Submitted:
23 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Protocol: Clinical Evaluation
2.2. Study Protocol: Laser Application
2.3. Study Protocol: Follow up
3. Results
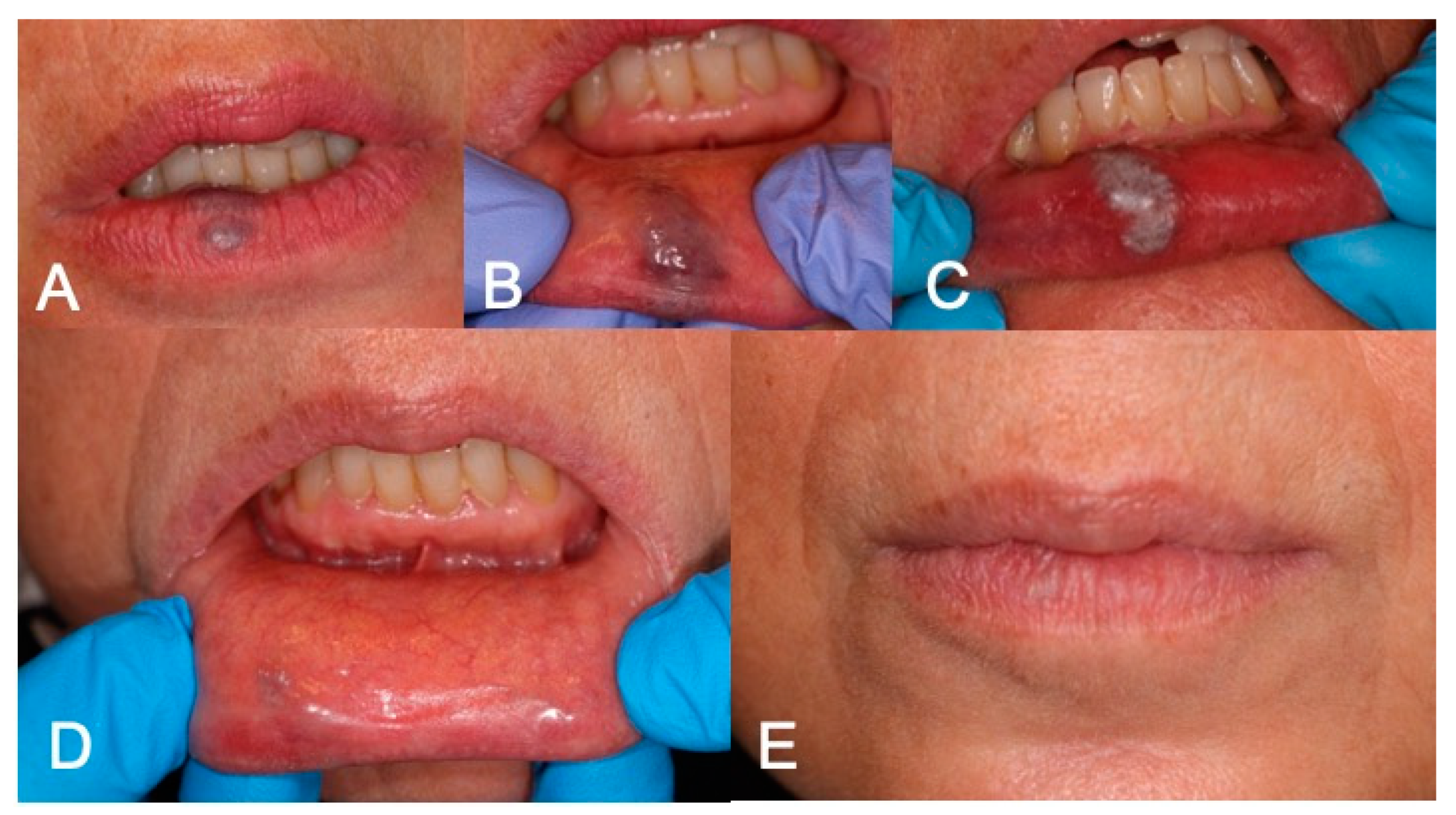
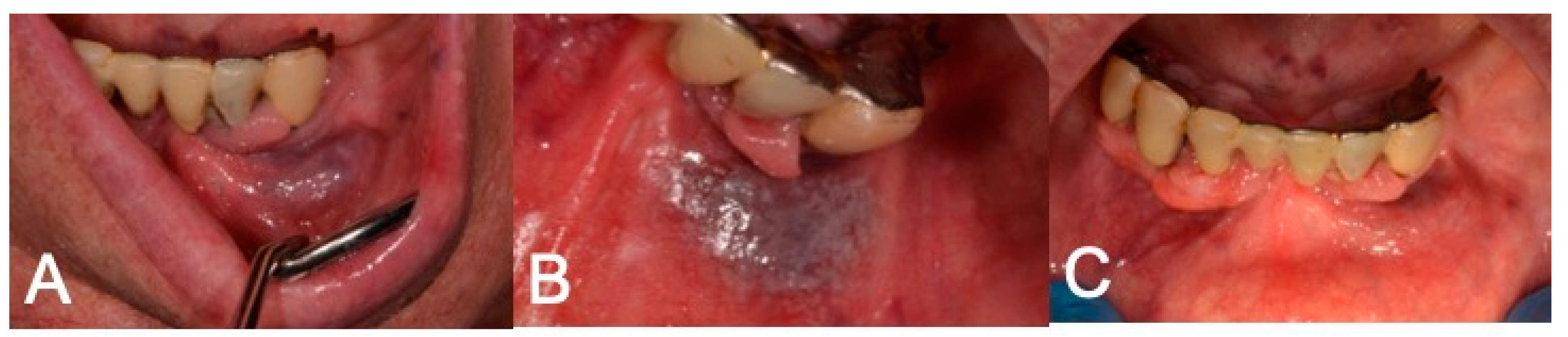
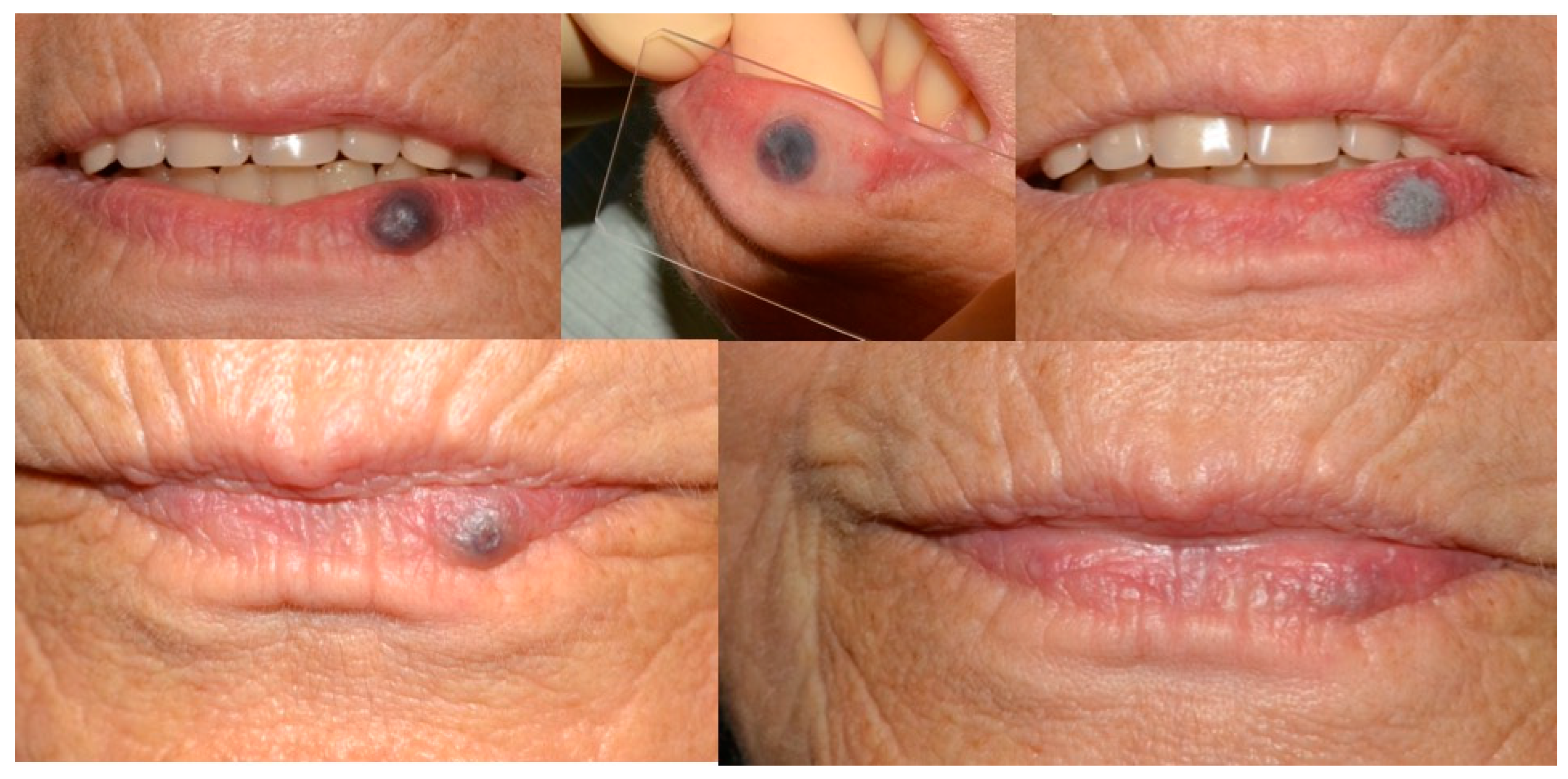
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kunimoto K, Yamamoto Y, Jinnin M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int J Mol Sci. February 2022;23(4):2358. [CrossRef]
- Dell’Olio F, De Falco D, Di Nanna S, Casorelli A, Favia G. Diode Laser Photocoagulation of Oral Venous Malformations in Patients on Anticoagulant Therapy Without Drug Discontinuation. Cureus. 20 march 2020;12(3):e7340. [CrossRef]
- John HE, Phen HS, Mahaffey PJ. Treatment of venous lesions of the lips and perioral area with a long-pulsed Nd:YAG laser. Br J Oral Maxillofac Surg. May 2016;54(4):376–8. [CrossRef]
- Jasper J, Camilotti RS, Pagnoncelli RM, Poli VD, da Silveira Gerzson A, Gavin Zakszeski AM. Treatment of lip hemangioma using forced dehydration with induced photocoagulation via diode laser: report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol. March 2015;119(3):e89-94. [CrossRef]
- Frigerio A, Tan OT. Laser applications for benign oral lesions. Lasers Surg Med. October 2015;47(8):643–50. [CrossRef]
- Miyazaki H, Ohshiro T, Romeo U, Noguchi T, Maruoka Y, Gaimari G, et al. Retrospective Study on Laser Treatment of Oral Vascular Lesions Using the «Leopard Technique»: The Multiple Spot Irradiation Technique with a Single-Pulsed Wave. Photomed Laser Surg. June 2018;36(6):320–5. [CrossRef]
- Zheng JW, Zhou Q, Yang XJ, Wang YA, Fan XD, Zhou GY, et al. Treatment guideline for hemangiomas and vascular malformations of the head and neck. Head Neck. August 2010;32(8):1088–98. [CrossRef]
- Johann ACBR, Aguiar MCF, do Carmo MAV, Gomez RS, Castro WH, Mesquita RA. Sclerotherapy of benign oral vascular lesion with ethanolamine oleate: an open clinical trial with 30 lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. November 2005;100(5):579–84. [CrossRef]
- Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. August 2000;37(8):517–84. [CrossRef]
- Medeiros R, Silva IH, Carvalho AT, Leão JC, Gueiros LA. Nd:YAG laser photocoagulation of benign oral vascular lesions: a case series. Lasers Med Sci. November 2015;30(8):2215–20. [CrossRef]
- Ulrich H, Bäumler W, Hohenleutner U, Landthaler M. Neodymium-YAG Laser for hemangiomas and vascular malformations -- long term results. J Dtsch Dermatol Ges. June 2005;3(6):436–40. [CrossRef]
- Landthaler M, Hohenleutner U. Laser therapy of vascular lesions. Photodermatol Photoimmunol Photomed. Dicember 2006;22(6):324–32. [CrossRef]
- Ohshiro T. Laser Treatment for Naevi. 1996a ed. New York: Wiley; 17415 p.
- Bradley PF. A review of the use of the neodymium YAG laser in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. February 1997;35(1):26–35. [CrossRef]
- Alani HM, Warren RM. Percutaneous photocoagulation of deep vascular lesions using a fiberoptic laser wand. Ann Plast Surg. August 1992;29(2):143–8. [CrossRef]
- Romeo U, Del Vecchio A, Russo C, Palaia G, Gaimari G, Arnabat-Dominguez J, et al. Laser treatment of 13 benign oral vascular lesions by three different surgical techniques. Med Oral Patol Oral Cir Bucal. March 2013;18(2):e279-284. [CrossRef]
- Miyazaki H, Romeo U, Ohshiro T, Kudo T, Makiguchi T, Kawachi N, et al. Treatment strategies for large oral venous malformations using intralesional laser photocoagulation. Lasers Med Sci. novembre 2014;29(6):1987–90. [CrossRef]
- Miyazaki H, Ohshiro T, Watanabe H, Kakizaki H, Makiguchi T, Kim M, et al. Ultrasound-guided intralesional laser treatment of venous malformation in the oral cavity. Int J Oral Maxillofac Surg. February 2013;42(2):281–7. [CrossRef]
- Górriz-Gómez E, Vicente-Barrero M, Loras-Caballero ML, Bocanegra-Pérez S, Castellano-Navarro JM, Pérez-Plasencia D, et al. Sclerotherapy of face and oral cavity low flow vascular malformations: our experience. Br J Oral Maxillofac Surg. January 2014;52(1):43–7. [CrossRef]
| ID | Gender | Age | Medical History | Medications | Smoking (n) |
|---|---|---|---|---|---|
| 1 | F | 63 | Carotid stenosis, hypertension, hypercolesterolemia, allergic to preservatives and fragrances | Antiaggregants, anticholesterolemics, anti-hypertensive | No |
| 2 | M | 66 | Myelodisplasia (2011), hypertension, prostate cancer (2015) | Xantin oxidase inhibitor, anti-hypertensive, anticholesterolemics | Ex (18 years) |
| 3 | M | 76 | Hypertension, hypercolesterolemia | Anti-hypertensive, anticholesterolemics, antiaggregants | No |
| 4 | M | 76 | Hypertension, discal hernia (intervention in 2017) | Anti-hypertensives | No |
| 5 | M | 69 | Allergic to penicillin | No | No |
| 6 | M | 60 | GERD | Proton pump inhibitor | No |
| 7 | F | 59 | Uterus cancer (2016), allergic to penicillin | Levotiroxine, benzodiazepine. | Ex (7 months) |
| 8 | F | 67 | Heart failure, hypertension, dyslipidemia, polyarhtrosis, osteoporosis, lung cancer (in 2000 subdued to surgical intervention and chemo-radiotherapy), thyroid nodules, hepatomegaly, AMI in 2002. Pace maker since 2004, OCBP. Allergic to penicillin, NSAIDS, fluoroquinolones and triciclic antidepressants. | Anticoagulants (Coumadin), bronchodilator, anti-hypertensives, diuretics, benzodiazepines, Proton pump inhibitor, digossin, anticholesterolemics, antilipidemics, antidepressants |
No |
| 9 | M | 41 | None | No | Yes (10 /die for 20 years) |
| 10 | F | 67 | Autoimmune hypothiroidism, breast cancer | Iodium | No |
| 11 | F | 67 | Autoimmune hypothiroidism | Iodium | No |
| 12 | F | 66 | None | None | Yes (10) |
| 13 | M | 75 | None | None | No |
| 14 | F | 66 | Osteoporosis | Colecalciferol | No |
| 15 | F | 69 | Thyroid Cancer, Hypertension, hypercolesterolemia | Levothyroxine, Anti-hypertensives Anticholesterolemics | No |
| 16 | F | 69 | Thyroid Cancer, hypertension, hypercolesterolemia | Levothyroxine, anti-hypertensives anticholesterolemics | No |
| 17 | M | 70 | Diabetes, hypercolesterolemia, gout, prostate cancer (2018) | Proton Pump Inhibitor, anti-hypertensive, Antiaggregants, Oral antidiabetics, Finasteride | No |
| 18 | M | 70 | Diabetes, hypercolesterolemia, gout, Prostate Cancer (2018) | Proton pump inhibitor, anti-hypertensive, antiaggregants, oral antidiabetics, finasteride | No |
| 19 | M | 86 | Benign prostate hypertrophia, chronic renal failure, atopic dermatitis eczema, hypertension. | Silodosin, anti-hypertensives, beta-blockers | No |
| 20 | M | 67 | Diabetes, parkinson’s disease, hypertension, hypercolesterolemia | Antidiabetics, diuretics, selegilin | Yes (20) |
| 21 | M | 67 | Diabetes, parkinson’s disease, hypertension, hypercolesterolemia | Antidiabetics, diuretics, selegilin | Yes (20) |
| 22 | M | 80 | Myocardic infarctuation (2018) | Antiaggregant, statins,collyrium | No |
| 23 | F | 63 | Thyroid cancer, hypertension, hypercolesterolemia | Levotiroxine | No |
| 24 | F | 62 | Melanoma, allergic to cats | None | No (ex 10 years) |
| 25 | F | Lung cancer, diabetes, syderopenia, vasculopathy with vertebral collapse | Antidiabetics, Proton pump inhibitors, bronchidilator, steroids, Dibase, oxygen therapy. | No | |
| 26 | F | 78 | Hypertension | NSAID, anti-hypertensive, diuretic | Ex (18) |
| 27 | F | 59 | Hypothyroidism, Hypertension | Levotiroxin, anti-hypertensives, diuretic | No |
| 28 | F | 74 | Gouge, hypercolesterolemia, allergic to penicillin | Allopurinol, anticholesterolemics | No |
| 29 | M | 73 | Hypertension, ischemic stroke, hyperlipidemia, osteoporosis | Antiaggregants, antilipidemics, anti-hypertensives, benzodiazepines, colecalciferol | No |
| 30 | F | 71 | Ipercolesterolemica, emicarnica | Anticholesterolemics, triciclinc antidepressants, beta blockers | No |
| ID | Site | Reason for intervention | Dimension (mm) | Technique | Pain | Bleeding | Scar | Side effects |
|---|---|---|---|---|---|---|---|---|
| 1 | Lower Lip | Aesthetic | 8 | LFD | NO | NO | NO | |
| 2 | Left Cheek | Bleeding | 10 | LFD | NO | NO | NO | Slight tingling immediately after application |
| 3 | Lower Lip | Bleeding | 3 | LFD | NO | NO | NO | |
| 4 | Left Cheek | Bleeding | 15 | LFD | NO | NO | NO | |
| 5 | Tongue | Clutter | 11 | LFD | NO | NO | NO | |
| 6 | Tongue | Clutter | 6 | LFD | YES | NO | YES | Slight visible scar, without pain or retraction |
| 7 | Left Cheek | Bleeding | 12 | LFD | NO | NO | NO | |
| 8 | Lower Lip | Bleeding | 20 | LFD | NO | SI | NO | Slight bleeding the day of the intervention |
| 9 | Left Cheek | Bleeding | 8 | LFD | NO | NO | NO | |
| 10 | Lower Lip | Aesthetic | 8 | LFD | NO | NO | NO | |
| 11 | Lower Lip | Clutter | 25 | LFD (2) | NO | NO | NO | |
| 12 | Lower Lip | Aesthetic | 3 | LFD | No | No | No | |
| 13 | Tongue dorsum | Bleeding | 3 | LFD | NO | NO | NO | Slight tingling the day of the intervention |
| 14 | Lower Lip | Aesthetic | 10 | LFD | NO | NO | NO | |
| 15 | Lower Lip | Aesthetic | 10 | LFD | NO | NO | NO | |
| 16 | Lower Lip | Aesthetic | 5 | LFD | NO | NO | NO | |
| 17 | Gingiva | Clutter | 15 | LFD | NO | NO | NO | |
| 18 | Lower Lip | Bleeding | 10 | LFD | NO | NO | NO | |
| 19 | Lower Lip | Aesthetic | 5 | LFD | NO | NO | NO | |
| 20 | Lower Lip | Aesthetic | 2 | LFD | NO | NO | NO | |
| 21 | Upper Lip | Aesthetic | 5 | LFD | NO | NO | NO | |
| 22 | Gingiva | Bleeding | 15 | LFD | NO | NO | NO | Ulcer after treatment |
| 23 | Lower Lip | Aesthetic | 4 | LFD | NO | NO | NO | |
| 24 | Lower Lip | Aesthetic | 7 | LFD | NO | NO | NO | |
| 25 | Palate | Bleeding | 8 | LFD | NO | NO | SI | |
| 26 | Lower Lip | Bleeding | 6 | LFD | NO | NO | NO | |
| 27 | Tongue Dorsum | Clutter | 3 | LFD | NO | NO | NO | |
| 28 | Lower Lip | Bleeding | 6 | LFD | NO | NO | NO | |
| 29 | Lower Lip | Bleeding | 3 | LFD | NO | NO | NO | |
| 30 | Lower Lip | Clutter | 4 | LFD | NO | NO | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




