Submitted:
24 April 2024
Posted:
25 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
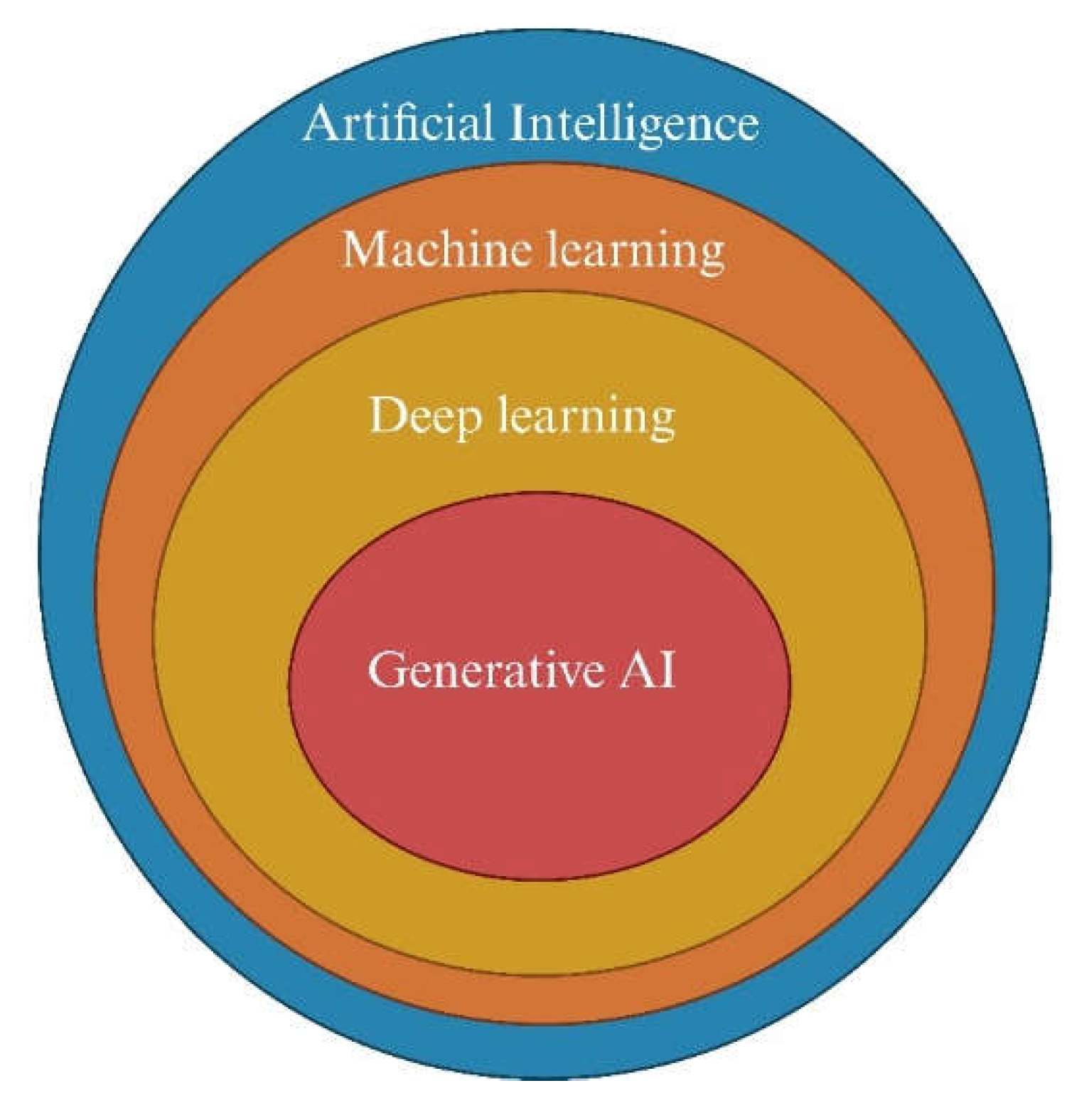
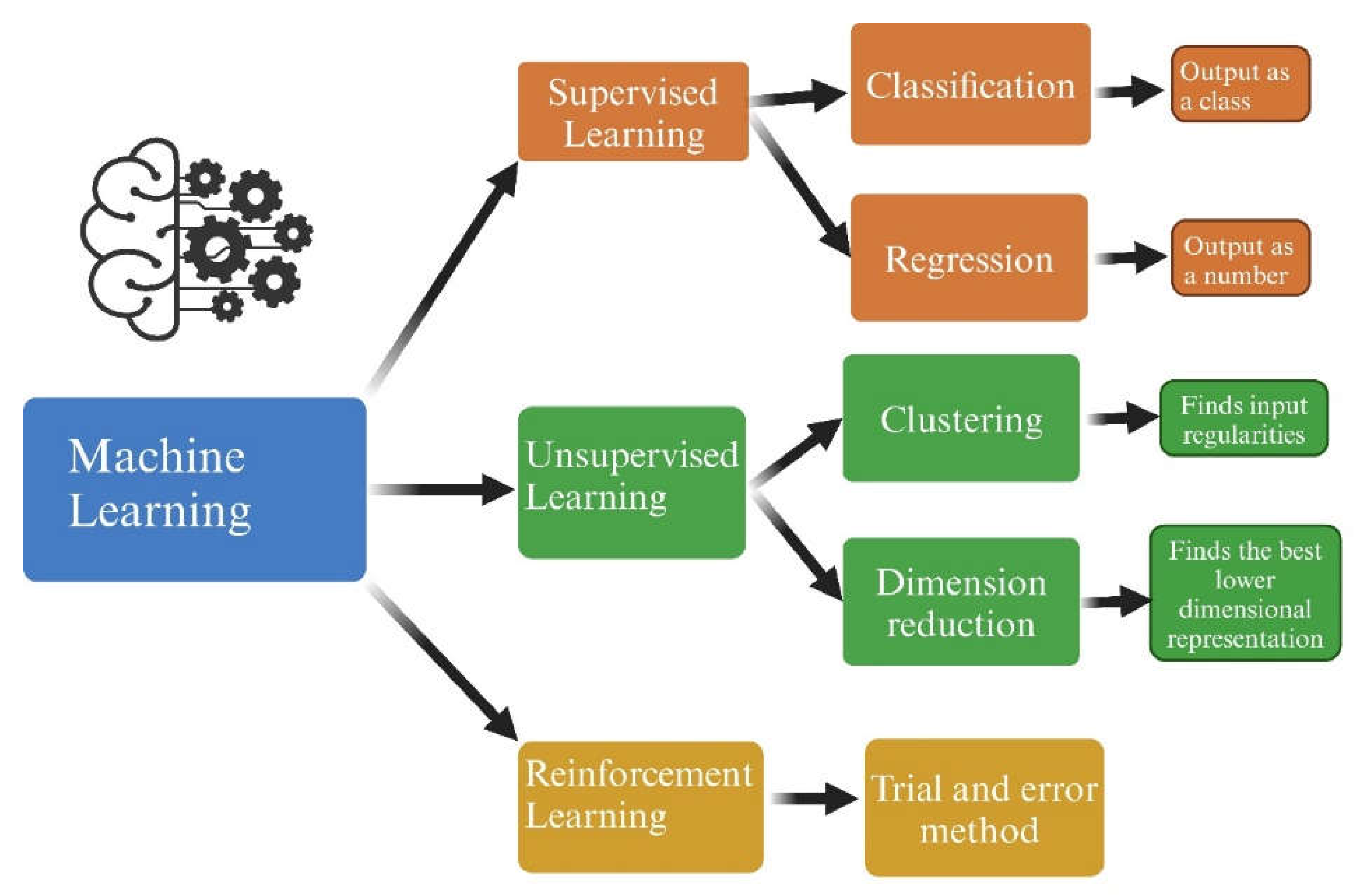
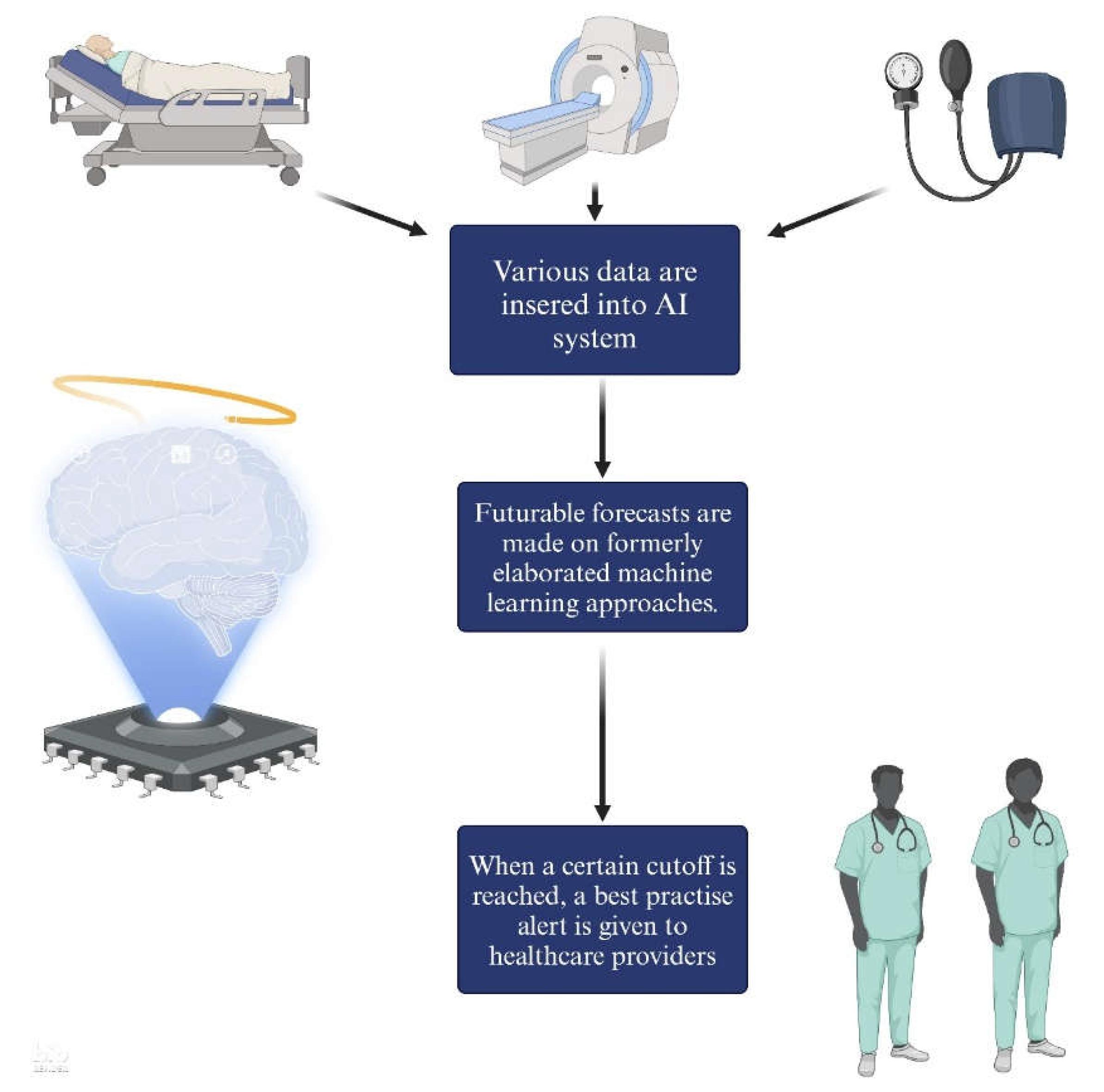
3. Artificial Intelligence and subfields
- Volume: the continuous and exponentially incremental flow of data spanning from personal medical records up to 3D imaging, genomics, and biometric sensor readings ought to be carefully managed[12]. Innovations in data management, such as virtualization and cloud computing, are enabling healthcare organizations to store and manipulate large amounts of data more efficiently and cost-effectively[14];
- Velocity: the prompt and rapid transmission of data is a pivotal item nowadays, especially in scenarios like trauma monitoring, anesthesia in operating rooms, and bedside heart monitoring, where timely data analysis can be life-saving[12]. Besides, future applications, such as early infection detection and targeted treatments based upon real-time data, have the potential to notably decrease morbidity, mortality, and ultimately impact on outcome[14,15];
- Variety: the ability to analyze large datasets, including multimedia and unstructured formats, represents an innovation in healthcare[12]. The wide range of structured, unstructured, and semi-structured data analyzed, stands as a revolutionary change that adds complexity to healthcare data management[16]. Structured data can be easily stored, recalled, elaborated and manipulated by machinery. They come from a variety of sources, including diagnoses, medications, instrument readings, and lab values, and can be sorted into numeric or categorical fields for easy analysis[12,17]. Unstructured data is commonly generated at the point of care, including free-form text such as medical notes or discharge summaries and multimedia content such as imaging[12,17]. The main challenge is to transform this data to make it suitable for AI analysis, but this process faces some obstacles. First, adding structure to unstructured data entails healthcare providers to manually review charts or images, sort the information out and enter it into the system[18]. This makes the process slow, inefficient, and prone to bias. New powerful tools such as Natural Language Processing can speed up and streamline the information extraction process[17]. Secondly, healthcare professionals' preference for the natural language simplicity of handwritten notes remains a major barrier to a widespread adoption of electronic health records, which require field coding at the point of care to provide structured inputs[12].
- Veracity: ensuring that big data is accurate and trustworthy is critical in healthcare, where accurate information can mean the difference between life and death[12]. Nevertheless, achieving veracity faces challenges, including variable quality and difficulties in ensuring accuracy, especially with handwritten prescriptions.
- Value consists of the worth of information to various stakeholders or decision makers[20].
4. Current Research and Applications
4.1. AI for Triage Optimization
4.2. AI for Stress Management
4.3. AI for Traumatic Brain Injury Assessment
4.4. AI for Pediatric Sepsis Prediction
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manickam P, Mariappan SA, Murugesan SM, et al. Artificial Intelligence (AI) and Internet of Medical Things (IoMT) Assisted Biomedical Systems for Intelligent Healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef]
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng 2018, 2, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Ramgopal S, Sanchez-Pinto LN, Horvat CM, et al. Artificial intelligence-based clinical decision support in pediatrics. Pediatr Res 2023, 93, 334–341. [Google Scholar] [CrossRef]
- Turing, AM. Computing Machinery and Intelligence. Mind 1950, 59, 433–460. [Google Scholar] [CrossRef]
- Rajula HSR, Verlato G, Manchia M, et al. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Med 2020, 56, 455. [Google Scholar] [CrossRef]
- Cirillo D, Valencia A. Big data analytics for personalized medicine. Curr Opin Biotechnol 2019, 58, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mesko, B. The role of artificial intelligence in precision medicine. Expert Rev Precis Med Drug Dev 2017, 2, 239–241. [Google Scholar] [CrossRef]
- Nijman J, Zoodsma RS, Koomen E. A Strategy for Artificial Intelligence With Clinical Impact—Eyes on the Prize. JAMA Pediatr 2024, 178, 219. [Google Scholar] [CrossRef]
- Hossain E, Rana R, Higgins N, et al. Natural Language Processing in Electronic Health Records in relation to healthcare decision-making: A systematic review. Comput Biol Med 2023, 155, 106649. [Google Scholar] [CrossRef]
- Benke K, Benke G. Artificial Intelligence and Big Data in Public Health. Int J Environ Res Public Health 2018, 15, 2796. [Google Scholar] [CrossRef]
- Mallappallil M, Sabu J, Gruessner A, et al. A review of big data and medical research. SAGE Open Med 2020, 8, 205031212093483. [Google Scholar] [CrossRef]
- Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Heal Inf Sci Syst 2014, 2, 3. [Google Scholar] [CrossRef]
- Frost, Sullivan. Drowning in Big Data? Reducing Information Technology Complexities and Costs for Healthcare Organizations. WSEAS Trans. Comput. Res. 2016, :4. 123-131.https://www.researchgate.net/publication/310416741_Healthcare_Big_Data_and_Cloud_Computing (accessed 12 Feb 2024).
- Feldman B, Martin E, Skotnes T. Big Data in Healthcare: Hype and Hope. 2012.https://www.yumpu.com/en/document/view/29226285/big-data-in-healthcare-hype-and-hope (accessed 13 Feb 2024).
- Hoover, W. Transforming Health Care Through Big Data: Strategies for leveraging big data in the health care industry. 2013.
- Ristevski B, Chen M. Big Data Analytics in Medicine and Healthcare. J Integr Bioinform 2018, 15. [Google Scholar] [CrossRef]
- Li I, Pan J, Goldwasser J, et al. Neural Natural Language Processing for unstructured data in electronic health records: A review. Comput Sci Rev 2022, 46, 100511. [Google Scholar] [CrossRef]
- Kamran, S. Natural Language Processing in Healthcare Explained. 2023.https://www.consensus.com/blog/natural-language-processing-in-healthcare/ (accessed 15 Feb 2024).
- SAS Big Data - What It Is and Why It Matters. https://www.sas.com/en_us/insights/big-data/what-is-big-data.html (accessed 21 Feb 2024).
- Hermon R, Williams PAH. Big data in healthcare: What is it used for? In: Australian Ehealth Informatics and Security Conference. Perth, Western Australia: : SRI Security Research Institute, Edith Cowan University 2014. 40–9. [CrossRef]
- Wang Y, Kung L, Byrd TA. Big data analytics: Understanding its capabilities and potential benefits for healthcare organizations. Technol Forecast Soc Change 2018, 126, 3–13. [Google Scholar] [CrossRef]
- Elgendy N, Elragal A. Big Data Analytics: A Literature Review Paper. In: Lecture Notes in Computer Science. 2014. 214–27. [CrossRef]
- Hasselgren A, Kralevska K, Gligoroski D, et al. Blockchain in healthcare and health sciences—A scoping review. Int J Med Inform 2020, 134, 104040. [Google Scholar] [CrossRef]
- Lax G, Russo A. Blockchain-Based Access Control Supporting Anonymity and Accountability. J Adv Inf Technol 2020, 11, 186–191. [Google Scholar] [CrossRef]
- Tagde P, Tagde S, Bhattacharya T, et al. Blockchain and artificial intelligence technology in e-Health. Environ Sci Pollut Res 2021, 28, 52810–52831. [Google Scholar] [CrossRef]
- Zheng Z, Xie S, Dai H, et al. An Overview of Blockchain Technology: Architecture, Consensus, and Future Trends. In: 2017 IEEE International Congress on Big Data (BigData Congress). IEEE 2017. 557–64. [CrossRef]
- Ali S, Abdullah, Armand TPT, et al. Metaverse in Healthcare Integrated with Explainable AI and Blockchain: Enabling Immersiveness, Ensuring Trust, and Providing Patient Data Security. Sensors 2023, 23, 565. [Google Scholar] [CrossRef]
- Jiang T, Gradus JL, Rosellini AJ. Supervised Machine Learning: A Brief Primer. Behav Ther 2020, 51, 675–687. [Google Scholar] [CrossRef]
- Maghami M, Sattari SA, Tahmasbi M, et al. Diagnostic test accuracy of machine learning algorithms for the detection intracranial hemorrhage: a systematic review and meta-analysis study. Biomed Eng Online 2023, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Schaffter T, Buist DSM, Lee CI, et al. Evaluation of Combined Artificial Intelligence and Radiologist Assessment to Interpret Screening Mammograms. JAMA Netw Open 2020, 3, e200265. [Google Scholar] [CrossRef]
- Zhang, F. Application of machine learning in CT images and X-rays of COVID-19 pneumonia. Medicine (Baltimore) 2021, 100, e26855. [Google Scholar] [CrossRef]
- Mueller B, Kinoshita T, Peebles A, et al. Artificial intelligence and machine learning in emergency medicine: a narrative review. Acute Med Surg 2022, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hernán MA, Hsu J, Healy B. A Second Chance to Get Causal Inference Right: A Classification of Data Science Tasks. CHANCE 2019, 32, 42–49. [Google Scholar] [CrossRef]
- Theodosiou AA, Read RC. Artificial intelligence, machine learning and deep learning: Potential resources for the infection clinician. J Infect 2023, 87, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bertsimas D, Dunn J. Optimal classification trees. Mach Learn 2017, 106, 1039–1082. [Google Scholar] [CrossRef]
- Decision Trees. https://www.ibm.com/topics/decision-trees (accessed 23 Feb 2024).
- Podgorelec V, Kokol P, Stiglic B, et al. Decision trees: an overview and their use in medicine. J Med Syst 2002, 26, 445–463. [Google Scholar] [CrossRef]
- Matsuo Y, LeCun Y, Sahani M, et al. Deep learning, reinforcement learning, and world models. Neural Networks 2022, 152, 267–275. [Google Scholar] [CrossRef]
- Bothe MK, Dickens L, Reichel K, et al. The use of reinforcement learning algorithms to meet the challenges of an artificial pancreas. Expert Rev Med Devices 2013, 10, 661–673. [Google Scholar] [CrossRef]
- Sidey-Gibbons JAM, Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction to natural language processing. BMC Med Res Methodol 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Stafie CS, Sufaru I-G, Ghiciuc CM, et al. Exploring the Intersection of Artificial Intelligence and Clinical Healthcare: A Multidisciplinary Review. Diagnostics 2023, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Zielinski C, Winker M, Aggarwal R, et al. Chatbots, generative AI, and scholarly manuscripts. WAME recommendations on chatbots and generative artificial intelligence in relation to scholarly publications. 2023.https://wame.org/page3.php?id=106. (accessed 16 Feb 2024).
- Stokel-Walker C, Van Noorden R. What ChatGPT and generative AI mean for science. Nature 2023, 614, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Birhane A, Kasirzadeh A, Leslie D, et al. Science in the age of large language models. Nat Rev Phys 2023, 5, 277–280. [Google Scholar] [CrossRef]
- Sallam, M. ChatGPT Utility in Healthcare Education, Research, and Practice: Systematic Review on the Promising Perspectives and Valid Concerns. Healthcare 2023, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Liverpool, L. AI intensifies fight against ‘paper mills’ that churn out fake research. Nature 2023, 618, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Gu J, Wang X, Li C, et al. AI-enabled image fraud in scientific publications. Patterns 2022, 3, 100511. [Google Scholar] [CrossRef] [PubMed]
- 48. Májovský M, Černý M, Kasal M, et al. Artificial Intelligence Can Generate Fraudulent but Authentic-Looking Scientific Medical Articles: Pandora’s Box Has Been Opened. J Med Internet Res. [CrossRef]
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- 51. Nitish S, Hinton G, Krizhevsky A, et al. Dropout: A Simple Way to Prevent Neural Networks from Overfitting. J Mach Learn Res.
- Si T, Bagchi J, Miranda PBC. Artificial Neural Network training using metaheuristics for medical data classification: An experimental study. Expert Syst Appl 2022, 193, 116423. [Google Scholar] [CrossRef]
- Sze V, Chen Y-H, Yang T-J, et al. Efficient Processing of Deep Neural Networks: A Tutorial and Survey. Proc IEEE 2017, 105, 2295–2329. [Google Scholar] [CrossRef]
- Masegosa AR, Cabañas R, Langseth H, et al. Probabilistic Models with Deep Neural Networks. Entropy 2021, 23, 117. [Google Scholar] [CrossRef]
- Grossberg, S. Recurrent neural networks. Scholarpedia 2013, 8, 1888. [Google Scholar] [CrossRef]
- Salehinejad H, Sankar S, Barfett J, et al. Recent Advances in Recurrent Neural Networks. arXiv Prepr Published Online First: 28 December 2017. [CrossRef]
- Choi BW, Kang S, Kim HW, et al. Faster Region-Based Convolutional Neural Network in the Classification of Different Parkinsonism Patterns of the Striatum on Maximum Intensity Projection Images of [18F]FP-CIT Positron Emission Tomography. Diagnostics 2021, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- 58. Popescu MC, Balas VE, L. P-P, et al. Multilayer perceptron and neural networks. WSEAS Trans Circuits Syst.
- Spiegelhalter D, Rice K. Bayesian statistics. Scholarpedia 2009, 4, 5230. [Google Scholar] [CrossRef]
- 60. Raita Y, Camargo CA, Liang L, et al. Big Data, Data Science, and Causal Inference: A Primer for Clinicians. Front Med. [CrossRef]
- Ji X, Chang W, Zhang Y, et al. Prediction Model of Hypertension Complications Based on GBDT and LightGBM. J Phys Conf Ser 2021, 1813, 012008. [Google Scholar] [CrossRef]
- Chen T, He T, Benesty M, et al. Xgboost: extreme gradient boosting. R Packag version 2015;:0.4-2, 1(4), 1-4.https://cran.ms.unimelb.edu.au/web/packages/xgboost/vignettes/xgboost.pdf.
- Rigatti, SJ. Random Forest. J Insur Med 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Berner ES, La Lande TJ. Overview of Clinical Decision Support Systems. In: Clinical Decision Support Systems. 2007. 3–22. [CrossRef]
- Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. npj Digit Med 2020, 3, 17. [Google Scholar] [CrossRef]
- Green NA, Durani Y, Brecher D, et al. Emergency Severity Index Version 4. Pediatr Emerg Care 2012, 28, 753–757. [Google Scholar] [CrossRef]
- Thomas D, Kircher J, Plint AC, et al. Pediatric Pain Management in the Emergency Department: The Triage Nurses’ Perspective. J Emerg Nurs 2015, 41, 407–413. [Google Scholar] [CrossRef]
- Di Sarno L, Gatto A, Korn D, et al. Pain management in pediatric age. An update. Pain management in pediatric age. An update. Acta Biomed 2023, 94, e2023174. [Google Scholar] [CrossRef]
- Hwang S, Lee B. Machine learning-based prediction of critical illness in children visiting the emergency department. PLoS One 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Levin S, Toerper M, Hamrock E, et al. Machine-Learning-Based Electronic Triage More Accurately Differentiates Patients With Respect to Clinical Outcomes Compared With the Emergency Severity Index. Ann Emerg Med 2018, 71, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kwon J, Jeon K-H, Lee M, et al. Deep Learning Algorithm to Predict Need for Critical Care in Pediatric Emergency Departments. Deep Learning Algorithm to Predict Need for Critical Care in Pediatric Emergency Departments. Pediatr Emerg Care 2021, 37, e988–94. [Google Scholar] [CrossRef]
- 72. Goto T, Camargo CA, Faridi MK, et al. Machine Learning–Based Prediction of Clinical Outcomes for Children During Emergency Department Triage. JAMA Netw Open. [CrossRef]
- Sarty J, Fitzpatrick EA, Taghavi M, et al. Machine learning to identify attributes that predict patients who leave without being seen in a pediatric emergency department. CJEM 2023, 25, 689–694. [Google Scholar] [CrossRef]
- Trost MJ, Ford AR, Kysh L, et al. Socially Assistive Robots for Helping Pediatric Distress and Pain. Clin J Pain 2019, 35, 451–458. [Google Scholar] [CrossRef]
- Sanchez Cristal N, Staab J, Chatham R, et al. Child Life Reduces Distress and Pain and Improves Family Satisfaction in the Pediatric Emergency Department. Clin Pediatr 2018, 57, 1567–1575. [Google Scholar] [CrossRef]
- Trost MJ, Chrysilla G, Gold JI, et al. Socially-Assistive Robots Using Empathy to Reduce Pain and Distress during Peripheral IV Placement in Children. Pain Res Manag 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chita-Tegmark M, Scheutz M. Assistive Robots for the Social Management of Health: A Framework for Robot Design and Human–Robot Interaction Research. Int J Soc Robot 2021, 13, 197–217. [Google Scholar] [CrossRef] [PubMed]
- 78. Nishat F, Hudson S, Panesar P, et al. Exploring the needs of children and caregivers to inform design of an artificial intelligence-enhanced social robot in the pediatric emergency department. J Clin Transl Sci. [CrossRef]
- Hudson S, Nishat F, Stinson J, et al. Perspectives of Healthcare Providers to Inform the Design of an AI-Enhanced Social Robot in the Pediatric Emergency Department. Children 2023, 10, 1–13. [Google Scholar] [CrossRef]
- 80. Mastrangelo M, Midulla F. Minor Head Trauma in the Pediatric Emergency Department: Decision Making Nodes. Curr Pediatr Rev. [CrossRef]
- Schutzman SA, Greenes DS. Pediatric minor head trauma. Ann Emerg Med 2001, 37, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Da Dalt L, Parri N, Amigoni A, et al. Italian guidelines on the assessment and management of pediatric head injury in the emergency department. Ital J Pediatr 2018, 44, 7. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 2009, 374, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Tunthanathip T, Oearsakul T. Application of machine learning to predict the outcome of pediatric traumatic brain injury. Chinese J Traumatol = Zhonghua chuang shang za zhi 2021, 24, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ellethy H, Chandra SS, Nasrallah FA. The detection of mild traumatic brain injury in paediatrics using artificial neural networks. Comput Biol Med 2021, 135, 104614. [Google Scholar] [CrossRef] [PubMed]
- Dayan PS, Ballard DW, Tham E, et al. Use of Traumatic Brain Injury Prediction Rules With Clinical Decision Support. Use of Traumatic Brain Injury Prediction Rules With Clinical Decision Support. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Hale AT, Stonko DP, Lim J, et al. Using an artificial neural network to predict traumatic brain injury. J Neurosurg Pediatr 2019, 23, 219–226. [Google Scholar] [CrossRef]
- Bertsimas D, Dunn J, Steele DW, et al. Comparison of Machine Learning Optimal Classification Trees with the Pediatric Emergency Care Applied Research Network Head Trauma Decision Rules. JAMA Pediatr 2019, 173, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa T, Saga M, Sasaki M, et al. Statistical and machine learning approaches to predict the necessity for computed tomography in children with mild traumatic brain injury. Statistical and machine learning approaches to predict the necessity for computed tomography in children with mild traumatic brain injury. PLoS One 2023, 18, e0278562. [Google Scholar] [CrossRef]
- Ellethy H, Chandra SS, Nasrallah FA. Deep Neural Networks Predict the Need for CT in Pediatric Mild Traumatic Brain Injury: A Corroboration of the PECARN Rule. J Am Coll Radiol 2022, 19, 769–778. [Google Scholar] [CrossRef]
- Wong A, Otles E, Donnelly JP, et al. External Validation of a Widely Implemented Proprietary Sepsis Prediction Model in Hospitalized Patients. JAMA Intern Med 2021, 181, 1065. [Google Scholar] [CrossRef] [PubMed]
- Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage Clin 2014, 4, 283–294. [Google Scholar] [CrossRef]
- Shah HA, Mehta NH, Saleem MI, et al. Connecting the connectome: A bibliometric investigation of the 50 most cited articles. Clin Neurol Neurosurg 2022, 223, 107481. [Google Scholar] [CrossRef] [PubMed]
- Payabvash S, Palacios EM, Owen JP, et al. White Matter Connectome Edge Density in Children with Autism Spectrum Disorders: Potential Imaging Biomarkers Using Machine-Learning Models. Brain Connect 2019, 9, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Raji CA, Wang MB, Nguyen N, et al. Connectome mapping with edge density imaging differentiates pediatric mild traumatic brain injury from typically developing controls: proof of concept. Pediatr Radiol 2020, 50, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur A, Weston J. A User’s Guide to Support Vector Machines. 2010. 223–39. [CrossRef]
- Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric Severe Sepsis. Pediatr Crit Care Med 2014, 15, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach LJ, Watson RS, Sorce LR, et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665. [Google Scholar] [CrossRef] [PubMed]
- Yu SC, Shivakumar N, Betthauser K, et al. Comparison of early warning scores for sepsis early identification and prediction in the general ward setting. JAMIA Open 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Uffen JW, Oosterheert JJ, Schweitzer VA, et al. Interventions for rapid recognition and treatment of sepsis in the emergency department: a narrative review. Clin Microbiol Infect 2021, 27, 192–203. [Google Scholar] [CrossRef]
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics*. Pediatr Crit Care Med 2005, 6, 2–8. [Google Scholar] [CrossRef]
- Balamuth F, Alpern ER, Grundmeier RW, et al. Comparison of Two Sepsis Recognition Methods in a Pediatric Emergency Department. Acad Emerg Med 2015, 22, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Kamaleswaran R, Akbilgic O, Hallman MA, et al. Applying Artificial Intelligence to Identify Physiomarkers Predicting Severe Sepsis in the PICU. Pediatr Crit Care Med 2018, 19, e495–503. [Google Scholar] [CrossRef] [PubMed]
- Le S, Hoffman J, Barton C, et al. Pediatric Severe Sepsis Prediction Using Machine Learning. Front Pediatr 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Stella P, Haines E, Aphinyanaphongs Y. Prediction of Resuscitation for Pediatric Sepsis from Data Available at Triage. In: AMIA Annu Symp Proc. 2021. 1129–38.http://www.ncbi.nlm.nih.gov/pubmed/35308977.
- Mercurio L, Pou S, Duffy S, et al. Risk Factors for Pediatric Sepsis in the Emergency Department. Pediatr Emerg Care 2023, 39, e48–56. [Google Scholar] [CrossRef]
- Rajkomar A, Hardt M, Howell MD, et al. Ensuring Fairness in Machine Learning to Advance Health Equity. Ann Intern Med 2018, 169, 866. [Google Scholar] [CrossRef] [PubMed]
- Amershi S, Weld D, Vorvoreanu M, et al. Guidelines for Human-AI Interaction. In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. New York, NY, USA: : ACM 2019. 1–13. [CrossRef]
- Wiens J, Saria S, Sendak M, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med 2019, 25, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Ventura CAI, Denton E. Artificial Intelligence Chatbots and Emergency Medical Services: Perspectives on the Implications of Generative AI in Prehospital Care. Open Access Emerg Med 2023, 15, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny MF, Robinson KM. Data quality: “Garbage in – garbage out”. Heal Inf Manag 2018, 47, 103–105. [Google Scholar] [CrossRef] [PubMed]
- He J, Baxter SL, Xu J, et al. The practical implementation of artificial intelligence technologies in medicine. Nat Med 2019, 25, 30–36. [Google Scholar] [CrossRef]
- Challen R, Denny J, Pitt M, et al. Artificial intelligence, bias and clinical safety. BMJ Qual Saf 2019, 28, 231–237. [Google Scholar] [CrossRef]
- Ramgopal S, Adler MD, Horvat CM. Application of the Improving Pediatric Sepsis Outcomes Definition for Pediatric Sepsis to Nationally Representative Emergency Department Data. Pediatr Qual Saf 2021, 6, e468. [Google Scholar] [CrossRef] [PubMed]
- Lee B, Kim K, Hwang H, et al. Development of a machine learning model for predicting pediatric mortality in the early stages of intensive care unit admission. Sci Rep 2021, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Padash S, Mohebbian MR, Adams SJ, et al. Pediatric chest radiograph interpretation: how far has artificial intelligence come? A systematic literature review. Pediatr Radiol 2022, 52, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- 117. Marshall TL, Rinke ML, Olson APJ, et al. Diagnostic Error in Pediatrics: A Narrative Review. Pediatrics. [CrossRef]
- Di Sarno L, Cammisa I, Curatola A, et al. A scoping review of the management of acute mastoiditis in children: What is the best approach? Turk J Pediatr 2023, 65, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Causio FA, Beccia F, Hoxhaj I, et al. Integrating China in the International Consortium for Personalized Medicine: A Position Paper on Personalized Medicine in Sustainable Healthcare. Public Health Genomics 2024, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paranjape K, Schinkel M, Nannan Panday R, et al. Introducing Artificial Intelligence Training in Medical Education. JMIR Med Educ 2019, 5, e16048. [Google Scholar] [CrossRef] [PubMed]
- Proposal For A Regulation Of The European Parliament And Of The Council Laying Down Harmonised Rules On Artificial Intelligence (Artificial Intelligence Act) And Amending Certain Union Legislative Acts. 2021.https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52021PC0206.
- Causio FA, Hoxhaj I, Beccia F, et al. Big data and ICT solutions in the European Union and in China: A comparative analysis of policies in personalized medicine. Digit Heal 2022, 8, 205520762211290. [Google Scholar] [CrossRef]
- Good Machine Learning Practice for Medical Device Development: Guiding Principles. U.S. Food Drug Adm. 2021.https://www.fda.gov/medical-devices/software-medical-device-samd/good-machine-learning-practice-medical-device-development-guiding-principles (accessed 15 Mar 2024).
- 124. Cascini F, Beccia F, Causio FA, et al. Scoping review of the current landscape of AI-based applications in clinical trials. Front Public Heal. [CrossRef]
- Michelson KN, Klugman CM, Kho AN, et al. Ethical Considerations Related to Using Machine Learning-Based Prediction of Mortality in the Pediatric Intensive Care Unit. J Pediatr 2022, 247, 125–128. [Google Scholar] [CrossRef]
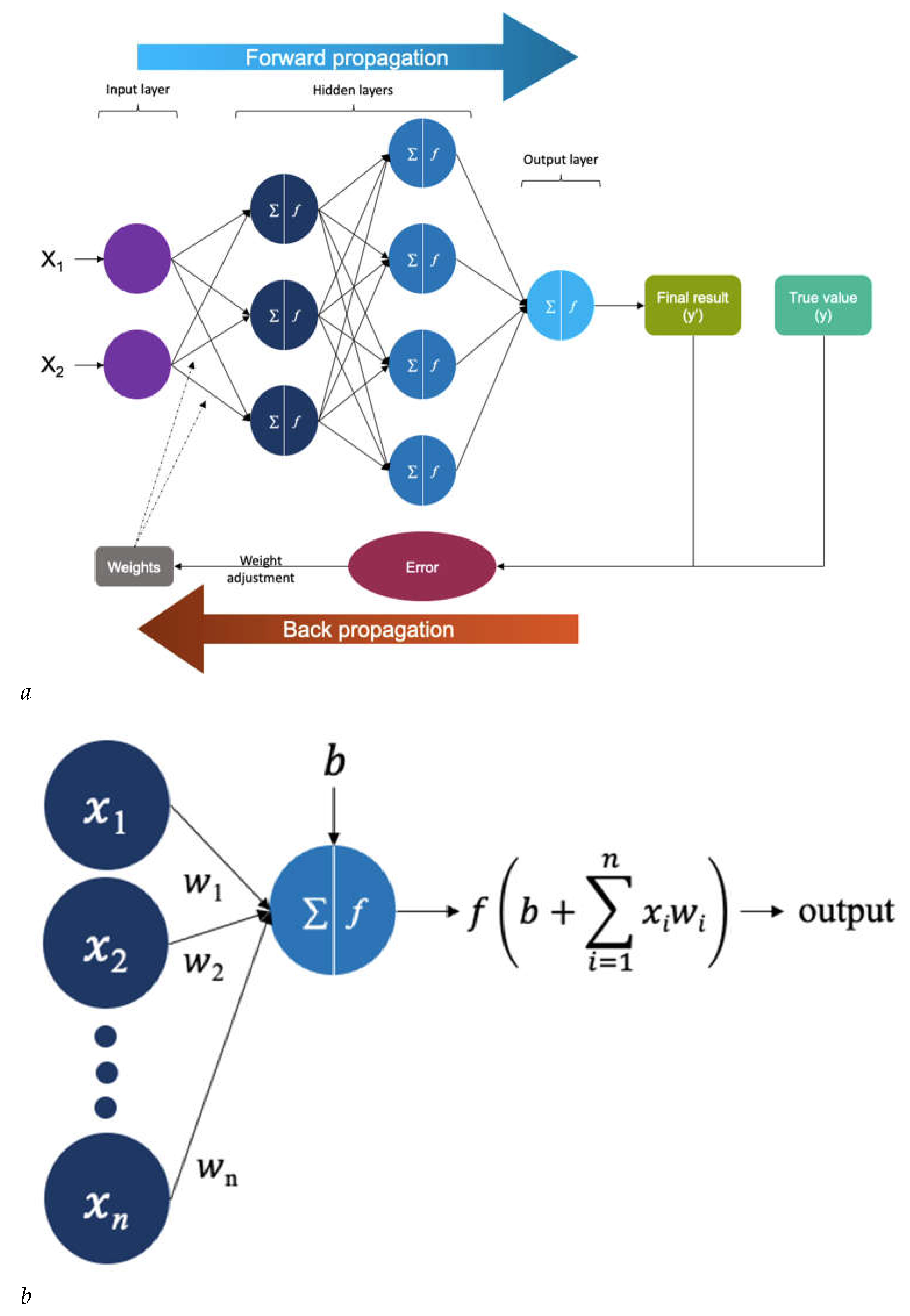
| System | Description |
|---|---|
| Artificial Neural Network (ANN) | Nodes, akin to neurons, process information, while connections between layers, termed edges, simulate synapses with weights. Output is computed via mathematical operations on input and hidden layers, with the learning algorithm adjusting weights to minimize errors between predicted and target outputs, forming probability-weighted associations stored within the network's structure[52]. |
| Backpropagation Neural Network | Backpropagation utilizes prediction errors to iteratively tune the weights, enabling the NN to learn patterns within the training data and enhance model accuracy over time[32]. |
| Convolutional Neural Network (CNN) | CNNs process data that comes in the form of multiple arrays such as signals, images, audio spectrograms and videos, and is applied in the recognition of objects[50]. |
| Deep Neural Network (DNN) | An ANN with numerous layers between the input and output layers which is capable of learning high-level features and requires high computational power[53]. |
| Probabilistic Neural Network (PNN) | An application of DNN within probabilistic models, able to capture complex non-linear stochastic relationships between random variables[54]. |
| Recurrent Neural Network (RNN) | A recurrent neural network (RNN) is any network whose neurons send feedback signals to each other, and are capable of modeling sequential data for sequence recognition and prediction[55,56]. |
| Region-based Convolutional Neural Network (R-CNN) | R-CNN models use region based networks, which are capable to detect an object in an image and holds great potential especially in diagnostic imaging[57]. |
| Multilayer Perceptron (MLP) | A feedforward type of powerful and dynamic ANN. The signals are transmitted within the network in one direction: from input to output[58]. |
| Bayesian Inference | Bayesian statistical methods are applied to algorithms. They start with existing 'prior' beliefs, which are then updated using data to give 'posterior' beliefs, which may be used as the basis for inferential decisions[59]. |
| Causal Associational Network (CASNET) | Three items constitute this model: patient observation, pathophysiological states, and disease classifications. Once documented, the observations are associated with the fitting states[60]. |
| Light Gradient Boosting Machine (LightGBM) | LightGBM employs a boosting strategy to combine numerous decision trees, with each tree utilizing the negative gradient of the loss function as the residual approximation for fitting. It is designed for optimal performance, particularly in distributed systems[61]. |
| Extreme Gradient Boosting (XGBoost) | XGBoost is a gradient boosting framework that is highly efficient and scalable. It features a proficient linear model solver and a tree learning algorithm. It enables diverse objective functions, such as regression, classification, and ranking. Its design allows for easy extension, enabling users to define custom objectives[62]. |
| Natural Language Processing (NLP) | NLP is a subfield of AI and ML used to interpret linguistic data (e.g. clinical note analysis and decision making) [9,40]. |
| Random Forest Models | Random forest models use randomization to create multiple decision trees, each contributing to the final output. In classification tasks, the trees' outputs are combined through voting, while in regression tasks, they are averaged to produce a single output[63]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





