (Southern Poland). Forests 2022, 13, 718. [Google Scholar] [CrossRef]
- Tinner, W.; Colombaroli, D.; Heiri, O.; Henne, P.; Steinacher, M.; Untenecker, J.; Vescovi, E.; Allen, J.; Carraro, G.; Conedera, M.; et al. The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol. Monogr. 2013, 83, 419–439. [Google Scholar] [CrossRef]
- Bošeľa, M.; Petráš, R.; Sitková, Z.; Priwitzer, T.; Pajtík, J.; Hlavatá, H.; Sedmák, R.; Tobin, B. Possible causes of the recent rapid increase in the radial increment of silver fir in the Western Carpathians. Environ. Pollut. 2014, 184, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Boettger, T.; Haupt, M.; Friedrich, M.; Waterhouse, J. S. Reduced climate sensitivity of carbon, oxygen and hydrogen stable isotope ratios in tree-ring celulose of silver fir (Abies alba Mill.) influenced by background SO2 in Franconia (Germany, Central Europe). Environ. Pollut. 2014, 185, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Vacek, S.; Černý, T.; Vacek, Z.; Podrázský, V.; Mikeska, M.; Králíček, I. Long-term changes in vegetation and site conditions in beech and spruce forests of lower mountain ranges of Central Europe. For. Ecol. Manag. 2017, 398, 75–90. [Google Scholar] [CrossRef]
- Gill, R. M. A. A review of damage by mammals in north temperate forests: 3. Impact on trees and forests. Forestry 1992, 65, 363–388. [Google Scholar] [CrossRef]
- Vacek, Z.; Bílek, L.; Kral, J.; Remeš, J.; Bulušek, D.; Králíček, I. Ungulate impact on natural regeneration in spruce-beech-fir stands in Černý důl Nature Reserve in the Orlické Hory Mountains, case study from Central Sudetes. Forests 2014, 5, 2929–2946. [Google Scholar] [CrossRef]
- Vacek, S.; Prokůpková, A.; Vacek, Z.; Bulušek, D.; Šimůnek, V.; Králíček, I.; Prausová, R.; Hájek, V. Growth response of mixed beech forests to climate change, various management and game pressure in Central Europe. J. For. Sci. 2019, 65, 331–345. [Google Scholar] [CrossRef]
- Huth, F.; Wehnert, A.; Tiebel, K.; Wagner, S. Direct seeding of silver fir (Abies alba Mill.) to convert Norway spruce (Picea Abies L.) forests in Europe: A review. For. Ecol. Manag. 2017, 403, 61–78. [Google Scholar] [CrossRef]
- Kupferschmid, A.; Zimmermann, S.; Bugmann, H. Browsing regime and growth response of naturally regenerated Abies alba saplings along light gradients. For. Ecol. Manag. 2013, 310, 393–404. [Google Scholar] [CrossRef]
- Kupferschmid, A. Selective browsing behaviour of ungulates influences the growth of Abies alba differently depending on forest type. For. Ecol. Manag. 2018, 429, 317–326. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.; Schelhaas, M.-J.; Nabuurs, G. J.; Zimmermann, N. Climate change may cause severe loss in economic value of European forestland. Nat. Clim. Change. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Konôpková, A.; Kurjak, D.; Kmeť, J.; Klumpp, R.; Longauer, R.; Ditmarová, Ľ.; Gömöry, D. Differences in photochemistry and response to heat stress between silver fir (Abies alba Mill.) provenances. Trees - Struct. Funct. 2018, 32, 73–86. [Google Scholar] [CrossRef]
- Linares, J. C.; Camarero, J. J. From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Change Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Büntgen, U.; Tegel, W.; Kaplan, J.; Schaub, M.; Hagedorn, F.; Bürgi, M.; Brázdil, R.; Helle, G.; Carrer, M.; Heussner, K.-U.; et al. Placing unprecedented recent fir growth in a European-wide and Holocene-long context. Front. Ecol. Environ. 2014, 12, 100–106. [Google Scholar] [CrossRef]
- Wolf, H. EUFORGEN Technical Guidelines for genetic conser-vation and use for silver fir (Abies alba). International PlantGenetic Resources Institute: Rome, 2003, p. 6.
- Musil, I.; Hamerník, J. Jehličnaté dřeviny: Přehled nahosemenných (i výtrusných) dřevin; Academia: Praha, 2007; p. 352. [Google Scholar]
- Svoboda, M.; Nagel, T. A. Gap disturbance regime in an old-growth Fagus-Abies forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 2008, 38, 2728–2737. [Google Scholar]
- Úradníček, L.; Madera, P.; Tichá, S.; Koblížek, J. Dřeviny České republiky; Lesnická práce, s.r.o.: Kostelec nad Černými lesy, 2009; p. 367. [Google Scholar]
- Xiang, X.-G.; Cao, M.; Zhou, Z.-K. Fossil history and modern distribution of the genus Abies (Pinaceae). Frontiers of Forestry in China 2007, 2, 355–365. [Google Scholar] [CrossRef]
- Debreczy, Z.; Rácz, I. Conifers Around the World: Conifers of the Temperate Zones and Adjacent Regions; Dendro Press: Wellesley, Massachusetts, USA, 2011. [Google Scholar]
- Žárník, M.; Holuša, O. Silver fir (Abies alba) in the forest-typological altitudinal vegetation zones of the Czech massif, Western and Eastern Carpathy Mts. In Jedle bělokorá – 2005. Proceedings of the Jedle bělokorá – 2005, Srní, Czech Republic, Oct 31–Nov 1, 2005; Neuhöferová, P., Ed.; ČZU FLE v Praze, Katedra pěstování lesů a Správa Národního parku a chráněné krajinné oblasti Šumava: Praha, 2005; pp. 83–87. [Google Scholar]
- Míchal, I. Dynamika přírodního lesa I. – VI. Vol. 31; Živa, 1983, 8–12, 48–51, 85–88, 128–133, 163–168, 233–238.
- Poleno, Z.; Vacek, S.; Podrázský, V.; Remeš, J.; Štefančík, I.; Mikeska, M.; Kobliha, J.; Kupka, I.; Malík, V.; Turčáni, M.; et al. Pěstování lesů III. Praktické postupy pěstování lesů; Lesnická práce, s.r.o.: Kostelec nad Černými lesy, 2009; p. 952. [Google Scholar]
- Zamora-Pereira, J. C.; Yousefpour, R.; Cailleret, M.; Bugmann, H.; Hanewinkel, M. Magnitude and timing of density reduction are key for the resilience to severe drought in conifer-broadleaf mixed forests in Central Europe. Ann. For. Sci. 2021, 78, 68. [Google Scholar] [CrossRef]
- Hilmers, T.; Avdagić, A.; Bartkowicz, L.; Bielak, K.; Binder, F.; Boncina, A.; Dobor, L.; Forrester, D.; Hobi, M.; Ibrahimspahic, A.; et al. The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. Forestry 2019, 92, 512–522. [Google Scholar] [CrossRef]
- Poleno, Z.; Vacek, S.; Podrázský, V.; Remeš, J.; Mikeska, M.; J, K.; Bílek, L. Pěstování lesů II. Teoretická východiska pěstování, lesů, Ed.; Lesnická práce, s.r.o.: Kostelec nad Černými lesy, 2007; p. 464.
- Botany.cz. Available online: https://botany.cz/cs/abies-alba/ (accessed on 4 July 2007).
- Dobrowolska, D. Structure of silver fir (Abies alba Mill.) natural regeneration in the `Jata' reserve in Poland. For. Ecol. Manag. 1998, 110, 237–247. [Google Scholar] [CrossRef]
- Novák, J.; Kacálek, D.; et al. Podpora a perskpetiva jedle bělokoré v Českých zemích; Lesnická práce: Kostelec nad Černými lesy, 2023; p. 240. [Google Scholar]
- Ferlin, F. The growth potential of understorey silver fir and Norway spruce for uneven-aged forest management in Slovenia. Forestry 2002, 75, 375–383. [Google Scholar] [CrossRef]
- Kacálek, D.; Mauer, O.; Podrázský, V.; Slodičák, M.; Houšková, K.; Špulák, O.; et al. Meliorační a zpěvňující funkce lesních dřevin; Lesnická práce: Kostelec nad Černými lesy, 2017; p. 300. [Google Scholar]
- Bercha, J. Konference: Jedle bělokorá - 2005. Lesnická práce 2006, 1, 10–11. [Google Scholar]
- Bledý, M. Využití jedle bělokoré (Abies alba Mill.) v přírodě blízkém hospodaření v podmínkách 2.-4. lesního vegetačního stupně. Dissertation thesis, Czech University of Life Sciences, Prague, 2023.
- Jović, G.; Dukić, V.; Stajic, B.; Kazimirović, M.; Petrović, D. A dendroclimatological analysis of fir (Abies alba Mill.) growth in the Borja Mountain area of Bosnia and Herzegovina. Glas. Sumar. Fak. 2018, 118, 27–45. [Google Scholar] [CrossRef]
- Prokůpková, A.; Vacek, Z.; Vacek, S.; Bulušek, D. Natural regeneration potential of mixed forests in Kronoše Mts. National Park: structure, dynamics and effect of game. Proceedings of Central European Silviculture; Houšková, K., Jan, D., Eds.; Publishing Centre of Mendel University in Brno: Brno, 2019; pp. 80–90. [Google Scholar]
- Hofmeister, Š.; Vacek, S.; Simon, J.; Minx, T. Struktura a vývoj přírodě blízkých porostů s jedlí bělokorou v genové základně Jánské Lázně v Krkonoších. In Increase of Close-to Nature Stand Component of Forests with Special Protection Status; Vacek, S., Ed.; Ústav hospodářské úpravy lesů LDF MZLU v Brně a Katedra pěstování lesů FLE ČZU v Praze: Brno, 2006. [Google Scholar]
- Šindelář, J.; Frýdl, J.; Novotný, P. Results of evaluation of the oldest provenance plot of the FGMRI Jíloviště-Strnady with silver fir established in 1961 on the locality Jíloviště, Baně. Reports of Forestry Research 2005, 50, 24–32. [Google Scholar]
- Motta, R.; Garbarino, F. Stand history and its consequences for the present and future dynamic in two silver fir (Abies alba Mill.) stands in the high Pesio Valley (Piedmont, Italy). Ann. For. Sci. 2003, 60, 361–370. [Google Scholar] [CrossRef]
- Jagodziński, A. M.; Dyderski, M. K.; Gęsikiewicz, K.; Horodecki, P. Tree and stand level estimations of Abies alba Mill. aboveground biomass. Ann. For. Sci. 2019, 76, 1–14. [Google Scholar] [CrossRef]
- Paluch, J. The influence of the spatial pattern of trees on forest floor vegetation and silver fir (Abies alba Mill.) regeneration in uneven-aged forests. For. Ecol. Manage. 2005, 205, 283–298. [Google Scholar] [CrossRef]
- Paluch, J. Ground seed density patterns under conditions of strongly overlapping seed shadows in Abies alba Mill. stands. Eur. J. For. Res. 2011, 130, 1009–1022. [Google Scholar] [CrossRef]
- Prokupková, A.; Brichta, J.; Vacek, Z.; Bielak, K.; Andrzejczyk, T.; Vacek, S.; Štefančík, I.; Bílek, L.; Fuchs, Z. Effect of vegetation on natural regeneration of mixed silver fir forests in lowlands: a case study from the Rogów region in Poland. Sylwan 2021, 165, 779–795. [Google Scholar]
- Tudoran, G.-M.; Avram, C.; Ciceu, A.; Dobre, A.-C. Growth relationships in silver fir stands at their lower-altitude limit in Romania. Forests 2021, 12, 439. [Google Scholar] [CrossRef]
- Kobal, M.; Grčman, H.; Zupan, M.; Levanič, T.; Simončič, P.; Kadunc, A.; Hladnik, D. Influence of soil properties on silver fir (Abies alba Mill.) growth in the Dinaric Mountains. For. Ecol. Manag. 2015, 337, 77–87. [Google Scholar] [CrossRef]
- Sopushynskyi, I. Intraspecific structural signs of curly silver fir (Abies alba Mill.) growing in the Ukrainian Carpathians. J. For. Sci. 2020, 66, 299–308. [Google Scholar] [CrossRef]
- Köppen, W. Das geographische System der Klimate. In Handbuch der Klimatologie, Köppen, W.; Geiger, R., Ed.; Gebrüder Borntraeger: Berlin, 1936; pp. 1–44. [Google Scholar]
- Peřina, V.; Kadlus, Z.; Jirkovský, V. Přirozená obnova lesních porostů; SZN: Praha, 1964; p. 167. [Google Scholar]
- Míchal, I.; Petříček, V. e. a. Péče o chráněná území, II. Lesní společenstva; AOPK: Praha, 1999; p. 713. [Google Scholar]
- Zakopal, V. Pěstovaní jedle ve světle nových poznatků. Reports of Forestry Research 1970, 16, 24–32. [Google Scholar]
- Schütt, P. Tannenarten Europas und Klein asiens; Ecomed Verlagsgesellschaft: Landsberg am Lech, 1994; pp. 1–132. [Google Scholar]
- Průša, E. Pěstování lesů na typologických základech; Lesnická práce, s.r.o.: Kostelec nad Černými lesy, 2001; p. 593. [Google Scholar]
- Korpeľ, Š. Pralesy Slovenska; Veda: Bratislava, 1989; p. 328. [Google Scholar]
- Tingey, D. T.; Phillips, D. L.; Johnson, M. G.; Rygiewicz, P. T.; Beedlow, P. A.; Hogsett, W. A. Estimates of douglas-fir fine root production and mortality from minirhizotrons. For. Ecol. Manag. 2005, 204, 359–370. [Google Scholar] [CrossRef]
- Weintraub, M. N.; Scott-Denton, L. E.; Schmidt, S. K.; Monson, R. K. The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 2007, 154, 327–338. [Google Scholar] [CrossRef]
- Röhrig, E.; Bartsch, N. Waldbau auf ökologischer Grundlage. 6 ed; Parey: Hamburg-Berlin, 1992. [Google Scholar]
- Whelan, M. J.; Sanger, L. J.; Baker, M.; Anderson, J. M. Spatial patterns of throughfall and mineral ion deposition in a lowland Norway spruce (Picea abies) plantation at the plot scale. Atmos. Environ. 1998, 20, 3493–3501. [Google Scholar] [CrossRef]
- Bartsch, N.; Bauhus, J.; Vor, T. Effects of group selection and liming on nutrient cycling in European beech forest on acidic soils. In Forest Development. Succession, Environmental Stress and Forest Management. Case Studies; Dohrenbush, A., Bartsch, N., Eds.; Springer: Berlin, 2002; pp. 109–166. [Google Scholar]
- Penne, C.; Ahrends, B.; Deurer, M.; Böttcher, J. The impact of the canopy structure on the spatial variability in forest floor carbon stocks. Geoderma 2010, 158, 282–297. [Google Scholar] [CrossRef]
- Morris, D. M.; Gordon, A. G.; Gordon, A. M. Patterns of canopy interception and throughfall along a topographic sequence for black spruce dominated forest ecosystems in northwestern Ontario. Can. J. For. Res. 2003, 33, 1046–1060. [Google Scholar] [CrossRef]
- Staelens, J.; De Schrijver, A.; Verheyen, K.; Verhoest, N. E. C. Spatial variability and temporal stability of throughfall water under a dominant beech (Fagus Sylvatica L.) tree in relationship to canopy cover. J. Hydrol. 2006, 330, 651–662. [Google Scholar] [CrossRef]
- Paluch, J.; Gruba, P. Inter-crown versus under-crown area: Contribution of local configuration of trees to variation in topsoil morphology, pH and moisture in Abies alba Mill. forests. Eur. J. For. Res. 2012, 131, 857–870. [Google Scholar] [CrossRef]
- Zhang, Q.; Zak, J. C. Effects of gap size on litter decomposition and microbial activity in a subtropical forest. Ecology 1995, 76, 2196–2204. [Google Scholar] [CrossRef]
- Collins, B. S.; Battaglia, L. L. Microenvironmental heterogeneity and Quercus michauxii regeneration in experimental gaps. For. Ecol. Manag. 2002, 155, 279–290. [Google Scholar] [CrossRef]
- Bauhus, J. C and N mineralization in an acid forest soil along a gap-stand gradient. Soil. Biol. Biochem. 1996, 28, 923–932. [Google Scholar] [CrossRef]
- Carvalheiro, K. O.; Nepstad, D. C. Deep soil heterogeneity and fine root distribution in forests and pastures of eastern Amazonia. Plant Soil 1996, 182, 279–285. [Google Scholar] [CrossRef]
- Pärtel, M.; Wilson, S. D. Root dynamics and spatial pattern in prairie and forest. Ecology 2002, 83, 1199–1203. [Google Scholar] [CrossRef]
- Vacek, S.; Nosková, I.; Bílek, L.; Vacek, Z.; Schwarz, O. Regeneration of forest stands on permanent research plots in the Krkonoše Mts. J. For. Sci. 2010, 56, 541–554. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Conifer seedling recruitment in a southeastern Canadian boreal forest: The importance of substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Třeštík, M.; Podrázský, V. Soil improving role of the silver fir (Abies alba Mill.): a case study. Reports of Forestry Research 2017, 62, 182–188. [Google Scholar]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, 1988; p. 731. [Google Scholar]
- Senn, J.; Suter, W. Ungulate browsing on silver fir (Abies alba) in the Swiss Alps: Beliefs in search of supporting data. For. Ecol. Manag. 2003, 181, 151–164. [Google Scholar] [CrossRef]
- Klopčič, M.; Simončič, T.; Bončina, A. Comparison of regeneration and recruitment of shade-tolerant and light-demanding tree species in mixed uneven-aged forests: experiences from the Dinaric region. Forestry 2015, 88, 552–563. [Google Scholar] [CrossRef]
- Schütz, J.-P. Silvicultural tools to develop irregular and diverse forest structures. Forestry 2002, 75, 329–337. [Google Scholar] [CrossRef]
- Korpeľ, Š.; Vinš, B. Pestovanie jedle; Slovenské vydavaťelstvo pódohospodárskej literatury: Bratislava, 1966; p. 342. [Google Scholar]
- Míchal, I. Obnova ekologické stability lesů; Academia: Praha, 1992; p. 169. [Google Scholar]
- Vacek, S.; Simon, J.; Remeš, J.; Podrázský, V.; Minx, T.; Mikeska, M.; Malík, V.; Jankopvský, L.; Turčáni, M.; Jakuš, R.; et al. Obhospodařování bohatě strukturovaných a přírodě blízkých lesů. [Management of structure-rich and close-to-nature forests]; Lesnická práce, s.r.o.: Kostelec nad Černými lesy, 2007; p. 447. [Google Scholar]
- Vacek, Z. Structure and dynamics of spruce-beech-fir forests in Nature Reserves of the Orlické hory Mts. in relation to ungulate game. Cent. Eur. For. J. 2017, 63, 23–34. [Google Scholar] [CrossRef]
- Vacek, S.; Vašina, V.; Mareš, V. Analýza autochtonních smrkobukových porostů SPR V bažinkách. [Analysis of autochthonous spruce-beech populations of the national nature reserve V bažinkách]. Opera Corcontica 1987, 24, 95–132. [Google Scholar]
- Korpeľ, Š. Die Urwälder der Westkarpaten; Gustav Fischer Verlag: Stuttgart, Jena, New York, 1995. [Google Scholar]
- Vacek, S.; Bílek, L.; Schwarz, O.; Hejcmanová, P.; Mikeska, M. Effect of air pollution on the health status of spruce stands. Mt. Res. Dev. 2013, 33, 40–50. [Google Scholar] [CrossRef]
- Hladík, M.; Korpeľ, Š.; Lukáč, T.; Tesař, V. Hospodárenie v lesoch horských oblastí; VŠZ – lesnická fakulta Praha a Matice lesnická Písek: Praha, Písek, 1993; p. 123. [Google Scholar]
- Vacek, Z.; Prokůpková, A.; Vacek, S.; Bulušek, D.; Šimůnek, V.; Hájek, V.; Králíček, I. Effect of Norway spruce and European beech mixing in relation to climate change: Structural and growth perspectives of mountain forests in Central Europe. For. Ecol. Manag. 2021, 488, 119019. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Cukor, J. European forests under global climate change: Review of tree growth processes, crises and management strategies. J. Environ. Manag. 2023, 332, 117353. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology. 3 ed; Sinauer Associates 2, 2002; pp. 690.
- Chmelař, J. Přirozená obnova jedle (Abies alba Mill.) v pralesové rezervaci „Mionší“ v Moravskoslezských Beskydech. Lesnictví 1959, 5, 225–238. [Google Scholar]
- Meyer, H. Beitrag zur Frage der Rückgängigkeitserscheinungen der Weisstanne (Abies alba Mill.) am Nordrand ihres Naturareals. Arch. Forstw. 1957, 6, 719–787. [Google Scholar]
- Podrázský, V.; Vacek, S.; Vacek, Z.; Raj, A.; Mikeska, M.; Boček, M.; Schwarz, O.; Hošek, J.; Šach, F.; Černohous, V.; et al. Půdy lesů a ekosystémů nad horní hranicí lesa v národních parcích Krkonoš; Lesnická práce, s. r. o.: Kostelec nad Černými lesy, 2010; p. 304. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen; Verlag Eugen Ulmer: Stuttgart, 1996, p. 1095; Vol. 6. [Google Scholar]
- Tinner, W.; Lotter, A. Holocene expansion of Fagus sylvatica and Abies alba in Central Europe: Where are we after eight decades of debate? Quat. Sci. Rev. 2006, 25, 526–549. [Google Scholar] [CrossRef]
- Kadlus, Z. Struktura a vývoj zmlazení smrku, jedle a buku v Orlických horách. Lesnictví 1969, 15, 381–399. [Google Scholar]
- Lieffers, V. J.; Macmillan, R. B.; MacPherson, D.; Branter, K.; Stewart, J. D. Semi-natural and intensive silvicultural systems for the boreal mixedwood forest. For. Chron. 1996, 72, 286–292. [Google Scholar] [CrossRef]
- Stancioiu, P. T.; O'Hara, K. L. Regeneration growth in different light environments of mixed species, multiaged, mountainous forests of Romania. Eur. J. Forest Res. 2006, 125, 151–162. [Google Scholar] [CrossRef]
- Paluch, J. Spatial distribution of regeneration in West-Carpathian uneven-aged silver fir forests. Eur. J. For. Res. 2005, 124, 47–54. [Google Scholar] [CrossRef]
- Košulič, M. Cesta k přírodě blízkému hospodářskému lesu; FSC ČR: Brno, 2010; p. 449. [Google Scholar]
- Forrester, D. I.; Albrecht, A. T. Light absorption and light-use efficiency in mixtures of Abies alba and Picea abies along a productivity gradient. For. Ecol. Manag. 2014, 328, 94–102. [Google Scholar] [CrossRef]
- Grassi, G.; Bagnaresi, U. Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol. 2001; 21, 959–967. [Google Scholar]
- Hynek, V. Opatření k záchraně a reprodukci genofondu jedle bělokoré v ČSR. Práce VÚLHM 1987, 71, 39–66. [Google Scholar]
- Heuze, P.; Schnitzler, A.; Klein, F. Consequences of increased deer browsing winter on silver fir and spruce regeneration in the Southern Vosges mountains: Implications for forest management. Ann. For. Sci. 2005, 62, 175–181. [Google Scholar] [CrossRef]
- Heuze, P.; Schnitzler, A.; Klein, F. Is browsing the major factor of silver fir decline in the Vosges Mountains of France? For. Ecol. Manag. 2005, 217, 219–228. [Google Scholar] [CrossRef]
- Forrester, D. I.; Bauhus, J.; Cowie, A. L.; Vanclay, J. K. Mixed-species plantations of Eucalyptus with nitrogen fixing trees: a review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Forrester, D. I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Pretzsch, H.; Steckel, M.; Heym, M.; Biber, P.; Ammer, C.; Ehbrecht, M.; Bielak, K.; Bravo, F.; Ordóñez, C.; Collet, C.; et al. Stand growth and structure of mixed-species and monospecific stands of Scots pine (Pinus sylvestris L.) and oak (Q. robur L., Quercus petraea (Matt.) Liebl.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2020, 139, 349–367. [Google Scholar] [CrossRef]
- Del Río, M.; Pretzsch, H.; Ruiz-Peinado, R.; Jactel, H.; Coll, L.; Löf, M.; Aldea, J.; Ammer, C.; Avdagić, A.; Barbeito, I.; et al. Emerging stability of forest productivity by mixing two species buffers temperature destabilizing effect. J. Appl. Ecol. 2022, 59, 2730–2741. [Google Scholar] [CrossRef]
- Pretzsch, H.; Block, J.; Dieler, J.; Dong, P.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zingg, A. Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Ann. For. Sci. 2010, 67, 712. [Google Scholar] [CrossRef]
- Coates, K. D.; Lilles, E. B.; Astrup, R. Competitive interactions across a soil fertility gradient in a multispecies forest. J. Ecol. 2013, 101, 806–818. [Google Scholar] [CrossRef]
- Forrester, D. I.; Kohnle, U.; Albrecht, A. T.; Bauhus, J. Complementarity in mixed-species stands of Abies alba and Picea abies varies with climate, site quality and stand density. For. Ecol. Manag. 2013, 304, 233–242. [Google Scholar] [CrossRef]
- Vrška, T.; Hort, L.; Odehnalová, P.; Adam, D.; Horal, D. Prales Mionší - historický vývoj a současný stav. J. For. Sci. 2000, 46, 411–424. [Google Scholar]
- Vrška, T.; Hort, L.; Odehnalová, P.; Adam, D.; Horal, D. Dynamika vývoje pralesovitých rezervací v České republice. Sv. 1 – Českomoravská vrchovina – Polom, Žákova hora. 1 ed; Academia: Praha, 2002; p. 213. [Google Scholar]
- Jaworski, A.; Kołodziej, Z.; Porada, K. Structure and dynamics of stands of primeval character in selected areas of the Bieszczady National Park. J. For. Sci. 2002, 48, 185–201. [Google Scholar] [CrossRef]
- Štefančík, I. Growth and development of fir (Abies alba Mill.) in mixed spruce, fir and beech stands. Ekológia 2004, 23, 144–151. [Google Scholar]
- Štefančík, I. Changes in tree species composition, stand structure, qualitative and quantitative production of mixed spruce, fir and beech stand on Stará Píla research plot. J. For. Sci. 2006, 52, 74–91. [Google Scholar] [CrossRef]
- Jaworski, A.; Podlaski, R. Processes of loss, recruitment, and increment in stands of a primeval character in selected areas of the Pieniny National Park (southern Poland). J. For. Sci. 2007, 53, 278–289. [Google Scholar] [CrossRef]
- Cramer, H. H. On the predisposition to disorders of MiddleEuropean forests. Pflanzenschutz-Nachrichten Bayer 1984, 37, 97–207. [Google Scholar]
- Larsen, J. B. Das Tannensterben: Eine neue Hypothese zur Klärung des Hintergrundes dieser rätselhaften Komplexkrankheit der Weißtanne (Abies alba Mill.). Forstw. Cbl. 1986, 105, 381–396. [Google Scholar] [CrossRef]
- Krehan, H. Das tannensterben in europa: eine literaturstudie mitkritischer stellungnahme; FBVA-Berichte 39, Forstliche Bundesversuchsanstalt in Wien, Österreichischer Agrarverlag: Wien, 1989. [Google Scholar]
- Kramer, W. Die Weißtanne (Abies alba Mill.) in Ost- und Südosteuropa; Gustav Fischer Verlag: Stuttgart, Jena, New York, 1992. [Google Scholar]
- Levanic, T. Growth depression of silver fir (Abies alba Mill.) in the Dinaric phytogeographic region between 1960-1995. Zbornik gozdarstva in lesarstva 1997, 52, 137–164. [Google Scholar]
- Svenning, J.-C.; Skov, F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004, 7, 565–573. [Google Scholar] [CrossRef]
- Hockenjos, W. Tannenbäume: Eine Zukunft für Abies alba; DRW-Verlag Weinbrenner GmbH & Co, KG: Leinfelden-Echterdingen, 2008; p. 232. [Google Scholar]
- Allen, J. R. M.; Huntley, B. Last interglacial palaeovegetation, palaeoenvironments and chronology: A new record from Lago Grande di Monticchio, southern Italy. Quat. Sci. Rev. 2009, 28, 1521–1538. [Google Scholar] [CrossRef]
- Opravil, E. Jedle bělokorá (Abies alba Mill.) v československém kvartéru. Časopis slezského muzea 1976, 25, 45–67. [Google Scholar]
- Tinner, W.; Hubschmid, P.; Wehrli, M.; Ammann, B.; Conedera, M. Long-term forest fire ecology and dynamics in southern Switzerland. J. Ecol. 1999, 87, 273–289. [Google Scholar] [CrossRef]
- Vacek, S.; Matějka, K.; Simon, J.; Malík, V.; Schwarz, O.; Podrázský, V.; Minx, T.; Tesař, V.; Anděl, P.; Jankovský, L.; et al. Zdravotní stav a dynamika lesních ekosystémů Krkonoš pod stresem vyvolaným znečištěním ovzduší; Folia forestalia Bohemica, Lesnická práce, s. r. o.: Kostelec nad Černými lesy, 2007; p. 216. [Google Scholar]
- Dannecker, K. Aus der hohen Schule Weisstannenwaldes; J.D. Sauerländer: Frankfurt, 1955; p. 206. [Google Scholar]
- Manion, P.; Lachance, D. Forest decline concepts; APS Press: University of Minnesota, 1992; p. 249. [Google Scholar]
- Elling, W.; Dittmar, C.; Pfaffelmoser, K.; Rötzer, T. Dendroecological assessment of the complex causes of decline and recovery of the growth of silver fir (Abies alba Mill.) in Southern Germany. For. Ecol. Manag. 2009, 257, 1175–1187. [Google Scholar] [CrossRef]
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) rotation in the Carpathians—A developmental cycle or a linear trend induced by man? For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Wentzel, K. F. Weissitanne = immissionsempfindlichste einheimische Baumart. Allg. Forstztg. 1980, 35, 373–374. [Google Scholar]
- Ulrich, B. Eine ökosystemare Hypothese über die Ursachen des Tannensterbens (Abies alba Mill.). Forstwiss. Cbl. 1981, 100, 228–236. [Google Scholar] [CrossRef]
- Longauer, R. Genetic variation of European silver fir (Abies alba Mill.) in the Western Carpathians. J. For. Sci. 2001, 47, 429–438. [Google Scholar]
- Ficko, A.; Boncina, A. Silver fir (Abies alba Mill.) distribution in Slovenian forests. Zbornik gozdarstva in lesarstva 2006, 79, 19–35. [Google Scholar]
- Diaci, J. Silver fir decline in mixed old-growth forests in Slovenia: an interaction of air pollution, changingforest matrix and climate. In AirPollution – New Developments; Moldoveanu, A., Ed.; InTech, 2011; pp. 263–274. [Google Scholar]
- Frank, G.; Mayer, H. Waldschadensinventur im Fichten-Tannen-Buchen-Urwaldrest Neuwald; Cbl. f. d. ges. Forstw: Wien, 1988; pp. 104–123. [Google Scholar]
- Berge, E.; Bartnicki, J.; Olendrzynski, K.; Tsyro, S. G. Long-termtrends in emissions and transboundary transport of acidifying airpollution in Europe. J. Environ. Manage. 1999, 57, 31–50. [Google Scholar] [CrossRef]
- Stern, D. I. Global sulfur emissions from 1850 to 2000. Chemosphere 2005, 58, 163–175. [Google Scholar] [CrossRef]
- Čavlović, J.; Bončina, A.; Božić, M.; Goršić, E.; Simončič, T.; Teslak, K. Depression and growth recovery of silver fir in uneven-aged Dinaric forests in Croatia from 1901 to 2001. For. Int. J. For. Res. 2015, 88, 586–589. [Google Scholar] [CrossRef]
- Balcar, V.; Vacek, S.; Henžlík, V. Poškození a úhyn lesních porostů v Sudetských horách. In Protection of Forest Ecosystems, Selected Problems of Forestry in Sudety Mts.; Paschalis, P., Zajaczkowski, S., Eds.; Biuro GEF: Warszawa, 1997; pp. 29–57. [Google Scholar]
- Vacek, S.; Lokvenc, T.; Balcar, V.; Henžlík, V. Obnova a stabilizace lesa v horských oblastech Sudet. In Protection of forest ecosystems, selected problems of forestry in Sudety Mts., Paschalis, P.; Zajaczkowski, S., Ed.; Biuro GEF: Warszawa, 1997; pp. 93–119. [Google Scholar]
- Cabrera, M. Evolución de abetares del Pirineo aragonés. Cuadernos de la SECF 2001, 11, 43–52. [Google Scholar]
- Molina, J. M.; Pique, M. Análisis de la regeneración natural en una masa irregular de abeto, pino negro y pino silvestre. Cuadernos de la SECF 2003, 15, 129–134. [Google Scholar]
- Diaci, J.; Roženbergar, D.; Anic, I.; Mikac, S.; Saniga, M.; Kucbel, S.; Višnjić, Ć.; Ballian, D. Structural dynamics and synchronous silver fir decline in mixed old-growth mountain forests in Eastern and Southeastern Europe. Forestry 2011, 84, 479–491. [Google Scholar] [CrossRef]
- Ficko, A.; Poljanec, A.; Boncina, A. Do changes in spatial distribution, structure and abundance of silver fir (Abies alba Mill.) indicate its decline? For. Ecol. Manag. 2011, 261, 844–854. [Google Scholar] [CrossRef]
- Brinar, M. Življenjska kriza jelke na slovenskem ozemlju v zvezi s klimatičnimi fluktuacijami. Gozdarski vestnik 1964, 22, 97–144. [Google Scholar]
- Schütt, P. Die gegenwärtige Epidemic des Tannensterbens. Eur. J. Plant Pathol. 1978, 7, 187–190. [Google Scholar]
- Wick, L.; Möehl, A. The mid-Holocene extinction of silver fir (Abies alba) in the Southern Alps: A consequence of forest fires? Palaeobotanical records and forest simulations. Veg. Hist. Archaeobot. 2006, 15, 435–444. [Google Scholar] [CrossRef]
- Anic, I.; Vukelic, J.; Mikac, S.; Baksic, D.; Ugarkovic, D. Utjecaj globalnih klimatskih promjena na ekološku nišu obične jele (Abies alba Mill.) u Hrvatskoj. Šumarski list 2009, 133, 135–144. [Google Scholar]
- Linares, J. C. Biogeography and evolution of Abies (Pinaceae) in the Mediterranean Basin: The roles of long-term climatic change and glacial refugia. J. Biogeogr. 2011, 38, 619–630. [Google Scholar] [CrossRef]
- Bošeľa, M.; Ionel, P.; Gömöry, D.; Longauer, R.; Tobin, B.; Kyncl, J.; Kyncl, T.; Nechita, C.; Petráš, R.; Sidor, C.; et al. Effects of postglacial phylogeny and genetic diversity on the growth variability and climate sensitivity of European silver fir. J. Ecol. 2016, 104, 716–724. [Google Scholar] [CrossRef]
- Leonarduzzi, C.; Piotti, A.; Spanu, I.; Giovanni Giuseppe, V. Effective gene flow in a historically fragmented area at the southern edge of silver fir (Abies alba Mill.) distribution. Tree Genet. Genomes 2016, 12, 95. [Google Scholar] [CrossRef]
- Ondrejčík, R.; Krajmerová, D.; Longauer, R. Genetické riziká v cykle produkcie lesného reprodukčného materiálu na príklade uznaného porastu a sadeníc jedle bielej. In Adaptívny manažment pestovania lesov v procese klimatickej zmeny a globálneho otepľovania: Adaptive management of silviculture in the process of climate change ang global warming; Jaloviar, P., Saniga, M., Eds.; Proceedings of Central European Silviculture: Zvolen, Technická univerzita vo Zvolene, 2017; pp. 87–94. [Google Scholar]
- Durand-Gillmann, M.; Cailleret, M.; Boivin, T.; Nageleisen, L.-M.; Davi, H. Individual vulnerability factors of silver fir (Abies alba Mill.) to parasitism by two contrasting biotic agents: Mistletoe (Viscum album L. ssp. Abietis) and bark beetles (Coleoptera: Curculionidae: Scolytinae) during a decline process. Ann. For. Sci. 2012, 71, 1–15. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Eberlé, P.; Mérian, P.; Seynave, I. Social status-mediated tree-ring responses to climate of Abies alba and Fagus sylvatica shift in importance with increasing stand basal area. For. Ecol. Manage. 2014, 328, 209–218. [Google Scholar] [CrossRef]
- Mrkva, R. . Korovnice kavkazska (Dreyfusia nordmannianae Eckstein), obrana proti ní a její podíl na ústupu jedle. Lesnictvi – Forestry 1994, 40, 361–370. [Google Scholar]
- Zubrík, M. Kôrovnica kaukazská – významný škodca jedle. Les 1994, 50, 21–22. [Google Scholar]
- Ujházy, K.; Križová, E.; Vančo, M.; Freňáková, E.; Ondruš, M. Herblayer dynamics of primeval fir–beech forests in central Slovakia. In Natural Forests in the Temperate Zone of Europe -Values and Utilisation, Commarmot, B.; Hamor, F. D., Ed.; Federal Research Institute WSL, Birmensdorf & Carpathian Biosphere Reserve: Rakhiv: Swiss, 2005. [Google Scholar]
- Ficko, A.; Roessiger, J.; Boncina, A. Can the use of continuous cover forestry alone maintain silver fir (Abies alba Mill.) in central European mountain forests? Forestry 2016, 89, 412–421. [Google Scholar] [CrossRef]
- Motta, R. Impact of wild ungulates on forest regeneration and tree composition of mountain forests in the Western Italian Alps. For. Ecol. Manag. 1996, 88, 93–98. [Google Scholar] [CrossRef]
- Dobrowolska, D. Growth and development of silver fir (Abies alba Mill.) regeneration and restoration of the species in the Karkonosze Mountains. J. For. Sci. 2008, 54, 398–408. [Google Scholar] [CrossRef]
- Klopčič, M.; Jerina, K.; Bončina, A. Long-term changes of structure and tree species composition in Dinaric uneven-aged forests: Are red deer an important factor? Eur. J. For. Res. 2010, 129, 277–288. [Google Scholar] [CrossRef]
- Čermák, P.; Grundmann, P. Effects of browsing on the condition and development of regeneration of trees in the region of Rýchory (KRNAP). Acta Univ. Agric. Silvic. Mendel. Brun. 2006, 54, 7–14. [Google Scholar] [CrossRef]
- Homolka, M.; Heroldová, M. Impact of large herbivores on mountain forest stands in the Beskydy Mountains. For. Ecol. Manag. 2003, 181, 119–129. [Google Scholar] [CrossRef]
- Slanař, J.; Vacek, Z.; Vacek, S.; Bulušek, D.; Cukor, J.; Štefančík, I.; Bílek, L.; Král, J. Long-term transformation of submontane spruce-beech forests in the Jizerské hory Mts.: Dynamics of natural regeneration. Cent. Eur. For. J. 2017, 63, 212–224. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Slanař, J.; Bílek, L.; Bulušek, D.; Štefančík, I.; Králíček, I.; Vančura, K. Adaption of Norway spruce and European beech forests under climate change: From resistance to close-to-nature silviculture. Cent. Eur. For. J. 2019, 65, 129–144. [Google Scholar] [CrossRef]
- Motta, R. Wild ungulate browsing, natural regeneration and silviculture in the Italian Alps. J. Sustain. For. 1998, 8, 35–53. [Google Scholar] [CrossRef]
- Borowski, Z.; Gil, W.; Bartoń, K.; Zajączkowski, G.; Łukaszewicz, J.; Tittenbrun, A.; Radliński, B. Density-related effect of red deer browsing on palatable and unpalatable tree species and forest regeneration dynamics. For. Ecol. Manag. 2021, 496, 119442. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A.; Muter, E.; Pielech, R.; Szewczyk, J.; Zięba, A.; Zwijacz-Kozica, T.; Wiertelorz, A.; Pachowicz, T.; Bodziarczyk, J. Effects of species and environmental factors on browsing frequency of young trees in mountain forests affected by natural disturbances. For. Ecol. Manag. 2020, 474, 118364. [Google Scholar] [CrossRef]
- Kupferschmid, A. D.; Greilsamer, R.; Brang, P.; Bugmann, H. Assessment of the impact of ungulate browsing on tree regeneration. Schweiz. Z. Forstwes. 2022, 170, 125–134. [Google Scholar] [CrossRef]
- Vasiliauskas, R. Damage to trees due to forestry operations and its pathological significance in temperate forests: A literature review. Forestry 2001, 74, 319–336. [Google Scholar] [CrossRef]
- Čermák, P.; Mrkva, R.; Horsák, P.; Špiřík, M.; Beranová, P.; Orálková, J.; Kadlec, M.; Zárybnický, O.; Svatoš, M. Impact of ungulate browsing on forest dynamics. Folia For. Bohem. 2011, 20, 80. [Google Scholar]
- Vlad, R.; Sidor, C. G. Research for the estimate of rotten stem wood volume in Norway spruce stands damaged by deer species. Rev. Pădurilor 2013, 128, 27–32. [Google Scholar]
- Cukor, J.; Vacek, Z.; Linda, R.; Sharma, R. P.; Vacek, S. Afforested farmland vs. forestland: Effects of bark stripping by Cervus elaphus and climate on production potential and structure of Picea abies forests. PLoS ONE 2019, 14, e0221082. [Google Scholar] [CrossRef] [PubMed]
- Cukor, J.; Vacek, Z.; Linda, R.; Vacek, S.; Marada, P.; Šimůnek, V.; Havránek, F. Effects of bark stripping on timber production and structure of Norway spruce forests in relation to climatic factors. Forests 2019, 10, 320. [Google Scholar] [CrossRef]
- Cukor, J.; Zeidler, A.; Vacek, Z.; Vacek, S.; Šimůnek, V.; Gallo, J. Comparison of growth and wood quality of Norway spruce and European larch: effect of previous land use. Eur. J. For. Res. 2020, 139, 459–472. [Google Scholar] [CrossRef]
- Bazzigher, G.; Schmid, P. Sturmschäden und Fäule. Vol. 119; Z. Forstwesen: Schweiz, 1969; pp. 1–15. [Google Scholar]
- Kohnle, U.; Kändler, G. Is Silver fir (Abies alba) less vulnerable to extraction damage than Norway spruce (Picea abies)? Eur. J. For. Res. 2007, 126, 121–129. [Google Scholar] [CrossRef]
- Metzler, B.; Hecht, U.; Nill, M.; Brüchert, F.; Fink, S.; Kohnle, U. Comparing Norway spruce and silver fir regarding impact of bark wounds. For. Ecol. Manag. 2012, 274, 99–107. [Google Scholar] [CrossRef]
- Oven, P.; Torelli, N. Wound response of the bark in healthy and declining silver firs (Abies alba). IAWA J. 1994, 15, 407–415. [Google Scholar] [CrossRef]
- Oven, P.; Torelli, N. Response of the cambial zone in conifers to wounding. Phyton - Ann. Rei Bot. 1999, 39, 133–137. [Google Scholar]
- Pach, M. Spałowanie jodły na terenie Leśnego Zakładu Doświadczalnego w Krynicy (Beskid Sądecki) oraz jego wpływ na wybrane cechy morfologiczne koron. Acta Agr. Silv. Ser. Silv. 2002, 40, 31–47. [Google Scholar]
- Pach, M. Wpływ spałowania powodowanego przez jelenie na szerokość słojów rocznych pni jodeł. Acta Agr. Silv. Ser. Silv. 2003, 41, 75–82. [Google Scholar]
- Pach, M. Wpływ spałowania powodowanego przez jelenie na przyrost wysokości i miąższości jodeł (Abies alba Mill.). Acta Agr. Silv. Ser. Silv. 2004, 42, 35–48. [Google Scholar]
- Pach, M. Zasięg i dynamika rozprzestrzeniania się zgnilizny wewnątrz pni jodeł w wyniku ich spałowania przez jeleniowate. Extent and dynamics of wood decay spreading inward fir stems as a result of bark stripping by ungulates. Sylwan 2005, 149, 23–35. [Google Scholar]
- Pach, M. Tempo zarastania spał na jodle oraz niektóre czynniki na nie wpływające. The rate of bark-stripping wound closure in fir and some factors affecting it. Sylwan 2008, 152, 46–57. [Google Scholar]
- Vacek, Z.; Vacek, S.; Cukor, J.; Mikulenka, P. Struktura, produkce a škody zvěří ve smrkojedlových porostech v genové základně Hochwald. In Pěstování jedle bělokoré v podmínkách klimatické změny, Remeš, J.; Vacek, Z., Ed.; Česká lesnická společnost: Stará Ves u Rýmařova, Potočná 396, Chata Severka, 2022; pp. 23–33. [Google Scholar]
- Isomäki, A.; Kallio, T. Consequences of injury caused by timber harvesting machines on the growth and decay of spruce (Picea abies (L.) Karst.). Acta For. Fenn. 1974, 136, 1–25. [Google Scholar] [CrossRef]
- Gregory, S. C. The development of stain in wounded Sitka spruce stems. Forestry 1986, 59, 199–208. [Google Scholar] [CrossRef]
- Barszcz, P.; Jamrozy, G. Deprecjacja drewna jodeł i jesionów spałowanych przez jelenie w lasach Beskidu Sądeckiego. Sylwan 2001, 145, 47–57. [Google Scholar]
- Vacek, Z.; Cukor, J.; Linda, R.; Vacek, S.; Šimůnek, V.; Brichta, J.; Gallo, J.; Prokůpková, A. Bark stripping, the crucial factor affecting stem rot development and timber production of Norway spruce forests in Central Europe. For. Ecol. Manag. 2020, 474, 118360. [Google Scholar] [CrossRef]
- Pietrzykowski, E.; McArthur, C.; Fitzgerald, H.; Goodwin, A. N. Influence of patch characteristics on browsing of tree seedlings by mammalian herbivores. J. Appl. Ecol. 2003, 40, 458–469. [Google Scholar] [CrossRef]
- Marada, P.; Cukor, J.; Linda, R.; Vacek, Z.; Vacek, S.; Havránek, F. Extensive orchards in the agricultural landscape: Effective protection against fraying damage caused by roe deer. Sustainability 2019, 11, 3738. [Google Scholar] [CrossRef]
- Stutz, R.; Croak, B.; Leimar, O.; Bergvall, U. Borrowed plant defences: Deterring browsers using a forestry by-product. For. Ecol. Manag. 2017, 390, 1–7. [Google Scholar] [CrossRef]
- Spake, R.; Bellamy, C.; Graham, L.; Watts, K.; Wilson, T.; Norton, L.; Wood, C.; Schmucki, R.; Bullock, J.; Eigenbrod, F. An analytical framework for spatially targeted management of natural capital. Nat. Sustain. 2019, 2, 90–97. [Google Scholar] [CrossRef]
- Carpio, A. J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: contexts, causes, monitoring and management recommendations. Mammal Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Valente, A. M.; Acevedo, P.; Figueiredo, A. M.; Fonseca, C.; Torres, R. T. Overabundant wild ungulate populations in Europe: management with consideration of socio-ecological consequences. Mammal Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Ruprecht, J. S.; Koons, D. N.; Hersey, K. R.; Hobbs, N. T.; MacNulty, D. R. The effect of climate on population growth in a cold-adapted ungulate at its equatorial range limit. Ecosphere 2020, 11, e03058. [Google Scholar] [CrossRef]
- Peláez, M.; San Miguel, A.; Rodríguez-Vigal, C.; Moreno-Gómez, Á.; del Rincón Garoz, A. G.; García-Calvo, R. P. Using retrospective life tables to assess the effect of extreme climatic conditions on ungulate demography. Ecol. Evol. 2022, 12, e8218. [Google Scholar] [CrossRef]
- Capretti, P.; Korhonen, K.; Mugnai, L.; Romagnoli, C. An Intersterility group of Heterobasidion annosum specialized to Abies alba. Eur. J. Plant Pathol. 1990, 20, 231–240. [Google Scholar] [CrossRef]
- Korhonen, K.; Capretti, P.; Karjaleinen, R.; Stenlid, J. Distribution of Heterobasidion annosum intersterility groups in Europe. In Heterobasidion annosum: biology, ecology, impact and control; Woodward, S. J., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, 1998; pp. 93–104. [Google Scholar]
- Oliva, J.; Colinas, C. Epidemiology of Heterobasidion abietinum and Viscum album on silver fir (Abies alba) stands of the Pyrenees. For. Pathol. 2010, 40, 19–32. [Google Scholar] [CrossRef]
- Korhonen, K.; Holdenrieder, O. Neue Erkenntnisse über den Wurzelschwamm (Heterobasidion annosum s. l.) – Eine Literaturübersicht. Forst Holz 2005, 60, 206–211. [Google Scholar]
- Kost, G.; Haas, H. Die Pilzflora von Bannwäldern in BadenWürttemberg. In Waldschutzgebiete; Buck-Feucht, G., Bücking, W., Haas, H., Gerhard, K., Müller, S., Winterhoff, W., Eds.; Mitt. FVA BadenWürttemberg, 1989; pp. 1–182. [Google Scholar]
- Krieglsteiner, G. J.; Kaiser, A. Die Großpilze Baden-Württembergs. Allgemeiner Teil: Ständerpilze: Gallert-, Rinden-, Stachel- und Porenpilze; Die Großpilze Baden-Württemberg, 1, Verlag Eugen Ulmer: Stuttgart, 2000. [Google Scholar]
- Janssen, T.; Wulf, A. Zur Bedeutung von Misteln im Forstschutz; Biologische Bundesanstalt für Land und Forstschutz: Berlin, Germany, 1999; p. 129. [Google Scholar]
- Procházka, F. A centre of occurrence of Viscum album subsp. album in eastern Bohemia and an overview of the diversity of its host plants in the Czech Republic. Preslia 2004, 76, 349–359. [Google Scholar]
- Iszkulo, G.; Armatys, L.; Dering, M.; Ksepko, M.; Tomaszewski, D.; Wazna, A.; Giertych, M. J. Mistletoe as a threat to the health state of coniferous forest. Sylwan 2020, 164, 226–236. [Google Scholar]
- Tubeuf, K. Monographie der Mistel; München & Berlin, 1923. [Google Scholar]
- Knuchel, H. Die Holzfehler; Classen: Zürich, 1947. [Google Scholar]
- König, E. Die Fehler des Holzes, Holz-Zent.bl. 83; 1957; pp. 222–234. [Google Scholar]
- Noetzli, K. P.; Müller, B.; Sieber, T. N. Impact of population dynamics of white mistletoe (Viscum album ssp. abietis) on European silver fir (Abies alba). Ann. For. Sci. 2003, 60, 773–779. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, J.; Bauhus, J.; Boncina, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Nagel, T. A.; Mikac, S.; Dolinar, M.; Klopčič, M.; Keren, S.; Svoboda, M.; Diaci, J.; Bončina, A.; Paulic, V. The natural disturbance regime in forests of the Dinaric Mountains: A synthesis of evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Koffi, B.; Koffi, E. Heat waves across Europe by the end of the 21st century: Multiregional climate simulations. Clim. Res. 2008, 36, 153–168. [Google Scholar] [CrossRef]
- Kyselý, J.; Gaál, L.; Beranová, R.; Plavcová, E. Climate change scenarios of precipitation extremes in Central Europe from ENSEMBLES regional climate models. Theor. Appl. Climatol. 2011, 104, 529–542. [Google Scholar] [CrossRef]
- Mihai, G.; Alexandru, A. M.; Stoica, E.; Birsan, M. V. Intraspecific growth response to drought of Abies alba in the Southeastern Carpathians. Forests 2021, 12, 387. [Google Scholar] [CrossRef]
- Bauhus, J.; Forrester, D. I.; Gardiner, B.; Jactel, H.; Vallejo, R.; Pretzsch, H. Ecological stability of mixed-species forests. In Mixed-Species Forests, Pretzsch, H.; Forrester, D. I., Bauhus, J., Eds.; Springer: Berlin, Heidelberg, 2017; pp. 337–382. [Google Scholar]
- Steckel, M.; Rio, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Černý, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) – Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Forrester, D.; Bauhus, J. A review of processes behind diversity—productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Olabarria, J.; Koricheva, J.; Meurisse, N.; Brockerhoff, E. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef]
- Zang, C.; Rothe, A.; Weis, W.; Pretzsch, H. Zur Baumarteneignung bei Klimawandel: Ableitung der Trockenstress-Anfälligkeit wichtiger Waldbaumarten aus Jahrringbreiten. Allg. Forst Jagdztg. 2011, 182, 98–112. [Google Scholar]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef] [PubMed]
- Klopčič, M.; Mina, M.; Bugmann, H.; Bončina, A. The prospects of silver fir (Abies alba Mill.) and Norway spruce (Picea abies (L.) Karst) in mixed mountain forests under various management strategies, climate change and high browsing pressure. Eur. J. For. Res. 2017, 136, 1071–1090. [Google Scholar] [CrossRef]
- Šindelář, J.; Frýdl, J. Perspektivy jedle bělokoré (Abies alba Mill.) v lesním hospodářství České republiky. In Jedle bělokorá – 2005. Proceedings of the Jedle bělokorá – 2005, Srní, Czech Republic, Oct 31–Nov 1, 2005; Neuhöferová, P., Ed.; ČZU FLE v Praze, Katedra pěstování lesů a Správa Národního parku a chráněné krajinné oblasti Šumava: Praha, 2005. [Google Scholar]
- Vencurik, J.; Sedmáková, D.; Bača, M.; Jaloviar, P.; Kucbel, S. Growth reactions of conifers on changing climate conditions in selection forest - a case study. Reports of Forestry Research 2022, 67, 203–212. [Google Scholar]
- Spittlehouse, D. L.; Stewart, R. B. Adaptation to climate change in forest management. Journal of Ecosystems and Management 2003, 4, 1–11. [Google Scholar] [CrossRef]
- Millar, C. I.; Stephenson, N. L.; Stephens, S. L. Climate change and forests of the future: managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- Hiltunen, M.; Strandman, H.; Kilpeläinen, A. Optimizing forest management for climate impact and economic profitability under alternative initial stand age structures. Biomass Bioenerg. 2021, 147, 106027. [Google Scholar] [CrossRef]
- Sterck, F.; Vos, M.; Hannula, S.; de Goede, S.; de Vries, W.; Ouden, J.; Nabuurs, G.-J.; var der Putten, W.; Veen, C. Optimizing stand density for climate-smart forestry: A way forward towards resilient forests with enhanced carbon storage under extreme climate events. Soil Biol. Biochem. 2021, 162, 108396. [Google Scholar] [CrossRef]
- ÚHÚL. Národní lesnický program pro období do roku 2013. Praha: Ústav pro hospodářskou úpravu lesů, 2008.
- European Commission. Strategie EU pro přizpůsobení se změně klimatu. COM 2016, 2014.
- Aitken, S. N.; Yeaman, S.; Holliday, J. A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Ripple, W. J.; Wolf, C.; Newsome, T. M.; Barnard, P.; Moomaw, W. R. World scientists' warning of a climate emergency. BioScience 2020, 70, 8–12. [Google Scholar] [CrossRef]
- Woodall, C. W.; Oswalt, C. M.; Westfall, J. A.; Perry, C. H.; Nelson, M. D.; Finley, A. O. An indicator of tree migration in forests of the eastern United States. For. Ecol. Manag. 2009, 257, 1434–1444. [Google Scholar] [CrossRef]
- Gömöry, D.; Krajmerova, D.; Hrivnák, M.; Longauer, R. Assisted migration vs. close-to-nature forestry: what are the prospects for tree populations under climate change? Cent. Eur. For. J. 2020, 66, 63–70. [Google Scholar] [CrossRef]
- Feurdean, A.; Tămaş, T.; Tanţău, I.; Farcas, S. Elevational variation in regional vegetation responses to late-glacial climate changes in the Carpathians. J. Biogeogr. 2011, 39, 258–271. [Google Scholar] [CrossRef]
- Feurdean, A.; Bhagwat, S.; Willis, K.; Birks, H.; Lischke, H.; Hickler, T. Tree migration-rates: narrowing the gap between inferred post-glacial rates and projected rates. PLoS ONE 2013, 8, e71797. [Google Scholar] [CrossRef] [PubMed]
- Mátyás, C. What do feld trials tell about the future use of forest reproductive material. In Climate Change and Forest Genetic Diversity: Implications for Sustainable Forest Management in Europe; Koskela, J., Buck, A., Teissier du Cros, E., Eds.; Bioversity International: Rome, 2007; pp. 53–69. [Google Scholar]
- Machar, I.; Vlčková, V.; Buček, A.; Voženílek, V.; Šálek, L.; Jeřábková, L. Modelling of climate conditions in forest vegetation zones as a support tool for forest management strategy in European beech dominated forests. Forests 2017, 8, 82. [Google Scholar] [CrossRef]
- Iverson, L.; McKenzie, D. Tree-species range shifts in a changing climate: Detecting, modeling, assisting. Landsc. Ecol. 2013, 28, 879–889. [Google Scholar] [CrossRef]
- Iverson, L.; Schwartz, M.; Prasad, A. How fast and far might tree species migrate in the eastern United States due to climate change? Glob. Ecol. Biogeogr. 2004, 13, 209–219. [Google Scholar] [CrossRef]
- Chroust, L.; et al. Pěstování lesa. Doplňkový učební text. Ústav pěstování lesa LDF MZLU, Brno, 2001. https://rumex.mendelu.cz/uzpl/pestovani_v_heslech/index.html.
- Hlavová, Z. Technologie skladování a předosevní příprava pro jedli bělokorou a buk lesní používané v lesnickém závodě Týniště nad Orlicí. In Pěstování sadebního materiálu z dlouhodobě skladovaného osiva buku a jedle. Proceedings of the Pěstování sadebního materiálu z dlouhodobě skladovaného osiva buku a jedle, Hradec Králové, 17 June 1999.; AVE Centrum: Opava, 1999; pp. 18–20. [Google Scholar]
- Chválová, K. Skúsenosti so spracováním, skladováním a predsejbovou prípravou buka a jedle na Slovensku. In Pěstování sadebního materiálu z dlouhodobě skladovaného osiva buku a jedle. Proceedings of the Pěstování sadebního materiálu z dlouhodobě skladovaného osiva buku a jedle, Hradec Králové, 17 June 1999.; AVE Centrum: Opava, 1999; pp. 27–31. [Google Scholar]
- Ruiz de la Torre, J. Flora Mayor; ICONA-OAPN: Madrid, 2006. [Google Scholar]
- Gradi, A. La conoscenza del contenuto d acqua degli strobili a dei semi faktore determinante per una razionale preparazione delle sementi di confere a per la loro conservazione. Monti a Bosch 1963, 14, 195–208. [Google Scholar]
- Walter, V. Rozmnožování okrasných stromů a keřů, novinářské závody, n.p. závod 6, Legerova 22, Praha 2, AA- 21,18, VA 21,62, publikace č. 29150. 1978.
- Leadem, C. L. Quick tests for tree seed viability. BC Ministry of Forests, Research Branch, Land Management report no. 18: Canada, 1984; pp. 45.
- Palátová, E. Zakládání lesa I. Lesní semenářství; MZLU: Brno, 2008; p. 119. [Google Scholar]
- Řezníčková, J.; Bezděčková, L.; Procházková, Z. Cone collection and processing, storing, pre-sowing treatment and quality of European silver fir (Abies alba) seeds: A literature review. Reports of Forestry Research 2010, 55, 180–186. [Google Scholar]
- Messer, H.; Hanau, W. Der Wassergehalt des Forstsaatgutes als Grundlage der Ernte-, Veredelungs- und Aufbewahrungs- massnahmen. Forst Holz. 1959, 9, 226–229. [Google Scholar]
- Musil, I.; Hamerník, J. Lesnická dendrologie 1: jehličnaté dřeviny: přehled nahosemenných (i výtrusných) dřevin; Česká zemědělská univerzita: Praha, 2002; pp. 79–98. [Google Scholar]
- Kantor, P. e. a. Zakládání lesů; Státní zemědělské nakladatelství: Praha, 1965; p. 490. [Google Scholar]
- Dušek, V.; Kotyza, F. Moderní lesní školkařství; Státní zemědělské nakladatelství: Praha, 1970; p. 480. [Google Scholar]
- Kupka, I. Reaction of Silver fir (Abies alba Mill.) plantation to fertilization. J. For. Sci. 2005, 51, 95–100. [Google Scholar] [CrossRef]
- Teodosiu, M.; Botezatu, A.; Ciocîrlan, E.; Mihai, G. Variation of cones production in a silver fir (Abies alba Mill.) clonal seed orchard. Forests 2023, 14, 17. [Google Scholar] [CrossRef]
- Hlásny, T.; Zimová, S.; Merganičová, K.; Štěpánek, P.; Modlinger, R.; Turčáni, M. Devastating outbreak of bark beetles in the Czech Republic: Drivers, impacts, and management implications. For. Ecol. Manag. 2021, 490, 119075. [Google Scholar] [CrossRef]
- Stejskalová, J.; Kupka, I. Forest vegetation zones influence on seed quality of silver fir (Abies alba Mill.). In Proceedigs of Central European Silviculture – 12th International Conference. Proceedings of Central European Silviculture 2011, Opočno, Czech Republic, June 28 - 29, 2011; Kacálek, D., Jurásek, A., Novák, J., Slodičák, M., Eds.; Forestry and Game Management Research Institute, Strnady - Opocno Research Station: Opočno, 2011; pp. 235–242. [Google Scholar]
- Bezděčkova, L.; Řezníčková, J. Effect of pre-sowing treatment on the germination and emergence of silver fir seeds. Reports of Forestry Research 2012, 57, 249–256. [Google Scholar]
- Morar, I. M.; Catalina, D.; Sestras, R. E.; Stoian-Dod, R. L.; Truța, A. M.; Sestras, A. F.; Sestras, P. Evaluation of different geographic provenances of silver fir (Abies alba) as seed sources, based on seed traits and germination. Forests 2023, 14, 1286. [Google Scholar] [CrossRef]
- Skořepa, H. Jedle bělokorá v našich lesích. Živa 2006, 3, 105–110. [Google Scholar]
- Boncaldo, E.; Bruno, G.; Tommasi, F.; Mastropasqua, L. Germinability and fungal occurrence in seeds of Abies alba Mill. populations in southern Italy. Plant Biosyst. 2010, 144, 740–745. [Google Scholar] [CrossRef]
- Gradečki-Poštenjak, M.; Ćelepirović, N. The influence of crown defoliation on the variability of some physiological and morphological properties of silver fir (Abies alba) seeds in the seed zone of Dinaric beech-fir forests in Croatia. Period. Biol. 2016, 117, 479–492. [Google Scholar] [CrossRef]
- Ledinský, J. Hnojení sazenic v lesních školkách průmyslovými hnojivy; Vol. 1. vydání; Výzkumný ústav lesního hospodářství a myslivosti – Bulletin TEI, série Pěstování, č. 2/87: Jíloviště-Strnady, 1987; p. 10. [Google Scholar]
- Šrámek, F.; Dubský, M.; Weber, M.; Dostálek, J.; Skaloš, J. Peat-reduced substrates with mineral components for growing of woody plants. Acta Hortic. 2010, 885, 361–366. [Google Scholar] [CrossRef]
- Sebastiana, M.; Pereira, V.; Alcântara, A.; Pais, M.; da Silva, A. Ectomycorrhizal inoculation with Pisolithus tinctorius increases the performance of Quercus suber L. (cork oak) nursery and field seedlings. New Forest. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Comandini, O.; Pacioni, G.; Rinaldi, A. C. Fungi in ectomycorrhizal associations of silver fir (Abies alba Miller) in Central Italy. Mycorrhiza 1998, 7, 323–328. [Google Scholar] [CrossRef]
- Eberhardt, U.; Oberwinkler, F.; Verbeken, M.; Rinaldi, A.; Pacioni, G.; Comandini, O. Lactarius ectomycorrhizae on Abies alba: morphological description, molecular characterization, and taxonomic remarks. Mycologia 2000, 92, 860–873. [Google Scholar] [CrossRef]
- Burda, P. Ověření pěstebních postupů a využití nových školkařských technologií při pěstování sadebního materiálu lesních dřevin a posouzení kvality vyprodukovaného sadebního materiálu. Dissertation thesis, Czech University of Life Sciences, Prague, 2009. [Google Scholar]
- Ivanković, M.; Marjanović, H.; Franjić, J.; Škvorc, Ž.; Bogdan, S. Variability of Silver fir (Abies alba Mill.) provenances in the number of lateral buds on terminal sprout and damage by the late frost. Period. Biol. 2007, 109, 55–59. [Google Scholar]
- Leugner, J.; Martincová, J.; Jurásek, A. Growth response of silver fir (Abies alba Mill.) young plants on desiccation during handling with respect to conditions following outplanting. Reports of Forestry Research 2014, 59, 28–34. [Google Scholar]
- Robakowski, P.; Pietrzak, T.; Kowalkowski, W.; Małecki, G. Survival, growth and photochemical efficiency of silver fir seedlings produced with different technologies. New Forest. 2021, 52, 1055–1077. [Google Scholar] [CrossRef]
- Mauer, O. Pěstování sadebního materiálu; Mendelova univerzita v Brně: Brno, 2013; p. 204. [Google Scholar]
- Burda, P.; Nárovcová, J.; Nárovec, V.; Kuneš, I.; Baláš, M.; Machovič, I. Technologie pěstování listnatých poloodrostků a odrostků nové generace v lesních školkách. Certifikovaná metodika., 1st ed.; Forestry and Game Management Research Institute: Strnady, Czech Republic, 2015; p. 56. [Google Scholar]
- Jurásek, A.; Martincová, J.; Leugner, J. Manipulace se sadebním materiálem lesních dřevin od vyzvednutí ve školce až po výsadbu. Certifikovaná metodika., 1st ed.; Forestry and Game Management Research Institute: Strnady, Czech Republic, 2010. [Google Scholar]
- South, D.; Enebak, S. Integrated pest management practices in southern pine nurseries. New Forest. 2006, 31, 253–271. [Google Scholar] [CrossRef]
- Kozlowski, T.; Pallardy, S. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Harayama, H.; Tobita, H.; Kitao, M.; Kon, H.; Ishizuka, W.; Kuromaru, M.; Kita, K. Enhanced summer planting survival of Japanese larch container-grown seedlings. Forests 2021, 12, 1115. [Google Scholar] [CrossRef]
- Englert, J.; Warren, K.; Fuchigami, L. H.; Chen, T. Antidesiccant compounds improve the survival of bare-root deciduous nursery trees. J. Am. Soc. Hortic. Sci. 1993, 118, 228–235. [Google Scholar] [CrossRef]
- ČSN 48 2115. Sadební materiál lesních dřevin. Český normalizační institut: Praha; pp. 17.
- Němec, L. Pěstování lesních sadby na vzduchovém polštáři. Lesnická práce 2001, 80, 426. [Google Scholar]
- Deans, J. D.; Lundberg, C.; Tabbush, P. M.; Cannell, M. G. R.; Sheppard, L. J.; Murray, M. B. The influence of desiccation, rough handling and cold storage on the quality and establishment of sitka spruce planting stock. Forestry 1990, 63, 129–141. [Google Scholar] [CrossRef]
- Balneaves, J.; Menzies, M. Water potential and subsequent growth of Pinus radiata seedlings: influence of lifting, packaging and storage conditions. N. Z. J. For. Sci. 1990, 20, 257–267. [Google Scholar]
- Genç, M. Effects of watering after lifting and exposure before planting on plant quality and performance in oriental spruce. Ann. Sci. Forest. 1996, 53, 139–143. [Google Scholar] [CrossRef]
- Jaworski, A. Charakterystyka hodowlana drzew leśnych; Gutenberg: Kraków, 1995; p. 237. [Google Scholar]
- Szymura, T. H. Silver fir sapling bank in seminatural stand: Individuals architecture and vitality. For. Ecol. Manag. 2005, 212, 101–108. [Google Scholar]
- Kantor, P. Obnova jedle bělokoré. In Pěstování a umělá obnova jedle bělokoré. Proceedings of the Pěstování a umělá obnova jedle bělokoré, Chudobín u Litovele, Czech Republic, 28 August 2001; Kotrla, K., Kyslík, P., Eds.; Česká lesnická společnost: Praha, 2001; pp. 5–13. [Google Scholar]
- Backman, G. Wachstum und organische Zeit; J. A. Barth: Leipzig, 1943. [Google Scholar]
- Bezačinský, H. Problém odumierania jedle na Slovensku z pestovateľského hľadiska; VŠLD: Zvolen, 1962; pp. 87–102. [Google Scholar]
- Vinš, B. Příspěvek k výzkumu proměnlivosti jedle (Abies alba Mill.). Rozpravy Čs. akademie věd, řada mat. a přír. věd 1966, 76, 1–82. [Google Scholar]
- Zakopal, V. Studie u nás vytvořených tvarů výběrného lesa. Lesnictví 1959, 5, 995–1012. [Google Scholar]
- Robakowski, P.; Bielinis, E. Needle age dependence of photosynthesis along a light gradient within an Abies alba crown. Acta Physiol. Plant. 2017, 39, 83. [Google Scholar] [CrossRef]
- Jarzyna, K. Climatic hazards for native tree species in Poland with special regards to silver fir (Abies alba Mill.) and European beech (Fagus sylvatica L.). Theor. Appl. Climatol. 2021, 144, 581–591. [Google Scholar]
- Piedallu, C.; Dallery, D.; Bresson, C.; Legay, M.; Gégout, J. C.; Pierrat, R. Spatial vulnerability assessment of silver fir and Norway spruce dieback driven by climate warming. Landsc. Ecol. 2023, 38, 341–361. [Google Scholar] [CrossRef]
- Kadlus, Z.; Zakopal, V. Pěstování jedle ve světle nových poznatků. Zprávy lesnického výzkumu 1970, 16, 24–32. [Google Scholar]
- Sokol, A. Jedľové porasty. In Pěstění lesů III, Polanský, D. et al., Eds.; SZN: Praha, 1956; pp. 439–446. [Google Scholar]
- Kadlus, Z. Obnova jedle v hospodářských porostech smrku a borovice na stanovištích jedlových doubrav. Práce VÚLHM 1970, 39, 79–102. [Google Scholar]
- Průša, E. Die böhmischen und mährischen Urwälder, ihre Struktur und Ökologie; Academia: Praha, 1985; p. 578. [Google Scholar]
- Klopčič, M.; Bončina, A. Stand dynamics of silver fir (Abies alba Mill.)-European beech (Fagus sylvatica L.) forests during the past century: A decline of silver fir? Forestry 2011, 84, 259–271. [Google Scholar] [CrossRef]
- Klopčič, M.; Bončina, A. A century long dynamics of silver fir population in mixed silver fir-European beech forests. Zbornik gozdarstva in lesarstva 2012, 97, 43–54. [Google Scholar]
- Polách, R.; Špulák, O. Prosperity of Silver fir planted under preparatory stands of pioneer broadleaves of different śtocking and age. Reports of Forestry Research 2022, 64, 269–277. [Google Scholar]
- Elliott, K. J.; Miniat, C.; Pederson, N.; Laseter, S. Forest tree growth response to hydroclimate variability in the southern Appalachians. Glob. Change Biol. 2015, 21, 4627–4641. [Google Scholar] [CrossRef] [PubMed]
- Uhl, E.; Hilmers, T.; Pretzsch, H. From acid rain to low precipitation: the role reversal of Norway spruce, silver fir, and European beech in a selection mountain forest and its implications for forest management. Forests 2021, 12, 894. [Google Scholar] [CrossRef]
- Ryan, M. G.; Phillips, N.; Bond, B. J. The hydraulic limitation hypothesis revisited. Plant Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Grote, R.; Gessler, A.; Hommel, R.; Poschenrieder, W.; Priesack, E. Importance of tree height and social position for drought-related stress on tree growth and mortality. Trees 2016, 30, 1467–1482. [Google Scholar] [CrossRef]
- Taccoen, A.; Piedallu, C.; Seynave, I.; Gégout-Petit, A.; Nageleisen, L.-M.; Nathalie, B.; Gégout, J.-C. Climate change impact on tree mortality differs with tree social status. For. Ecol. Manag. 2021, 489, 119048. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Jansons, A.; Matisons, R.; Jansone, L.; Neimane, U.; Jansons, J. Relationships between climatic variables and tree-ring width of European beech and European larch growing outside of their natural distribution area. Silva Fenn. 2015, 49, 1255. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology; Springer: Berlin, 1995; p. 513. [Google Scholar]
- Kolář, T.; Čermák, P.; Trnka, M.; Žid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in Central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Pretzsch, H.; Forrester, D. I. Stand dynamics of mixed-species stands compared with monocultures. In Mixed-species Forests, Pretzsch, H. et al. Eds.; Springer: Berlin, Heidelberg, 2017; pp. 117–209. [Google Scholar]
- Lindner, M.; Fitzgerald, J. B.; Zimmermann, N. E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.-J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Spiecker, H.; Hansen, J.; Klimo, E.; Skovsgaard, J. P.; Sterba, H.; von Teuffel, K. (Eds.) Norway spruce conversion – options and consequences; European Forest Institute Research Report: Brill, Leiden – Boston, 2004; p. 269. [Google Scholar]
- Bílek, L.; Peña, J. F.; Remeš, J. National Nature Reserve Voděradské bučiny – 30 years of forestry research; Lesnická práce: Kostelec nad Černými lesy, 2013; pp. 1–86. [Google Scholar]
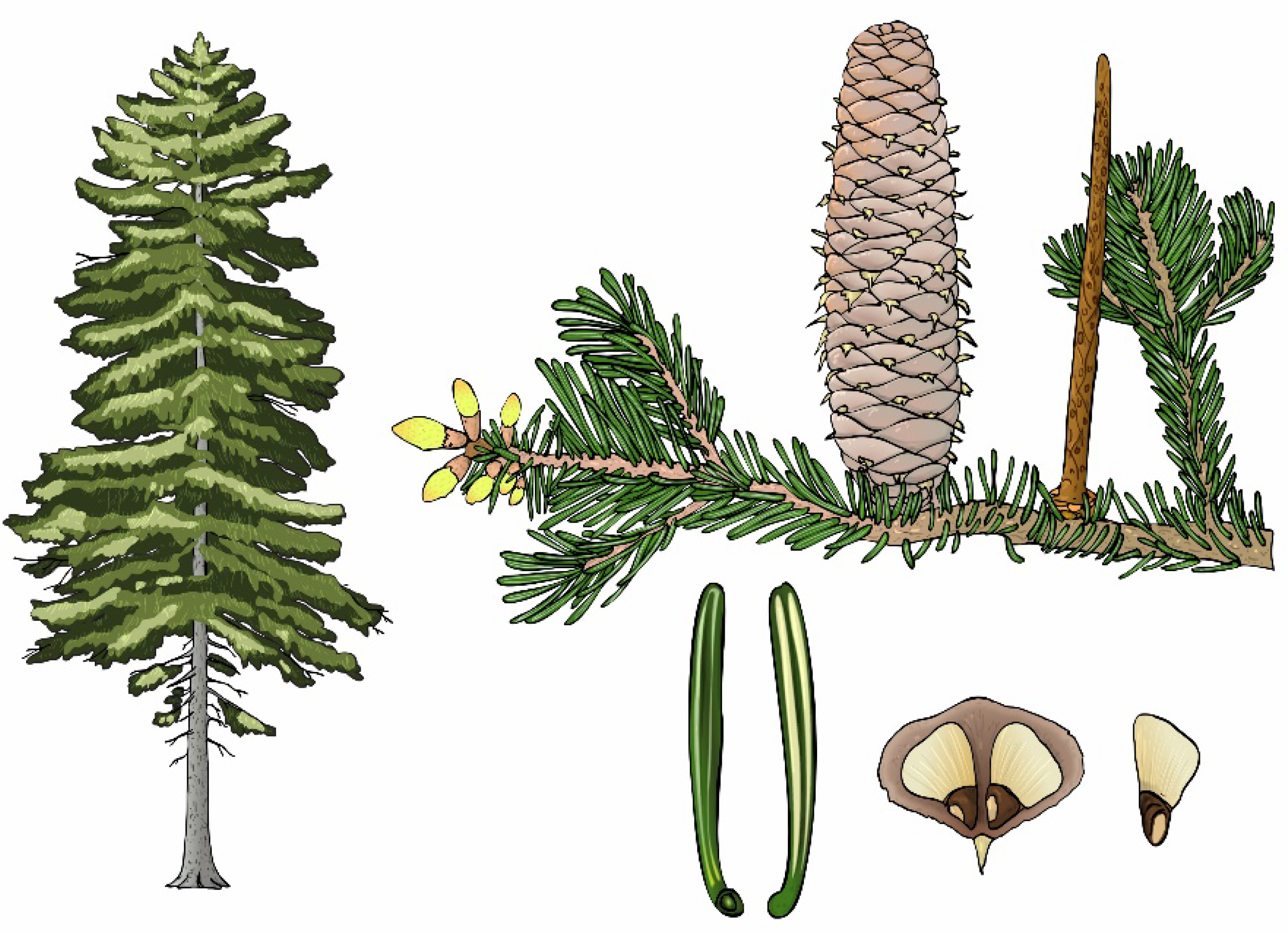

| Study | Country | Altitude [m a.s.l.] |
Age [y] |
DBH [cm] |
Height [m] |
Basal area [m2 ha−1] |
Volume [m3 ha−1] |
Density [trees ha−1] |
Climate classification |
|---|---|---|---|---|---|---|---|---|---|
| [58] | Bosnia and Herzegovina |
to 1078 | max. 165 | 28.7–45.7 | 274–590 | 588–732 | Dfb | ||
| [10] | Croatia | 876–978 | 73–76 | 28.4–32.2 | 19.4–22.6 | 227–361 | Cfa | ||
| [9] | Czechia | 660–710 | 56–146 | 28.9–40.7 | 18.0–26.0 | 43.4–53.3 | 486–594 | 336–816 | Dfb |
| [59] | Czechia | 940–1100 | 158–189 | 23.0–27.0 | 13.0–15.0 | 450–560 | Dfb | ||
| [60] | Czechia | 640–800 | 68 | 29–34 | 333 | Dfb | |||
| [10] | Czechia | 660–790 | 63–76 | 25.8–31.8 | 21.2–23.6 | 346–398 | Dfb | ||
| [61] | Czechia | 330 | 45 | 18.0–22.0 | 17.0–19.0 | 164–372 | 714–1042 | Cfb | |
| [8] | Czechia | 670–730 | 108–126 | 15.4–34.9 | 22.8–37.6 | 20.6–43.5 | 237–598 | 456–1624 | Dfb |
| [62] | Italy | 1200 | max. 130 | 35.0–45.9 | 336–356 | 1175–1215 | Cfb/Csb | ||
| [10] | Italy | 944–1324 | 66–75 | 23.0–28.1 | 18.7–25.4 | 220–289 | Csb | ||
| [63] | Poland | 337–889 | 40–115 | 15.9–46.5 | 14.1–31.5 | 35.5–43.8 | 322–657 | 211–1852 | Cfb/Dfb |
| [64] | Poland | 550–600 | 68–78 | 24.6–30.0 | 24.9–25.5 | 27.6–37.9 | 362–533 | 569–752 | Dfb |
| [65] | Poland | 300–725 | 40–150 | 21.0–39.0 | 27.3–31.0 | 34.0–70.0 | 382–715 | Cfb/Dfb | |
| [66] | Poland | 194 | 60 | 33.1 | 22.2 | 301 | 378 | Cfb | |
| [8] | Poland | 520 | 124 | 34.5 | 35.2 | 43.3 | 591 | 464 | Dfb |
| [67] | Romania | 700–950 | 100–130 | 7.6–45.0 | 5.8–31.6 | 35.0–45.0 | 299 | Dfb | |
| [68] | Slovenia | 850 | 108–132 | 33.4 | 207 | Dfb | |||
| [69] | Ukraine | 750–1045 | 94–132 | 36.0–48.0 | 28.0–30.0 | 324–550 | Dfb |
| Study | Country | Climate classification | Altitude | Fir | Beech | Spruce | Rowan | Maple | Ash | Pine |
|---|---|---|---|---|---|---|---|---|---|---|
| [185] | Czechia | Dfb | 450–1033 | 41 | 16 | 14 | 31 | 22 | 42 | 0 |
| [186] | Czechia | Dfb | 1000–1257 | 8 | 44 | 0 | 35 | 64 | ||
| [187] | Czechia | Dfb | 725–765 | 36 | 12 | 3 | 57 | 100 | ||
| [59] | Czechia | Dfb | 940–1100 | 100 | 78 | 48 | 76 | 91 | ||
| [8] | Czechia | Dfb | 520–730 | 88 | 30 | 9 | ||||
| [102] | Czechia | Dfb | 740–920 | 100 | 56 | 18 | 94 | |||
| [31] | Czechia | Dfb | 420–440 | 53 | 23 | 25 | 50 | 34 | 23 | |
| [188] | Czechia | Dfb | 640–810 | 68 | 6 | 2 | 82 | 100 | ||
| [182] | Italy | Dfc/Dfb | 900–2300 | 42 | 14 | 2 | ||||
| [189] | Italy | Dfc/Dfb | 800–2500 | 41 | 15 | 13 | 46 | 46 | 39 | 14 |
| [190] | Poland | Dfb | 223–364 | 19 | 15 | 48 | ||||
| [191] | Poland | Dfb | 800–1600 | 33 | 1 | 1 | 40 | 39 | ||
| [192] | Switzerland | Dfc/Dfb | 380–3000 | 6 | 3 | 1 | 62 | 29 | 19 | |
| Mean | 49 | 25 | 12 | 57 | 57 | 31 | 5 |
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Production (thous. kg) | 42.1 | 59.0 | 30.5 | 95.3 | 7.7 | 48.6 | 19.5 | 15.2 | 79.0 | 5.9 | 115.7 | 42.7 | 134.5 |
| Seed amount(thous. kg) | 4.2 | 5.9 | 3.1 | 9.5 | 0.8 | 4.9 | 1.9 | 1.5 | 7.9 | 0.6 | 11.6 | 4.3 | 13.4 |
| Seedling numbers (mil. pcs) | 12.6 | 17.7 | 9.2 | 28.6 | 2.3 | 14.6 | 5.8 | 4.6 | 23.7 | 1.8 | 34.7 | 12.8 | 40.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





