1. Introduction
Fused silica is widely used in the field of high power laser system due to its excellent physical and optical properties [
1,
2]. And the laser damage resistance performance has become the primary goal due to the damage and failure of optical components caused by high-flux laser irradiation. In order to improve the laser-induced damage threshold (LIDT) of fused silica, many scholars internationally are committed to researching the processing methods for obtaining the intrinsic surface of high power optics [
3,
4,
5,
6,
7]. With the deepening of researches, researchers have gradually realized that chemical structure defects generated by pre-processing are one of the main damage precursors under higher flux (12-16J/cm
2) laser irradiation. However, relevant researches are not sufficient, the generation rules are not clear, and there is also a lack of effective suppression methods. How to further improve the laser damage resistance performance of fused silica has become a hot and major scientific problem, and the manufacturing research for higher flux laser irradiation is imminent.
Current researches have shown that it is difficult to achieve comprehensive and effective suppression of damage precursors by a single post-treatment process [
4,
5,
6,
7,
8]. Therefore, exploring the theories and methods of combining multiple post-treatment processes to suppress defects has become an inevitable choice to realize the low-defect surface manufacturing of high power optics. Based on the high removal efficiency of HF acid etching and the low damage characteristics of IBS, our research team has explored this combined process. When IBS is used to remove a certain depth (<1000nm) of HF acid etched surface, it can reduce the concentration of ODC, NBOHC and OH- groups on surface, weaken the photothermal weak absorption intensity, and increase the LIDT of fused silica components by 30% compared with that of single HF acid etching method. This preliminarily verifies the good effect of IBS in suppressing chemical structure defects[
9,
10,
11,
12]. IBS has also become one of the most promising new processing method for suppressing chemical structure defects. However, with the emergence of new phenomena and the deepening of research, it has been gradually realized that IBS has two sides in the regulation of chemical structure defects, which can not only remove chemical structure defects generated by pre-processing, but also produce sputtering damage on its intrinsic surface, but the process conditions and generation mechanism of the two-sided effect are still unclear. The regulation law of IBS on chemical structure defects is not well understood, and the research on the combination sequence and process interface conditions of the combined process are not fully studied, which cannot provide technical guidance for effectively suppressing surface chemical structure defects.
The OH- groups in fused silica are both impurity defects and chemical structure defects. They break the Si-O bonds in the silica network, reduce the chemical stability of the substrate surface, and also affect the existence of other types of structure defects, seriously reducing its laser damage resistance performance[
13]. Our team have studied the evolution law of chemical structure defects on fused silica surface during the combined process of HF acid etching and IBS. It was found that the hydroxyl content on the sputtering surface increased after being cleaned with deionized water, and the photothermal weak absorption intensity also increased, while the LIDT decreased significantly. However, there is a lack of deep understanding of the mechanism and rules of this phenomenon. In this paper, the theoretical analysis of the hydroxylation process of fused silica surface is carried out, and the mechanism of IBS to change the material structural characteristics and accelerate the surface hydroxylation process is revealed. Based on the mapping relationship between surface hydroxylation and photothermal weak absorption intensity, the influence of surface hydroxylation on laser damage resistance performance is investigated, providing technical guidance for effectively suppressing chemical structure defects, forming a low damage surface manufacturing process for optical components, and achieving the improvement of laser damage resistance performance under high-flux laser irradiation.
2. Influence of OH- Groups on Material Characteristics
During the processing of fused silica components, along with the chemical bonds breakage in the silicon-oxygen tetrahedral network structure [SiO
4], a large number of chemical structure defects will occur in the material, mainly due to oxygen deficiency and doping of other elements (such as Cl, -OH, etc.) [
14]. The chemical stability of fused silica depends on the firmness of the Si-O-Si network connection structure, and the hydroxyl defects break the Si-O bonds, changes the continuity of the network structure, and reduces its chemical stability.
The introduction of impurity ions in the SiO
2 network structure leads to breakpoints in tits complete grid structure.
Figure 1 shows the chemical structure defects generated by the introduction of OH- and F-ion impurities [
15]. Chemical structure defects can form energy absorption points in high-flux laser irradiation and reduce the LIDT. The absorption effect of individual defects is equivalent to that of Ce element. Therefore, it is necessary to strictly control the content of OH- groups inside fused silica to improve its optical uniformity and increase its LIDT under 351 nm ultraviolet laser irradiation.
3. Experimental Method
The fused silica samples (Heraeus 312) with a size of 50mm×50mm×10mm were polished by the same supplier to avoid the uncertain effects of different polishing qualities, and all samples had bright surface with no obvious brittle scratches. Two samples (marked as 1#, 2#) were firstly ultrasonically etched with 5% HF acid solution supplemented by megasonic vibration. The etching process parameters have been optimized, and each round of operation includes 30 minutes of HF acid etching and 30 minutes of megasonic cleaning by deionized water. Then the samples were dried with absolute alcohol. The ion sputtering parameters are shown in
Table 1.
Both the two surfaces of sample 1# were sputtered with the same removal depth of 900nm, 1500nm and 2000nm respectively before damage performance test. An R-on-1 procedure was used in the LIDT test in which a ramping fluence focused on a single area until damage was registered on the imaging CCD. Ten positions were chosen randomly during each LIDT test and taken the average value as the final damage threshold. A table-top 3ω Nd: AG laser with pulse width of 7ns operated at a repetition rate of 1Hz was employed in the test. The spatial beam distribution was almost flat Gaussian with diameter of ~1.2 mm. The laser beam was focused on the backside of the sample. After the damage performance test, sample 2# was dried with absolute ethanol after 30min megasonic cleaning, and the LIDT tests were carried out again by R-on-1 method.
4. Experimental Results and Analysis
4.1. Damage Performance Test
The LIDT test results with different IBS removal depths are shown in
Table 2. It is found that the damage threshold of the sputtered surfaces after ultrasonic cleaning are significantly lower than that of the surface only treated with IBS, and the damage threshold test results are reduced by 21%, 23% and 14%, respectively. In addition, the damage threshold of the sputtered surface after ultrasonic cleaning are almost all lower than that of the HF acid etched surfaces.
The experimental results give us a thought: What is the reason for the damage threshold reduction of about 20% after the sputtered surface is cleaned with deionized water? Revealing the mechanism of this change plays an important role in in-depth research on the influence of IBS on optical material characteristics and its laser damage resistance performance, and then optimizes the IBS process and provides technical guidance for the optimization of combined process parameters.
4.2. Measurement of OH- Groups
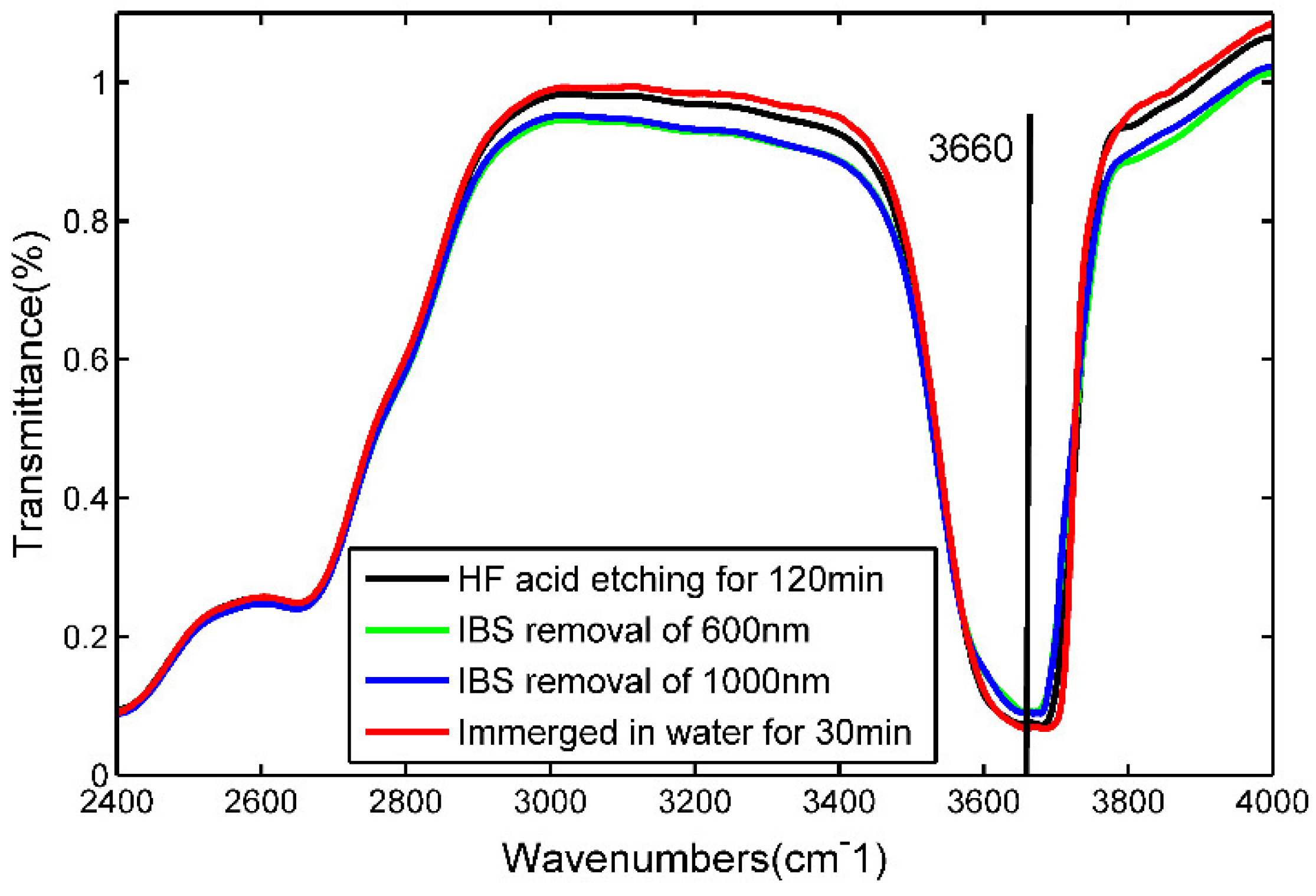
Infrared spectroscopy is the main means to study the structure characteristics of OH- group in fused silica, which has an absorption peak near 3660 cm-1 in infrared spectra. A Bruker Vertex70 high resolution fourier transform infrared spectroscopy (FTIR) was used to measure the OH- groups content after different treatment processes.
As shown in
Figure 2, fused silica sample after different processes show strong absorption peaks at 3660 cm
-1. After IBS removal depth of 600nm and 1000nm, the hydroxyl absorption peak intensity decrease to a certain extent compared with that of the HF etched surface. After IBS removal depth of 1000nm, the sample was soaked in deionized water for 30min and then dried by high pressure nitrogen gas and immediately measured by the infrared spectroscopy. It can be seen from
Figure 2 that the hydroxyl absorption peak intensity is significantly enhanced after immersed in deionized water, even stronger than that of HF etched surface.
The hydroxyl content is calculated as follows [
16]:
Where
C is the hydroxyl content of the sample, ppm (10
-6).
d is the thickness of the sample, cm.
I0 is the distance from the baseline to the zero line at wavelength 2.73 μm, mm.
I is the distance from the absorption peak to the zero line at wavelength 2.73 μm, mm. According to the infrared spectral transmittance curve shown in
Figure 2, the hydroxyl content is 93.2ppm with IBS removal depth of 1000nm and 110.0ppm after being cleaned by deionized water, in which the hydroxyl content increased by 18%. It should be noted that the calculation of hydroxyl content is greatly affected by the thickness of the sample, and the transmittance of the hydroxyl peak increases as the thickness increases. Therefore, the change rate in hydroxyl content here is only used as a reference to characterize the degree of surface hydroxylation.
4.3. Relationship between Surface Hydroxylation and Photothermal Weak Absorption Effects
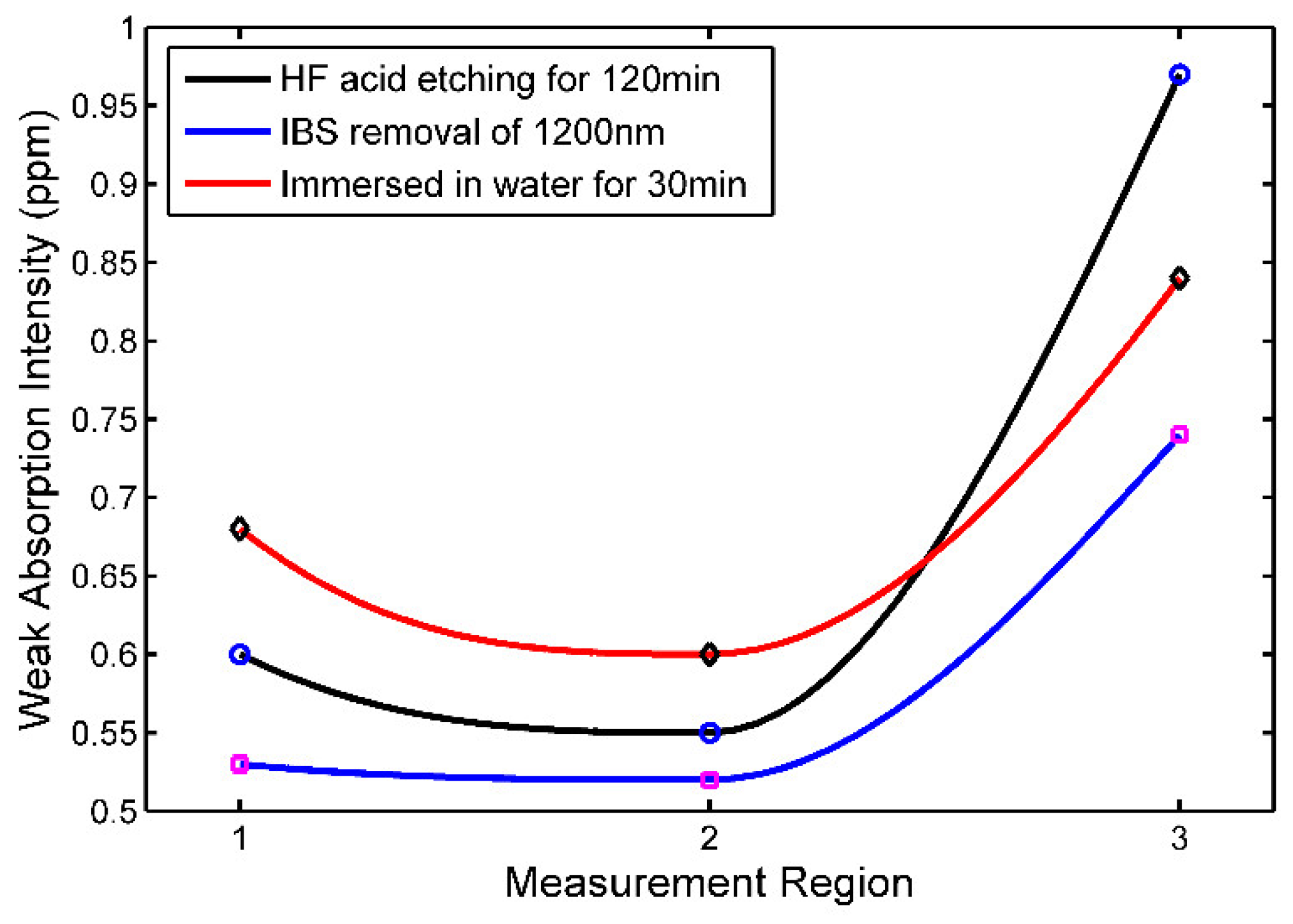
As a nondestructive test method, the photothermal weak absorption detection is directly related to the damage precursors, and reflects the damage resistance performance of optics. In order to further reveal the damage mechanism of the hydroxylation layer to reduce the laser damage resistance, the photothermal weak absorption distribution of fused silica surface were measured. The changes of photothermal weak absorption intensity of three marking points on fused silica surface before and after immersion were tested, and the size of each area was 3 mm×3 mm.
Figure 3 shows the change curves of photothermal weak absorption intensity corresponding to the three marked areas on the sample surface.
As can be seen from
Figure 3, the photothermal weak absorption intensity of IBS surface is significantly enhanced after being immersed in deionized water, indicating that chemical reaction has occurred after the IBS surface is immersed in water, resulting in a change in the surface material properties.
Previous studies have shown that when the IBS removal depth is greater than 1000nm, ion sputtering damage will occur in the shallow layer of fused silica[
9]. Although the overall photothermal weak absorption intensity of HF acid etched surface can be reduced by IBS, the structure damage on the shallow surface caused by sputtering accelerates the change process of material characteristics, which further improves the photothermal weak absorption intensity of the corresponding area after immersion.
5. Discussion: Mechanism of Surface Hydroxylation of Fused Silica
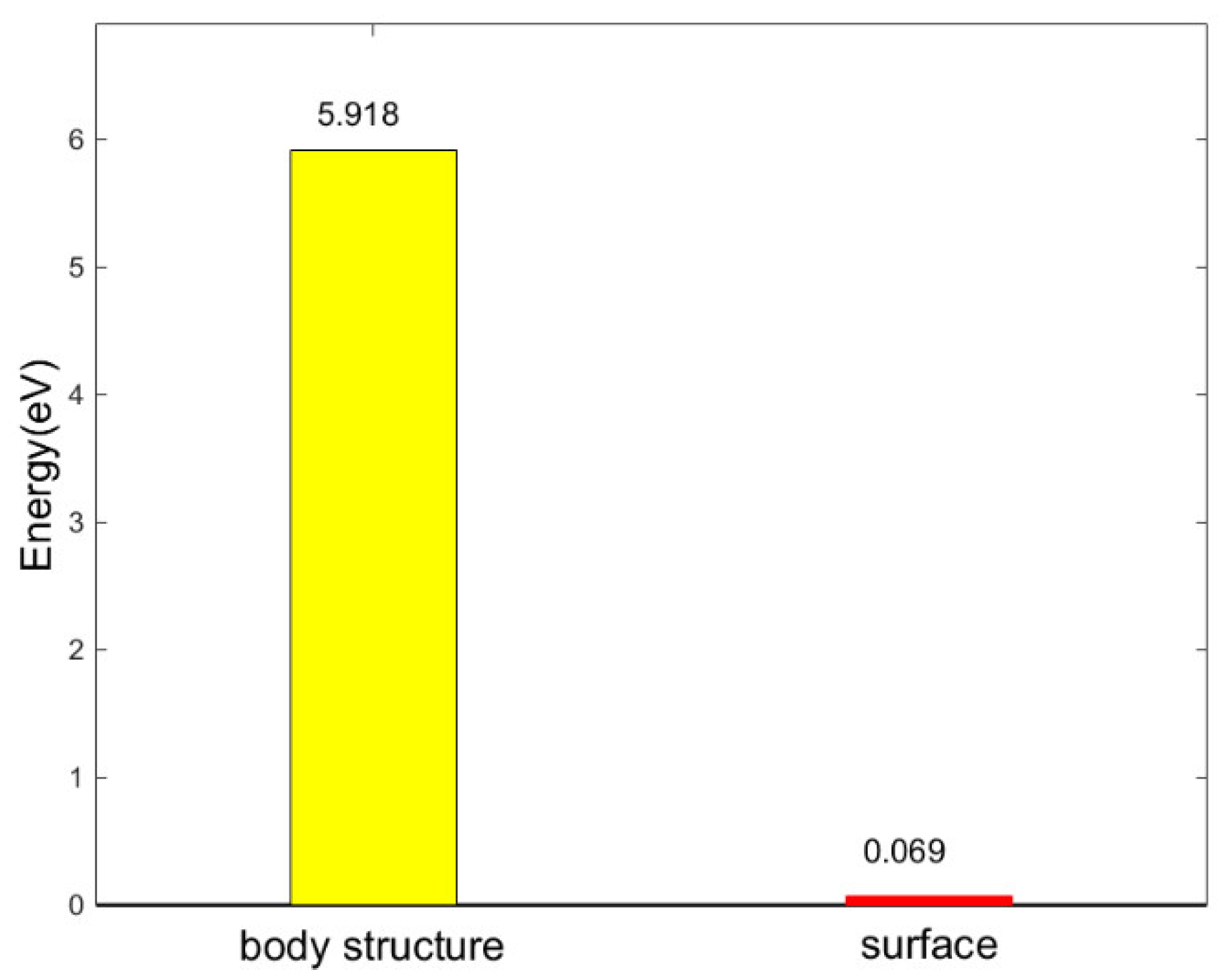
There is a force field around the atoms that make up the solid, and the force field of the atoms inside the solid is saturated due to the force symmetry. However, the atoms on the solid surface are at a higher energy level due to the unbalanced force, which makes the solid surface has a higher reactivity. Molecular dynamics simulations were used in Ref. [
16] to show that the band gap on silica surface is significantly smaller than that of the bulk structure (
Figure 4), indicating that surface atoms are more likely to have chemical reactions with external atoms than the bulk atoms.
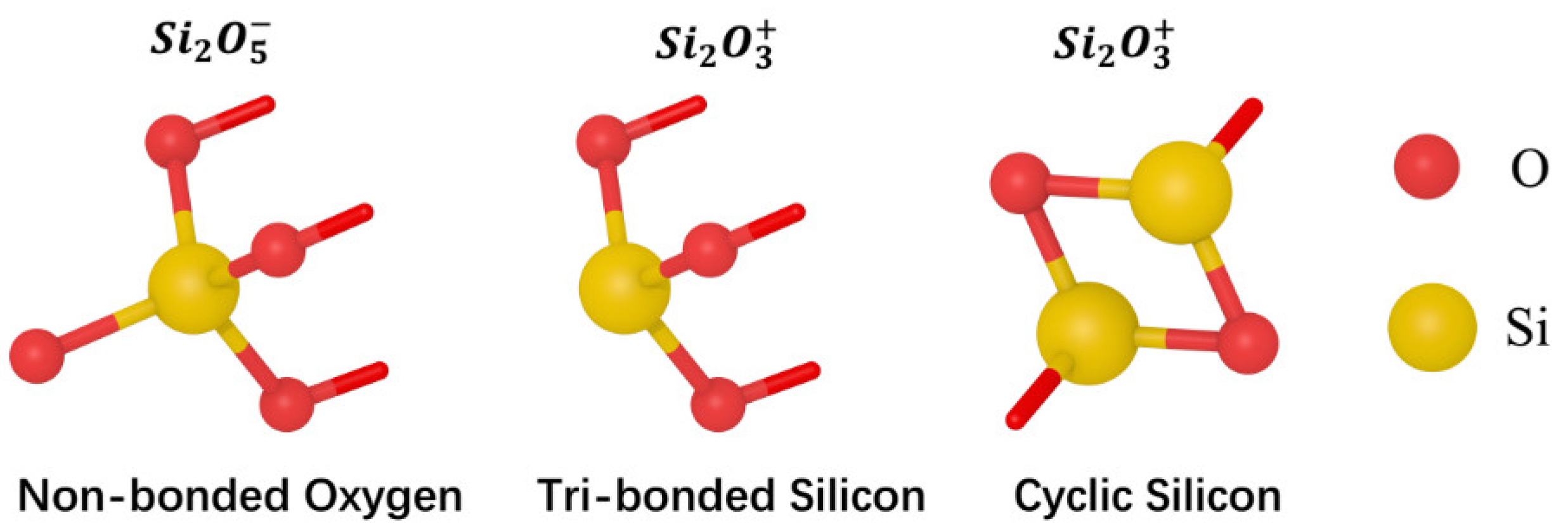
On the other hand, the waviness of microscopic fluctuations on the solid surface is mainly manifested in the form of lattice defects, vacancies and dislocations at the atomic scale. The chemical state of surface atoms is also different due to the different structural forms of atoms at different surface defect locations. The silicate glass surface composed of [SiO
4] mainly contains unsaturated structures such as non-bonded oxygen, tri-bonded silicon, and cyclic silicon, as shown in
Figure 5.
During the sputtering process, many atoms leave its lattice position under the bombardment of incident ions, resulting in the fracture of chemical bonds or the formation of new chemical bonds of surface atoms, which will cause chemical structure defects such as lattice defects, vacancies and dislocations, making the chemical bonding state of surface atoms significantly different from that of the internal bulk atoms. The asymmetry of the chemical bond energy of the surrounding atoms and the existence of multiple unbond electrons in the outer layer of the atoms lead to the enhancement of physical properties and chemical activity of surface atoms. The chemical properties of fused silica are very stable and water-soluble. However, there are many unsaturated structures on surface after IBS treatment, which has a strong adsorption capacity for polar molecules.
Michalske et al. [
17] found that the broken or unbroken Si-O-Si bonds can undergo electrophilic or nucleophilic reactions with water molecules to weaken the Si-O bond strength, so that the silica structure is continuously destroyed to form a surface adsorption layer of OH- groups. Water plays an extremely important role in SiO
2 medium, and the water molecules can penetrate into the SiO
2 molecule under hydrostatic force at room temperature, to break the Si-O bond, making it change into a Si-OH bond with lower bond energy. The diffusion of water molecule causes surface hydroxylation of SiO
2 molecules thus forming a soft layer of Si(OH)
4, which can be expressed as:
Cook [
18] believed that the hydroxylation genarated by the chemical reaction between siloxane (Si-O-Si) and water is mainly determined by the chemical properties of the processed surface. When the IBS surface of fused silica is immersed in water, the polar water molecules are easy to adsorb with surface atoms and hydroxylate these surface atoms, thus forming a layer of Si (OH)
n structure on the silica surface , so that the Si atoms on surface form a relatively stable structure. Due to the presence of H+ and OH- ions in aqueous solution, the two ions chemically interact with different types of unsaturated structures on the surface, respectively. Leed et al. [
19] found that the surface hydroxylation process is that H+ reacts with non-bonded oxygen in aqueous solution, while OH- reacts with unsaturated silicon to form OH- groups.The whole action process can be expressed as follows:
Generally speaking, the more unsaturated of the bonding structure, the stronger of its adsorption capacity for water molecules, thus the stronger of its surface hydroxylation ability, and correspondingly the higher the number of OH- groups formed on its surface. Due to the limitation of the bonding ability of Si atoms, the number of OH- groups of a single Si atom after hydroxylation is not more than 3. Bassett et al. [
20] found that the average area density of OH- groups on the traditional polished fused silica surface after water cleaning is 4-6/nm
2.
After the surface hydroxylation process, the binding force between surface atoms will be relatively weakened due to the introduction of OH- groups, and the binding energy of surface atoms after surface hydroxylation can be expressed as [
21]:
Where
Eas is the binding energy of surface atoms after surface hydroxylation.
Ebs is the binding energy of surface atoms before surface hydroxylation.
nOH is the number of OH- groups generated by a single Si atom (
nOH=1, 2, 3).
k is the Boltzmann constant, and
T is the ambient temperature. The more OH- groups adsorbed during surface hydroxylation process, the more severe the weakening of atomic binding energy of surface layer, resulting in the reduction of surface mechanical strength. The hydroxylation process on silica surface destroys its original spatial network structure of the silica bulk, thereby reducing its mechanical strength[
22].
The strong hydroxyl characteristic peak of fused silica sample treated by HF acid etching is mainly due to that the acid etching process occurs in water environment. Once the fresh etched surface is exposed, it is easy to have surface hydroxylation with the surrounding water molecules, resulting in the formation of hydroxyl on silica surface. After a removal of 1000nm by ion sputtering, the hydroxylation layer on surface was removed, resulting in the decrease of hydroxyl characteristic peak intensity. When the ion sputtered surface is completely immersed in deionized water, the chemical activity of surface atoms is enhanced by ion sputtering, which improves the probability of reaction between the unsaturated structure of silica surface and water molecules, resulting in the further increase of surface hydroxyl content. This is the reason for the enhancement of hydroxyl characteristic peak after immersion in deionized water. In addition, the hydroxyl characteristic peak intensity of IBS surface after immersion in deionized water for 30min is stronger than that of HF acid etching for 120min, indicating that ion sputtering increases the unsaturated structure, enhances the activity of Si and O, and thus making the surface hydroxylation reaction easier to occur.
Due to the corrosive effect of OH- ions on the silica surface, chemical adsorption will occur and destroy the original spatial network structure of silica bulk, resulting in the reduction of mechanical strength and the decrease of laser damage resistance performance. Therefore, the ion sputtering surface should be strictly avoided from contacting with water environment to avoid the decrease of laser damage threshold caused by surface hydroxylation.
6. Conclusion
During the material removal process of IBS, due to the ion sputtering effect, the lattice atoms are separated from their original positions, and a large number of unsaturated structures are formed, which improves the chemical activity of Si and O atoms, accelerates the chemical reaction process between surface atoms and water molecules on the sputtered surface, increases the OH- groups content in the shallow layer and enhances the photothermal weak absorption effect. However, the presence of OH- groups reduces the bonding strength of Si-O, destroys the spatial network structure of silica bulk, and reduces its chemical stability and surface mechanical strength, resulting in a decrease in its laser damage resistance performance. Therefore, the IBS surface should be strictly prevented from contacting with water environment in order to avoid surface hydroxylation during the combined process of HF acid etching and IBS cleaning, so as to avoid the reduction of laser damage threshold caused by surface hydroxylation. The paper reveals for the first time the mechanism of IBS to change the structure characteristics of silica material, accelerate the surface hydroxylation process, and thereby reduces the laser damage resistance performance. This work provides a theoretical basis for the combination sequence and process interface conditions of the combined process, and also provides technical guidance for effectively suppressing chemical structure defects on silica surface and improving laser damage resistance performance of optical components under high-flux laser irradiation.
Author Contributions
Conceptualization, M.X.; methodology, W.W. and Y.Z.; investigation, X.L.; writing—original draft preparation, M.X.; writing—review and editing, W.W. and Y.K.; supervision, X.G.; funding acquisition, M.X. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of China (52105496), Natural Science Foundation of Hubei Province (2022CFB866), National Key Laboratory on Ship Vibration and Noise (JCKY2021207CI03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was financially supported through Natural Science Foundation of China (52105496), Natural Science Foundation of Hubei Province (2022CFB866), National Key Laboratory on Ship Vibration and Noise (JCKY2021207CI03).
Conflicts of Interest
The authors declare no conflict of interest.
References
- N.Shen,J. D. Bude, and C. W. Carr, “Model laser damage precursors for high quality optical materials,” Optics Express 22(3),3393-3404(2014).
- Manenkov, A.A. Fundamental mechanisms of laser-induced damage in optical materials: today’s state of understanding and problems. Opt. Eng. 2014, 53, 010901–010901, . [CrossRef]
- Bude, J.; Miller, P.; Baxamusa, S.; Shen, N.; Laurence, T.; Steele, W.; Suratwala, T.; Wong, L.; Carr, W.; Cross, D.; et al. High fluence laser damage precursors and their mitigation in fused silica. Opt. Express 2014, 22, 5839–5851, . [CrossRef]
- Shi, F.; Zhong, Y.; Dai, Y.; Peng, X.; Xu, M.; Sui, T. Investigation of surface damage precursor evolutions and laser-induced damage threshold improvement mechanism during Ion beam etching of fused silica. Opt. Express 2016, 24, 20842–54, . [CrossRef]
- Liu, H.; Ye, X.; Zhou, X.; Huang, J.; Wang, F.; Zhou, X.; Wu, W.; Jiang, X.; Sui, Z.; Zheng, W. Subsurface defects characterization and laser damage performance of fused silica optics during HF-etched process. Opt. Mater. 2014, 36, 855–860, . [CrossRef]
- Ding, W.; Zhao, L.; Chen, M.; Cheng, J.; Yin, Z.; Liu, Q.; Chen, G.; Lei, H. Concentration characterization of underlying intrinsic defects accompany with surface structural defects and their effect on laser damage resistance. Appl. Surf. Sci. 2024, 643, . [CrossRef]
- Heinke, R.; Ehrhardt, M.; Bauer, J.; Lotnyk, A.; Lorenz, P.; Morgenstern, R.; Lampke, T.; Arnold, T.; Zimmer, K. Low surface damage laser processing of silicon by laser-induced plasma etching (LIPE). Appl. Surf. Sci. 2022, 597, . [CrossRef]
- Xu, M.; Dai, Y.; Zhou, L.; Peng, X.; Chen, S.; Liao, W. Evolution mechanism of surface roughness during ion beam sputtering of fused silica. Appl. Opt. 2018, 57, 5566–5573, . [CrossRef]
- Xu, M.; Shi, F.; Zhou, L.; Dai, Y.; Peng, X.; Liao, W. Investigation of laser-induced damage threshold improvement mechanism during ion beam sputtering of fused silica. Opt. Express 2017, 25, 29260–29271, . [CrossRef]
- Zhong, Y.; Shi, F.; Tian, Y.; Dai, Y.; Song, C.; Zhang, W.; Lin, Z. Detailed near-surface nanoscale damage precursor measurement and characterization of fused silica optics assisted by ion beam etching. Opt. Express 2019, 27, 10826–10838, . [CrossRef]
- Zhong, Y.; Dai, Y.; Shi, F.; Song, C.; Tian, Y.; Lin, Z.; Zhang, W.; Shen, Y. Effects of Ion Beam Etching on the Nanoscale Damage Precursor Evolution of Fused Silica. Materials 2020, 13, 1294, . [CrossRef]
- Zhong, Y.; Dai, Y.; Tian, Y.; Shi, F. Effect on nanoscale damage precursors of fused silica with wet etching in KOH solutions. Opt. Mater. Express 2021, 11, 884–894, . [CrossRef]
- Y. Su, Y. Zhou, W. Huang, Z. Gu. Study on reaction kinetics between silica glass and hydrofluoric acid. Journal of the chinese ceramic society, 2004, 32(3):287-293.
- Skuja, L.; Hirano, M.; Hosono, H.; Kajihara, K. Defects in oxide glasses. Phys. Status solidi (c) 2005, 2, 15–24, . [CrossRef]
- Liu Wei, Wang Lili, Liu Lianli, Zhang Yanping, Xu Xinxin. Determination of hydroxy in quartz glass by infrared spectrology. Journal of Bohai University (Natural Science Edition),2008, 29(4):332-335.
- W. Peng, “Study on the key technology of ultra-smooth surface fabrication based on the material removal in elastic mode,” Ph.D. dissertation (National University of Defense Technology, 2014) (in Chinese).
- Michalske, T.A.; Freiman, S.W. A molecular interpretation of stress corrosion in silica. Nature 1982, 295, 511–512, . [CrossRef]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Crystalline Solids 1990, 120, 152–171, . [CrossRef]
- A Leed, E.; Pantano, C.G. Computer modeling of water adsorption on silica and silicate glass fracture surfaces. J. Non-Crystalline Solids 2003, 325, 48–60, . [CrossRef]
- Bassett, D.; Boucher, E.; Zettlemoyer, A. Adsorption studies on hydrated and dehydrated silicas. J. Colloid Interface Sci. 1968, 27, 649–658, . [CrossRef]
- Song, X.Z.; Zhang, Y.; Zhang, F.H. Study on Removal Mechanism of Nanoparticle Colloid Jet Machining. Adv. Mater. Res. 2008, 53-54, 363–368, . [CrossRef]
- Y. Su, Y. Zhou, W. Huang, Z. Gu. Study on reaction kinetics between silica glass and hydrofluoric acid. Journal of the chinese ceramic society, 2004, 32(3): 287-293.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).