Submitted:
23 April 2024
Posted:
25 April 2024
You are already at the latest version
Abstract
Keywords:
The Scoping Review and Analysis Has Observed the Following Strengths and Limitations
Introduction
Methods and Methods
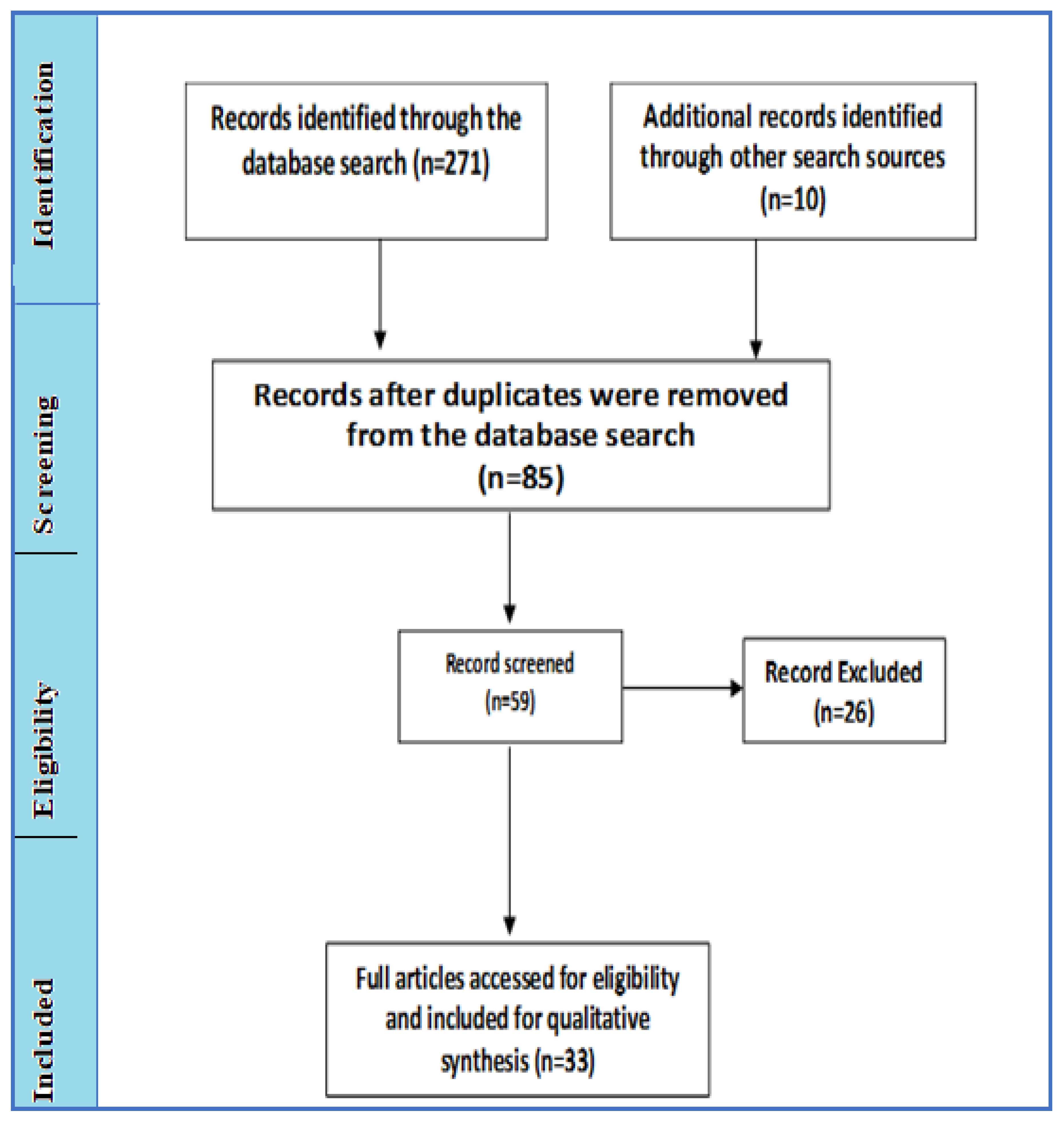
Results
| Author Name | Sample Size & (%) | Country | Year | Article type | %Qi | Evaluation | Citation |
|---|---|---|---|---|---|---|---|
| Abaver et al.,2012 | 480(1.04) | Nigeria | 2012 | Clinical Reviews | 50.0 | low | [42] |
| Abo-Madyan et al.,2004 | 1,019(1.67) | Egypt | 2004 | clinical trial | 50.0 | low | [43] |
| Abou-Basha et al.,2000 | 2,492(4.45) | Egypt | 2000 | Clinical Reviews | 40.0 | low | [44] |
| Adarosy et al.,2013a | 1,868(0,59) | Egypt | 2013 | clinical trial | 30.0 | low | [45] |
| Adarosy et al.,2013b | 1,868(1.07) | Egypt | 2013 | clinical trial | 30.0 | low | [45] |
| Adarosy et al.,2013c | 1,868(0.75) | Egypt | 2013 | clinical trial | 30.0 | low | [45] |
| Arinola et al.,2015 | 349(0.29) | Nigeria | 2015 | Impact Evaluation | 37.5 | high | [46] |
| Black et al. 2013a | 3(33.33) | South Africa | 2013 | Case report article | 30.0 | low | [47] |
| Black et al.,2013b | 3(66.67) | South Africa | 2013 | Case report article | 30.0 | low | [47] |
| Curtale et al.,2000 | 8,854(2.88) | Egypt | 2000 | Parasitological Reviews | 62.5 | moderate | [48] |
| Curtale et al.,2003 | 1,331(5.41) | Egypt | 2003 | Crossectional Survey | 80.0 | low | [49] |
| de Alegría et al.,2017 | 372(0.27) | Angola | 2017 | Crossectional Survey | 100.0 | moderate | [50] |
| El Shazly et al.,2006 | 3,180(4.78) | Egypt | 2006 | Parasitological Survey | 12.5 | high | [51] |
| El-Karaksy et al.,2018 | 4,475(0.85) | Egypt | 2018 | Clinical Reviews | 37.5 | high | [52] |
| el-Shazly et al.,2001 | 605(52.56) | Egypt | 2001 | clinical trial | 37.5 | moderate | [53] |
| El-Shazly et al.,2002a | 1,000(8.20) | Egypt | 2002 | Serological Evaluation | 50.0 | moderate | [54] |
| El-Shazly et al.,2002b | 6(33.33) | Egypt | 2002 | Rectal Biospy | 50.0 | moderate | [55] |
| el-Shazly et al.,2006 | 1,000(0.40) | Egypt | 2006 | Clinical Reviews | 37.5 | low | [54] |
| El-Shazly et al.,2009 | 3,000(3.27) | Egypt | 2009 | Clinical Reviews | 33.3 | low | [53] |
| Esteban et al.,2003 | 678(19.03) | Egypt | 2003 | Clinical Reviews | 70.0 | low | [56] |
| Fawzi et al.,2004 | 575(2.43) | Egypt | 2004 | Public Health Reviews | 55.6 | moderate | [57] |
| Fawzi et al.,2004 | 575 | Egypt | 2004 | Crossectional Survey | 50.0 | high | [57] |
| Fentie et al.,2013 | 520(3.27) | Ethiopia | 2013 | Crossectional Survey | 90.0 | moderate | [58] |
| Hammami et al.,2007 | 30(6.67) | Tunisia | 2007 | Crossectional Survey | 25.0 | high | [59] |
| Ihesiulor et al.,2013 | 570(0.88) | Nigeria | 2013 | Parasitological Reviews | 37.5 | moderate | [60] |
| Ijagbone et al.,2006 | 533(0.56) | Nigeria | 2006 | Parasitological Survey | 44.4 | moderate | [61] |
| Keiser et al.,2011 | 1,215(3.37) | Egypt | 2011 | clinical trial | 62.5 | moderate | [62] |
| Lukambagire et al.,2015 | 1,460(20.89) | Tanzania | 2015 | Crossectional Survey | 55.6 | moderate | [12] |
| Mas-Coma.2004 | 7,071(6.84) | Africa | 2004 | research article | 30.0 | low | [39] |
| Mekky et al.,2015a | 23(8.70) | Egypt | 2015 | research article | 25.0 | low | [63] |
| Mekky et al.,2015b | 23(100) | Egypt | 2015 | research article | 80.0 | high | [63] |
| Mekky et al.,2015c | 23(100) | Egypt | 2015 | research article | 80.0 | high | [63] |
| Mekky et al.,2015d | 23(65.22) | Egypt | 2015 | research article | 80.0 | high | [63] |
| Mekky et al.,2015e | 23(13.04) | Egypt | 2015 | research article | 37.5 | low | [63] |
| Mekky et al.,2015f | 23(13.04) | Egypt | 2015 | research article | 37.5 | low | [63] |
| Na’acha et al.,2017 | 438(0.46) | Nigeria | 2017 | Crossectional Survey | 50.0 | high | [64] |
| Nxasana et al.,2013 | 162(0.62) | South Africa | 2013 | Crossectional Survey | 87.5 | low | [65] |
| Okaka et al.,2000 | 6,430(2.32) | Nigeria | 2000 | Public Health Reviews | 25.0 | moderate | [66] |
| Osman et al.,2011 | 6,214(2.29) | Egypt | 2011 | Impact Evaluation | 62.5 | low | [67] |
| Periago et al.,2021 | 6,657(1.44) | Egypt | 2021 | Crossectional Survey | 12.5 | high | [68] |
| Shitta et al.,2017 | 254(33.07) | Nigeria | 2017 | Crossectional Survey | 50.0 | low | [69] |
| Soliman,2008 | 3,000(3.0) | Egypt | 2008 | research article | 30.0 | low | [70] |
| Squire et al.,2018 | 95(1.05) | Ghana | 2018 | Parasitological Survey | 50.0 | moderate | [71] |
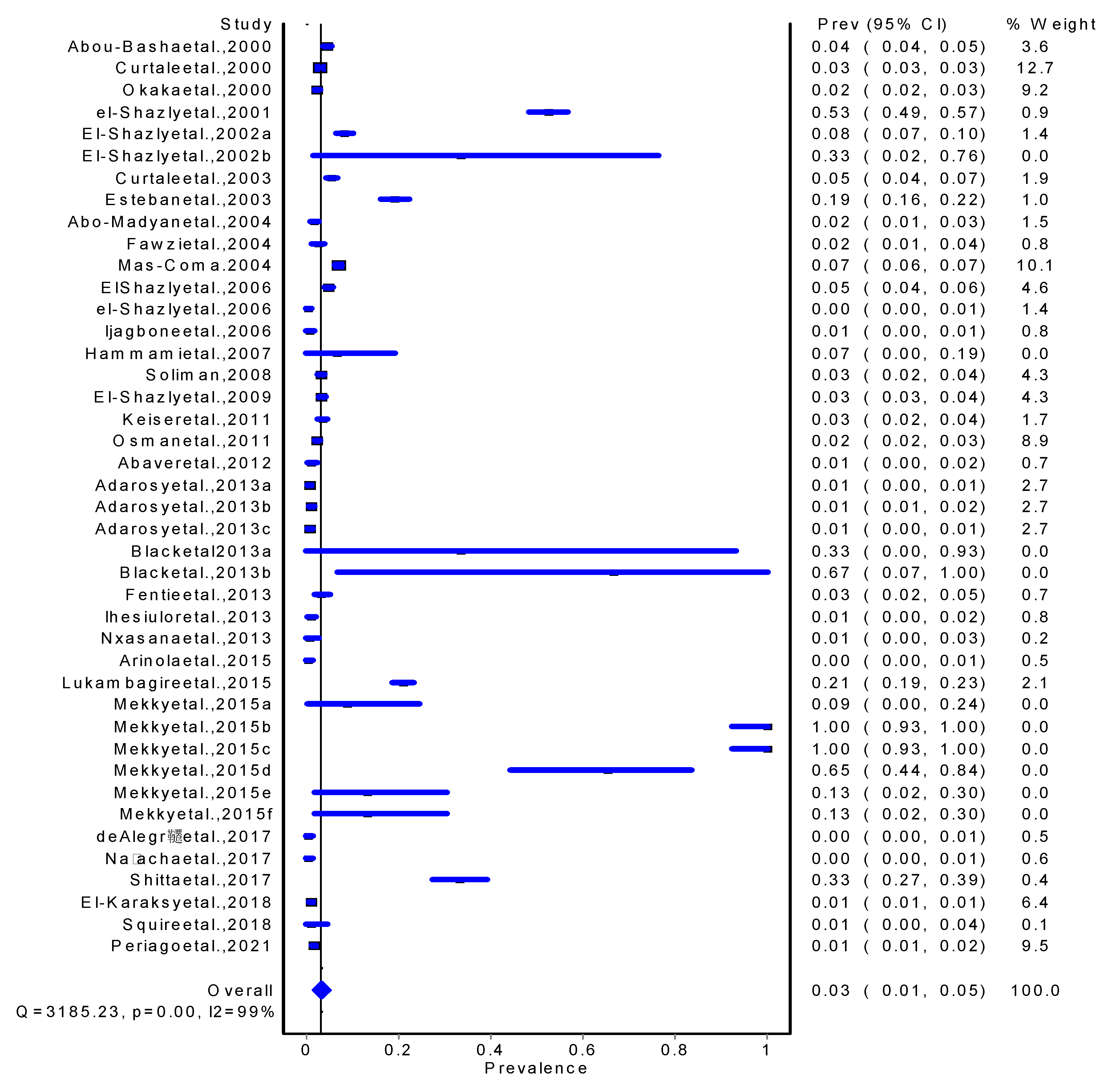
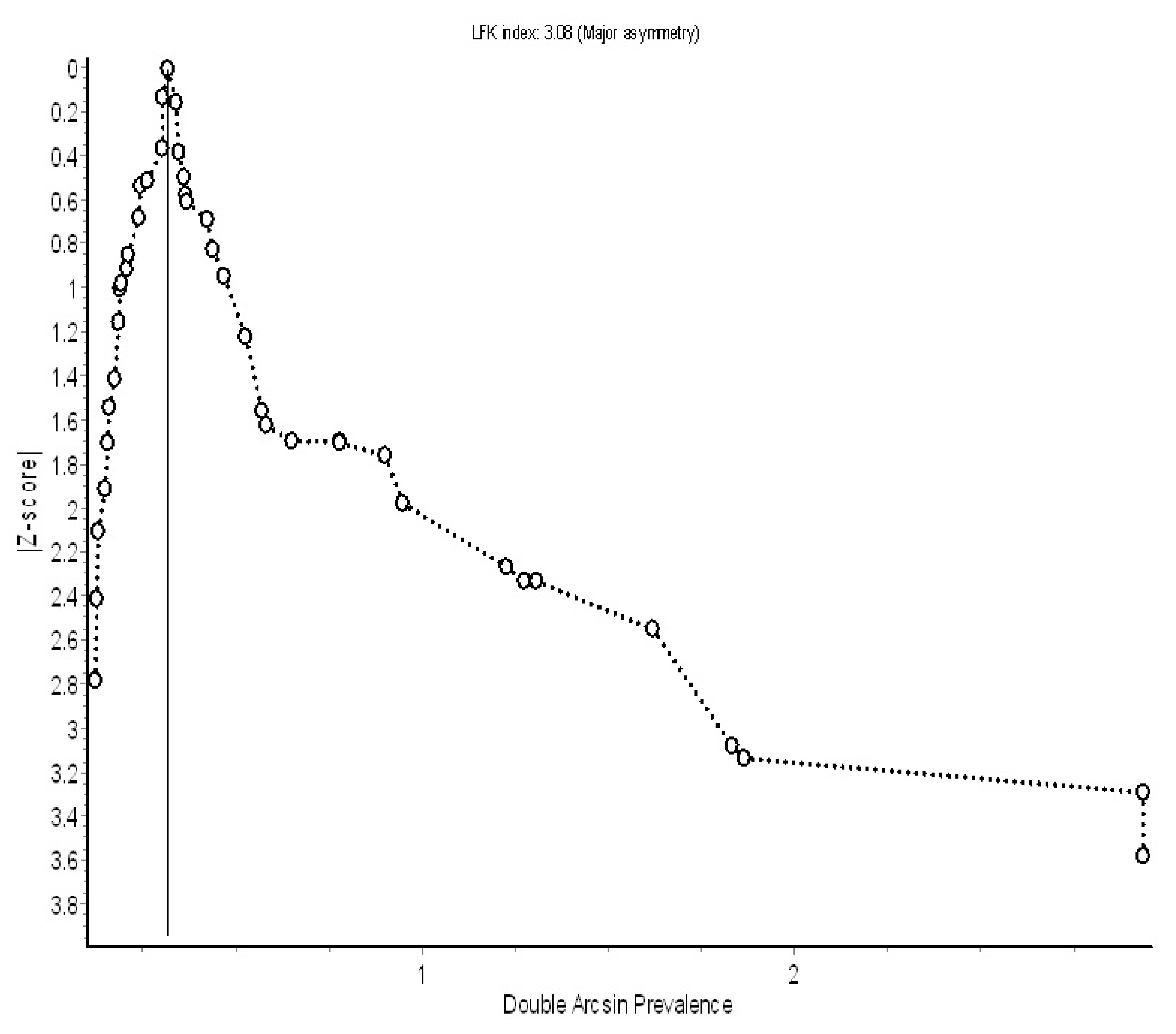
Discussions
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehmood, K., et al., A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microbial pathogenesis, 2017. 109: p. 253-262. [CrossRef]
- Cwiklinski, K., et al., A prospective view of animal and human Fasciolosis. Parasite immunology, 2016. 38(9): p. 558-568. [CrossRef]
- Saleha, A., Liver fluke disease (fascioliasis): epidemiology, economic impact and public health significance. Southeast Asian J Trop Med Public Health, 1991. 22(361): p. 4.
- Newell, D.G., et al., Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. International journal of food microbiology, 2010. 139: p. S3-S15. [CrossRef]
- Nyindo, M. and A.H. Lukambagire, Fascioliasis: An Ongoing Zoonotic Trematode Infection. Biomed Res Int, 2015. 2015: p. 786195. [CrossRef]
- Johansen, M.V., et al., Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Advances in Parasitology, 2010. 73: p. 171-195. [CrossRef]
- Dermauw, V., et al., Human fascioliasis in Africa: A systematic review. PLoS One, 2021. 16(12): p. e0261166. [CrossRef]
- Farag, H.F., Human fascioliasis in some countries of the Eastern Mediterranean Region. EMHJ-Eastern Mediterranean Health Journal, 4 (1), 156-160, 1998, 1998. [CrossRef]
- Ashrafi, K. and S. Mas-Coma, Fasciola gigantica transmission in the zoonotic fascioliasis endemic lowlands of Guilan, Iran: experimental assessment. Vet Parasitol, 2014. 205(1-2): p. 96-106. [CrossRef]
- Malatji, M., D. Pfukenyi, and S. Mukaratirwa, Fasciola species and their vertebrate and snail intermediate hosts in East and Southern Africa: a review. Journal of helminthology, 2020. 94. [CrossRef]
- Ashrafi, K., et al., Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel Med Infect Dis, 2014. 12(6 Pt A): p. 636-49. [CrossRef]
- Lukambagire, A.H., D.N. McHaile, and M. Nyindo, Diagnosis of human fascioliasis in Arusha region, northern Tanzania by microscopy and clinical manifestations in patients. BMC Infect Dis, 2015. 15: p. 578. [CrossRef]
- Nyindo, M. and A.-H. Lukambagire, Fascioliasis: an ongoing zoonotic trematode infection. BioMed research international, 2015. 2015. [CrossRef]
- Addy, F., T. Romig, and M. Wassermann, Genetic characterisation of Fasciola gigantica from Ghana. Veterinary Parasitology: Regional Studies and Reports, 2018. 14: p. 106-110. [CrossRef]
- Calvani, N.E.D., et al., Scrambled eggs: A highly sensitive molecular diagnostic workflow for Fasciola species specific detection from faecal samples. Plos Neglected Tropical Diseases, 2017. 11(9): p. e0005931. [CrossRef]
- Kajugu, P.-E., et al., Fasciola hepatica: Specificity of a coproantigen ELISA test for diagnosis of fasciolosis in faecal samples from cattle and sheep concurrently infected with gastrointestinal nematodes, coccidians and/or rumen flukes (paramphistomes), under field conditions. Veterinary parasitology, 2015. 212(3-4): p. 181-187. [CrossRef]
- Salimi-Bejestani, M., et al., Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Research in veterinary science, 2005. 78(2): p. 177-181. [CrossRef]
- Cabán-Hernández, K., et al., Development of two antibody detection enzyme-linked immunosorbent assays for serodiagnosis of human chronic fascioliasis. Journal of Clinical Microbiology, 2014. 52(3): p. 766-772. [CrossRef]
- Khaitsa, M.L., J. Hammond, and J. Opuda-Asibo, Use of meat inspection records in veterinary planning. Bulletin of animal health and production in Africa, 1994.
- Kendall, S., Relationships between the species of Fasciola and their molluscan hosts. Advances in parasitology, 1970. 8: p. 251-258. [CrossRef]
- Periago, M., et al., Phenotypic comparison of allopatric populations of Fasciola hepatica and Fasciola gigantica from European and African bovines using a computer image analysis system (CIAS). Parasitology research, 2006. 99(4): p. 368-378. [CrossRef]
- McGarry, J., et al., PCR-based differentiation of Fasciola species (Trematoda: Fasciolidae), using primers based on RAPD-derived sequences. Annals of Tropical Medicine & Parasitology, 2007. 101(5): p. 415-421. [CrossRef]
- Rokni, M.B., et al., Identification and differentiation of Fasciola hepatica and Fasciola gigantica using a simple PCR-restriction enzyme method. Experimental Parasitology, 2010. 124(2): p. 209-213. [CrossRef]
- Ai, L., et al., Specific PCR-based assays for the identification of Fasciola species: their development, evaluation and potential usefulness in prevalence surveys. Annals of Tropical Medicine & Parasitology, 2010. 104(1): p. 65-72. [CrossRef]
- Mas-Coma, S., M.A. Valero, and M.D. Bargues, Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Advances in parasitology, 2009. 69: p. 41-146. [CrossRef]
- FAO, Foodborne parasitic infections: Fascioliasis Fact sheet. https://www.fao.org/3/cb1127en/cb1127en.pdf, accessed on 22/12/2021. 2021.
- WHO, A key role for veterinary authorities and animal health practitioners in preventing and controlling neglected parasitic zoonoses: a handbook with focus on Taenia solium, Trichinella, Echinococcus and Fasciola. 2021.
- Correa, A.C., et al., Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis. BMC evolutionary biology, 2010. 10(1): p. 1-12. [CrossRef]
- Arias-Pacheco, C., et al., Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Tropical animal health and production, 2020: p. 1-6. [CrossRef]
- Khan, M.K., et al., The global burden of fasciolosis in domestic animals with an outlook on the contribution of new approaches for diagnosis and control. Parasitology research, 2013. 112(7): p. 2421-2430. [CrossRef]
- Mas-Coma, S., M.A. Valero, and M.D. Bargues, Fascioliasis. Digenetic trematodes, 2014: p. 77-114. [CrossRef]
- Schols, R., et al., Invasive snails, parasite spillback, and potential parasite spillover drive parasitic diseases of Hippopotamus amphibius in artificial lakes of Zimbabwe. BMC biology, 2021. 19(1): p. 1-21. [CrossRef]
- Jean-Richard, V., et al., Estimating population and livestock density of mobile pastoralists and sedentary settlements in the south-eastern Lake Chad area. Geospatial health, 2015. 10(1). [CrossRef]
- Jaja, I.F., et al., Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite epidemiology and control, 2017. 2(4): p. 27-34. [CrossRef]
- Liba, J.W., N.N. Atsanda, and M.I. Francis, Economic loss from liver condemnation due to fasciolosis in slaughtered ruminants in Maiduguri abattoir, Borno State, Nigeria. Journal of Advanced Veterinary and Animal Research, 2017. 4(1): p. 65-70. [CrossRef]
- Schweizer, G., et al., Estimating the financial losses due to bovine fasciolosis in Switzerland. Veterinary Record, 2005. 157(7): p. 188-193. [CrossRef]
- Marcos, L., et al., Risk factors for Fasciola hepatica infection in children: a case–control study. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2006. 100(2): p. 158-166. [CrossRef]
- LA, M.R., Clinical characteristics of chronic infection by Fasciola hepatica in children. Revista de gastroenterologia del Peru: organo oficial de la Sociedad de Gastroenterologia del Peru, 2002. 22(3): p. 228-233.
- Mas-Coma, S., Human fascioliasis: epidemiological patterns in human endemic areas of South America, Africa and Asia. Southeast Asian J Trop Med Public Health, 2004. 35(Suppl 1): p. 1-11.
- Moher, D., et al., Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol, 2009. 62(10): p. 1006-12. [CrossRef]
- Munn, Z., et al., The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag, 2014. 3(3): p. 123-8. [CrossRef]
- Abaver, D.T., et al., Enteric parasitic infections in HIV-infected patients with low CD4 counts in Toto, Nigeria. 2012.
- Abo-Madyan, A.A., et al., Clinical trial of Mirazid in treatment of human fascioliasis, Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J Egypt Soc Parasitol, 2004. 34(3): p. 807-818.
- Abou-Basha, L., et al., Hepatic fibrosis due to fascioliasis and/or schistosomiasis in Abis 1 village, Egypt. Eastern Mediterranean Health Journal= La Revue de Sante de la Mediterranee Orientale= Al-majallah Al-sihhiyah Li-sharq Al-mutawassit, 2000. 6(5-6): p. 870-878.
- Adarosy, H.A., et al., Changing pattern of fascioliasis prevalence early in the 3rd millennium in Dakahlia Governorate, Egypt: an update. J Egypt Soc Parasitol, 2013. 43(1): p. 275-86. [CrossRef]
- Arinola, G.O., et al., Serum Micronutrients in Helminth-infected Pregnant Women and Children: Suggestions for Differential Supplementation During Anti-helminthic Treatment. Ann Glob Health, 2015. 81(5): p. 705-10. [CrossRef]
- Black, J., et al., Human fascioliasis in South Africa. South African Medical Journal, 2013. 103(9): p. 658-659. [CrossRef]
- Curtale, F., Anaemia among young male workers in Alexandria, Egypt. EMHJ-Eastern Mediterranean Health Journal, 6 (5-6), 1005-1016, 2000, 2000. [CrossRef]
- Curtale, F., et al., Clinical signs and household characteristics associated with human fascioliasis among rural population in Egypt: a case-control study. Parassitologia, 2003. 45(1): p. 5-11.
- de Alegría, M.L.A.R., et al., Prevalence of Strongyloides stercoralis and other intestinal parasite infections in school children in a rural area of Angola: a cross-sectional study. The American journal of tropical medicine and hygiene, 2017. 97(4): p. 1226. [CrossRef]
- El Shazly, A.M., et al., Intestinal parasites in Dakahlia governorate, with different techniques in diagnosing protozoa. J Egypt Soc Parasitol, 2006. 36(3): p. 1023-34.
- el-Shazly, A.M., et al., Clinico-epidemiological study of human fascioliasis in an endemic focus in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol, 2001. 31(3): p. 725-36.
- El-Shazly, A.M., et al., Past and present situation of human fascioliasis in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol, 2009. 39(1): p. 247-62.
- el-Shazly, A.M., et al., The reflection of control programs of parasitic diseases upon gastrointestinal helminthiasis in Dakahlia Governorate, Egypt. Journal of the Egyptian Society of Parasitology, 2006. 36(2): p. 467-480.
- el-Shazly, A.M., et al., Evaluation of two serological tests in diagnosis of human cases of biliary and ectopic fascioliasis. J Egypt Soc Parasitol, 2002. 32(1): p. 79-90.
- Esteban, J.G., et al., Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg, 2003. 69(4): p. 429-37. [CrossRef]
- Fawzi, M., et al., Vegetable-transmitted parasites among inhabitants of El-Prince, Alexandria and its relation to housewives’ knowledge and practices. J Egypt Public Health Assoc, 2004. 79(1-2): p. 13-29.
- Fentie, T., et al., Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Trans R Soc Trop Med Hyg, 2013. 107(8): p. 480-6. [CrossRef]
- Hammami, H., N. Hamed, and A. Ayadi, Epidemiological studies on Fasciola hepatica in Gafsa Oases (south west of Tunisia). Parasite, 2007. 14(3): p. 261-4. [CrossRef]
- Ihesiulor, G.U., et al., Helminths of the gastrointestinal tract among children in Kano, Northern Nigeria. Asian Journal of Biological and Life Science, 2013. 2(2).
- Ijagbone, I.F. and T.F. Olagunju, Intestinal helminth parasites in school children in Iragbiji, boripe local government, Osun state, Nigeria. African Journal of Biomedical Research, 2006. 9(1). [CrossRef]
- Keiser, J., et al., Efficacy and safety of artemether in the treatment of chronic fascioliasis in Egypt: exploratory phase-2 trials. PLoS Negl Trop Dis, 2011. 5(9): p. e1285. [CrossRef]
- Mekky, M.A., et al., Human fascioliasis: a re-emerging disease in upper Egypt. Am J Trop Med Hyg, 2015. 93(1): p. 76-9. [CrossRef]
- Na’acha, E., P. Vandi, and G. Chessed, Species and prevalence determination of Human Intestinal Parasites among Patients attending two Medical Centers in Yola, Adamawa State, Nigeria. Journal of Applied Sciences and Environmental Management, 2017. 21(3): p. 431-437. [CrossRef]
- Nxasana, N., et al., Prevalence of intestinal parasites in primary school children of Mthatha, Eastern Cape Province, South Africa. Annals of medical and health sciences Research, 2013. 3(4): p. 511-516. [CrossRef]
- Okaka, C., A. Awharitoma, and J. Okonji, Gastrointestinal parasites of school children in Benin city, Nigeria. Iranian Journal of Public Health, 2000. 29(1-4): p. 1-12.
- Osman, M., et al., Evaluation of two doses of triclabendazole in treatment of patients with combined schistosomiasis and fascioliasis. EMHJ-Eastern Mediterranean Health Journal, 17 (4), 266-270, 2011, 2011. [CrossRef]
- Periago, M.V., et al., Very high fascioliasis intensities in schoolchildren from Nile Delta Governorates, Egypt: The Old World highest burdens found in lowlands. Pathogens, 2021. 10(9): p. 1210. [CrossRef]
- Shitta, K., H. Audu, and A. Usman, Prevalence of geohelminthes in school children in some parts of Lokoja, Kogi State, North-Central Nigeria. Bayero Journal of Pure and Applied Sciences, 2017. 10(1): p. 151-154. [CrossRef]
- Soliman, M.F., Epidemiological review of human and animal fascioliasis in Egypt. The Journal of Infection in Developing Countries, 2008. 2(03): p. 182-189. [CrossRef]
- Squire, S.A., et al., Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitology research, 2018. 117: p. 3183-3194. [CrossRef]
- Mas-Coma, M., J. Esteban, and M. Bargues, Epidemiology of human fascioliasis: a review and proposed new classification. Bulletin of the World Health Organization, 1999. 77(4): p. 340.
- Rokni, M.B., et al., Diagnosis of human fasciolosis in the Gilan province of Northern Iran: application of cathepsin L-ELISA. Diagnostic microbiology and infectious disease, 2002. 44(2): p. 175-179. [CrossRef]
- Moghaddam, A., et al., Human and animal fascioliasis in Mazandaran province, northern Iran. Parasitology Research, 2004. 94: p. 61-69. [CrossRef]
- Esteban, J.-G., et al., Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. The American journal of tropical medicine and hygiene, 2003. 69(4): p. 429-437. [CrossRef]
- De, N.V., et al., The food-borne trematode zoonoses of Vietnam. The Southeast Asian Journal of Tropical Medicine and Public Health, 2003. 34: p. 12-34.
- Mas-Coma, S., Epidemiology of fascioliasis in human endemic areas. Journal of helminthology, 2005. 79(3): p. 207-216. [CrossRef]
- El-Shazly, A., et al., Clinico-epidemiological study of human fascioliasis in an endemic focus in Dakahlia Governorate, Egypt. Journal of the Egyptian Society of Parasitology, 2001. 31(3): p. 725-736.
- Ashrafi, K., et al., Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel medicine and infectious disease, 2014. 12(6): p. 636-649. [CrossRef]
- Fentie, T., et al., Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Transactions of The Royal Society of Tropical Medicine and Hygiene, 2013. 107(8): p. 480-486. [CrossRef]
- Bayu, B., et al., Cases of human fascioliasis in North-West Ethiopia. Ethiopian Journal of Health Development, 2005. 19(3): p. 237-240. [CrossRef]
- Lukambagire, A.-H.S., D.N. Mchaile, and M. Nyindo, Diagnosis of human fascioliasis in Arusha region, northern Tanzania by microscopy and clinical manifestations in patients. BMC infectious diseases, 2015. 15: p. 1-8. [CrossRef]
- Noel, K., et al., Fasciolosis risk factors for public health in Huambo, Angola. Revista de Salud Animal, 2013. 35(3): p. 164-173.
- Isah, U.M. and A. Dalhatu, Epidemiological studies of human fascioliasis among selected individuals in northern Bauchi state, Nigeria.
- Futagbi, G., et al., Assessment of Helminth Infections in Goats Slaughtered in an Abattoir in a suburb of Accra, Ghana. West African Journal of Applied Ecology, 2015. 23(2): p. 35-42.
- Chougar, L., et al., Genetically ‘pure’Fasciola gigantica discovered in Algeria: DNA multimarker characterization, trans-Saharan introduction from a Sahel origin and spreading risk into north-western Maghreb countries. Transboundary and Emerging Diseases, 2020. 67(5): p. 2190-2205. [CrossRef]
- Zaimi, I., et al., Hepatic fascioliasis in Tunisia. Tunisie Medicale, 1971. 49(1): p. 39-49.
- Slifko, T.R., H.V. Smith, and J.B. Rose, Emerging parasite zoonoses associated with water and food. International journal for parasitology, 2000. 30(12-13): p. 1379-1393. [CrossRef]
- Malatji, M.P., J. Lamb, and S. Mukaratirwa, Molecular characterization of liver fluke intermediate host lymnaeids (Gastropoda: Pulmonata) snails from selected regions of Okavango Delta of Botswana, KwaZulu-Natal and Mpumalanga provinces of South Africa. Veterinary Parasitology: Regional Studies and Reports, 2019. 17: p. 100318. [CrossRef]
- Malatji, M.P., D.M. Pfukenyi, and S. Mukaratirwa, Fasciola species and their vertebrate and snail intermediate hosts in East and Southern Africa: a review. J Helminthol, 2019. 94: p. e63. [CrossRef]
- Singh, K., et al., Special Issues. Statistics, 2022. 3(3).
- Keiser, J. and J. Utzinger, Emerging foodborne trematodiasis. Emerging infectious diseases, 2005. 11(10): p. 1507. [CrossRef]
- WHO, Control of foodborne trematode infections: report of a WHO study group. 1995: World Health Organization.
- Mas-Coma, S., M. Bargues, and M. Valero, Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures–CORRIGENDUM. Parasitology, 2020. 147(5): p. 601-601. [CrossRef]
- Webb, C.M. and M.M. Cabada, Recent developments in the epidemiology, diagnosis, and treatment of Fasciola infection. Curr Opin Infect Dis, 2018. 31(5): p. 409-414. [CrossRef]
- Fitzpatrick, J., Global food security: the impact of veterinary parasites and parasitologists. Veterinary Parasitology, 2013. 195(3-4): p. 233-248. [CrossRef]
- Caravedo, M.A. and M.M. Cabada, Human fascioliasis: current epidemiological status and strategies for diagnosis, treatment, and control. Research and reports in tropical medicine, 2020: p. 149-158. [CrossRef]
- WHO, A key role for veterinary authorities and animal health practitioners in preventing and controlling neglected parasitic zoonoses: A handbook with focus on Taenia solium, Trichinella, Echinococcus and Fasciola. 2021: Food & Agriculture Org.
- Mas-Coma, S., M. Valero, and M. Bargues, Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech, 2008. 27(2): p. 443-57.
- Mas-Coma, S., M.A. Valero, and M.D. Bargues, Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Veterinary parasitology, 2009. 163(4): p. 264-280. [CrossRef]
- Fox, N.J., et al., Predicting impacts of climate change on Fasciola hepatica risk. PLoS One, 2011. 6(1): p. e16126. [CrossRef]
- Afshan, K., et al., Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospatial Health, 2014. 8(2): p. 317-334. [CrossRef]
- Webb, C.M. and M.M. Cabada, Recent developments in the epidemiology, diagnosis, and treatment of Fasciola infection. Current opinion in infectious diseases, 2018. 31(5): p. 409-414. [CrossRef]
- Infantes, L.R.R.-H., et al., The Global Prevalence of Human Fascioliasis: A Systematic Review and Meta-Analysis. 2023. [CrossRef]
- Ahmad, T., et al., A bibliometric analysis and global trends in fascioliasis research: A neglected tropical disease. Animals, 2021. 11(12): p. 3385. [CrossRef]
- Momčilović, S., et al., Rapid diagnosis of parasitic diseases: current scenario and future needs. Clinical Microbiology and Infection, 2019. 25(3): p. 290-309. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





