Submitted:
25 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Staining and Light Microscopy
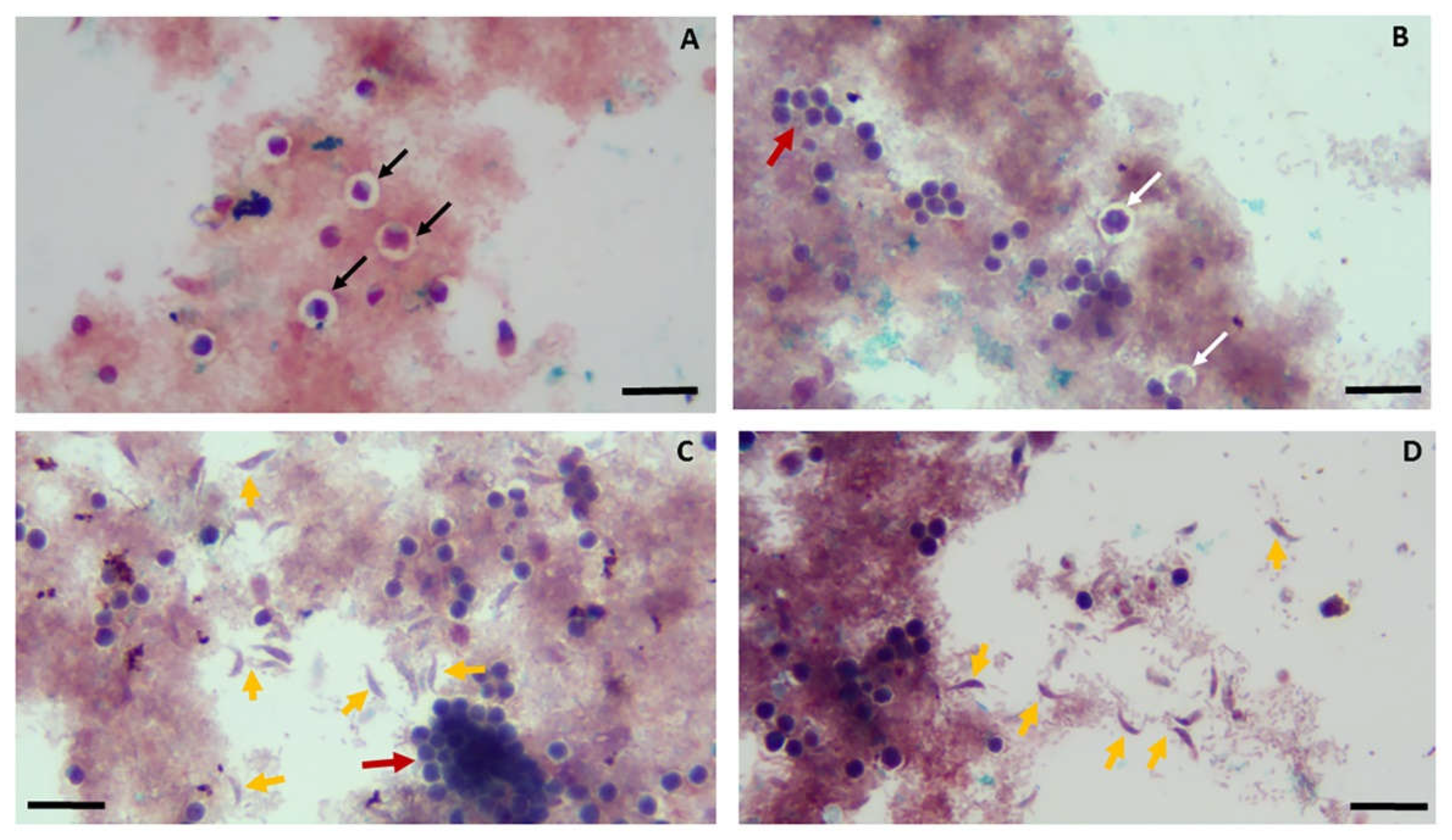
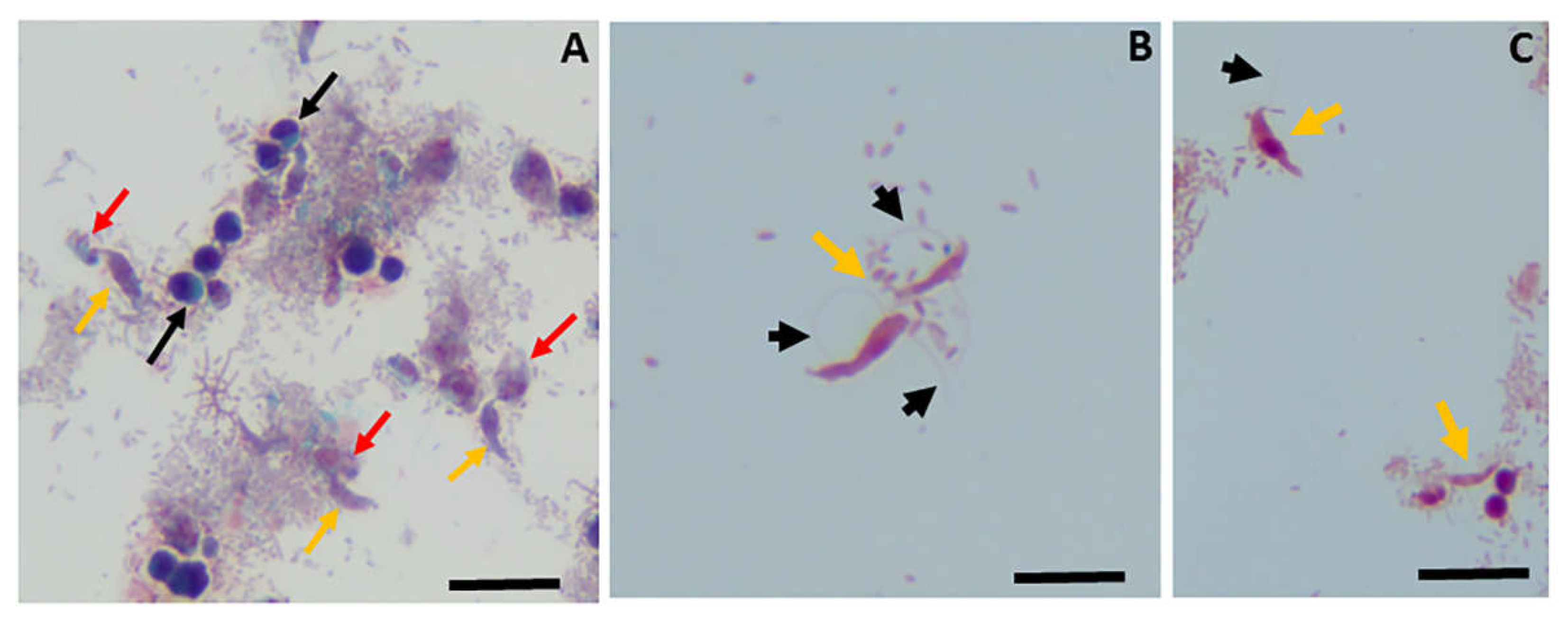
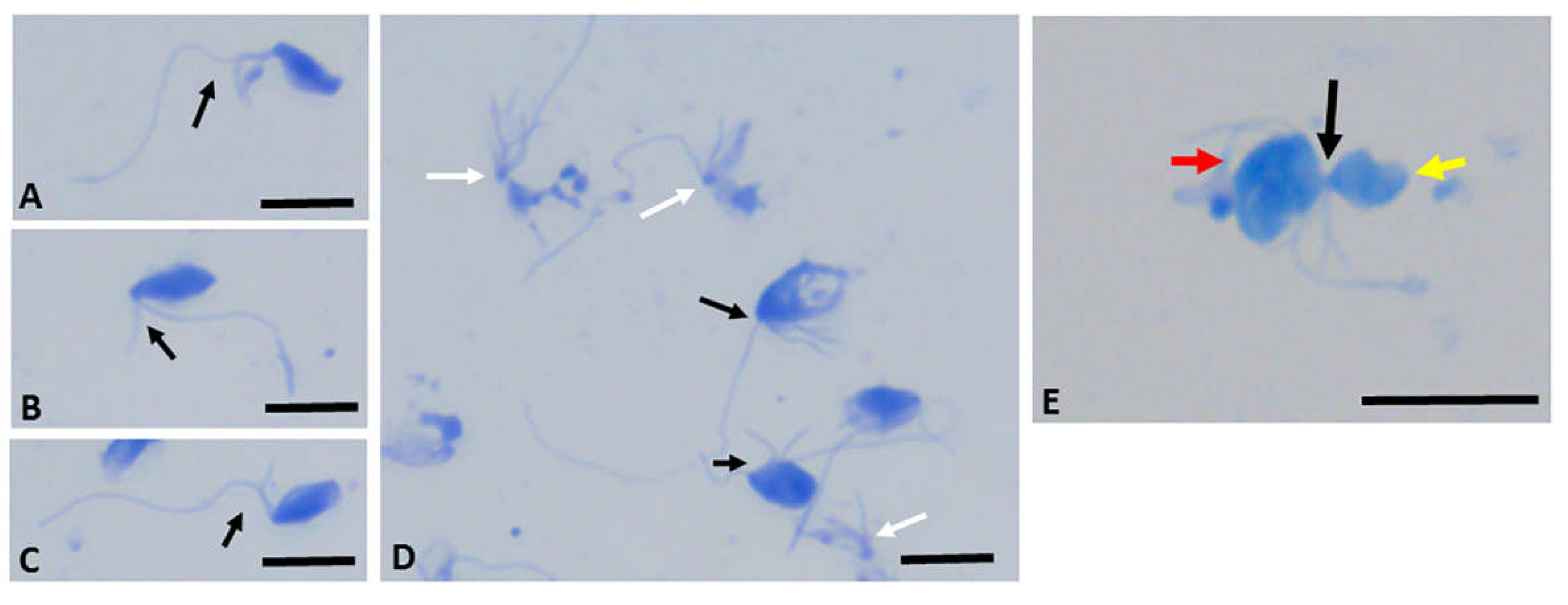
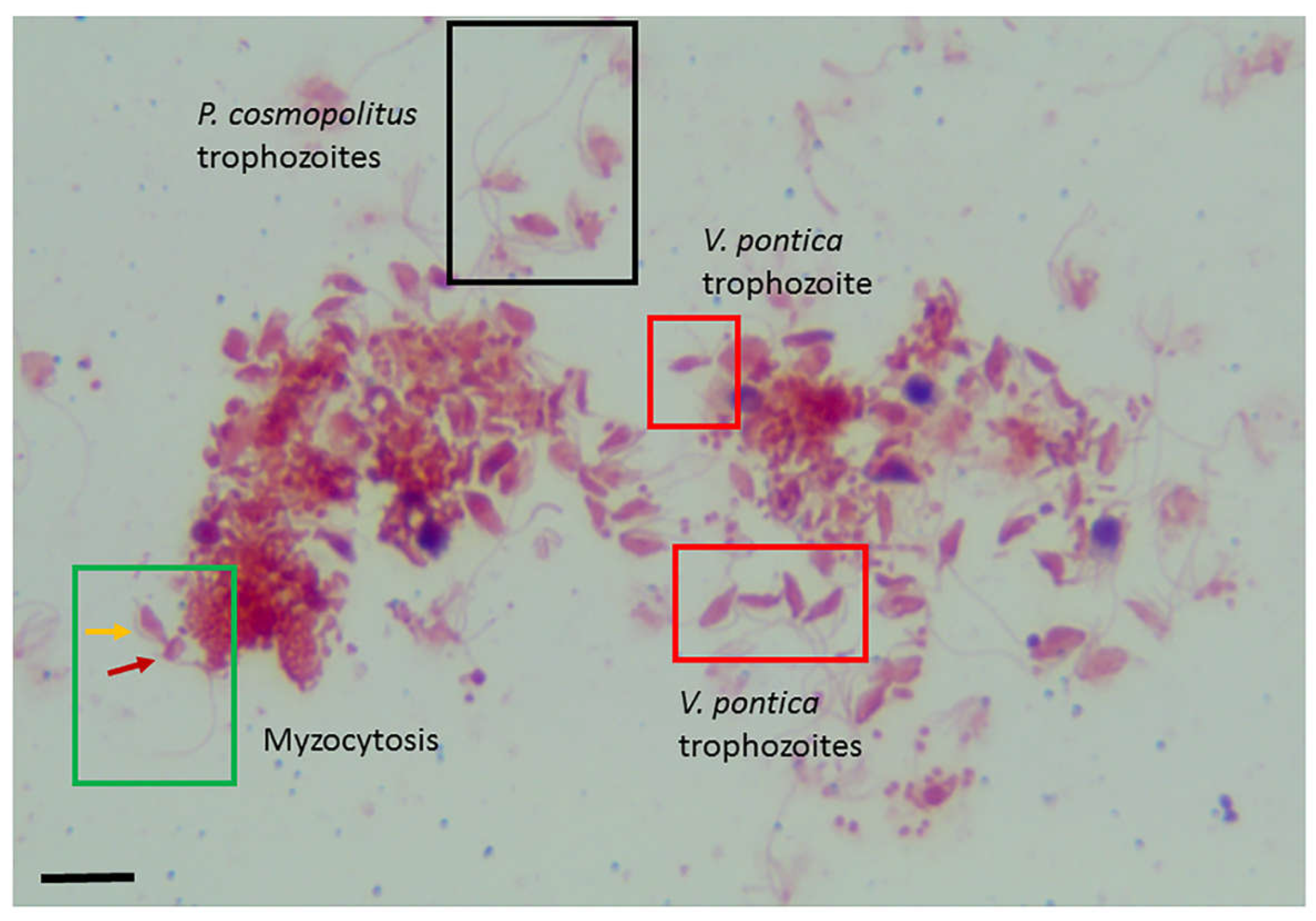
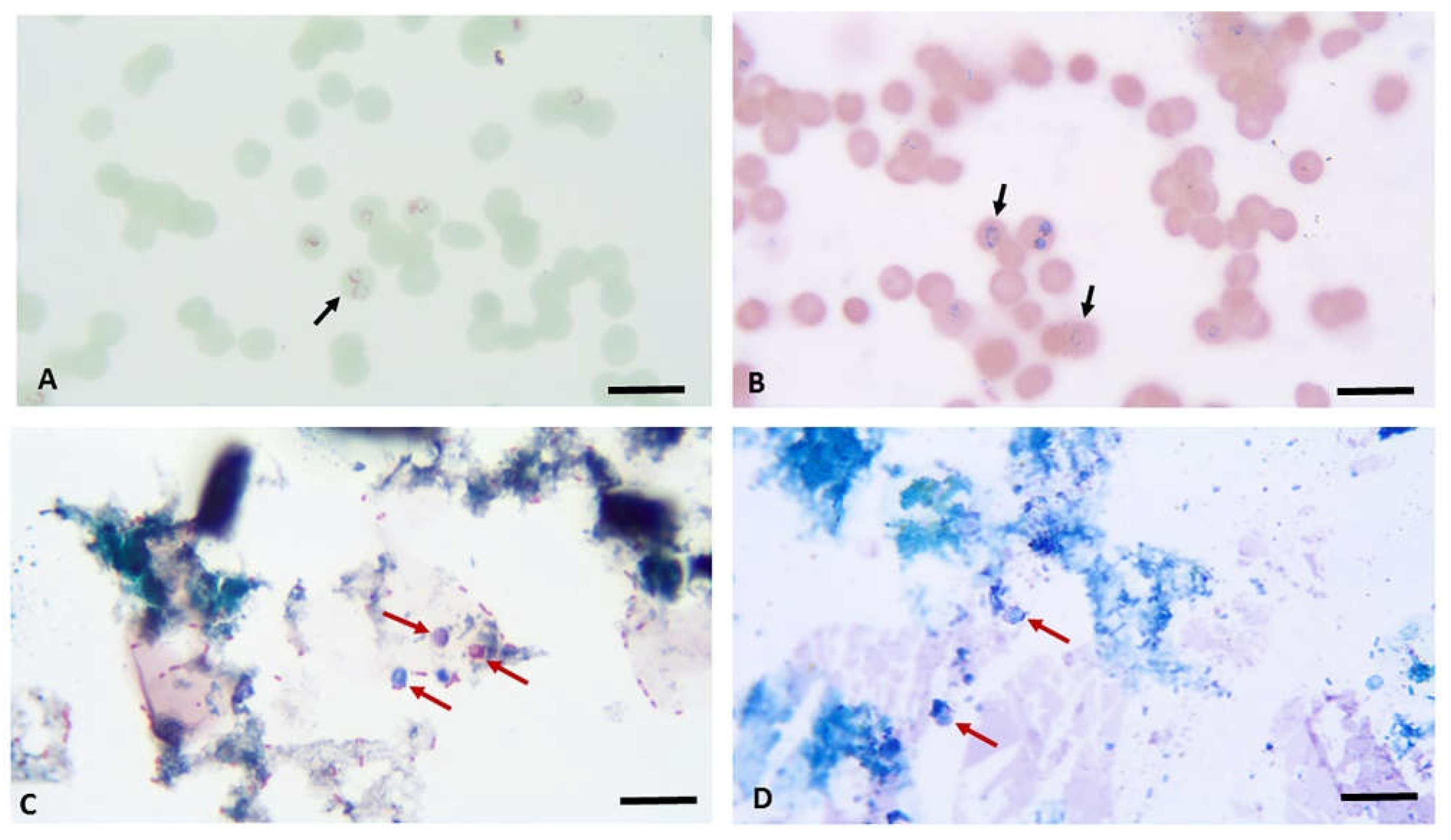
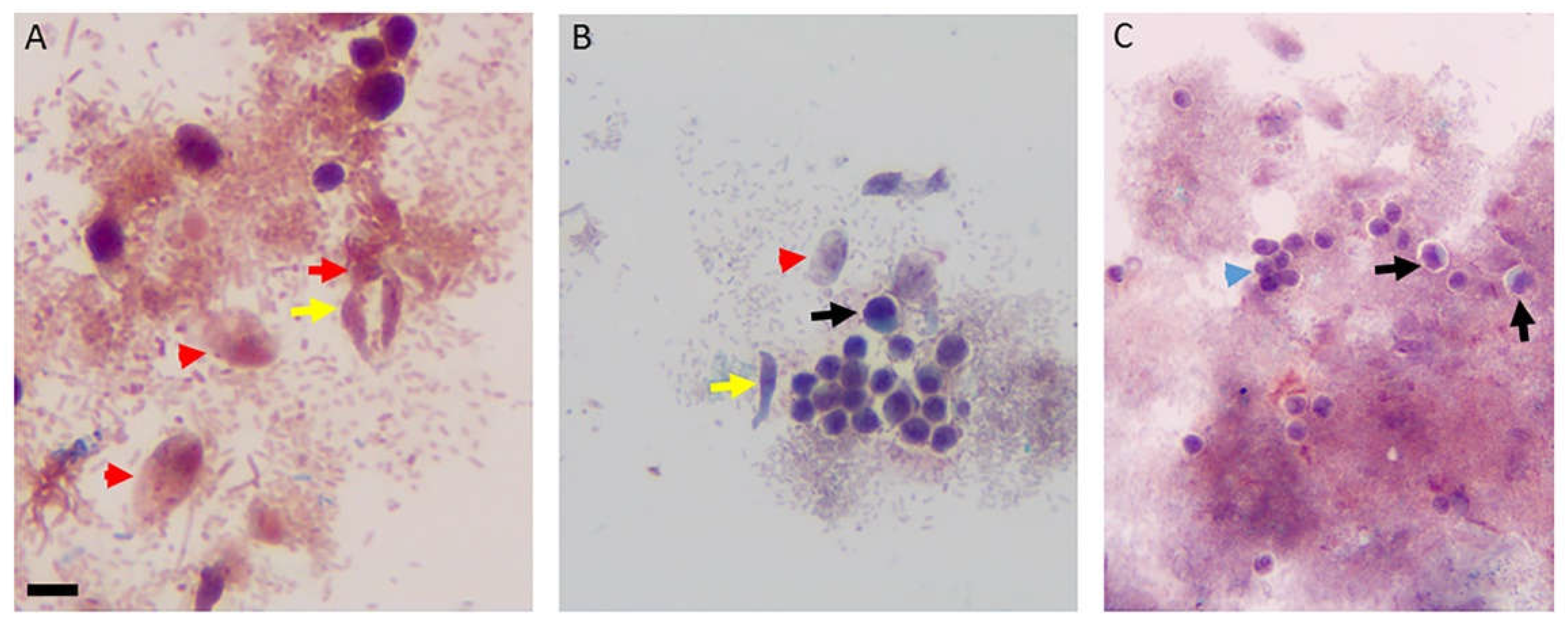
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuvardina, O.N.; Leander, B.S.; Aleshin, V.V.; Myl’nikov, A.P.; Keeling, P.J.; Simdyanov, T.G. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J Eukaryot Microbiol. 2002, 49:498-504. [CrossRef]
- Gile, G.H.; Slamovits, C.H. Transcriptomic analysis reveals evidence for a cryptic plastid in the colpodellid Voromonas pontica, a close relative of chromerids and apicomplexan parasites. PLoS One, 2014, 9(5):e96258. [CrossRef]
- Olmo, J.L.; Esteban, G.F.; Finlay, B.J. New records of the ectoparasitic flagellate Colpodella gonderi on non-Colpoda ciliates. J Int Microbiol, 2011, 14:207-211. [CrossRef]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. Nov.). Eur J Protistol, 2004, 40:185-212. [CrossRef]
- Simpson, A.G.B.; Patterson, D.J. Ultrastructure and identification of the predatory flagellate Colpodella pugnax Cienkowski (Apicomplexa) with a description of Colpodella turpis n. sp. and a review of the genus. Syst Parasitol, 1996, 33:187-198.
- Brugerolle, G. Colpodella vorax: Ultrastructure, predation, life-cycle, mitosis, and phylogenetic relationships. Europ. J. Protistol., 2002, 38:113-125. [CrossRef]
- Mylnikov, A.P.; Mylnikova, Z.M. Feeding spectra and pseudoconoid structure in predatory alveolate flagellates. Inland Water Biol. 2008, 1:210-216. [CrossRef]
- Sam-Yellowe, T.Y.; Fujioka, H.; Peterson, J.W. Ultrastructure of Myzocytosis and Cyst Formation, and the Role of Actin in Tubular Tether Formation in Colpodella sp. (ATCC 50594). Pathogens, 2022, 11:455.
- Mylnikov, A.P. Ultrastructure and phylogeny of colpodellids (Colpodellida, Alveolata). Biol Bulletin, 2009, 36:582-590. [CrossRef]
- Yuan, C.L.; Keeling, P.J.; Krause, P.J. et al. Colpodella spp.–like Parasite Infection in Woman, China. Emerg Infect Dis. 2012, 18:125-127.
- Jiang, J-F.; Jiang, R-R.; Chang, Q-C.; Zheng, Y-C.; Jiang, B-G.; Sun, Y.; Jia, N.; Wei, R.; Bo, H-B.; Huo, Q-B.; Wang, H.; von Fricken, M. E.; Cao, W-C. Potential novel tick-borne Colpodella species parasite infection in patient with neurological symptoms. PLOS Negl. Trop. Dis., 2018, 12(8):e0006546. [CrossRef]
- Neculicioiu, V. S.; Colosi, I. A.; Toc, D. A.; Lesan, A.; Costache, C. When a ciliate meets a flagellate: A rare case of Colpoda spp. and Colpodella spp. isolated from the urine of a human patient. Case report and brief review of the literature. Biology (Basel) 2021 Jun; 10 (6):476.
- Squarre, D.; Nakamura, Y.; Hayashida, K.; Kawai, N.; Chambaro, H.; Namangala, B.; Sugimoto, C.; Yamagishi, J. Investigation of the piroplasm diversity circulating in wildlife and cattle of the greater Kafue ecosystem, Zambia. Parasit Vectors. 2020,13:599. [CrossRef]
- Solarz, W.; Najberek, K; Wilk-Wozniak, E.; Biedrzycka, A. Raccoons foster the spread of freshwater and terrestrial microorganisms-mammals as source of microbial eDNA. Divers. Distrib. 2020, 26 453-459. [CrossRef]
- Wheatley, M.A.; Shamoun, J.; Maggi, R.; Breitschwerdt, E.B.; Sommer, S.L.; Cullen, J.M.; Stowe, D.M. Eosinophilic pericardial effusion and pericarditis in a cat. JFMS Open Rep., 2023 9:20551169231213498. [CrossRef]
- Huggins, L. G.; Colella, V.; Koehler, A. V.; Schunack, B.; Traub, R. J. A multipronged next-generation sequencing metabarcoding approach unearths hyperdiverse and abundant dog pathogen communities in Cambodia. Transbound Emerg Dis. 2022, 69:1933-1950. [CrossRef]
- Qi, Y.; Wang, J.; Lu, N.; Qi, X.; Yang, C.; Liu, B.; Lu, Y.; Gu, Y.; Tan, W.; Zhu, C.; Ai, L.; Rao, J.; Mao, Y.; Yi, H.; Li, Y.; Yue, M. Potential novel Colpodella spp. (phylum Apicomplexa) and high prevalence of Colpodella spp. in goat-attached Haemaphysalis longicornis ticks in Shandong province, China. Ticks Tick Borne Dis. 2024, 15:102328. [CrossRef]
- Xu, M.; Hu, Y.; Qiu, H.; Wang, J.; Jiang, J. Colpodella sp. (Phylum Apicomplexa) Identified in Horses Shed Light on Its Potential Transmission and Zoonotic Pathogenicity. Front Microbiol. 2022, 13:857752. [CrossRef]
- Chiu, H.C.; Sun, X.; Bao, Y.; Fu, W.; Lin, K.; Chen, T.; Zheng, C.; Li, S.; Chen, W.; Huang, C. Molecular identification of Colpodella sp. of South China tiger Panthera tigris amoyensis (Hilzheimer) in the Meihua Mountains, Fujian, China. Folia Parasitol (Praha). 2022, 69:2022.019. [CrossRef]
- Hussein, S.; Li, X.; Bukharr, S. M.; Zhou, M.; Ahmad, S.; Amhad, S.; Javid, A.; Guan, C.; Hussain, A.; Ali, W.; Khalid, N,;Ahmad, U, Tian, L.; Hou, Z. Cross-genera amplification and identification of Colpodella sp. with Cryptosporidium primers in fecal samples of zoo felids from northeast China. Braz J Biol. 2021 Sep 6; 83:e247181.doi.10.1590/1519-6984.247181 eCollection 2021. [CrossRef]
- Neupane, S.; Saski, C.; Nayduch, D. House fly larval grazing alters dairy cattle manure microbial communities. BMC Microbiol. 2021, 21:346. [CrossRef]
- Phetkarl, T.; Fungwithaya, P.; Udompornprasith, S.; Amendt, J. Sontigun, N. Preliminary study on prevalence of hemoprotozoan parasites harbored by Stomoxys (Diptera: Muscidae) and tabanid flies (Diptera: Tabanidae) in horse farms in Nakhon Si Thammarat province, Southern Thailand. Vet World. 2023, 16:2128-2134. [CrossRef]
- Getty, T.A.; Peterson, J.W.; Fujioka, H.; Walsh, A.M.; Sam-Yellowe, T.Y. Colpodella sp. (ATCC 50594) Life Cycle: Myzocytosis and Possible Links to the Origin of Intracellular Parasitism. Trop Med Infect Dis., 2021, 6:127. [CrossRef]
- Sam-Yellowe, T.Y.; Getty, T.A.; Addepalli, K.; Walsh, A.M.; Williams-Medina, A.R.; Fujioka, H.; Peterson, J.W. Novel life cycle stages of Colpodella sp. (Apicomplexa) identified using Sam-Yellowe’s trichrome stains and confocal and electron microscopy. Int Microbiol., 2022, 25:669-678.
- Sam-Yellowe, T.Y.; Addepalli, K.; Yadavalli, R.; Peterson, J.W. New trichrome stains identify cysts of Colpodella sp. (Apicomplexa) and Bodo caudatus. J Int Microbiol, 2019, 23:303-311. [CrossRef]
- Yadavalli, R.; Sam-Yellowe, T.Y. Developmental stages identified in the trophozoite of the free-living Alveolate flagellate Colpodella sp. (Apicomplexa). J Int Microbiol, 2017, 20:178-183.
- Sam-Yellowe, T.Y.; Yadavalli, R. Voromonas pontica Identified by Giemsa Staining and Anti-RhopH3 Protein Reactivity. J Microbiol Modern Tech, 2019, 4(1): 103.
- Yadavalli, R.; Peterson, J.W.; Drazba, J.A.; Sam-Yellowe, T.Y. Trafficking and Association of Plasmodium falciparum MC-2TM with the Maurer’s Clefts. Pathogens, 2021, 10:431. [CrossRef]
- Sam-Yellowe, T. Y.; Asraf, M. M.; Peterson, J. W.; Fujioka, H. Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594. Microorganisms. 2023, 11:1945.
- Matsimbe, A. M.; Magaia, V.; Sanchez, G. S.; Neves, L.; Noormahomed, E.; Antunes, S.; Domingos, A. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91-102. [CrossRef]
- Zhao, G.P., Wang, Y.X.; Fan ZW, Ji Y, Liu MJ, Zhang WH, Li XL, Zhou SX, Li H, Liang S, Liu W, Yang Y, Fang LQ. Mapping ticks and tick-borne pathogens in China. Nat Commun. 2021 Feb 17;12(1):1075.
- Wu, S. Meng, J.; Yu, F.; Zhou, C.; Yang, B.; Chen, X.; Yang, G.; Sun, Y.; Cao, W.; Jiang, J.; Wu, J.; Zhan, L. Molecular epidemiological investigation of piroplasms carried by pet cats and dogs in an animal hospital in Guiyang, China. Front Microbiol. 2023,14:1266583.
- El-Sayed, N.M.; Hikal, W.M. Several staining techniques to enhance the visibility of Acanthamoeba cysts. Parasitol Res, 2015, 114:823-830. [CrossRef]

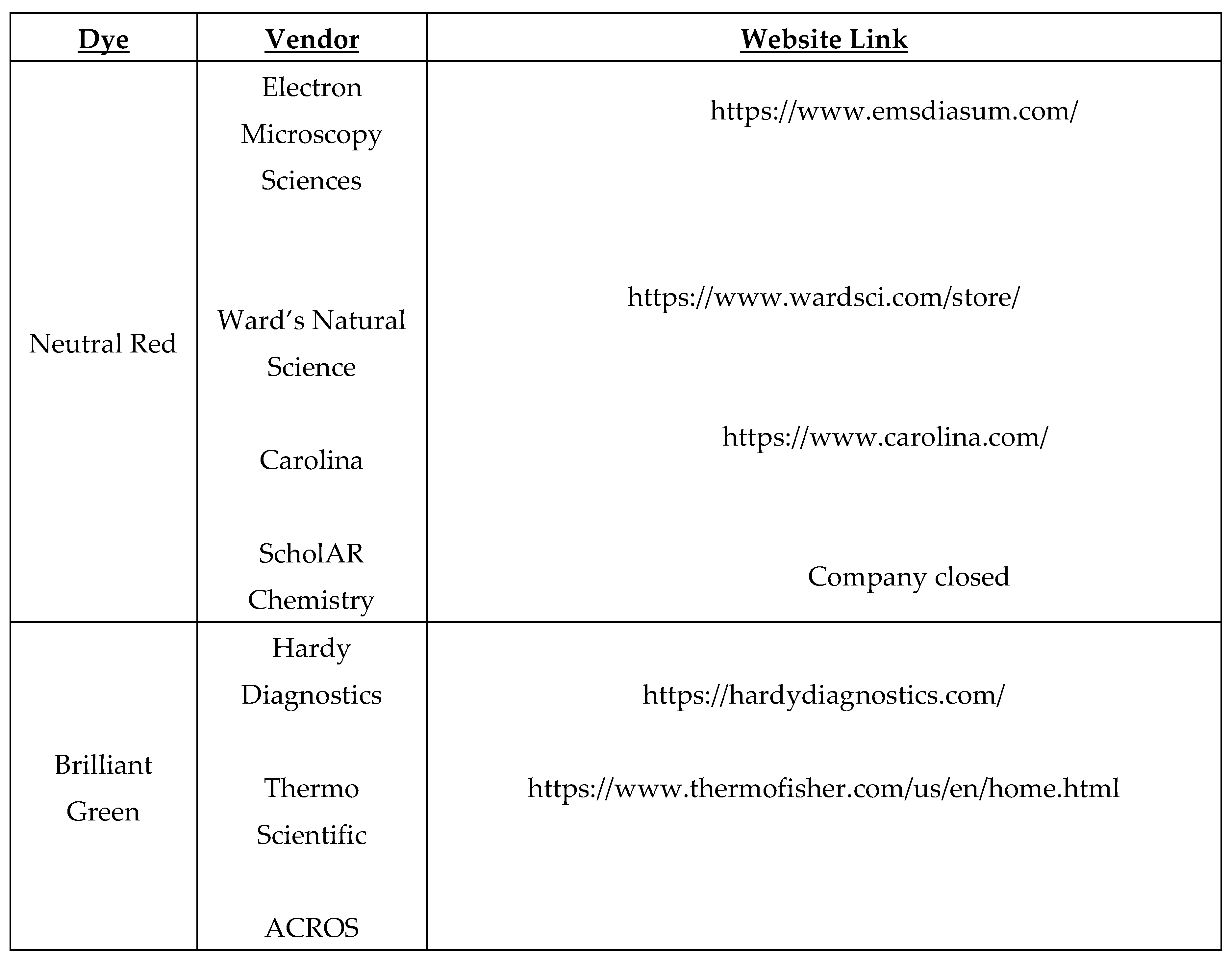
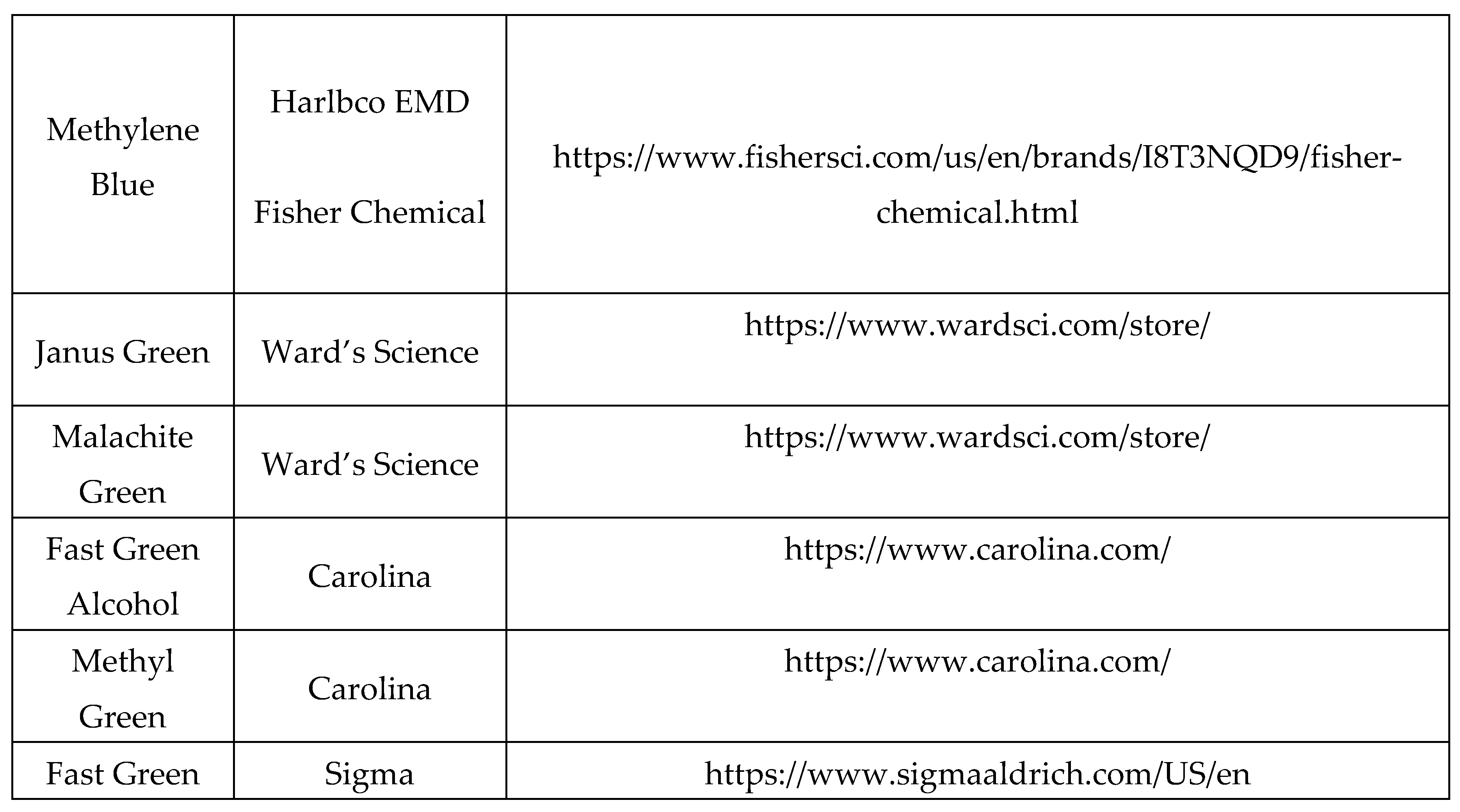
| 1 Minute | 5 Minutes | 1 Minute |
|---|---|---|
| Fisher Chemical 0.3% Methylene Blue | ACROS 1% Brilliant Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | ACROS 1% Brilliant Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | ACROS 1% Brilliant Green | Ward’s Science 1% Neutral Red |
| EMD 0.3% Methylene Blue | ACROS 1% Brilliant Green | Ward’s Science 1% Neutral Red |
| EMD 0.3% Methylene Blue | ACROS 1% Brilliant Green | ScholAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | ACROS 1% Brilliant Green | Carolina 1% Neutral Red |
| EMD 0.3% Methylene Blue | Thermo Scientific 1% Brilliant Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Thermo Scientific 1% Brilliant Green | ScholAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | Hardy Diagnostic 1% Brilliant Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Hardy Diagnostic 1% Brilliant Green | SchoAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | Sigma 1% Fast Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Sigma 1% Fast Green | ScholAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | Carolina 1% Methyl Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Carolina 1% Methyl Green | ScholAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | Ward’s Science 1% Malachite Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Ward’s Science 1% Janus Green | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Ward’s Science 1% Janus Green | ScholAR Chemistry 1% Neutral Red |
| EMD 0.3% Methylene Blue | Carolina 0.5% Fast Green Alcohol | EMS 1% Neutral Red |
| EMD 0.3% Methylene Blue | Carolina 0.5% Fast Green Alcohol | ScholAR Chemistry 1% Neutral Red |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





