Submitted:
26 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- https://www.who.int/health-topics/tuberculosis#tab=tab_1 accessed on 11 April 2024.
- Sester, M et al. “Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis.” The European respiratory journal vol. 37,1 (2011): 100-11. [CrossRef]
- Shah, Maunank, and Susan E Dorman. “Latent Tuberculosis Infection.” The New England journal of medicine vol. 385,24 (2021): 2271-2280. [CrossRef]
- Person, Anna K, and Timothy R Sterling. “Treatment of latent tuberculosis infection in HIV: shorter or longer?.” Current HIV/AIDS reports vol. 9,3 (2012): 259-66. [CrossRef]
- https://www.eacsociety.org/media/guidelines-12.0.pdf accessed on 11 April 2024.
- Goletti, Delia et al. “Latent tuberculosis infection screening in persons newly-diagnosed with HIV infection in Italy: A multicentre study promoted by the Italian Society of Infectious and Tropical Diseases.” International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases vol. 92 (2020): 62-68. [CrossRef]
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2022–2020 data. https://www.ecdc.europa.eu/sites/default/files/documents/Tuberculosis-surveillance-monitoring-europe-2022_0.pdf.
- Pipitò, Luca et al. “Hospitalizations for tuberculosis in Sicily over the years 2009-2021: Clinical features, comorbidities, and predictors of mortality.” Journal of infection and public health vol. 16,9 (2023): 1518-1524. [CrossRef]
- Petruccioli, Elisa et al. “Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection.” The Journal of infection vol. 80,5 (2020): 536-546. [CrossRef]
- Nziza, Nadege et al. “Defining Discriminatory Antibody Fingerprints in Active and Latent Tuberculosis.” Frontiers in immunology vol. 13 856906. 20 Apr. 2022. [CrossRef]
- Chendi, Bih H et al. “CCL1 and IL-2Ra differentiate Tuberculosis disease from latent infection Irrespective of HIV infection in low TB burden countries.” The Journal of infection vol. 83,4 (2021): 433-443. [CrossRef]
- Bastos, Mayara Lisboa et al. “The latent tuberculosis cascade-of-care among people living with HIV: A systematic review and meta-analysis.” PLoS medicine vol. 18,9 e1003703. 7 Sep. 2021. [CrossRef]
- Kall, Meaghan M et al. “Latent and subclinical tuberculosis in HIV infected patients: a cross-sectional study.” BMC infectious diseases vol. 12 107. 4 May. 2012. [CrossRef]
- Bourgarit, Anne et al. “Latent Tuberculosis Infection Screening and 2-Year Outcome in Antiretroviral-Naive HIV-Infected Patients in a Low-Prevalence Country.” Annals of the American Thoracic Society vol. 12,8 (2015): 1138-45. [CrossRef]
- White, Helena A et al. “The impact, effectiveness and outcomes of targeted screening thresholds for programmatic latent tuberculosis infection testing in HIV.” AIDS (London, England) vol. 36,14 (2022): 2035-2044. [CrossRef]
- Runels, Tessa et al. “Testing and treatment for latent tuberculosis infection in people living with HIV and substance dependence: a prospective cohort study.” BMJ open vol. 12,3 e058751. 10 Mar. 2022. [CrossRef]
- Matulyte, Elzbieta et al. “Latent Tuberculosis Infection and Associated Risk Factors among People Living with HIV and HIV-Uninfected Individuals in Lithuania.” Pathogens (Basel, Switzerland) vol. 12,8 990. 28 Jul. 2023. [CrossRef]
- Carlos Silveira Machado, António et al. “Adverse Events of Latent Tuberculosis Treatment With Isoniazid in People Living With HIV: A Case-Control Study in a Resource-Rich Setting.” Cureus vol. 15,7 e41647. 10 Jul. 2023. [CrossRef]
- van Geuns, Dorine et al. “Screening for tuberculosis infection and effectiveness of preventive treatment among people with HIV in low-incidence settings.” AIDS (London, England) vol. 38,2 (2024): 193-205. [CrossRef]
- Temu, Tecla M et al. “Latent tuberculosis is associated with heightened levels of pro-and anti-inflammatory cytokines among Kenyan men and women living with HIV on long-term antiretroviral therapy.” AIDS (London, England) vol. 37,7 (2023): 1065-1075. [CrossRef]
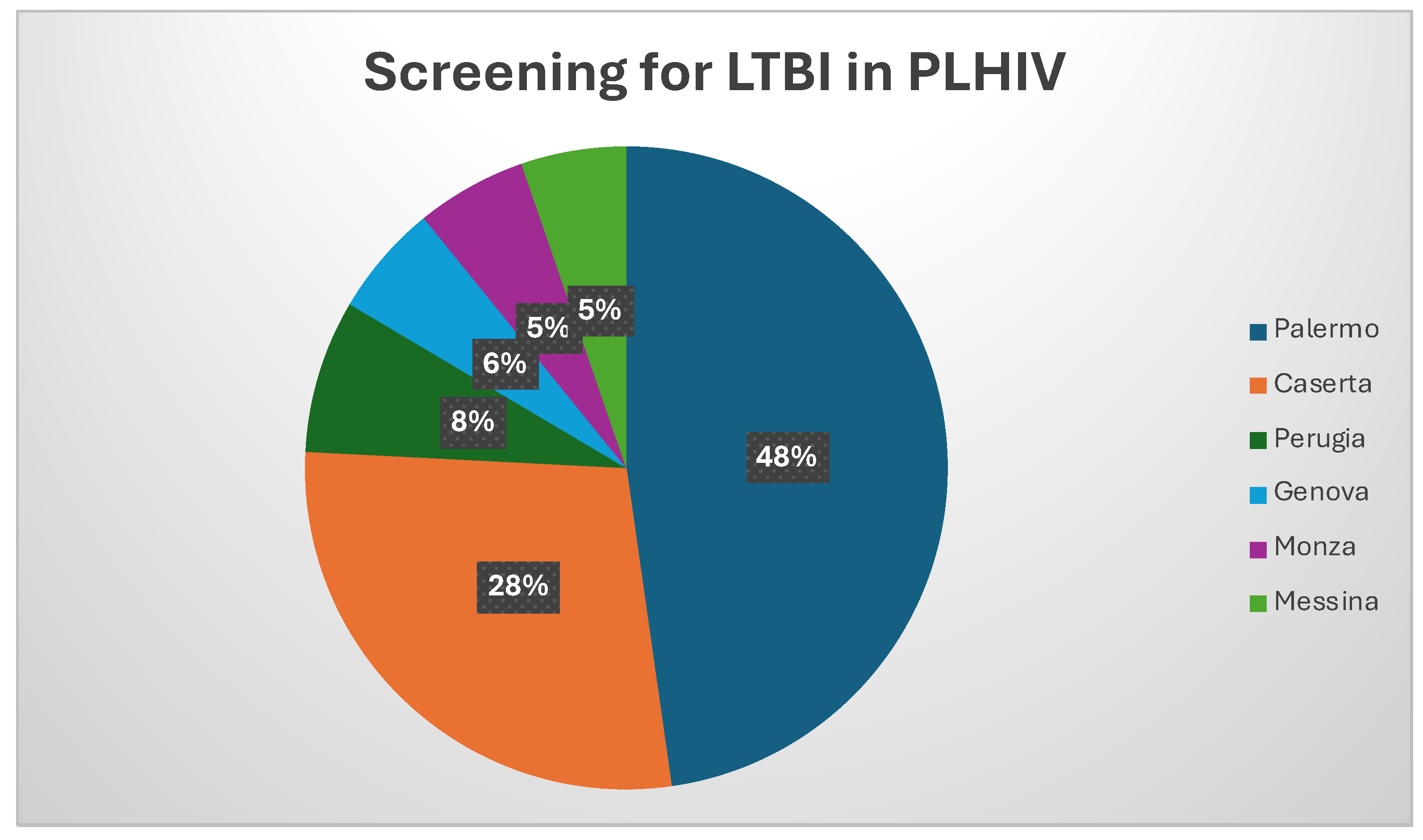
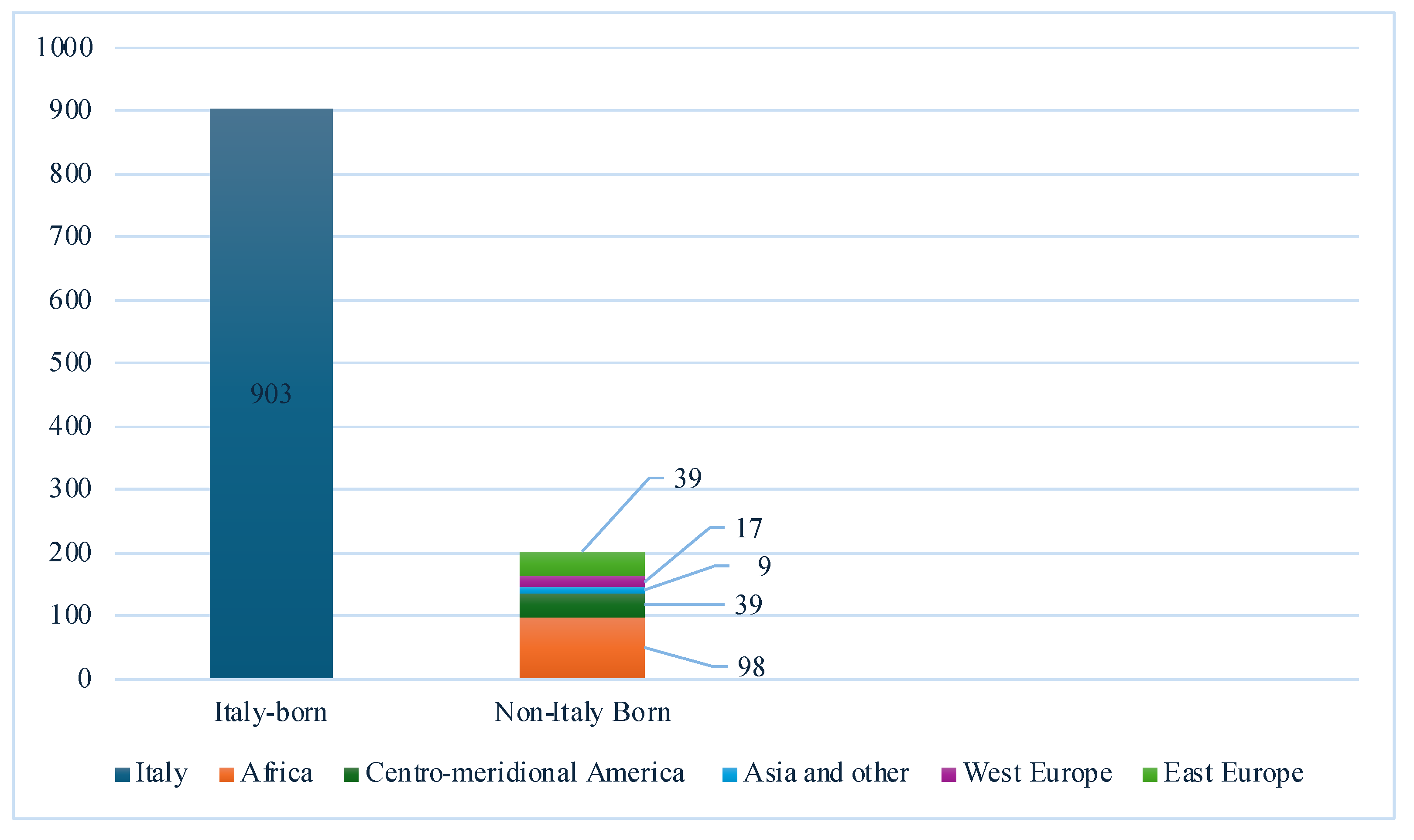
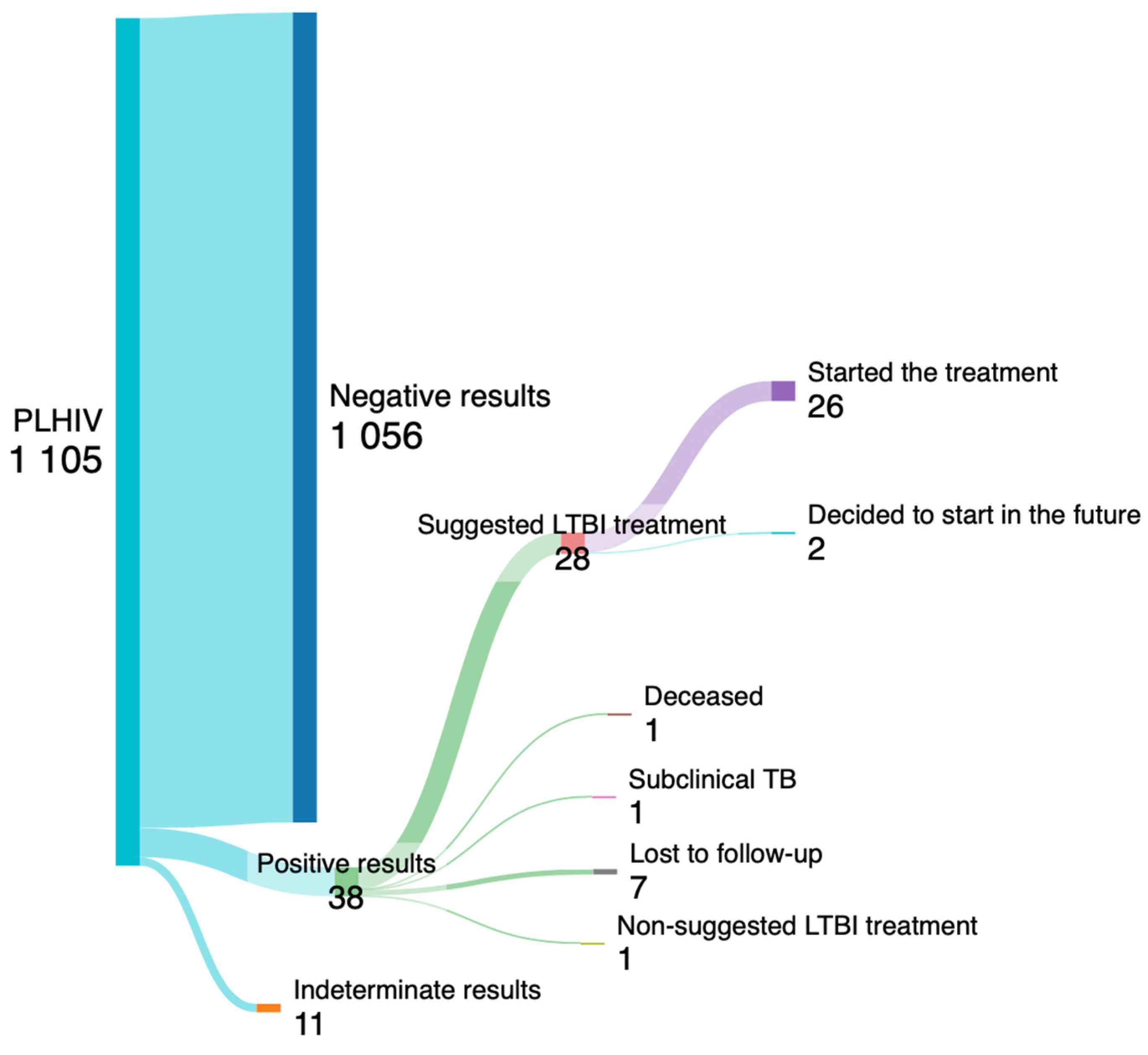
| Variables | All (n=1087) |
IGRA positive (n=31, 2.8%) |
IGRA negative (n=1056, 97.2%) |
p |
|---|---|---|---|---|
|
Sex, n (%) Female Male Other |
254 (23.4) 817 (75.2) 16 (1.5) |
9 (29.0) 22 (71.0) 0 |
245 (23.2) 795 (75.3) 16 (1.5) |
0.61 |
|
Country of origin, n (%) Italy Non-Italy-born |
892 (82.1) 195 (17.9) |
19 (61.3) 12 (38.7) |
873 (82.7) 183 (17.3) |
0.002 |
| Age, median (IQR) | 49 (40-56) | 49 (35-57) | 48.5 (40-56) | 0.90 |
|
Drug users, n (%) No Yes Missing |
842 (77.5) 124 (11.4) 121 (11.1) |
22 (71.0) 5 (16.1) 4 (12.9) |
820 (77.6) 119 (11.3) 117 (11.1) |
0.64 |
|
MSM, n (%) No Yes Missing |
686 (63.1) 270 (24.8) 131 (12.1) |
18 (58.1) 8 (25.8) 5 (16.1) |
668 (63.3) 262 (24.8) 126 (11.9) |
0.75 |
|
Alcoholism, n (%) No Yes Missing |
619 (57.0) 45 (4.1) 423 (38.9) |
19 (61.3) 5 (16.1) 7 (22.6) |
600 (56.8) 40 (3.8) 416 (39.4) |
0.001 |
| Years from HIV diagnosis, median (IQR) | 7.2 (2.3-17.3) | 5.0 (1.1-13.9) | 7.3 (2.3-17.3) | 0.34 |
| CD4, median (IQR) | 618 (426-840) | 647 (460-802) | 617 (426-841) | 0.57 |
| Nadir CD4, median (IQR) | 300 (104-484) | 406 (275-570) | 300 (103-482) | 0.06 |
|
Comorbidities, n (%) 0 1 ≥2 |
786 (72.3) 256 (23.6) 45 (4.1) |
26 (83.9) 3 (9.7) 2 (6.4) |
760 (72.0) 253 (24.0) 43 (4.1) |
0.16 |
| Cardiovascular diseases, n (%) | 239 (22.0) | 4 (12.9) | 235 (22.2) | 0.27 |
| Diabetes, n (%) | 61 (5.6) | 1 (3.2) | 60 (5.7) | 1.00 |
| Solid neoplasia, n (%) | 40 (3.7) | 1 (3.2) | 39 (3.7) | 1.00 |
| Hematologic malignancy, n (%) | 10 (0.9) | 1 (3.2) | 9 (0.8) | 0.25 |
| Variables | All (n=26) |
Italy-born (n=14) |
Non-Italy-born (n=12) |
p |
|---|---|---|---|---|
|
Pretreatment Ast Median IQR Alt Median IQR Bilirubin Median IQR |
18 (14-21) 18 (15-22) 0.40 (0.29-0.52) |
17 (14-19) 17 (15-21) 0.39 (0.29-0.50) |
22 (16-25) 18 (15-24) 0.52 (0.28-0.80) |
0.10 0.54 0.48 |
|
Last measured value* Ast Median IQR Alt Median IQR Bilirubin Median IQR |
21 (18-25) 22 (13-27) 0.41 (0.34-0.72) |
21 (18-29) 26 (15-30) 0.54 (0.33-0.72) |
21 (17-24) 17 (13-23) 0.39 (0.35-0.41) |
0.68 0.09 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





