1. Introduction
The Tissue engineering (TE) is a multidisciplinary field that integrates life sciences and engineering to develop biological substitutes that replace, repair, and enhance tissue functions [
1]. Central to TE is the triad of cells, scaffold and growth factors. Cells play a key role in synthesizing the matrix of the new tissue, while scaffolds provide an optimal environment for cell proliferation or differentiation, and growth factors aid in the for-mation of new tissue [
2]. A scaffold is a three-dimensional, porous structure that sup-ports the growth, proliferation, and interconnection of cells. It also facilitates the effi-cient transportation of nutrients, oxygen, and waste metabolites [
3,
4]. The fabrication of the scaffold is crucial for the success of implants. To achieve this, careful selection of materials and manufacturing techniques tailored to specific requirements is necessary [
5,
6,
7,
8,
9]. This involves considering factors such as the shape, size, and properties of the scaffold. In particular, the materials used must be biocompatible, biodegradable with an adequate degradation time and not release toxic degradation products [
3]. Furthermore, during the design phase of a scaffold, special attention must be paid to the mechanical and physical properties of the porous matrix [
4]. Once the most suitable material has been selected, the fabrication methods used to make scaffolds are varied [
5,
8].
Thermally Induced Phase Separation is an advanced manufacturing process known for its efficiency in producing a well-connected polymer network [
9]. The process is based on a homogeneous polymer solution whose solubility equilibrium varies with temperature [
10]. On cooling, phase separation occurs, leading to the formation of pores and the growth of a highly porous and interconnected structure. Precise control is achieved through carefully designed protocols that control the temperature and time during these phases [
10,
11]. Known for its versatility, this approach is characterized by its simplicity, speed and adaptability, particularly in producing polymeric structures with different pore sizes and high interconnectivity [
11]. Poly-L-lactic acid is a biode-gradable thermoplastic polymer derived from lactic acid [
12]. It is produced by ring-opening polymerization of lactide monomers and is particularly suited to the TIPS method.
PLLA scaffolds offer tunable mechanical properties and biodegradability, provid-ing critical mechanical support to facilitate tissue regeneration [
13]. However, despite the use of biocompatible materials, scaffold implantation inevitably triggers an immune response, leading to inflammation and potential scarring that could compromise the success of the implant [
14]. One of the key features of the inflammatory response is a phenomenon known as oxidative stress [
14,
15]. Oxidative stress is manifested by an overabundance of reactive oxygen species (ROS), which are characterized by an un-paired electron in their outermost orbital, making them unstable and capable of causing cellular damage by reacting readily with other molecules [
17]. The use of natural anti-oxidants, thanks to their scavenging properties, allows the neutralization of excessive ROS, restoring the correct redox balance and reducing the inflammatory response [
17,
19].
Rosmarinic acid, is a polyphenolic constituent found in many plants such as La-miaceae family and the subfamily Nepetoideae [
20,
22]. As documented, it is the ester of caffeic acid and 3,4-dihydroxyphenyllactic acid and it has various biological effects, in-cluding antioxidant, anti-inflammatory, antibacterial, and anticancer properties, sup-ported by numerous in vivo and in vitro studies [
21,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. Moreover, rosmarinic acid demonstrates lipophilic characteristics, rendering it highly soluble in several organic solvents such as ethanol (EtOH), dimethylformamide (DMF), and dimethyl sulfoxide (DMSO), while displaying poor solubility in water. The anti-inflammatory actions of RA are believed to stem from its scavenging abilities, inhibition of neutrophil activity, suppression of metalloproteinase-9 (MMP-9) activity, and modulation of the NF-jB pathway [
34]. These processes suggest that RA may have potential as a treatment for inflammatory conditions through its ability to reduce inflammation and prevent tissue damage.
This information suggests that it is worth exploring the therapeutic use of RA in the development of treatments to minimize the inflammatory process caused by scaffold implantation. Of particular importance is the striking lack of studies proposing com-posite polymeric structures incorporating RA. To our knowledge, information about polymeric scaffolds doped with natural antioxidants is limited. Previous research by Chen et al. demonstrated the possibility of incorporating other antioxidant molecules into 3D porous matrices for tissue regeneration. In their study, a 3D-printed PLLA scaffold was coated with a layer of polydopamine (PD) and then functionalized with varying concentrations of quercetin (Qu). This resulted in Qu/PD-PLLA scaffolds that showed potential for bone repair, as demonstrated by their application in MC3T3-E1 cells. Furthermore, the study conducted by Lihao et al. further underscores the potential of this scaffold and antioxidant molecule approach. Utilizing 3D printing technology, they created a porous SAB-SA-Gel composite scaffold by incorporating salvianolic acid (SAB) into a matrix of sodium alginate (SA) and gelatin (Gel). This scaffold exhibited antioxidant, anti-inflammatory, and pro-angiogenic properties, reducing the expression of inflammatory factors while enhancing tissue regeneration and collagen deposition, thereby promoting diabetic wound healing. This paper aims to explore the possibility of manufacturing composite PLLA-RA scaffolds. A protocol was designed to include var-ying amounts of RA in PLLA scaffolds produced through the TIPS technique. The scaffolds were characterized using a range of analyses, such as gravimetric, microscopic, and spectroscopic analyses, to evaluate their morphological, thermal, and surface properties. In addition, contact angle tests were conducted to determine their hydro-philicity, providing a comprehensive assessment of their potential for tissue engineer-ing applications.
2. Materials and Methods
2.1. Materials
Poly-L-lactic-acid (PLLA, Resomer, L 209 S, Evonik Industries, Essen, Germany) and 1,4-dioxane (Sigma-Aldrich, Munich, Germany) were used for scaffold preparation. Rosmarinic acid (RA, 96% pure, Sigma Aldrich) and Ethanol absolute anhydrous (Carlo Erba Reagents) were used for ethanol/RA solution preparation.
2.2. Scaffold’s Preparation
PLLA scaffolds were prepared according to a previous work of the authors Lombardo et al. Briefly, the polymer was dissolved in 1,4-dioxane at a concentration of 4% (wt/wt) at a temperature of 120°C. Distilled water was then added to obtain a final dioxane/water weight/weight ratio of 87/13. 5 mL of the solution, kept at 60 °C, were poured into a cylindrical high-density polyethylene sample holder (inner diameter 17.6 mm and height 35.7 mm). The sample holder was then immersed in a thermostatic water bath at 20 °C (demixing temperature) for 15 minutes (demixing time). At the same time, a cylindrical polytetrafluoroethylene (PTFE) coating, used to obtain a homogeneous temperature distribution in the sample holder, was pre-cooled to -20°C. Finally, the sample holder was inserted into the PTFE cylinder and the system was rapidly quenched by immersion in an ethyl alcohol bath at a temperature of -20°C for at least 15 minutes to stop the demixing process and freeze the structure obtained. The obtained samples were washed in deionized water and dried at 60°C to remove any remaining traces of solvent completely. The cylindrical scaffolds were then first cut transversely into 2 mm discs and finally shaped into cylinders of 4 mm diameter and 2 mm height using a biopsy punch.
2.3. Rosmarinic Acid Additivation
The samples were first weighed using an ABT220-D5M (Kern) analytical balance.
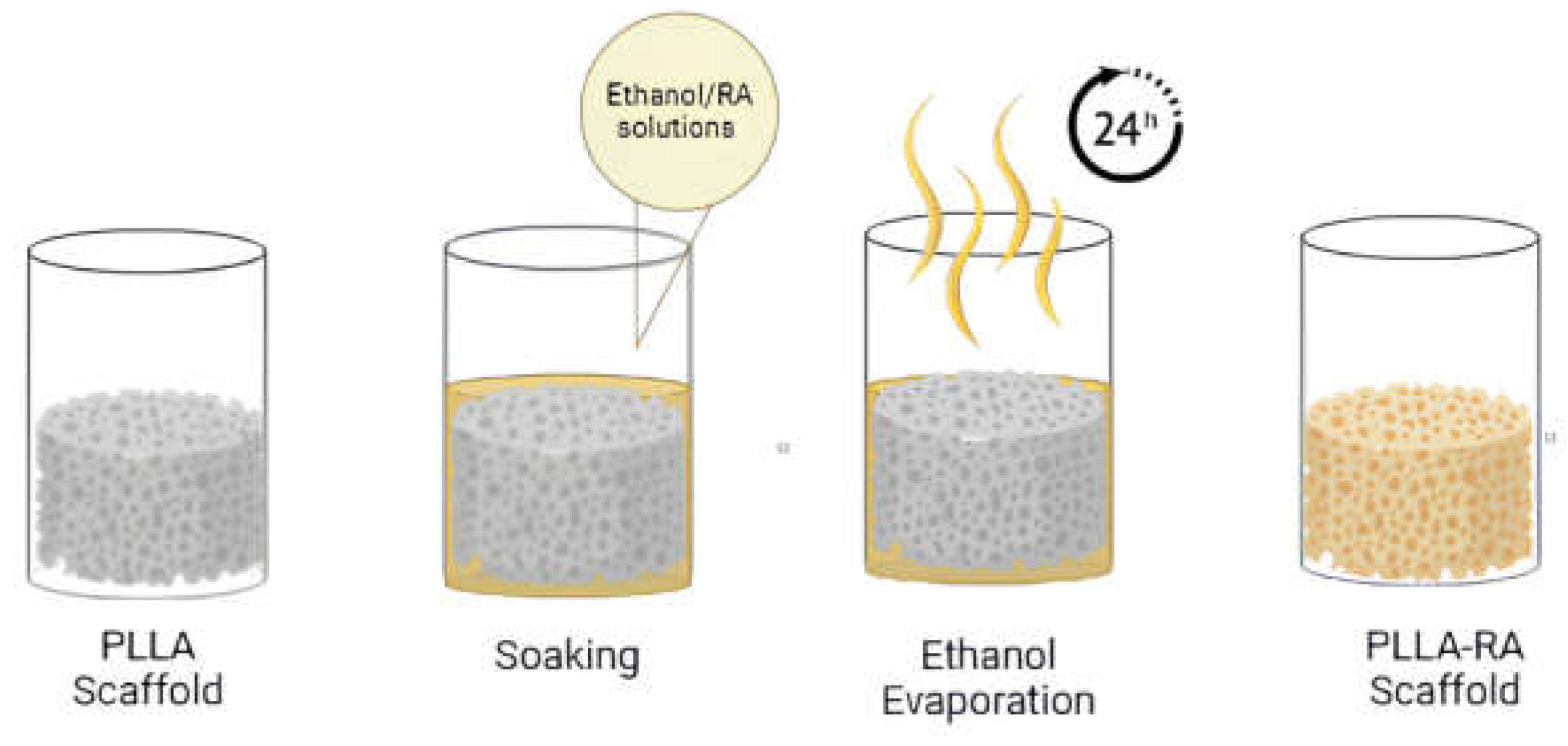
Subsequently, the weighed scaffolds were placed into a 96 multiwell plate and soaked in pure ethanol under vacuum for 2 minutes, to ensure complete penetration of the solvent into the pores. Once the entire surface was penetrated, the ethanol was removed. For the addition of RA, two ethanol/RA wt/wt solutions were prepared, one containing 2% wt/wt RA and the other containing 4% wt/wt RA. Then, 200 microlitres of the solution were added to each well containing scaffolds. After evaporation of the ethanol (at least 24 hours), the dry samples were extracted from the well and reweighed. The procedure used is schematised in
Figure 1.
2.4. Characterization
The percentage of additivated RA with respect to total weight was calculated as follow:
Where WA is the weight of the sample after RA additivation process and WB is the initial weight of the sample.
The microstructure of the scaffold was observed by Scanning Electron Microscopy (SEM) using a Philips Quanta 200 F SEM at 10 kV. The external surfaces of the samples were visualized after a gold deposition (Sputtering Scancoat Six, Edwards) for 150 seconds.
Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) spectroscopy using a Spectrum One spectrometer from PerkinElmer, Waltham, MA, USA, was used to study the molecular deposition and surface structural characteristics of the material. This technique is used to investigate the vibrational modes and chemical bonds within the sample to be analysed. For each scaffold studied, ATR-FTIR analysis was performed on both the top and bottom surfaces to determine the presence of the RA molecule. A total of 16 scans were performed at 4 cm-1 resolution. The ATR-FTIR spectra presented have been carefully selected based on normalized results obtained from a minimum of three samples.
The samples were analyzed calorimetrically using a DSC Setaram 131evo. Pure PLLA, RA powder and additivated PLLA-RA samples and an RA film obtained via solvent casting were analyzed. Each sample was subjected to two heating scans. The samples were carefully weighed and placed in aluminum crucibles for analysis and the following thermal protocol was applied: first heating from 25°C to 220°C at 10°C/min, held at 220°C for 10 minutes, cooling to 50 °C at 10°C/min, held at 50 °C for 10 minutes and second heating from 25°C to 220°C at 10°C/min. Melting enthalpies and temperatures were determined using Calisto software.
The static contact angle test was performed using an FTA 1000 (First Ten Ångstroms, Cambridge, UK) instrument with distilled water (DW) as the liquid. Specifically, a drop of DW (~4 μL) was dropped onto the scaffold and images were taken 10 s after DW deposition.
3. Results
3.1. Gravimetrical Analysis
In this study, we used the solution deposition method starting with two solutions at 2% and 4% wt/wt, as reported in the Materials and Methods section.
Figure 2 shows the picture of pure PLLA and RA additivated samples. A clear change in the color of the scaffold can be attributed to the presence of RA in the samples.
In order to evaluate the presence of RA incorporated into the scaffold, 5 samples of each type were weighed dry before and after the deposition of RA. The obtained data are presented in
Table 1. A significant amount of biological molecules of RA are clearly incorporated into the polymeric structure, leading to a substantial increase in weight.
3.2. SEM Analysis
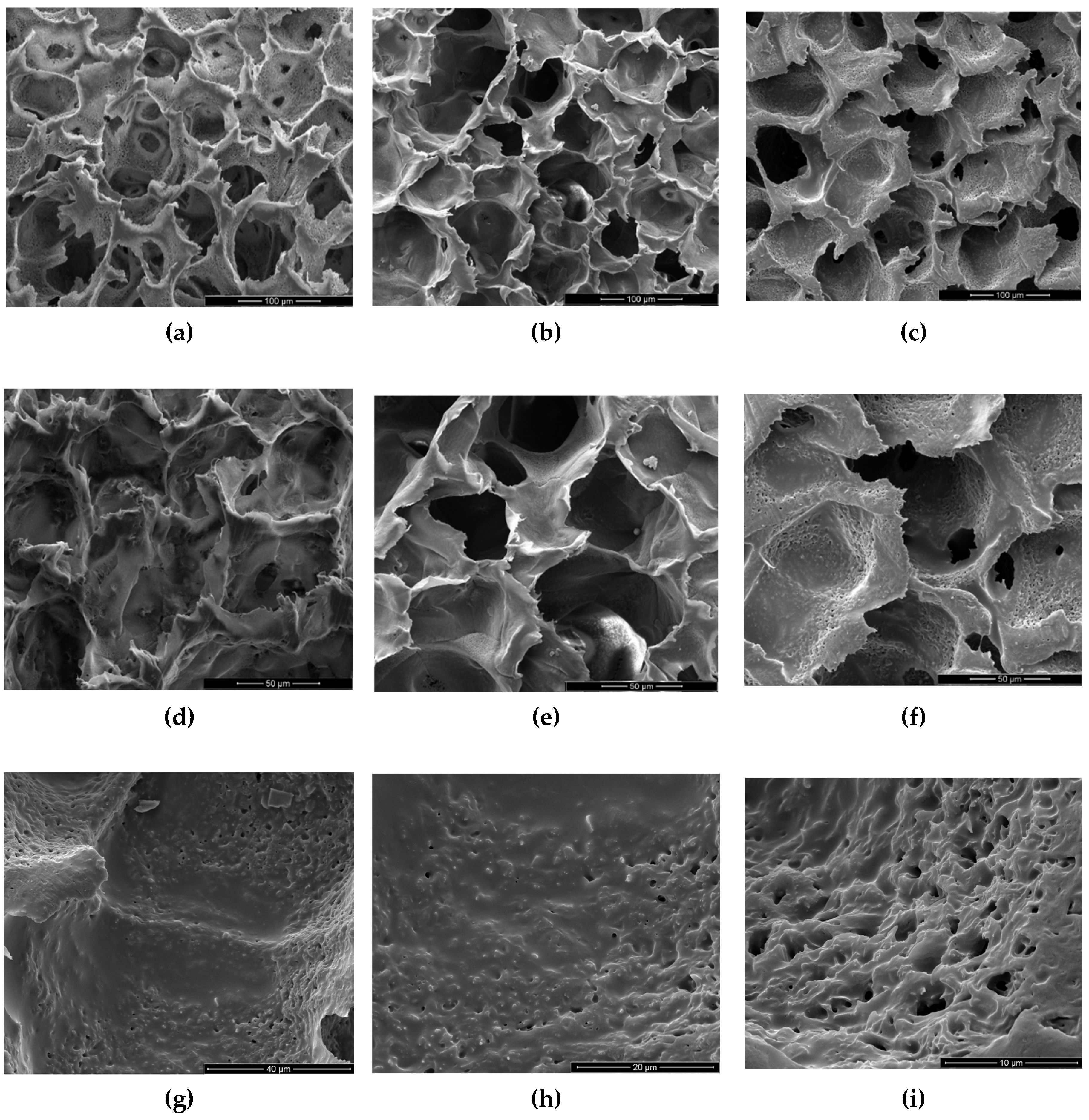
Scanning Electron Microscopy (SEM) is a powerful analytical technique widely utilized for examining the surface morphology and microstructure of scaffolds at a microscopic level. In this study, SEM was employed to investigate the morphology of samples.
Figure 3 (a-f) shows SEM images of three investigated scaffolds at different magnifications, while
Figure 3 (g-i) provides a closer look at the high magnification of a PLLA-RA 4% scaffold. As noticeable, the dimensions of pores could be estimated ranging from 50-70 μm with a good interconnectivity.
The images in
Figure 3a-f showed that the pores’ morphology remained unchanged despite the presence of RA. Moreover, the micrographs at high magnification of PLLA-RA 4% samples revealed the presence of an RA layer (see
Figure 3g-i) at the level of the pore’s surfaces. Additionally, the presence of the RA layer can be observed in
Figure 3e, although it was less pronounced.
3.3. Attenuated Total Reflectance Fourier Transform Infrared Analysis (ATR-FTIR)
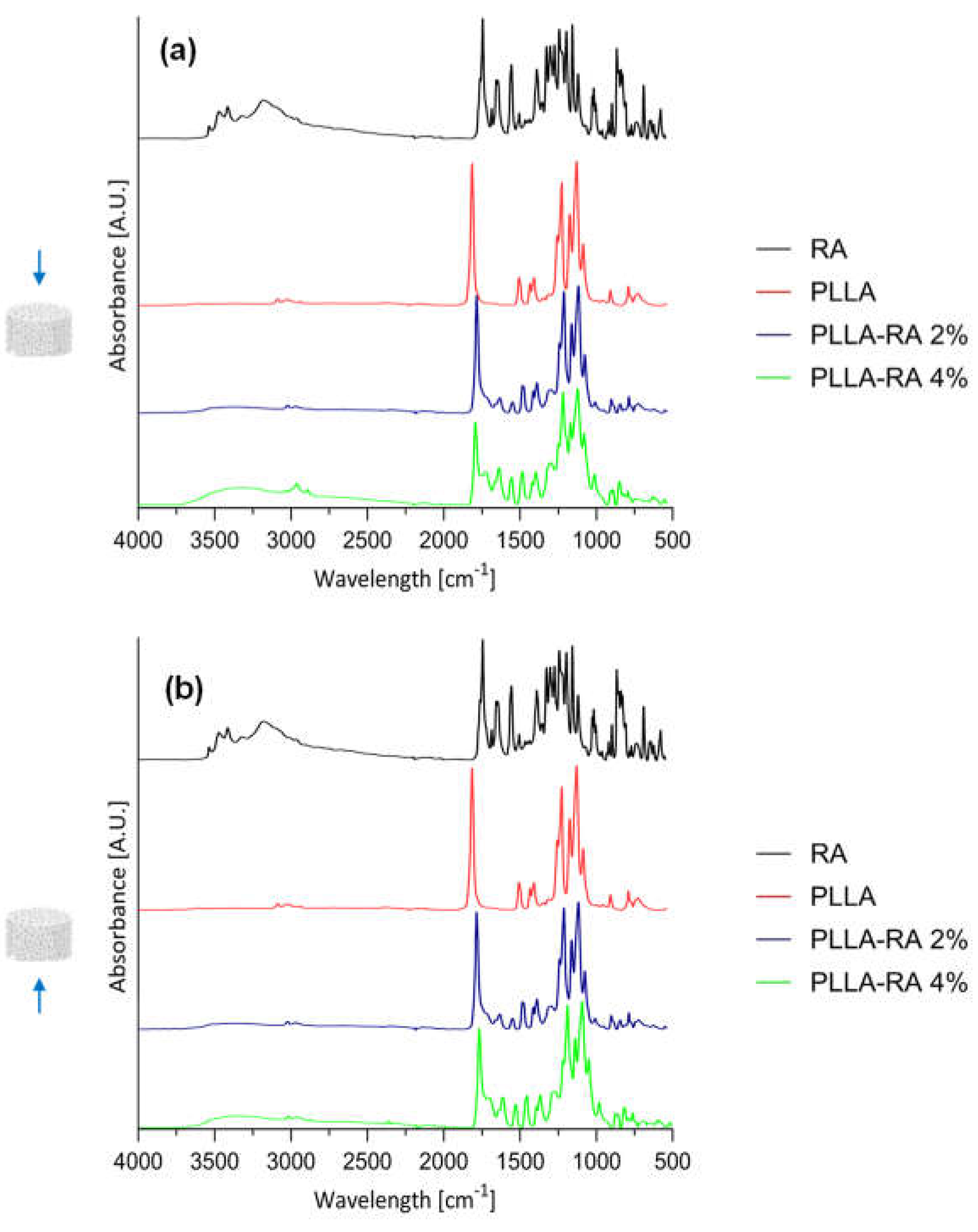
Results of ATR-FTIR analysis on RA powder and PLLA, PLLA-RA 2%, and PLLA-RA 4% scaffolds are shown in
Figure 4a-b for top and bottom surfaces.
In addition, according to the literature,
Table 2 contains the assignments of characteristic peaks of both PLLA [
37] and RA [
38]. As expected, the pure PLLA sample, on both top and bottom surfaces, showed no peaks in the spectral region between 3500 and 3000 cm
-1, while RA itself showed several peaks in this region related to phenolic -OH stretching, occurring at c.a. 3500 cm
-1, and C-H stretching, occurring at the frequencies above 3000 cm
-1. Further, within the range at 1700-1000 cm
-1, distinct peaks appeared in RA spectra, specifically, one at 1700 cm
-1 corresponding to the stretching vibration of >C=O, followed by peaks around 1605 and 1520 cm
-1 indicating stretching of the aromatic ring. In addition, two other signals appeared at 1360 cm
-1 and 1180 cm
-1 due to O-H and C-O stretching, respectively.
In the spectra of PLLA-RA 2% and PLLA-RA 4% samples, changes in peaks in the regions around 3500-3000 cm-1 and 1700-1000 cm-1 were observed. In particular, PLLA-RA 2% and PLLA-RA 4% showed a small shoulder around 3500-3000 cm-1. Furthermore, PLLA showed various intrinsic peaks in the range of 1700-1000 cm -1, and as noticeable, the spectra of PLLA-RA 2% and 4% showed more complex peaks in this region.
Interestingly, some specific characteristic peaks in PLLA shifted to lower frequencies due to the presence of RA molecules. Specifically, the -C-O- stretch at 1086 cm-1 in the spectrum of PLLA shifted to 1081 cm-1 in the spectrum of PLLA/RA 4%, and the -CH and CH3 stretches at 1386 cm-1 and 1456 cm-1, shifted to 1379 cm-1 and 1448 cm-1, respectively. Further, small shifts for the bands at ca. 3000 and 2945 cm-1 attributed to the stretching of -CH groups were also noticed. All these changes suggested that in the samples containing RA, the interactions between the RA biomolecules and PLLA scaffold structure occurred.
3.4. Differential Scanning Calorimetry (DSC) Analysis
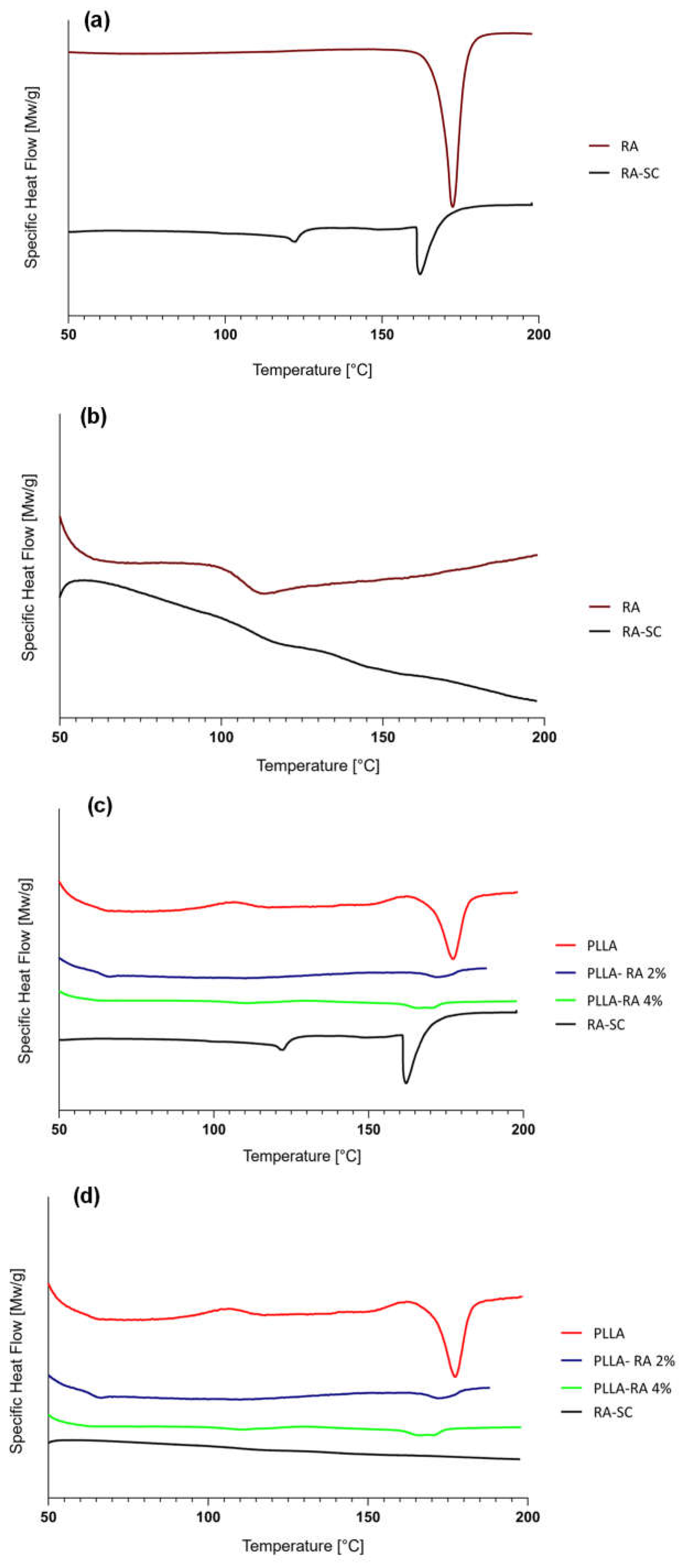
Since the RA is additivated to the scaffolds through a solvent casting procedure, a thin film of RA, obtained with the same technique, was prepared, analyzed, and compared to RA powder
. Figure 5a and
Figure 5b illustrate, respectively, the thermograms of first and second heating of the RA powder and RA solution casted film (RA-SC). The thermograms of PLLA, PLLA-RA 2%, PLLA-RA 4%, and RA-SC of first and second heating are shown in
Figure 5c and
Figure 5d.
The data obtained from the tests are shown in
Table 3. It can be observed that there is a large difference in terms of melting enthalpy and temperature between the two samples. No peaks were detected in the second heating, due to the degradation of RA over 200°C, which agrees with the value reported in the literature [
39].
A concentration-dependent decrease in melting enthalpies and temperatures of PLLA was observed with increasing RA. The data that was obtained has been displayed in Table 3.
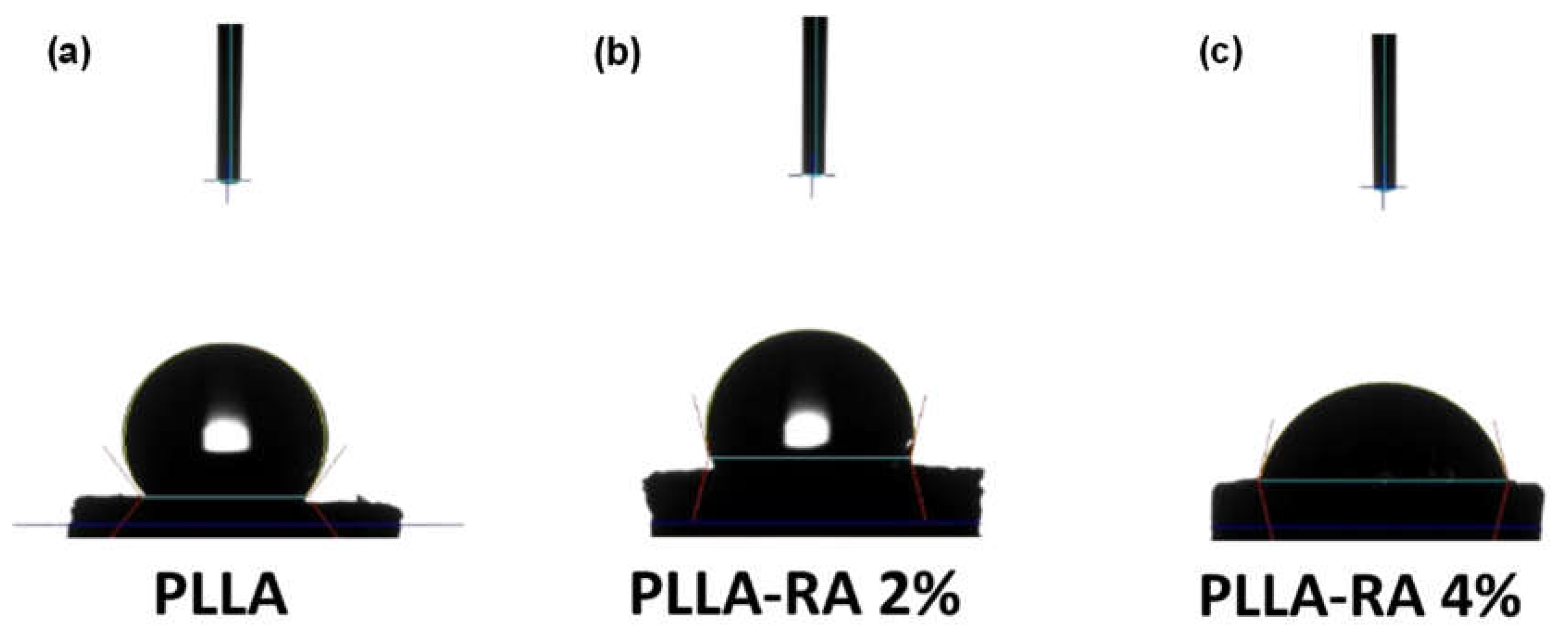
2.3. Water Contact Angle Measurements
Figure 6 shows the water contact angle (WCA) values obtained with distilled water on PLLA, PLLA-RA 2% and PLLA-RA 4% samples. The test was performed on three samples of each type to assess the change in the hydrophilicity of the scaffold.
As can be noticed from the
Figure 6, the water contact angle decreases when increasing RA concentration, highlighting that the presence of RA induces the hydrophilicity change. Interestingly, the water contact angles of PLLA samples remain unchanged over time. For example, immediately after water deposition, the value is 128.40°. After one minute, it decreases slightly to 128.28°, and after 5 minutes, it further decreases to 127.86°. This suggests that PLLA scaffold maintains this hydrophobicity in the time. The water angle contact of PLLA-RA 2% and PLLA-RA 4% samples was 103,96° and 76,51° respectively. After 1 minute, the water drop disappeared. The water absorption is in favor of RA presence, and the measurement cannot be performed.
4. Discussion
In this work, the solution deposition method was employed to incorporate an antioxidant molecule into polymeric scaffolds produced via TIPS. Scaffolds, with a diameter of 4 mm and a thickness of 2 mm, were obtained and characterized. Ascribing to the presence of RA, the color of the whole surface of the scaffolds changed from white to yellow. Moreover, when the concentration of RA increases, the pigmentation of the samples is more evident. A gravimetric analysis revealed that the percentual of RA incorporated in the samples doubles when passing from 2% to 4% solution. The data obtained from the analysis show that samples of PLLA-RA 2% and PLLA-RA 4% contain high concentrations of the antioxidant molecule, approximately 47.7%, and 81.9% respectively.
An analysis of SEM micrographs revealed the presence of an interconnected porous network in the samples. As known, the pore dimension is widely regarded as one of the most important requirements of a scaffold for tissue regeneration. A study by Bergonzi et al. showed that increasing the concentration of antioxidants, particularly vitamin E, led in changes in pore size and a wider range of pore sizes in the scaffold. The deposition method used in this study to incorporate natural antioxidant molecules into the scaffolds preserved the interconnectivity and maintained the original pore size. Moreover, as the concentration of antioxidants on the scaffold increases, a visible layer of RA becomes more evident at the level of the surface of the structure. All things considered, the thermally induced phase separation method with solvent casting deposition allowed the production of 3D porous structures capable of accommodating high concentrations of antioxidant molecules while precisely controlling pore size and interconnectivity.
ATR-FTIR analysis was conducted to determine the presence of RA molecules on both the top and bottom surfaces of the scaffold. All obtained results suggests that the RA solution has penetrated and permeated the entire three-dimensional polymeric structure. The neat RA shows typical intrinsic peaks in the regions around 3500-3000 cm
-1 and 1700-500 cm
-1, assignment to the presence of phenolic and carboxylic functionalities [
38]. Neat PLLA sample shows typical peaks in 1700-1000 cm
-1 according to the literature [
37].
The spectra of both PLLA-RA samples show an evident presence of phenolic functionalities and more complex peaks around 1700-1000 cm-1 in comparison to PLLA sample. These changes were attributed to the presence of RA molecules, especially in the PLLA-RA 4% sample, due to the lower amount of PLLA, which was only 18% of the total weight.
Furthermore, it can be assumed that the biomolecule interacts physically with the polymer through the formation of hydrogen bonds; this kind of interaction is confirmed by several papers in which polymer/polyphenol systems were analyzed through the same technique[
41,
42,
43].
These considerations confirm the presence of RA molecules and the occurrence of the interactions between PLLA and RA molecules, according to the SEM images.
Calorimetric analyses substantially confirm the integration of RA and its distribution throughout the PLLA-RA scaffolds. The RA powder shows a melting enthalpy about three times that of the RA-SC film, and additionally, RA shows higher fusion temperatures than the RA-SC one. These results show that after solubilization of the powder in ethanol, the formation of crystalline structures by the RA molecule is disfavored, and as expected, the RA molecules are organized in a predominantly amorphous state with low crystalline content, in comparison to RA powder. Once the RA powder sample reaches the upper temperature of 200 °C, the molecules probably undergo irreversible degradation. The second rise of the RA powder and RA-SC samples shows small humps at a temperature of about 120 °C probably due to the reorganization of the decomposition products of the RA molecule.
PLLA scaffolds show a reduction in the fusion enthalpy of second heating of fusion of about 20.8% in comparison to the first fusion enthalpy, while the temperature of fusion changes from 181 °C to 177°C.
The presence of RA in the PLLA-RA 2% and PLLA-RA 4% samples leads to a drastic reduction of melting enthalpy and temperature of fusion both in the first heating and in the second heating.
It should be noted that in the PLLA-RA 2% and PLLA-RA 4% samples it is not possible to identify two distinct peaks for RA and PLLA, but only one peak is observed due to the fusion of the composite structure.
A decrease in their melting enthalpies and temperatures with respect to pure PLLA is observed, when increasing the concentration of RA this value is very similar to RA-SC value. The latter could be understood considering the high concentration of RA molecules in the composite scaffolds, with respect to the PLLA component.
Hydrophilicity is considered to play an important role in the interaction between the scaffold and the tissue. For tissue engineering applications, good scaffold hydrophilicity is required for cell adhesion and proliferation. Several studies reported in the literature have shown that the use of polyphenolic coatings was able to improve the hydrophilicity of the scaffold surfaces [
44].
The WCA values of PLLA scaffold was revealed at three different times (immediately after water drop deposition, after 1 and 5 minutes of deposition). The result suggests that the PLLA sample maintains in the time its hydrophobic nature. Different results were obtained for the samples after the surface modification by RA. In PLLA-RA 2% and PLLA-RA 4% scaffolds the WCA values decrease when increasing the RA concentration. Moreover, it was noticed that the droplet deposited on the samples in a few seconds appeared distributed over the entire surface. These phenomena could be explained by the presence of polyphenolic compounds in the RA according to the data found in the literature [
44].
All obtained results suggest that the considered ad hod protocol allows the successful production of PLLA scaffolds, incorporating large amounts of RA molecules. As previously stated, our main objective was to produce a composite scaffold and effectively incorporate RA molecules, without specifically examining the biological activities of RA. As a result, this particular topic will be the center of attention for our upcoming study, which will serve as a natural extension of the current work.
5. Conclusions
In our study, we successfully incorporated Rosmarinic acid, a natural antioxidant, into Poly-L-Lactic acid scaffolds. To achieve this, we introduced a novel protocol to incorporate a natural biomolecule, which is soluble in organic solvents, into the polymeric scaffolds produced via TIPS. This approach is not only cost-effective but also customizable with different biomolecules. The resulting scaffolds showed well-defined pore networks with good interconnectivity, even in those containing different amounts of rosmarinic acid (up to 81,9% of RA). Notably, these scaffolds not only exhibit a favorable morphology but also excellent hydrophilicity, meeting the requirements for tissue engineering. The water contact angle of the samples decreased from 128.40° to 76.51°. Our focus on composite PLLA-RA scaffolds yielded promising results, indicating that incorporating natural antioxidant molecules into polymeric structures could be a potential solution to mitigate implant-associated inflammation, opening new avenues for future development in this field.
Author Contributions
Conceptualization, V.S. and N.T.D.; methodology, F.C.P., V.L.C. and N.T.D.; validation, F.C.P., V.L.C., N.T.D. and V.B.; formal analysis, V.S. and F.C.P.; investigation, V.S., F.C.P. and N.T.D.; resources, N.T,D., V.L.C. and V.B.; data curation, V.S. and F.C.; writing—original draft preparation, V.S., F.C.P. and N.T.D; writing—review and editing, V.S. and N.T.D.; supervision, F.C.P. and N.T.D.; funding acquisition, V.B. and N.T.D.
Funding
Please add: The APC was funded by N.Tz. Dintcheva.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
V.S. would thank the University of Palermo, Engineering Department, PhD Course in “Chemical, Environmental, Biomedical, Hydraulic and Materials Engineering” for the financial support of her PhD study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Advances in Materials Science and Engineering 2019, 1–13. [Google Scholar] [CrossRef]
- Gautam, S.; Ambwani, S. Tissue Engineering: New Paradigm of Biomedicine. Biosci Biotechnol Res Asia 2019, 16. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur Spine J. 2008, 17. [Google Scholar] [CrossRef] [PubMed]
- Abpeikar, Z.; Milan, P.B.; Moradi, L.; Anjomshoa, M.; Asadpour, S. Influence of Pore Sizes in 3D-Scaffolds on Mechanical Properties of Scaffolds and Survival, Distribution, and Proliferation of Human Chondrocytes. Mech. Adv. Mater. Struct. 2022, 29. [Google Scholar] [CrossRef]
- Subia, B., J. Kundu, e S. C. «Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications». In “Tissue Engineering”, a cura di Daniel Eberli. InTech, 2010. [CrossRef]
- Hutmacher, D.W. Scaffold Design and Fabrication Technologies for Engineering Tissues - State of the Art and Future Perspectives. J Biomater Sci Polym Ed 2001, 12. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Tandon, B.; Dalton, P.D. Scaffold Design and Fabrication. In Tissue Engineering; Elsevier, 2023; pp. 355–385 ISBN 978-0-12-824459-3.
- Mi, H.Y.; Jing, X.; Turng, L.S. Fabrication of Porous Synthetic Polymer Scaffolds for Tissue Engineering. J. Cell. Plast. 2014, 51. [Google Scholar] [CrossRef]
- Zeinali, R.; Del Valle, L.J.; Torras, J.; Puiggalí, J. Recent Progress on Biodegradable Tissue Engineering Scaffolds Prepared by Thermally-Induced Phase Separation (TIPS). IJMS 2021, 22, 3504. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, R.; Yousefi, A. Effects of Processing Parameters in Thermally Induced Phase Separation Technique on Porous Architecture of Scaffolds for Bone Tissue Engineering. J Biomed Mater Res 2014, 102, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- A., C.; Olivas-Armendariz, I.; S., J.; E., P. A., C.; Olivas-Armendariz, I.; S., J.; E., P. Scaffolds for Tissue Engineering Via Thermally Induced Phase Separation. In Advances in Regenerative Medicine; Wislet-Gendebien, S., Ed.; InTech, 2011 ISBN 978-953-307-732-1.
- Kumar Panda, P.; Jebastine, J.; Ramarao, M.; Fairooz, S.; Reddy, C.K.; Nasif, O.; Alfarraj, S.; Manikandan, V.; Jenish, I. Exploration on Mechanical Behaviours of Hyacinth Fibre Particles Reinforced Polymer Matrix-Based Hybrid Composites for Electronic Applications. Advances in Materials Science and Engineering 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-L-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polym. 2022, 14. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants – A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. COMPREHENSIVE INVITED REVIEW Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal 2014, 20, 1126–67. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxidants & Redox Signaling 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind J Clin Biochem 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus Alba L.). Molecules 2021, 26. [Google Scholar] [CrossRef]
- Wójciak, M.; Drozdowski, P.; Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Kubrak, T.; Sowa, I. ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes. Molecules 2024, 29, 955. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Dintcheva, N.Tz.; D’Anna, F. Anti-/Pro-Oxidant Behavior of Naturally Occurring Molecules in Polymers and Biopolymers: A Brief Review. ACS Sustainable Chem. Eng. 2019, 7, 12656–12670. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of Active Rosmarinic Acid-Gelatin Biodegradable Films with Antioxidant and Long-Term Antibacterial Activities. Food Hydrocoll 2018, 83. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Jin, D.; Xin, Y.; Tian, L.; Wang, T.; Zhao, D.; Wang, Z.; Wang, J. Rosmarinic Acid and Related Dietary Supplements: Potential Applications in the Prevention and Treatment of Cancer. Biomolecules 2022, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic Acid and Its Derivatives: Current Insights on Anticancer Potential and Other Biomedical Applications. Biomedicine & Pharmacotherapy 2023, 162, 114687. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci Rep 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Kernou, O.-N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Amoah, S.; Sandjo, L.; Kratz, J.; Biavatti, M. Rosmarinic Acid – Pharmaceutical and Clinical Aspects. Planta Med 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical Features and Therapeutic Potential of Rosmarinic Acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan – Rosmarinic Acid Conjugates with Antioxidant, Anti-Inflammatory and Photoprotective Properties. Carbohydr Polym 2021, 273. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med 2021, 87. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-Inflammatory Effect of Rosmarinic Acid and an Extract of Rosmarinus Officinalis in Rat Models of Local and Systemic Inflammation. Basic Clin Pharmacol Toxicol 2015, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, L.; Wen, W.; Lu, L.; Zhou, C.; Luo, B. Fabrication and Evaluation of 3D Printed Poly(l -Lactide) Scaffold Functionalized with Quercetin-Polydopamine for Bone Tissue Engineering. ACS Biomater Sci Eng 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Lihao, Q.; Tingting, L.; Jiawei, Z.; Yifei, B.; Zheyu, T.; Jingyan, L.; Tongqing, X.; Zhongzhi, J. 3D Bioprinting of Salvianolic Acid B-Sodium Alginate-Gelatin Skin Scaffolds Promotes Diabetic Wound Repair via Antioxidant, Anti-Inflammatory, and Proangiogenic Effects. Biomed. Pharmacother. 2024, 171. [Google Scholar] [CrossRef]
- Thangaraju, E.; Srinivasan, N.T.; Kumar, R.; Sehgal, P.K.; Rajiv, S. Fabrication of Electrospun Poly L-Lactide and Curcumin Loaded Poly L-Lactide Nanofibers for Drug Delivery. Fibers Polym 2012, 13, 823–830. [Google Scholar] [CrossRef]
- Stehfest, K.; Boese, M.; Kerns, G.; Piry, A.; Wilhelm, C. Fourier Transform Infrared Spectroscopy as a New Tool to Determine Rosmarinic Acid in Situ. Journal of Plant Physiology 2004, 161, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Veras, K.S.; Fachel, F.N.S.; Pittol, V.; Garcia, K.R.; Bassani, V.L.; dos Santos, V.; Henriques, A.T.; Teixeira, H.F.; Koester, L.S. Compatibility Study of Rosmarinic Acid with Excipients Used in Pharmaceutical Solid Dosage Forms Using Thermal and Non-Thermal Techniques. Saudi Pharm. J. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3d Printed Chitosan-Based Scaffolds Containing α-Tocopherol Showing Antioxidant and Antimicrobial Activity. Appl. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Yen, K.C.; Mandal, T.K.; Woo, E.M. Enhancement of Bio-Compatibility via Specific Interactions in Polyesters Modified with a Bio-Resourceful Macromolecular Ester Containing Polyphenol Groups. J Biomed Mater Res A 2008, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, G.; Guo, Q.; Li, R.; Li, C.; He, H. Fabrication and Characterization of Polylactic Acid Electrospun Wound Dressing Modified with Polyethylene Glycol, Rosmarinic Acid and Graphite Oxide. Nanomaterials 2023, 13. [Google Scholar] [CrossRef]
- Zhu, B.; Li, J.; He, Y.; Yamane, H.; Kimura, Y.; Nishida, H.; Inoue, Y. Effect of Steric Hindrance on Hydrogen-Bonding Interaction between Polyesters and Natural Polyphenol Catechin. J Appl Polym Sci 2004, 91. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol Scaffolds in Tissue Engineering. Mater Horiz 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.E.; Carfì Pavia, F.; Vitrano, I.; Ghersi, G.; Brucato, V.; Rosei, F.; La Carrubba, V. PLLA Scaffolds with Controlled Architecture as Potential Microenvironment for in Vitro Tumor Model. Tissue Cell 2019, 58, 33–41. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).