1. Introduction
Application of electroanalysis allows analytical information to be obtained as a result of an electrochemical or ion-exchange reaction occurring at the
electrode surface/
solution interface. In voltammetric analysis, quantitative and qualitative information is obtained from the peak height and specific voltammetric potential. Improvement of the voltammetric signal includes the introduction of new electrode materials [
1,
2,
3,
4,
5] and the modification of the electrode surface [
6,
7,
8]. The concept of electrode surface modification can bring two main advantages for electroanalysis: i) facilitating ion/electron exchange between electrode and analyte; ii) increasing the selectivity through the binding of the signal molecule by the modified materials. Conjugated polymers have emerged as promising candidates for electrode surfaces modification, because their surface can be used as an electrochemical transducer for the molecular recognition process. Therefore, selective surfaces based on conjugated polymers and their derivatives attract a great deal of attention. The rational molecular design of polymers with certain structures allows enhance binding affinity and specificity of the electrode towards the analytes of interest. An advantage of the conjugated polymer-modified surface is that the relatively rigid conjugated polymer backbone can act as a scaffold capable of arraying a number of recognizing sites along the backbone, which is important for realizing enhanced molecular recognition. Thus, the incorporation of specific binding sites into the side chain of a conjugated polymer opens a new perspective in the field of development a new selective surface. A number of conjugated polymers derived from polyaniline (PANI), polythiophene (PTh), and polypyrrole (PPy) have been designed and synthesized for the development of sensors make them fascinating materials in various areas of application [
9]. Electrochemical polymerizations are alternative ways to synthesize CPs and to modify electrode surface.
Polythiophene and its derivatives electrochemically deposited on various substrates have been increased the selectivity and sensitivity of a number of electrochemical sensors [
10]. Electrodes modified by the electropolymerization of thiophene and its derivatives were used for voltammetric detection of some organic and biological molecules of industrial and medicinal interest [
11]. Wang and Lin described the poly(3-methylthiophene (P3MT)-modified glassy carbon electrodes that have received a great attention in the modification of electrodes [
12]. The electroactive conducting polymer P3MT reduced the response to ascorbic acid and enhanced the selectivity towards acetaminophen. As a PTh derivative, poly(3,4-ethylenedioxythiophene) (PEDOT) in the form of polymeric and molecular-imprinted films has been a large scale applied in the electroanalysis morphine [
13,
14,
15], diphenylamine [
16] and ephedrine [
17,
18]. N. Atta et al. have been found that morphine could be effectively adsorbed and accumulated on PEDOT/Pt electrode in the presence of anionic surfactant sodium dodecyl sulfate and successfully determined its content in commercial tablets using a voltammetric approach [
15]. In contrast to previously developed potentiometric sensors for the detection of diphenylamine [
19], the voltammetric sensor based on PEDOT/MIP membranes has been displayed significantly lower detection limits (5.4 μM vs. 290–350 μM) and consequently a wider working range [
16].
The term “Forensic Electrochemistry” explains that voltammetric methods have received well-deserved recognition in the field of forensic analysis. There are a number of works devoted to the voltammetric determination of gunshot residues or new psychoactive substances (NPS). In recent years, a trend has been observed to replace drugs of natural origin (such as cocaine) by NPS [
20]. Several electrochemical strategies have been reported in the literature for the modification of the electrode surfaces for the detection of cocaine, which is one of the most widely used drugs worldwide. The potential use of Schiff base complexes that form stable films on the electrode surface in voltammetric analysis of cocaine was evident from the investigations cited in references [
21,
22,
23]. M.F.M. Ribeiro et al. [
21] designed a screen-printed electrode modified with uranyl Schiff base to determine cocaine with limits of detection (LOD) and quantification (LOQ) of 110 and 390 μmol L
−1, respectively. T.Y. Sengel et al. modified a glassy carbon electrode with antibody and poly-l-phenylalanine bearing electroactive EDOT for detection of cocaine between 0.5–25 μM [
24]. Affinity polymers such
o-phenylenediamine (OPD) and
p-aminobenzoic acid (PABA) were successfully integrated onto the surface of graphene-modified screen printed electrodes to improve the selectivity of cocaine detection in street-level seized samples and to suppress interference of levamisole [
25].
Synthetic cathinones and aminoindane are synthetic stimulants that represent NPS and are monitored by the United Nations Office on Drugs and Crime (UNODC) and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [
26]. They are designed to replicate the effects of traditional controlled drug stimulants and can be synthesized into a variety of formulations. The new type of electrode materials, nanomaterials, molecularly imprinted polymers [
27,
28,
29] improve the specificity and sensitivity of the voltammetric detection of NPS [
30]. Recently, it has been confirmed that polymer-based materials that are capable to bind the analyte of interest through a combination of electrostatic, hydrogen and π−π interactions might be a selective modifier of the electrode surface [
31]. Deposition of thiophene derivatives with –PhCF
3 group by means of electrochemical oxidation on the electrode surface is a promising alternative for molecularly imprinted polymers. Previous investigations by L. Forlani have shown some specific interactions of nitro-halogenobenzenes and amines that can lead to the formation of various molecular complexes, such as charge-transfer or hydrogen bonding complexes [
32,
33]. Different kinds of associations were observed depending on the nature of the amine. Moreover, the F atom in the CF3 group is prone to interact with aromatic H atoms [
34].
Herein, we report the electrochemical deposition of fluoro derivative thiophene on surface of graphite electrode and discuss its interaction with 2-aminoindane (2-AI) and two representatives from category synthetic cathinones, namely buphedrone and naphyrone.
2. Materials and Methods
2.1. Chemicals
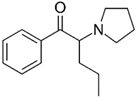
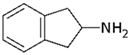
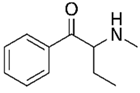
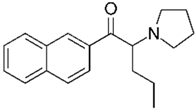
The following chemicals were used in this work: 99% tetrabutylammonium tetrafluoroborate (TBATFB; Sigma-Aldrich, St. Louis, MO, USA), 98% thiophene (Th; Sigma-Aldrich, St. Louis, MO, USA), 99.5 % acetonitrile (ACN; Penta, Prague, Czech Republic), potassium hexacyanoferrate and potassium ferricyanide (K4[Fe(CN)6] / K3[Fe(CN)6]) (Lachema, Brno, Czech Republic). 3-(4-trifluoromethyl)-phenyl)-thiophene (ThPhCF3) used as a monomer for electrochemical polymerization, was synthesized at the Department of Organic Chemistry, UCT Prague (Czech Republic). Analytes in hydrochloride form were: 2-aminoindane (2-AI; Sigma-Aldrich, St. Louis, MO, USA) and synthetic cathinones (buphedrone and naphyron). Synthetic cathinones were supplied by the Laboratory of Forensic Analysis of Biologically Active Substances (BAFA) (University of Chemistry and Technology, Prague, Czech Republic).
2.2. Modification of Electrode Surface
Electrode surface modification was carried out using cyclic voltammetry (CV) with Palmsens 3 (PalSmSens BV, Houten, Netherlands) in a three-electrode system. A graphite electrode (G, 1 mm2, Elektrochemické detektory s.r.o., Turnov, Czech Republic) was used as the working electrodes for electrochemical measurements. Ag/AgCl (3 mol L−1 KCl) and a Pt plate (81 mm2) served as reference and counter electrodes, respectively. The polymeric film was electrochemically deposited on the G-electrode surface from 0.01 mol L–1 ThPhCF3, dissolved in the supporting electrolyte ACN with 0.05 mol L−1 TBATFB, the potential window was from 0.0 V to +2.0 V with a scan rate of 100 mV s–1, 10 cycles.
2.3. Raman Spectroscopy
Raman spectra were collected on a Thermo Scientific DXR Raman microscope using 633 nm laser excitation (power 0.1 mW). The scattered light was analyzed by a spectrograph with holographic gratings (600 lines per mm) with an aperture of 50 μm and an EMCCD detector. The spot size of the laser focused by the 50× objective was ∼1 μm in diameter. The acquisition time was 10 s with 10 repetitions.
2.4. Electrochemical Impedance Spectroscopy
The adsorption constants between the G-electrode coated with un- (Th) /substituted (ThPhCF3) thiophene and target analytes were measured by the EIS technique in the supporting electrolyte with/without the target analytes using a Palmsens 3. Phosphate buffer with the addition of 140 mmol L−1 NaCl (PBS) was used as a supporting electrolyte in the same three-electrode system (2.2. Modification of electrode surface). The EIS spectra were collected at a potential of 0.0 V in the frequency range of 10 kHz to 10 mHz (62 points), with an applied sinusoidal potential (10 mV amplitude). A Randles circuit including resistance to charge transfer (Rct) and double-layer capacitance (CDL) in parallel, the solution resistance was used for curve fitting of the impedance spectra in the PSTrace 5.4 software package.
2.5. Square Wave Voltammetry
Calibration dependencies for the monitored analytes were measured in the range from 1.0–1170 μmol.l–1 using square wave voltammetry (SWV). The supporting electrolyte was 5 mmol L−1 K4[Fe(CN)6] / K3[Fe(CN)6]. The SWV technique was performed in the potential range of – 0,1 V do + 1,6 V, at a frequency of 10 Hz, with a pulse amplitude of 25 mV and a potential step of 5 mV. For better visualization, all the square wave voltammograms (SWVs) presented here were baseline-corrected using the moving average filter included in the PSTrace 5.4 software without affecting the results.
3. Results and Discussion
3.1. Cyclic Voltammetry
The 3′-substituted thiophenes are generally more suitable for electrochemical oxidation and surface modification due to their high stability and ease of preparation [
35]. The effect of the substituent on the polymerization process has been noted in the electrochemical polymerization of the terthiophene derivative, 3´-(2-aminopyrimidyl)-2,2´:5´,2´´-terthiophene (oxidized peak at + 1.25 V) [
36] and 15-crown-5 substituted thiophene that was in direct n-conjugation with the macrocycle (oxidized peak at + 1.4 V) [
37].
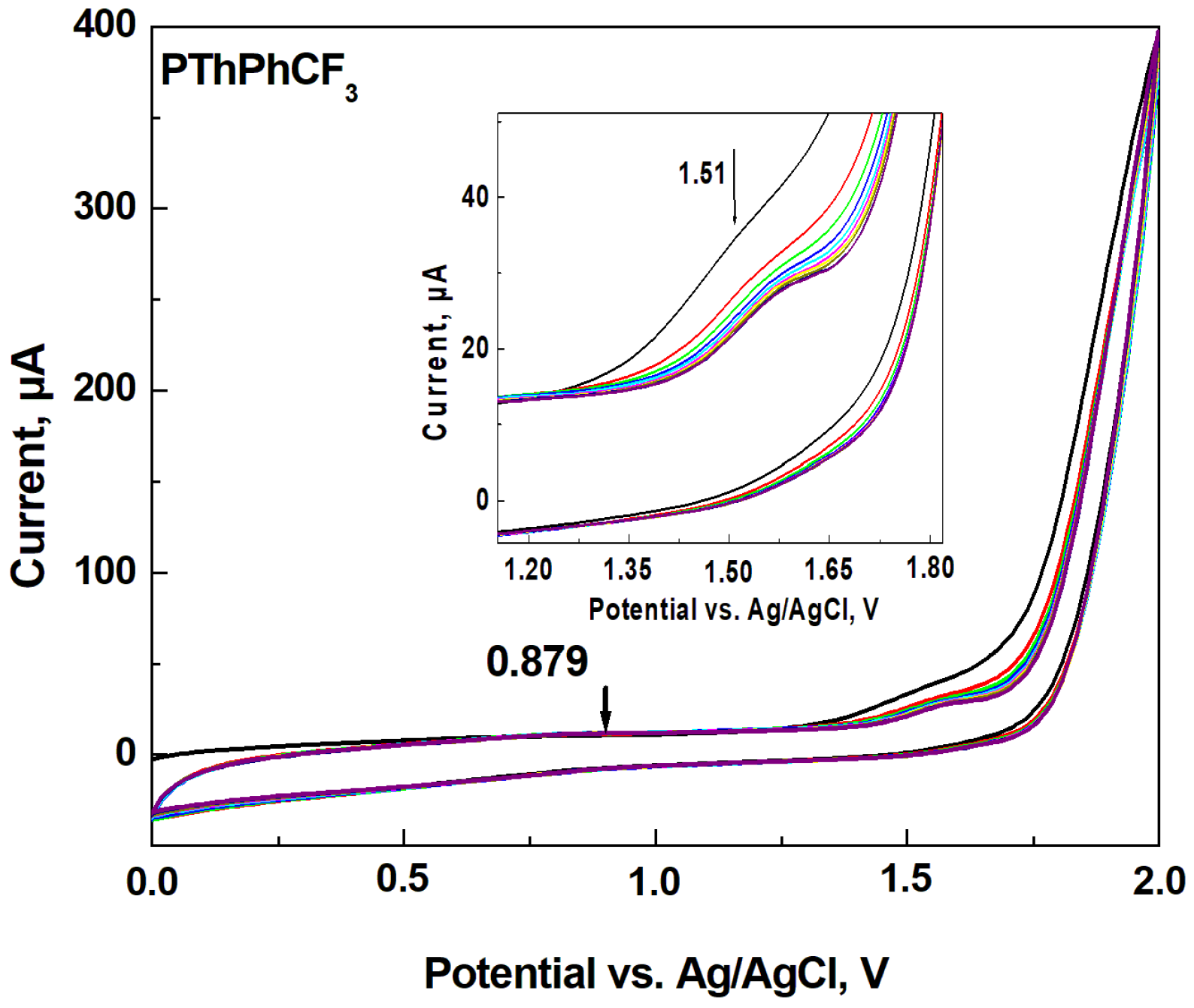
Figure 1 shows the cyclic voltammograms obtained during the electropolymerization of ThPhCF
3. Two irreversible anodic peaks
Ea1 = 0.879 V and
Ea2 = 1.51 V were observable. The current intensity for
Ea1 increased up to 3 scan, while the current intensity for
Ea2 decreased. An oxidation peak of 1.56 V was recently reported in the electrochemical synthesis of a copolymer based on EDOT and 1-(3,5-bis(trifluoromethyl) phenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole [
38]. There are no significant changes in the cyclic voltamogram depending on the number of cycles, but the anodic peak
Ea2 has slightly shifted towards more positive potentials. J. C. Ahumada et al attributed this phenomenon to the electron-withdrawing nature of the –PhCF
3 substituent of the polymer [
39]. The reason of the observed shift of anodic peak can be attributed to different conformational states of the polymer chains formed by growing through thiophene units. As noted by Tanaka et al., the increase in thickness of the polymer film also increases the electrical resistance and leads to a peak shift [
40]. The drop in current at 1.56 V is due to the decrease of monomer species to be oxidized around the working electrode.
3.2. Raman Spectroscopy
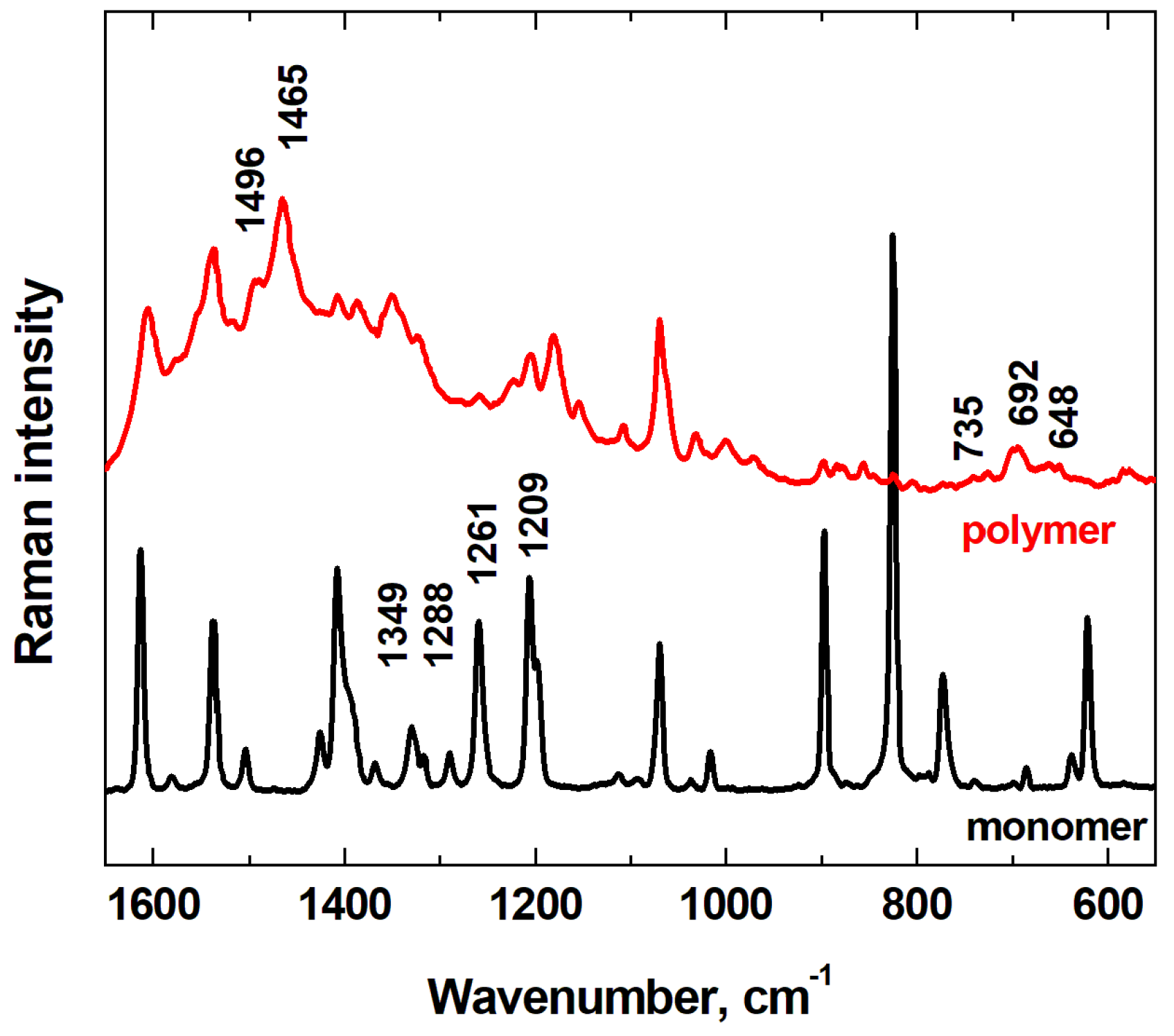
In field of development of selective surface it is significance their characterization using spectroscopic methods. The application of Raman spectroscopy made possible to confirm the electrochemical modification of graphite electrode surface with a thiophene-derived polymeric layer bearing group –PhCF
3 for recognition of synthetic stimulants.
Figure 2 presents the Raman spectra of the monomeric and polymeric form ThPhCF
3. The characteristic vibrations of polythiophene skeleton from Raman spectra were described by number of authors [41-45].The shoulder band at 1496 cm
–1 and the intensive band at 1465 cm
–1 are attributed to the C=C stretching of PTh ring, their whole widths being dependent on the film thickness [
46]. R.R. Subbulakshmi et al. have been carried out theoretical studies on molecular structure benzene derivatives with C–F group [
47]. It has been found that the C–F stretching vibration can be observed over a wide frequency range of 1360-1000 cm
–1 because there is significant influence of neighboring atoms or groups. In our case, in the region of 735 to 648 cm
–1, less intense bands of C–S–C ring deformation and kinks attributed to PTh are observed. The regions 1205 to 1420 cm
–1 are assigned to the –PhCF
3 mode [
48]. Raman bands 1209, 1261, 1288, 1349 cm
–1 of different intensity observed in the spectrum of monomer and polymer confirm the presence of –PhCF
3 substituent in the prepared polymer layer.
3.3. Affinity Properties of the Modified Electrodes
Evaluation of the affinity properties of the modified surface can provide an insight into the selectivity of the deposited layer to the tested analytes. The interaction of the analyte with the modified electrode surface is dependent on the surface coverage and, consequently, on the occupancy of both PThPhCF
3 binding sites (specific interactions) and uncoated segments of the electrode surface (non-specific interactions). Therefore, the adsorption constants for PTh/G and PThPhCF
3/G electrodes were estimated (
Table 1). The highest affinity and the best discrimination between the tested analytes were confirmed for PThPhCF
3 modified electrodes. Importantly, the adsorption constant for 2-AI (1,87 x 10
6) as the primary amine, which is able to form the largest number of hydrogen bonds with –CF
3 groups, is higher compared to buphedrone (MABP) (9,79 x 10
5) and naphyrone (O-2482) (1,57 x 10
6). In the case of PTh/G electrodes, these are of course non-specific interactions that can be the result of not only lipofilicity of the analyte (log P). The comparable adsorption constant for primary and tertiary amine can be explained by the findings reported in reference [
34] which indicate the ability of the F atom in the –PhCF
3 group to interact with the aromatic H atoms.
3.4. Square Wave Voltammetry
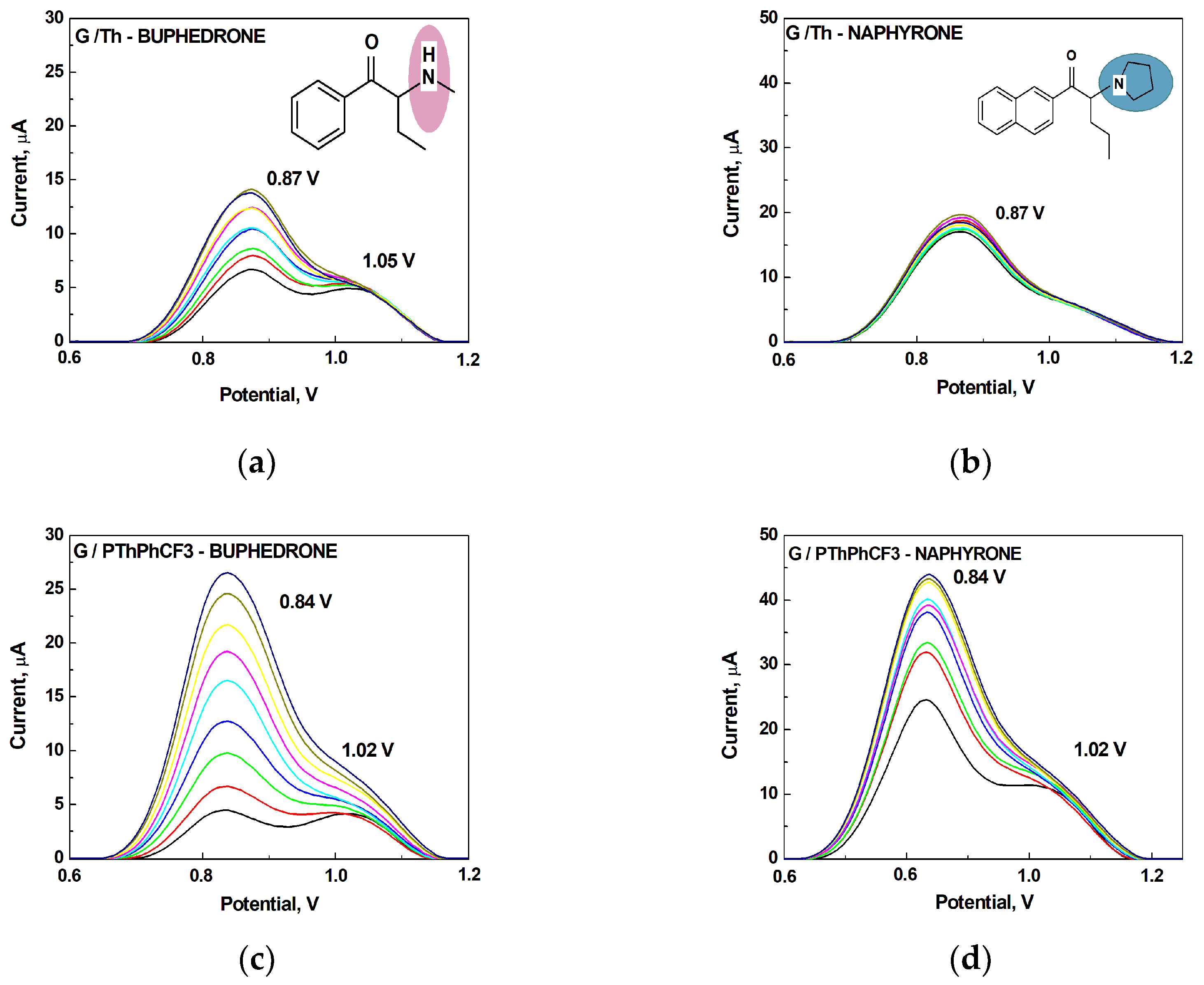
All analytes differ according to the type of amine group. 2-Aminoindane is a cyclic analogue of amphetamine, its primary group cannot be directly oxidized in the potential region of graphite electrode. Buphedrone (MABP) and naphyrone (O-2482) differ in the type of amine group and the substitution on the aromatic ring. Among the selected analytes, buphedrone has a more electroactive group, a secondary amine group.
The electrochemical profile of buphedrone and naphyrone at PTh- and PThPhCF
3- modified G-electrodes by SWV is revealed at an anodic region potential between 0.8 and 1.1 V (
Figure 3). Voltammetric studies of synthetic cathiones as one representative group in the NPS category have received the most attention. Therefore, we decided to comment/discuss our results in comparison with results published in the literature (
Table 2). For this purpose, the analogues of buphedrone and naphyrone were selected, namely N-ethylhexedrone (NEH) and alpha-pyrrolidinovalerophenone (PVP), respectively (
Table 2). Selected cathinones (NEH and PVP) were characterized using the SWV technique on the surfaces of graphite SPE electrodes modified with such nanomaterials as graphene (GPH/SPE) and multiwalled carbon nanotubes (MWCNT/SPEs) [
50]. Nanomaterials are particularly attractive for electrochemical sensing due to their unique electrocatalytic properties, conductivity, and strong adsorption capacity, possibility of oxidation or reduction of the analyte on the electrode [
51]. It should be noted that the effect of nanomaterials on the electrochemical signal was observable in a remarkably increasing anodic current intensity for the secondary amine NEH: G/SPE (3.07 μA) < MWCNT/SPE (10.24 μA) < GPH/SPE (3.07 μA).
In this study, the intensity of anodic current was increased for PThPhCF
3/G-electrodes towards the tertiary amine, naphyrone. In contrary to literature data, both synthetic catinones showed the split peaks: 0.84 / 1.02 V for buphedrone and 0.84 / 1.02 V for naphyrone. For 2-AI, only an anodic peak at 1.095 V was observed. We were interested in the origin of the appearance of these split peaks for synthetic cathinones and its absence for 2-AI at the PThPhCF
3/G-electrode. A.-M. Dragan et al. found that the electrochemical oxidation of NEH proceeds during the oxidative dealkylation of the secondary amine and the formation of the primary amine [
50]. Profiling of the electrochemical oxidation products of of 4-Cl- alpha PVP showed transformation of the pyrrolidine ring to the primary amine at 1.10 V via a ring-opening intermediate [
52]. We can assume that the weak peaks at 1.02 as shoulders (
Figure 3c-d) might be the result of the formation and subsequent oxidation of the primary amine as the oxidation product of buphedrone (MABP) and naphyrone (O-2482). In fact, the anodic peak of greater intensity that was observable in the SWV-voltammogram at 1.095 V for 2-AI can be an evidence for this assumption.
3.5. Analytical Application
Chromatographic methods are preferable by forensic laboratories for an identification of synthetic cathinones although they require expensive equipment, and/or complicated sample preparation. With the point of view of development of screening tests, electrochemical sensors using selective surfaces could be an alternative to colorimetric tests. In this context, the recognition of synthetic stimulants using ThPhCF
3/G-modified electrodes might be crucial for forensic analysis. The electrochemical oxidation of thiophene modified with -PhCF
3 substituent and its deposition on the electrode surface extends our ongoing research focused on improving the properties of electrochemical sensors for the detection of synthetic stimulants [53, 54] regarding the application of monomeric and polymeric units for electrode modification. The current results of our investigations are summarized in
Table 3. Doses of synthetic cathinones ranging from 5 to 20 mg are taken, usually orally, but also intranasally, rectally, and intravenously [
55].
4. Conclusions
A polymeric film derived from 3-(4-trifluoromethyl)-phenyl)-thiophene (ThPhCF3) deposited on the surface of graphite electrode was applied to study the interaction with synthetic stimulants containing primary (2-AI), secondary (buphedrone) and tertiary (naphyrone) amino groups. The relationship between the adsorption constants and the structure of selected synthetic stimulants allowed to determine the role of the –PhCF3 group for their recognition. It was found the importance of both hydrogen bonding and aromatic H atoms at interaction with PThPhCF3/G-electrodes and their effect on the increase of the electrochemical signal. Experimental finding show the importance of finding and studying new materials for the detection of commonly abused new psychoactive substances.
Author Contributions
Conceptualization, T.V.S.; methodology, T.V.S.; investigation, N.Š.; data curation, T.V.S. and N.Š.; writing—original draft preparation and editing, T.V.S. and G.B.; Review and editing M.V. and G.B.; Project administration, M.V.; Resources, T.V.S and M.V.
Funding
This work was supported by a specific University research grant number 402850061 (UCT Prague, CZ) from Ministry of Education, Youth and Sports of the Czech Republic and by OP JAC financed by ESIF and the MEYS (Project No. SENDISO - CZ.02.01.01/00/22_008/0004596)
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to T. Tobrman for synthesis of receptor, M. Trchová for spectroscopic measurements, Dr. M. Kuchař for providing the synthetic cathinones. This work was supported by Institutional Resources (Department of Analytical Chemistry, UCT Prague, CZ; Grant Number: 402850061) and by OP JAC financed by ESIF and the MEYS (Project No. SENDISO - CZ.02.01.01/00/22_008/0004596).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Porada, R.; Jedlińska, K.; Lipińska, J.; Baś, B. Review—voltammetric sensors with laterally placed working electrodes: A review. J. Electrochem. Soc. 2020, 167, 037536. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, M.T.N.; Lee, J.S. Carbon-based materials and their applications in sensing by electrochemical voltammetry. Inorganics 2023, 11, 81. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: a review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Melo, L.M.A.; de Faria, L.V.; Arantes, L.C.; Vojs, M.; Marton, M.; Brocenschi, R.F.; Richter, E.M.; Munoz, R.A.A.; dos Santos, W.T.P. Use of a lab-made screen-printed sensor with chemically deposited boron-doped diamond for simple and selective electrochemical detection of the synthetic cathinone N-ethylpentylone in forensic samples. Electrochim. Acta 2023, 465, 142996. [Google Scholar] [CrossRef]
- Araújo, D.S.; Arantes, L.C.; Faria, L.V.; Souza, K.A.O.; Pimentel, D.M.; Barbosa, S.L.; Richter, E.M.; Muñoz, R.A.A.; dos Santos, W.T.P. Electrochemistry of 5F-MDMB-PICA synthetic cannabinoid using a boron-doped diamond electrode with short anodic-cathodic pretreatment: A simple screening method for application in forensic analysis. Electrochim. Acta 2023, 454, 142356. [Google Scholar] [CrossRef]
- Ren, S.; Zeng, J.; Zheng, Z.; Shi, H. Perspective and application of modified electrode material technology in electrochemical voltammetric sensors for analysis and detection of illicit drugs. Sens. Actuator A-Phys. 2021, 329, 112821. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Foster, C.W.; Banks, C.E. The electrochemical performance of graphene modified electrodes: An analytical perspective. Analyst 2012, 137, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Fakude, C.T.; Modise, R.P.; Haruna, A.B.; Pillay, J.; Ozoemena, K.I. Advances in the application of nanomaterials for the electrocatalytic detection of drugs of abuse. Advanced Sensor and Energy Materials 2023, 2, 100056. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, characterization and applications of conductive polymers: a brief review. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- AL-Refai, H.H.; Ganash, A.A.; Hussein, M.A. Polythiophene and its derivatives—based nanocomposites in electrochemical sensing: a mini review. Mater. Today Commun. 2021, 26, 101935. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Karagözler, A.E.; Russell, G.C.; Zimmer, H.; Mark, H.B., Jr. Electrochemistry and detection of some organic and biological molecules at conducting poly(3-methylthiophene) electrodes. Biosens. Bioelectron. 1991, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R. Highly stable voltammetric measurements of phenolic compounds at poly(3-methylthiophene)-coated glassy carbon electrodes. Anal. Chem. 1989, 61, 2809–2811. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Yeh, W.M.; Tung, T.S.; Liao, J.Y. Amperometric detection of morphine based on poly(3,4-ethylenedioxythiophene) immobilized molecularly imprinted polymer particles prepared by precipitation polymerization. Anal. Chim. Acta 2005, 542, 90–96. [Google Scholar] [CrossRef]
- Weng, C.-H.; Yeh, W.-M.; Ho, K.-C.; Lee, G.-B. A microfluidic system utilizing molecularly imprinted polymer films for amperometric detection of morphine. Sensor. Actuator. B Chem. 2007, 121, 576–582. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Direct and simple electrochemical determination of morphine at PEDOT modified Pt electrode. Electroanalysis 2011, 23, 737–746. [Google Scholar] [CrossRef]
- Granado, V.L.V.; Gutiérrez-Capitán, M.; Fernández-Sánchez, C.; Gomes, M.T.S.R.; Rudnitskaya, A.; Jimenez-Jorquera, C. Thin-film electrochemical sensor for diphenylamine detection using molecularly imprinted polymers. Anal. Chim. Acta 2014, 809, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.E.; Branger, C.; Iordache, T.-V.; Iovu, H.; Vitrik, O.B.; Dyshlyuk, A.V.; Sarbu, A.; Brisset, H. Application of unusual on/off electrochemical properties of a molecularly imprinted polymer based on an EDOT–thiophene precursor for the detection of ephedrine. Electrochem. Commun. 2018, 94, 45–48. [Google Scholar] [CrossRef]
- Hui, Y.; Bian, C.; Xia, S.; Tong, J.; Wang, J. Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review. Anal. Chim. Acta 2018, 1022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Granado, V.L.V.; Rudnitskaya, A.; Oliveira, J.A.B.P.; Gomes, M.T.S.R. Design of molecularly imprinted polymers for diphenylamine sensing. Talanta 2012, 94, 133–139. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. The synthetic drug phenomenon. Available online: https://www.unodc.org/res/WDR-2023/WDR23_B3_CH1_Synthetic_drugs.pdf (accessed on July 2023).
- Ribeiro, M.F.M.; da Cruz Júnior, J.W.; Dockal, E.R.; McCord, B.R.; de Oliveira, M.F. Voltammetric determination of cocaine using carbon screen printed electrodes chemically modified with uranyl Schiff base films. Electroanalysis 2016, 28, 320–326. [Google Scholar] [CrossRef]
- Castro, A.S.; de Menezes, M.M.T.; Alves, G.M.; de Oliveira, M.F. Voltammetric analysis of cocaine hydrochloride at carbon paste electrode chemically modified with N,N’-ethylene-bis-(salicylideneiminato) manganese(II) Schiff base complex. Microchem. J. 2020, 153, 104399. [Google Scholar] [CrossRef]
- Castro, A.S.; Rodrigues, C.H.P.; de Menezes, M.M.T.; da Silva, A.B.D.; Bruni, A.T.; de Oliveira, M.F. Fe(II), Ni(II), Cu(II), and Co(II) salen Schiff base complexes: Proposal for a voltammetric sensor to analyze cocaine hydrochloride and its interferents. Forensic Chemistry 2021, 25, 100347. [Google Scholar] [CrossRef]
- Sengel, T.Y.; Guler, E.; Gumus, Z.P.; Aldemir, E.; Coskunol, H.; Akbulut, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S.; Endo, T.; Yagci, Y. An immunoelectrochemical platform for the biosensing of ‘Cocaine use’. Sensor. Actuator. B Chem. 2017, 246, 310–318. [Google Scholar] [CrossRef]
- Florea, A.; Cowen, T.; Piletsky, S.; De Wael, K. Polymer platforms for selective detection of cocaine in street samples adulterated with levamisole. Talanta 2018, 186, 362–367. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: a review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Costa, S.S.; Mounssef, B.; Pacheco, J.G.; Fernandes, E.; Carvalho, F.; Rodrigues, C.M.P.; Delerue-Matos, C.; Braga, A.A.C.; Moreira Gonçalves, L.; Quinaz, M.B. Electrochemical sensing of ecstasy with electropolymerized molecularly imprinted poly(o-phenylenediamine) polymer on the surface of disposable screen-printed carbon electrodes. Sensor. Actuator. B Chem. 2019, 290, 378–386. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, F.; Huang, X.; He, S.; Han, Q.; Zhang, Z.; Liu, W. Fabrication of a molecularly-imprinted-polymer-based graphene oxide nanocomposite for electrochemical sensing of new psychoactive substances. Nanomaterials 2023, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Merli, D.; Lio, E.; Protti, S.; Coccia, R.; Profumo, A.; Alberti, G. Molecularly imprinted polymer-based voltammetric sensor for amino acids/indazole derivatives synthetic cannabinoids detection. Anal. Chim. Acta 2024, 1288, 342151. [Google Scholar] [CrossRef]
- Florea, A.; de Jong, M.; de Wael, K. Electrochemical strategies for the detection of forensic drugs. Curr. Opin. Electrochem. 2018, 11, 34–40. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Perspectives of Polymers in Forensic Analysis. Macromol 2023, 3, 108–119. [Google Scholar] [CrossRef]
- Forlani, L. Catalysis in nucleophilic aromatic substitution reactions. The presence of molecular complexes on the pathway of reactions between 1-fluoro- and 1-chloro-2,4-dinitrobenzene and aliphatic amines. J. Chem. Soc. Perkin Trans. 2, 1993; 1525–1530. [Google Scholar] [CrossRef]
- Forlani, L.; Mezzina, E. Interactions between amines and aromatic fluoro derivatives. 19F NMR Investigation in [2H8]toluene. J. Chem. Soc. Perkin Trans. 2 1995, 11, 2019–2021. [Google Scholar] [CrossRef]
- Boitsov, S.; Songstad, J.; Törnroos, K.W. [4-(Trifluoromethyl)phenyl]aceto-nitrile. Acta Cryst. C 2002, 58, o145–o147. [Google Scholar] [CrossRef] [PubMed]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Kim, D.-M.; Shim, K.-B.; Son, J.I.; Reddy, S.S.; Shima, Y.-B. Spectroelectrochemical and electrochromic behaviors of newly synthesized poly[3′-(2-aminopyrimidyl)-2,2′:5′,2″-terthiophene]. Electrochim. Acta 2013, 104, 322–329. [Google Scholar] [CrossRef]
- Bauerle, P.; Scheib, St. Synthesis and characterization of thiophenes, oligothiophenes and polythiophenes with crown ether units in direct n-conjugation. Acta Polym. 1995, 46, 124–129. [Google Scholar] [CrossRef]
- Tutuncu, E.; Ozkut, M.I.; Balci, B.; Berk, H.; Cihaner, A. Electrochemical and optical characterization of a multielectrochromic copolymer based on 3,4-ethylenedioxythiophene and functionalized dithienylpyrrole derivative. Eur. Polym. J. 2019, 110, 233–239. [Google Scholar] [CrossRef]
- Ahumada, J.C.; Soto, J.P.; Alemán, C.; Torras, J. Synthesis and characterization of a new benzobisoxazole/thiophene derivative polymer and the effect of the substituent on the push/pull properties. J. Polymer Sci. 2021, 59, 3167–3180. [Google Scholar] [CrossRef]
- Tanaka, K.; Shichiri, T.; Wang, S.; Yamabe, T. A study of the electropolymerization of thiophene. Synth. Met. 1988, 24, 203–215. [Google Scholar] [CrossRef]
- Furukawa, Y.; Akimoto, M.; Harada, I. Vibrational key bands and electrical conductivity of polythiophene. Synth. Met. 1987, 18, 151–156. [Google Scholar] [CrossRef]
- Louarn, G.; Buisson, J.P.; Lefrant, S.; Fichou, D. Vibrational studies of a series of α-oligothiophenes as model systems of polythiophene. J. Phys. Chem. 1995, 99, 11399–11404. [Google Scholar] [CrossRef]
- Chen, R.; Chen, S.; Zhou, Y.; Wei, Z.; Wang, H.; Zheng, Y.; Li, M.; Sun, K.; Li, Y. Unsubstituted polythiophene film deposited via in-situ sequential solution polymerization for chemo-/electrochromism. Macromolecules 2020, 53, 4247–4254. [Google Scholar] [CrossRef]
- Kinno, Y.; Omachi, H.; Nakanishi, Y.; Shinohara, H. Synthesis of long-chain polythiophene inside carbon nanotubes. Chem. Lett. 2018, 47, 1022–1025. [Google Scholar] [CrossRef]
- Bouabdallaoui, M.; Aouzal, Z.; Ben Jadi, S.; El Jaouhari, A.; Bazzaoui, M.; Lévi, G.; Aubard, J.; Bazzaoui, E.A. X-ray photoelectron and in situ and ex situ resonance Raman spectroscopic investigations of polythiophene overoxidation. J. Solid State Electrochem. 2017, 21, 3519–3532. [Google Scholar] [CrossRef]
- Fu, M.; Shi, G.; Chen, F.; Hong, X. Doping level change of polythiophene film during its electrochemical growth process. Phys. Chem. Chem. Phys. 2002, 4, 2685–2690. [Google Scholar] [CrossRef]
- Subbulakshmi, R.R.; Palanichamy, E.; Arivazhagan, M.; Manivel, S. Theoretical studies on molecular structure and vibra-tional spectra of 2,4-difluoro-1-methoxy benzene and 1-chloro-3-methoxy benzene. Int. J. Sci. Res. Phys. Appl. Sci. 2019, 7, 34–48. [Google Scholar] [CrossRef]
- Jo, S.G.; Park, D.H.; Kim, B.-G.; Seo, S.; Lee, S.J.; Kim, J.; Kim, J.; Joo, J. Dual-mode waveguiding of Raman and lumines-cence signals in a crystalline organic microplate. J. Mater. Chem. C 2014, 2, 6077–6083. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Králík, F.; Synytsya, A. Voltammetric detection of vanillylmandelic acid and homovanillic acid using urea-derivative-modified graphite electrode. Sensors 2023, 23, 3727. [Google Scholar] [CrossRef]
- Dragan, A.M.; Feier, B.G.; Tertiș, M.; Bodoki, E.; Truta, F.; Ștefan, M.-G.; Kiss, B.; Van Durme, F.; De Wael, K.; Oprean, R.; Cristea, C. Forensic analysis of synthetic cathinones on nanomaterials-based platforms: chemometric-assisted voltametric and UPLC-MS/MS investigation. Nanomaterials 2023, 13, 2393. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-based electrochemical sensors: mechanism, preparation, and application in bio-medicine. Adv. NanoBiomed Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- Schram, J.; Parrilla, M.; Sleegers, N.; Van Durme, F.; Van Den, J.; van Nuijs, A.L.N.; De Wael, K. Electrochemical profiling and LC-MS characterization of synthetic cathinones: from methodology to detection in forensic samples. Drug Test. Anal. 2021, 13, 1282–1294. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Vatrsková, L.; Spálovská, D.; Králík, F.; Cuřínová, P.; Winkler, M.; Budka, J.; Jurásek, B.; Kuchař, M.; Setnička, V. Complexation of cathinones by 4-tert-butylcalix[4]arene tetraacetate as a possible technique for forensic analysis. Forensic Toxicol. 2020, 38, 70–78. [Google Scholar] [CrossRef]
- Shishkanova, T.V.; Štěpánková, N.; Tlustý, M.; Tobrman, T.; Jurásek, B.; Kuchař, M.; Trchová, M.; Fitl, P.; Vrňata, M. Electro-chemically oxidized 15-crown-5 substituted thiophene and host-guest interaction with new psychoactive substances. Elec-trochim. Acta 2021, 373, 137862. [Google Scholar] [CrossRef]
- Ross, E.A.; Watson, M.; Goldberger, B. “Bath salts“ intoxication. N. Engl. J. Med. 2011, 365, 967–968. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).