Submitted:
25 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
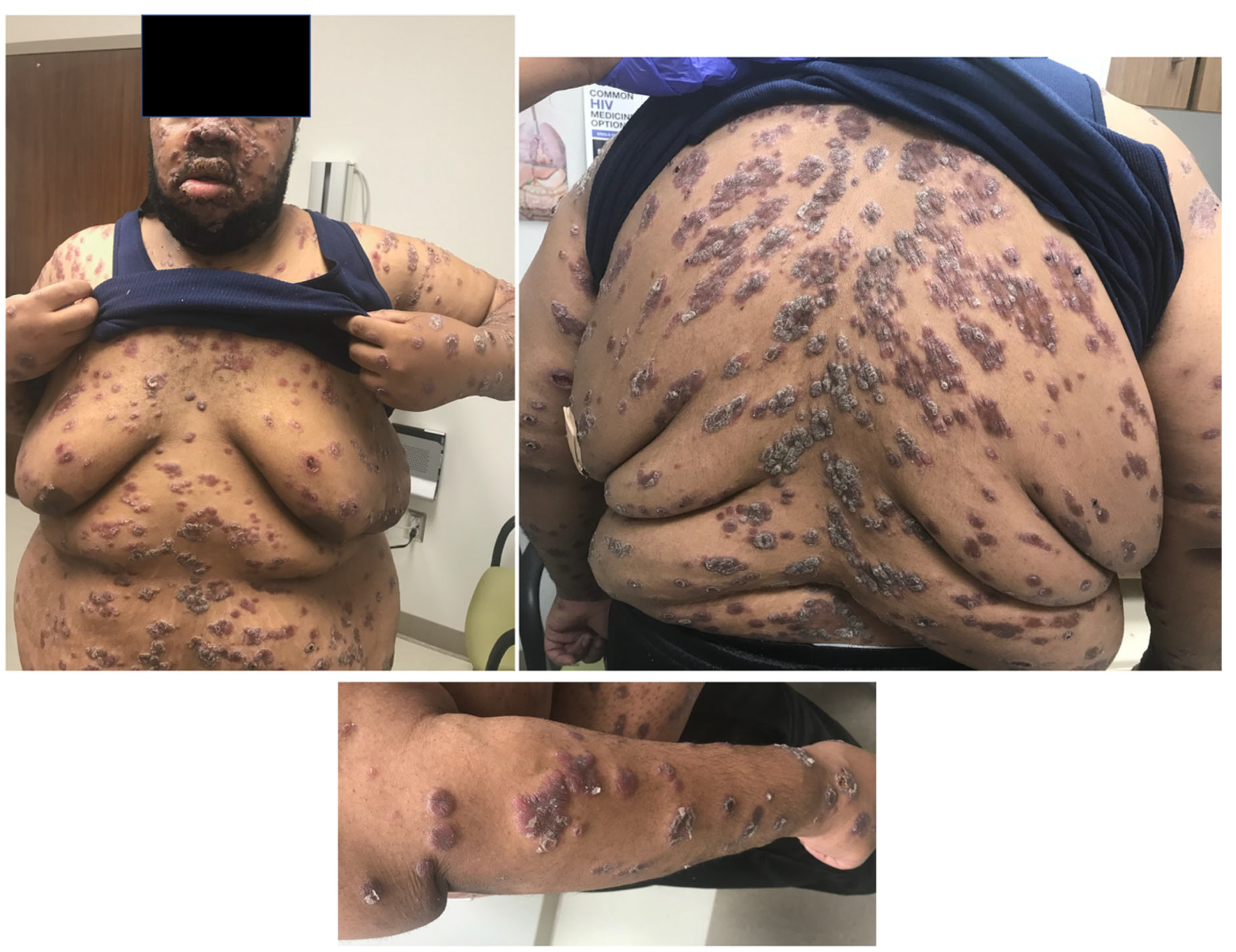
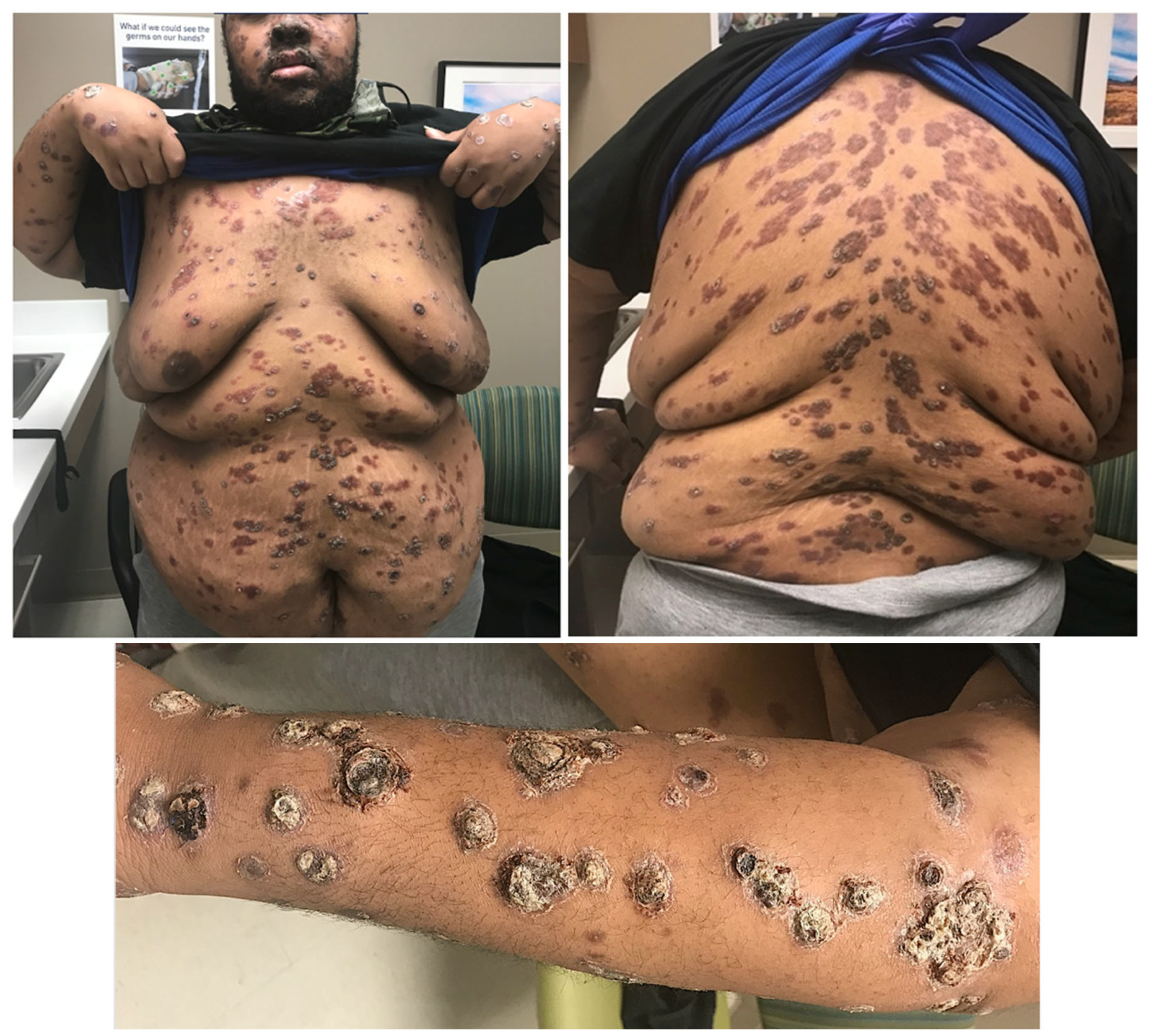
3. Case
4. Results: Case Series Summary
5. Discussion
5.1. Nomenclature
5.2. Historical Perspective
5.3. Description and Evolution of the Rash
5.4. Differential Diagnosis.
5.5. Epidemiology, Risk Factors, and Pathogenesis of Ulceronodular-Rupioid Syphilis
5.6. Syphilis Complications Associated with Ulceronodular-Rupioid Syphilis
5.7. Treatment of Ulceronodular-Rupioid Syphilis.
5.8. Previous Diagnostic Criteria of Ulceronodular-Rupioid Syphilis
5.9. A High Serological Titer as a Diagnostic Criterion
5.10. Rejection of the Jarisch-Herxheimer Reaction (JHR) as a Diagnostic Criteria
5.11. Rapid Response to Treatment as a Diagnostic Criteria
5.12. Histopathologic Criteria for Ulceronodular-Rupioid Syphilis
5.13. Revised Diagnostic Criteria for Ulceronodular-Rupioid Syphilis
- (1)
- Dermatologic. Round or oval pleomorphic skin lesions: papulopustules, nodules, ulcerations, ulcers with brown-black rupioid crusts, and healing lesions (same as Neisser [43]).
- (2)
- Histopathologic. Typically, there is a dermal infiltrate of lymphocytes, histiocytes, and plasma cells, often with vascular involvement and/or granuloma formation. Immunohistochemical staining provides confirmation of the presence of spirochetes in about 80% of cases.
- (3)
- Serologic. A positive RPR or VDRL titer, with RPR and VDRL titers of at least 1:8 and a positive treponemal test. Greater than 90% of patients will have an RPR or VDRL titer of 1:32 or higher.
- (4)
- Response to Therapy. Improvement or resolution of the dermatologic manifestations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A: Glossary of Dermatopathology Terms
References
- Pleimes, M.; Hartschuh, W.; Kutzner, H.; Enk, A.H.; Hartmann, M. Malignant syphilis with ocular involvement and organism-depleted lesions. Clin. Infect. Dis. 2009, 48, 83–85. [Google Scholar] [CrossRef]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The immunopathobiology of syphilis: the manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W. 3rd. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.; Zambare, U.; Nayak, C. Nodulo-ulcerative and erythrodermic secondary syphilis in human immunodeficiency virus-infected individuals. Int. J. STD AIDS 2019, 30, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Barit, J.-V.J.; Hiroyasu, S.; Yamada, K.; Tsuruta, D. Malignant syphilis in a young immunocompetent patient presenting as ulceronecrotic lesions on the lower extremities. Int. J. Dermatol. 2023, 62, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Chang, L.W.; Tuffanelli, D.L. Lues maligna. Presentation of a case and a review of the literature. Arch. Dermatol. 1969, 99, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Wibisono, O.; Idrus, I.; Djawad, K. Malignant syphilis: a systematic review of the case reports published in 2014-2018. Actas Dermosifiliogr. (Engl Ed). 2021, S0001-7310(21)00135-6. [CrossRef]
- Sá Lopes, R.; Monteiro, A.S.; Saez, R.; Candeias, C.; Mendonça, C. Malignant syphilis: a rare case of early secondary syphilis in an immunocompetent patient. Eur. J. Case Rep. Intern. Med. 2023, 10, 003721. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Shaw, F.M.; Li, M.M.; Blalock, T.W. A rare case of lues maligna in an HIV-negative woman. Dermatol. Online J. [CrossRef]
- Correia, C.; Borges-Costa, J.; Soares-de-Almeida, L.; Filipe, P. Lues maligna in an immunocompetent patient. Sex. Transm. Dis. 2022, 49, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Lv, W. Malignant syphilis. N. Engl. J. Med. 2023, 388, 1991. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Idrogo, J.J.; Muñante, R.; López-Fuentes, M.; Sanz-Castro, M.; Ventura-León, A.; Chávez-Esparza, G.; García-Cortez, Y. Malignant syphilis as the presenting complaint of advanced HIV. Int. J. STD AIDS 2023, 34, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Cavallo, F.; Susca, S.; Mastorino, L.; Trunfio, M.; Bonora, S.; Rugge, W.; Calleri, G.; Conti, L.; Senetta, R.; et al. Oral doxycycline in HIV-related synchronous malignant syphilis and condyloma lata. Ital. J. Dermatol. Venerol. 2022, 157, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Melian-Olivera, A.; Jimenez-Cauhe, J.; Moya-Martinez, C. Malignant syphilis. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 462. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kamath, V.; Martinello, M.; Overton, K. Case report of a man with HIV presenting with malignant syphilis. Sex. Health 2023, 20, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Cao, Y.L.; Man, X.Y. Malignant syphilis in a young woman: a case report. J. Int. Med. Res. 2022, 50, 3000605221131368. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-Z.; Jia, L.-L.; Li, Y. A case of malignant syphilis complicated with myiasis in Northeast China. Indian J. Derm. Venereol. Leprol. 2020, 86, 470. [Google Scholar] [CrossRef] [PubMed]
- Djawad, K. Malignant syphilis as an initial presentation of HIV infection: a case report. Internat. J. Derm.Venereol. 2021, 4, 192–194. [Google Scholar] [CrossRef]
- Dimnik, J.; Benko, M.; Hosta, V.; Murnik Rauh, A.; Pagon, A.; Špik, V.C.; Battelino, S.; Vozel, D. Malignant syphilis in a female patient: a case report and mini-review. Trop. Med. Infect. Dis. 2022, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Ma, D.L. Oyster shell-like skin lesions in a young man. Mayo Clin Proc. 2021, 96, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.; Patel, S.P.; Motaparthi, K. Ulceronecrotic rash in an immunocompetent individual. JAAD Case Rep. 2022, 27, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Dhaliwal, P.; Readinger, A. Vasculitis in a case of rupioid syphilis in HIV. Proc (Bayl Univ Med Cent). 2022, 35, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res. Hum. Retroviruses. 2021, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tanojo, N.; Murtiastutik, D.; Sari, M.; Astindari, *!!! REPLACE !!!*; Widyantari, S.; Nurul Hidayati, A.; Indramaya, D.M. A single dose of benzathine penicillin G as an effective treatment for malignant syphilis in an HIV-positive patient: a case report. Acta Dermatovenerol. Alp. Pannonica Adriat. 2022, 31, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Lozada, A.; Monteiro, A.F. Malignant syphilis in a patient with acquired Human Immunodeficiency Virus (HIV) infection. An. Bras. Dermatol. 2022, 97, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A.; Şikar Aktürk, A.; Odyakmaz Demirsoy, E.; Kıran, R.; Bayramgürler, D.; Sayman, N.; Açıkbaş, E.; Vural, Ç. Lues maligna in an immunocompetent male: a case report. J. Cosmet. Dermatol. 2022, 21, 3160–3162. [Google Scholar] [CrossRef]
- Lueking, R.; Lazarte, S. Malignant syphilis. N. Engl. J. Med. 2022, 386, e26. [Google Scholar] [CrossRef]
- Ghanian, S.; Dalla Costa, R.; Singer, H.; Robinson-Bostom, L. Extensive lues maligna syphilis in an immunocompromised male. Int. J. Dermatol. 2022, 61, e410–e411. [Google Scholar] [CrossRef]
- Bosch-Amate, X.; Fustà-Novell, X.; Morgado-Carrasco, D. Cutaneous ulcers in an untreated HIV patient. Dermatol. Pract. Concept. 2021, 11, e2021007. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, M.C.; Trila, C.; Heffner, L.; Cantón, M.E.; Quadrana, F.; Zylberman, M. [Malignant syphilis in a patient with HIV infection]. Medicina (B Aires) 2020, 80, 714–717. [Google Scholar] [PubMed]
- Dos Santos, C.A.; Benevides, L.C.; Cardili, R.N.; Pileggi, G.C.S.; Ferriani, V.P.L.; Roselino, A.M.F. Malignant syphilis in a young patient with juvenile idiopathic arthritis under biological therapy. J. Clin. Rheumatol. 2021, 27, S382–S383. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Li, D.M.; Qiu, Y.; Fu, H.J.; Zhang, X.Y.; Shi, D.M. Malignant syphilis Accompanied with neurosyphilis in a malnourished patient: a case report. World J. Clin. Cases. 2019, 7, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Yildizhan, I.K.; Şanli, H.E.; Çetinkaya, H.; Akay, B.N.; Koçyiğit, P.; Kundakçi, N. A rare case of malignant syphilis after adalimumab therapy due to Crohn's Disease associated with bariatric surgery. Microbiol. Infect. Dis. 2019, 95, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, J.; Padma, M.; Ma, Z.; Tian, Y. Cutaneous leukocytoclastic vasculitis as the first manifestation of malignant syphilis coinfected with Human Immunodeficiency Virus. J. Cutan. Pathol. 2019, 46, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Spivak, A.M. Lues maligna. Open Forum Infect. Dis. 2017, 4, ofx139. [Google Scholar] [CrossRef] [PubMed]
- Braue, J.; Hagele, T.; Yacoub, A.T.; Mannivanan, S.; Sokol, L.; Glass, F.; Greene, J.N. A case of rupioid syphilis masquerading as aggressive cutaneous lymphoma. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015026. [Google Scholar] [CrossRef] [PubMed]
- Devkota, A.R.; Ghimire, R.; Sam, M.; Aung, O. Malignant syphilis as an initial presentation of underlying HIV infection: a case report. Brit. J. Med. Pract. 2015, 8, a816. [Google Scholar]
- Kelly, J.D.; LeLeux, T.M.; Citron, D.R.; Musher, D.M.; Giordano, T.P. Ulceronodular syphilis (lues maligna praecox) in a person newly diagnosed with HIV infection. BMJ Case Rep. 2011, 2011, bcr1220103670. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Marley-Kemp, D.; Keller, M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis 2014, 94, 119–121. [Google Scholar] [PubMed]
- Schöfer, H.; Imhof, M.; Thoma-Greber, E.; Brockmeyer, N.H.; Hartmann, M.; Gerken, G.; Pees, H.W.; Rasokat, H.; Hartmann, H.; Sadri, I.; et al. Active syphilis in HIV infection: a multicentre retrospective survey. The German AIDS Study Group (GASG). Genitourin. Med. 1996, 72, 176–181. [Google Scholar] [CrossRef]
- Yu, T.; Che, J.; Song, J.; Duan, X.; Yang, J. Annular rupioid secondary syphilis confined to the face. Int. J. Infect. Dis. 2022, 122, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Bazin, P.-A.-E. Leçons Théoriques et Cliniques sur la Syphilis et les Syphilides. Adrien Delahaye, Paris, France, 1859.
- Neisser, A. Malignant syphilis. Brit. J. Derm. 1897, 9, 11–26. [Google Scholar]
- Haslund, A. Syphilis maligna. Archiv fur Dermatologie und Syphilis 1897, 38, 345–392. [Google Scholar] [CrossRef]
- Requena, C.B.; Orasmo, C.R.; Ocanha, J.P.; Barraviera, S.R.C.S.; Marques, M.E.A.; Marques, S.A. Malignant syphilis in an immunocompetent female patient. An. Bras. Dermatol. 2014, 89, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Rallis, E.; Paparizos, V. Malignant Syphilis as the first manifestation of HIV infection. Infect. Dis. Rep. 2012, 4, e15. [Google Scholar] [CrossRef] [PubMed]
- Fustà-Novell, X.; Morgado-Carrasco, D.; Barreiro-Capurro, A.; Manzardo, C.; Alsina-Gibert, M.; et al. Syphilis maligna: a presentation to bear in mind. Actas Dermosifiliogr. (Engl Ed) 2019, 110, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Flamm, A.; Alcocer, V.M.; Kazlouskaya, V.; Kwon, E.J.; Elston, D. Histopathologic features distinguishing secondary syphilis from its mimickers. J. Am. Acad. Dermatol. 2020, 82, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, D.; Tripoli, L.; Abell, E. Lues maligna in a patient with Human Immunodeficiency Virus infection. Am. J. Med. 1988, 85, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Faraone, A.; Fortini, A. An elderly woman with ulceronodular rash. Eur. J. Intern. Med. 2017, 44, e3–e4. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019. Atlanta, GA 2020. Available online: https://www.cdc.gov/std/statistics/2019/std-surveillance-2019.pdf (accessed on 27 Jan 2024).
- Statistics Kingdom. Two Sample T-Test Calculator (Pooled-Variance). Available online: https://www.statskingdom.com/140MeanT2eq.html. (accessed on 15-AUG-2023).
- Wendland, T.; Furrer, H.; Vernazza, P.L.; Frutig, K.; Christen, A.; Matter, L. , et al. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 1999, 13, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Barros D’Elia Zanella, L.G.F.A.; Facchini Lellis, R.; Khoury, Z.; Keiko Matsuka Oyafuso, L.; Figueiredo-Mello, C. Rupioid lesions, PLEVA and superposition phenomenon in malignant syphilis: two case reports in HIV-infected patients. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e91–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, M.; Peng, R.R.; Gu, X.; Guan, Z.; Xu, H.; Zhou, P. Neurosyphilis is more common in malognant syphilis: a case series and review of the literature. Int. J. STD AIDS 2019, 30, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Karanfilian, K.M.; Almohssen, A.A.; Kapila, R.; Schwartz, R.A. Malignant syphilis: a new and revised definition. Int. J. Dermatol. 2023, 62, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.C.; Ali, R.A.; Isache, C.L.; Sharma, R.K.; Perniciaro, C. Malignant syphilis: ostraceous, ulceronecrotic lesions in a patient with Human Immunodeficiency Virus. Dermatol. Online J. 2017, 23, 13030/qt3ps899bh. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.M.; White, J.M.; Salisbury, J.R.; Creamer, D. Lues maligna. Clin. Exp. Dermatol. 2004, 29, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Sammet, S.; Draenert, R. Case report of three consecutive lues maligna infections in an HIV-infected patient. Int. J. STD AIDS, 2017, 28, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.L.; Ren, H.; Xu, B.F.; Zhang, J.P.; Zhang, R.L.; Wang, Q.Q.; Zhang, T.T. Evaluation of IL-17A, IL-17F, IL-23R, VDR, CCL2, CCL5, CCR2, and CCR5 gene polymorphisms and expression in Chinese individuals with syphilis. J. Cell. Biochem. 2018, 119, 10151–10164. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.M.; Sahi, S.K.; Tantalo, L.C.; Ho, E.L.; Dunaway, S.B.; Jones, T.; Hawn, T.R. Toll-like receptor polymorphisms are associated with increased neurosyphilis risk. Sex. Transm. Dis. 2014, 41, 440–446. [Google Scholar] [CrossRef] [PubMed]
- de Unamuno Bustos, B.; Sánchez, R.B.; Carazo, J.L.; de Míquel, V.A. Malignant syphilis with ocular involvement in an immunocompetent patient. Int. J. Dermatol. 2014, 53, e258–e260. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.H.; Ricciardo, B.; Manjri Tiwari, S.; French, M.A.; Italiano, C.M.; Vinciullo, C. A rare case of lues maligna with ocular involvement presenting as an unmasking immune reconstitution inflammatory syndrome in a patient with HIV infection. Australas. J. Dermatol. 2018, 59, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, B.; Almeidinha, Y.; Borelli, N.; Esteves, E.; Olivera, A.R.; Cestari, M.D.; Garbelini, L.; Eid, R.; Michalany, A.; de Oliveira Filho, J. Malignant syphilis and neurosyphilis in an immunocompetent patient. J. Am. Acad. Dermatol. 2016, 74, AB152. [Google Scholar] [CrossRef]
- Rockwood, N.; Nwokolo, N. Syphilis the great pretender: when is cancer not cancer? Sex. Transm. Infect. 2018, 94, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M. Bolan, G.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Domantay-Apostol, G.P.; Handog, E.B.; Gabriel, M.T. Syphilis: the international challenge of the great imitator. Dermatol. Clin. 2008, 26, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Lin, L.R.; Tong, M.L.; Zhang, H.L.; Huang, S.J.; Chen, Y.Y.; Guo, X.J.; Xi, Y.; Liu, L.; Chen, F.Y.; Zhang, Y.F.; Zhang, Q.; Yang, T.C. Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin. Infect. Dis. 2014, 59, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.A.; Steiner, B.M.; Rudolph, A.H. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 1995, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rompalo, A.M.; Cannon, R.O.; Quinn, T.C.; Hook, E.W. 3rd. Association of biologic false-positive reactions for syphilis with Human Immunodeficiency Virus infection. J. Infect. Dis. 1992, 165, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Lynn, W.A.; Lightman, S.L. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004, 4, 456–66. [Google Scholar] [CrossRef] [PubMed]
- Jordaan, H.F. Secondary syphilis. A clinicopathological study. Am. J. Dermatopathol. 1988, 10, 399–409. [Google Scholar] [PubMed]
- Kolber, S.E.; Manz, H.J.; Schwartz, D.A. Syphilis. In Pathology of Infectious Diseases; Connor, D.H., Chandler, F.W., Manz, H.J., Schwartz, D.A., Lack, E.E., Eds., Appleton and Lange, Stamford, CT, USA, 1997; pp. 833–846.
- Yamashita, M.; Fujii, Y.; Ozaki, K.; Urano, Y.; Iwasa, M.; Nakamura, S.; Fujii, S.; Abe, M.; Sato, Y.; Yoshino, T. Human Immunodeficiency Virus positive secondary syphilis mimicking cutaneous T-cell lymphoma. Diagn. Pathol. 2015, 10, 185. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta, GA 2019. Available online: https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf (accessed on 28 Jan 2024).
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Atlanta, GA: 2021. Available online: https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf (accessed on 27 Jan 2024).
- Johnson, K.A.; Snyder, R.E.; Tang, E.C.; de Guzman, N.S.; Plotzker, R.E.; Murphy, R.; Jacobson, K. Geospatial social determinants of health correlate with disparities in syphilis and congenital syphilis cases in California. Pathogens 2022, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta, GA 2017. Available online: https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf. (accessed on 28 Jan 2024).
- McGuire, T. Practice update: Sexually transmitted infections: implications for pharmacists. Aust. J. Pharmacists 2019, 100, 80–85. [Google Scholar]
- Luetkemeyer, A.F.; Donnell, D.; Dombrowski, J.C.; Cohen, S.; Grabow, C.; Brown, C.E.; Malinski, C.; Perkins, R.; Nasser, M.; Lopez, C.; Vittinghoff, E.; Buchbinder, S.P.; Scott, H.; Charlebois, E.D.; Havlir, D.V.; Soge, O.O.; Celum, C. DoxyPEP Study Team. Postexposure doxycycline to prevent bacterial sexually transmitted infections. N. Engl. J. Med. 2023, 388, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Stedman, T.L. Stedman's Medical Dictionary, 28th ed. [electronic]. Williams & Wilkins: Baltimore, MD (USA), 2006.
- Murphy, M.; Kerr, P.; Grant-Kels, J.M. The histopathologic spectrum of psoriasis. Clin. Dermatol. 2007, 25, 524–528. [Google Scholar] [CrossRef] [PubMed]
| Case | Age (yrs)/ sex/ [ref] |
HIV status/ CD4/ HIV VL/ Underlying conditions/ RPR or VDRL titer |
Description of lesions/ Location/ Duration prior to presentation/ Other diagnoses | Histopathologic Findings/ Spirochete Visualization by Warthin-Starry (W-S) stain or Immunostain |
Rx; Jarisch-Herxheimer Reaction (JHR) = Yes or No; Outcome |
|---|---|---|---|---|---|
|
Fi |
34/M/[6]e | Pre-HIV era (1967)/ Malnutrition/ RPR 1:256 |
Ulcerated lesions w/ rupioid crusts/ leg, R inguinal area, buttock, R dorsal and ventral lower trunk, and R hand/24 wks/ neurosyphilis/S. aureus infection | Necrotizing vasculitis; Necrosis of epidermis, upper dermis. Endothelial swelling and proliferation. Perivascular infiltrate in dermis of lymphocytes, plasma cells, some neutrophils; RBC extravasation. Fibrinoid material causing partial to complete lumen obliteration of most of the vessels/ Immunostain negative | Tetracycline, 10-days; JHR = No; Resolved over 2 wks |
|
1 |
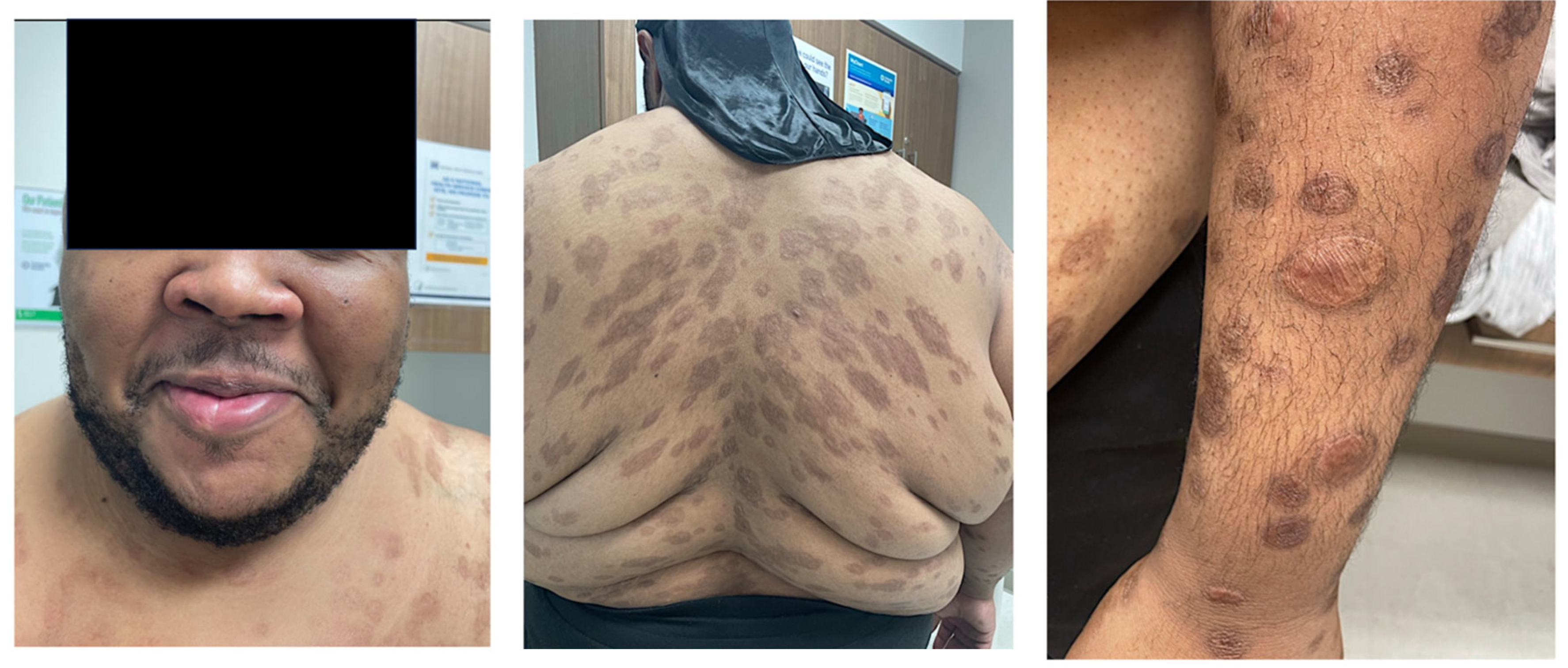
28/M/ This case |
HIV-positive/ CD4 411/ VL 35,200/ RPR 1:512 |
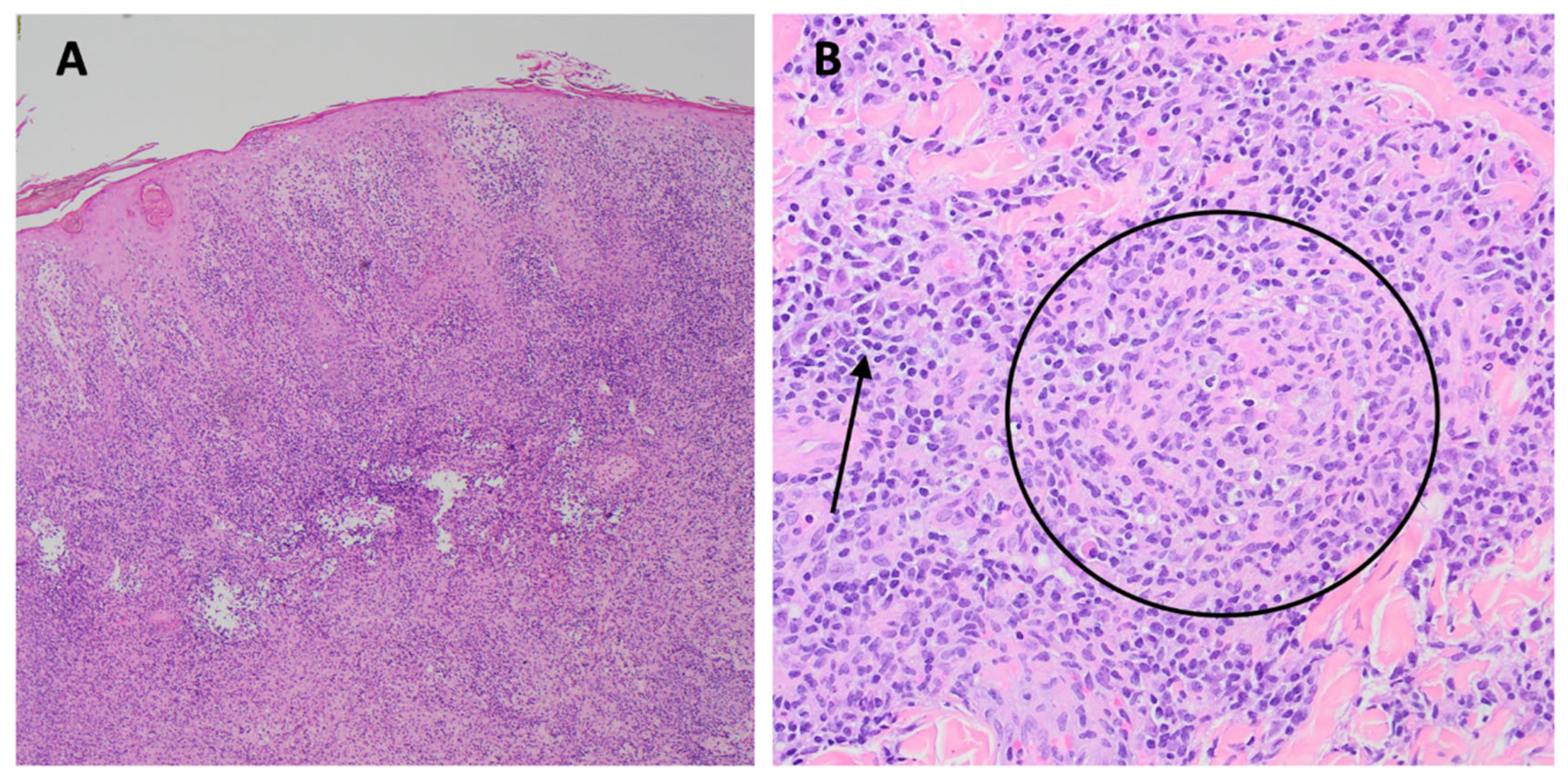
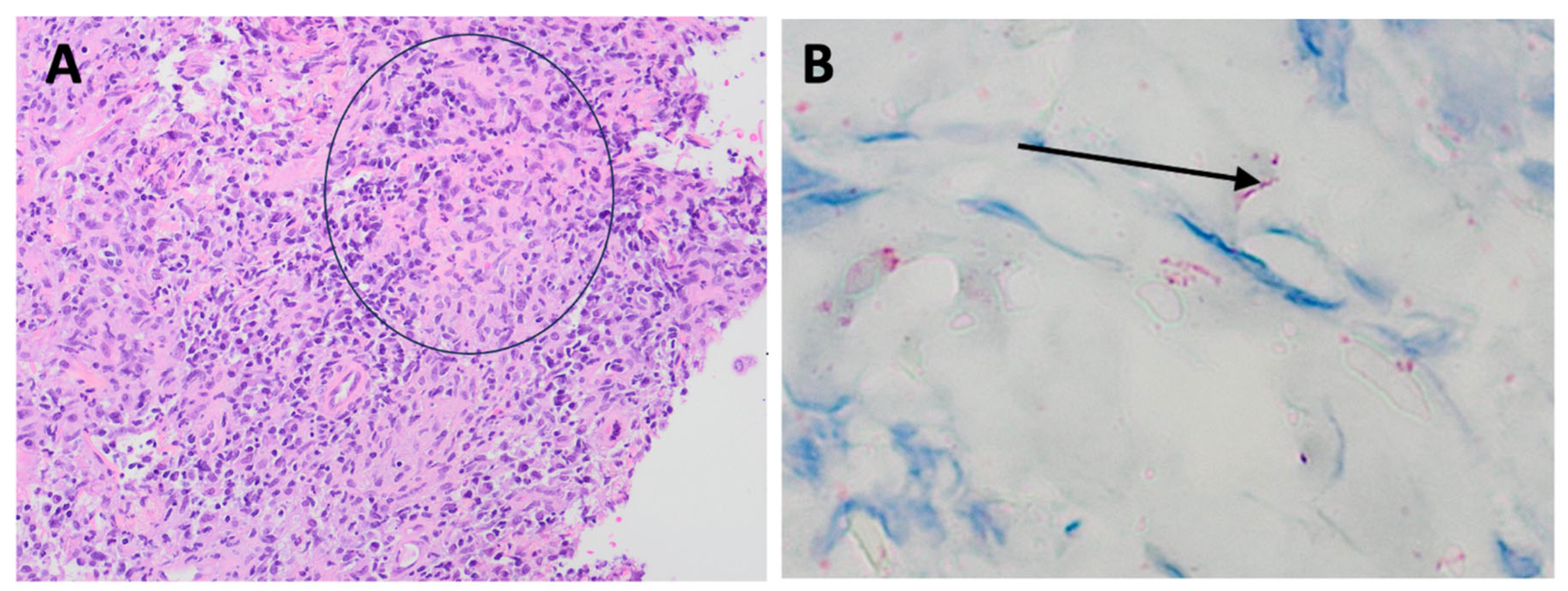
Nodular and ulcerated lesions with rupioid crusts/face, trunk, arms, legs/12 wks/ S. aureus, rectal chlamydia, gonorrhea | Lichenoid psoriasiform dermatitis with infiltrate of lymphocytes, plasma cells, and histiocytes; granuloma present/ Immunostain positive | IV penicillin X 1 dose; Benzathine PCN weekly X 3 weeks; doxycycline X 17 days; JHR = Yes; Resolved with hyperpigmented scarring |
|
2 |
26/M/[5] | HIV-negative/ RPR positive |
Multiple ulcerative lesions/ legs/ 8 wks | Dense infiltrates w/ plasma cells and fibrinoid degeneration/ Immunostain positive | Minocycline, then amoxicillin x 4 wks; JHR = No; Outcome not reported |
|
3 |
29/F/ [8] |
HIV-negative/ Hypothyroidism RPR 1:128 |
Erythematous nodules/ scalp, face, neck, axilla, trunk, back, palms and soles, and perineum/ 6 wks/ fever, myalgias | Subcorneal pustules; dermal granulomas with macrophages, Langerhans cells, lymphocytes, plasma cell infiltrate/ Immunostain not specified | IV Benzathine PCN weekly x 3 doses; JHR = No; Resolved over 1 mo |
|
4 |
43/F/ [9] |
HIV-negative/ DM II, Schizophrenia/ RPR 1:128 |
Nodules and plaques; some ulcerated and crusted/face, neck, trunk, legs, arms/4 wks | Epidermal acanthosis with diffuse dermal infiltrate of plasma cells/Immunostain positive | IM Benzathine PCN X 1 dose; JHR = No; Outcome not reported |
|
5 |
33, M/ [10] |
HIV-negative/ no PMH/ RPR 1:256 |
Erythematous-violaceous, ulcerated nodules, and plaques with rupioid crusts/ 8 wks | Dermal infiltrate of plasma cells, histiocytes, and lymphocytes with granuloma/ Immunostain positive | IV Benzathine PCN weekly X 3; JHR = Yes; Resolved over 6 wks with hyperpigmented macules |
|
6 |
44/M/[11] | HIV-positive/ CD4 86/ VL 35,900/RPR 1:32 |
Blackish-brown lamellated plaques/ limbs and scalp/ 4 wks | Diffuse dermal lymphocytes and histiocytes admixed with plasma cells/Immunostain not specified | IV ceftriaxone for 2 wks; JHR = not reported; Improved over 2 weeks |
|
7 |
41/F/ [12] |
HIV-positive/ CD4 164/ VL 223,000/ Alcoholism/ RPR 1:128 |
Plaques with a peripheral inflammatory-necrotic reaction, raised scabs, and pustular areas with violaceous erythematous background/thorax, extremities, neck, and face/ 4 wks/ uveitis | Lymphoplasmacytic-histiocytic perivascular and periadnexal dermatitis; epidermis w/pseudo- epitheliomatous hyperplasia, parakeratosis, neutrophilic exocytosis, spongiosis, supra- basal vascular degeneration/ Immunostain positive |
IV penicillin for 14-days and ciprofloxacin/ dexamethasone eye drops for 10-days; JHR = No; Improved |
|
8 |
48/M/[13] | HIV-positive/ CD4 88/ VL 495,000/ VDRL 1:32 |
Erythematous and ulcerated nodules with well-demarcated borders on the face, trunk, and upper arms/ 8 wks | Psoriasiform hyperplasia, parakeratosis, dense lichenoid and superficial perivascular lymphoplasmacytic infiltrate/ Immunostain positive | Doxycycline for 4 weeks; JHR = No; Resolved |
|
9 |
21/ F/ [14] |
HIV-positive/ CD4 23/ VL 89,125/ RPR 1:16 |
Erythematous papules and plaques, some with a central necrotic eschar/ trunk, arms/ 3 wks/ none | Not performed | Benzathine PCN IM x 1 dose; JHR = Yes; Resolved within a month with residual scarring |
|
10 |
47/M/ [15] |
HIV-positive/ CD4 320/ VL 60,500/ RPR 1:256 |
Eroded plaques/cheek and forearm/2 wks/ neurosyphilis, R eye uveitis, L eye papillitis |
Psoriasiform and vacuolar interface reaction w/ superficial and deep dermal perivascular/ periadnexal lymphoplasmacytic infiltrate dominated by plasma cells/ Immunostain positive | IV benzyl PCN, 15-d and corticosteroids; JHR = No (corticosteroids); Full recovery after 2 mos |
|
11 |
26/F/ [16] |
HIV-neg/ Modified VDRL 1:128 |
Erythematous scaly plaques/forehead, jaw eyelids, axilla, fingers, anogenital area/ 2 wks | Plasma cell-rich granulation tissue/Immunostain not described |
IM Benzathine PCN weekly X 3; JHR = Yes; resolved after 2 weeks |
|
12 |
55/M/[17] | HIV-neg/ alcohol abuse/ RPR 1:128 |
Ulcers with rupioid crusts/face, scalp, trunk, extremities/ 2 wks/ myiasis | Epidermal hyperplasia and infiltration with plasma cells and lymphocytes in dermis/ Immunostain not described | IM benzathine PCN weekly X3; JHR = No (corticosteroids); Resolved in one mo, with scarring |
|
13 |
35/M/ [18] |
HIV-positive CD4 291/ VL untreated/ RPR 1:128 |
Nodules and ulcers with thick brown-black crusts/ axilla, trunk, back, inguinal, penis, soles/ 4 wks | Epidermal hyperkeratosis and acanthosis. In the dermis, lymphocytes, and histiocytes and dense perivascular and periadnexal plasma cells/ Immunostain not described | IM benzathine PCN weekly X 3; JHR = No; Resolved over 3 weeks with hyperpigmented scarring |
|
14 |
41/F/ [19] |
HIV-neg/ malnutrition, alcoholic hepatitis/ RPR 1:128 |
Plaques with crusts and erosions on nose, cheeks, neck, scalp, trunk, limbs/ 12 wks/ tonsillar mucus patch/S. aureus | Dermal infiltrate of atypical T-cells, numerous plasma cells, and histiocytes/W-S stain positive | IV benzylpenicillin for 14 days; JHR = No (cortico- steroids); Lesions resolved, mild hyperpigmented scarring |
|
15 |
30/M/[20] | HIV-neg/ healthy/ RPR 1:32 |
Oyster shell-like skin lesions on his scalp, face, trunk, arms, and legs/ 4 wks/ condyloma lata | Dense infiltrate of lymphocytes, plasma cells, and neutrophils in the dermis/W-S stain positive | IM benzathine PCN weekly X 3 doses; JHR = No; Resolution within 3 weeks without scarring |
|
16 |
28/F/ [21] |
HIV -neg/ baseline health not stated/ RPR 1:128 |
Ulcerated papules and plaques involving the face, shins, knees, and thighs/6 wks/uveitis | Lichenoid granulomatous dermatitis with plasma cells/ Immunostain not described |
IV penicillin G for 14 days; JHR not specified; Outcome not described |
| 17 | 61/M/[22] | HIV-positive/ (‘poorly controlled’; no CD4, VL stated)/ RPR 1:128 |
Oval plaques with central necrosis, crust, and ulceration w/surrounding erythema/distribution not described/3 wks/arthalgia | Abscess w/ blood vessels with fibrinoid necrosis of vessel wall and neutrophilic infiltrate in and around the vessel/Immunostain negative | IV penicillin for 4 weeks; JHR not specified; Resolution over one month |
|
18 |
22/M/[23] | HIV-positive/ CD4 117/ VL 420,000/ RPR 1:64 |
Maculopapules/blisters on face, trunk, limbs; treated w/ levofloxacin w/o improvement; blisters ruptured; skin ulcerated and scabbed with brown–black rupioid crusts/4 wks | Dense infiltration of dermis w/ neutrophils, lymphocytes, and histocytes; perivascular infiltration of lymphocytes and plasma cells/Immunostain not described | IM Benzathine PCN weekly X 3 doses; JHR not specified; rash resolved. Re-infected with syphilis 13 mos later; similar rash, RPR 1:128 (CD4 657); doxycycline for 4 wks, rash resolved |
|
19 |
24/M/[24] | HIV-positive/ CD4 470/ VL untreated VDRL 1:512 |
Ulcers with rupioid surface/ face, trunk, inguinal area, arms/8 weeks/Condyloma lata | Spongiotic dermatitis w/ many dermal neutrophils; endarteritis, and microthrombi in blood vessels/Darkfield positive | IM Benzathine PCN IM X 1 dose; JHR = No; resolved in one month with hyperpigmentation |
|
20 |
57/M/[25] |
HIV-positive/ CD4 504/ VL <20 RPR 1:128 |
Ulcerated plaques and nodules, with lamellar crusts/scalp, face, trunk, limbs; 4 weeks | Lymphohistiocytic infiltrate in superficial dermis, no plasma cells/Immunostain positive |
IM Benzathine PCN weekly X 3 wks; JHR = No; Resolved with hypopigmented scarring |
|
21 |
35/M/[26] | HIV-negative/ Hepatitis B/ RPR 1:16 |
Ulcerative lesions with hemorrhagic crusts/trunk, extremities, genitals/ 3 wks |
Parakeratosis, acanthosis, prominent spongiosis, lymphocyte exocytosis, and dermal lymphohistiocytic infiltrate and perivascular, periadnexal, perineural plasma cells/Immunostain not stated | IM Benzathine PCN weekly X 3 wks; JHR = No; Improvement within 4-days after first dose |
|
22 |
42/M/[27] | HIV-positive/ CD4 399/ VL 102,000/ RPR 1:512 |
Ulcers w/ keratosis and crusting/scalp, face, perineum, limbs/4 wks/ neurosyphilis | Dermal infiltrate of plasma cells, lymphocytes, and histiocytes, in a lichenoid pattern with psoriasiform hyperplasia/ Immunostain positive |
IV PCN; JHR not specified; Lost to f/u |
|
23 |
31/M/[28] | HIV-positive/ CD4 481/ VL 89,200 Hepatitis C, IV drug abuse/ RPR 1:256 |
Ulcerated papules plaques w/ purulent and sanguineous drainage and adherent crusts/ trunk, limbs, scalp, face/ “weeks old”/ S. aureus | Suppurative granulomatous and lymphoplasmacytic inflammation w/ overlying lichenoid and spongiotic dermatitis/Immunostain negative | IM Benzathine PCN X 1 dose; JHR not specified; Lost to follow-up |
|
24 |
22/M/[29] | HIV-positive/ CD4 284/ VL 243,000/ VDRL 1:32 |
Erythematous and ulcerated plaques (some with necrotic crusts)/ trunk, arms, legs/ 4 wks | Not performed | IM Benzathine PCN X 1 dose; JHR not specified; Complete resolution |
|
25 |
50/F/ [30] |
HIV-positive CD4 338/ VL undetected/ VDRL 1:32 |
Ulcers, plaques and nodules/neck, face, arm, thigh/12 wks |
Granulomas w/ neutrophils, histiocytes, lymphocytes, plasma cells; dermal vessels w/ endothelial swelling. fibrinoid necrosis and leukocytoclasia in a small dermal vessel/W-S negative/ Immunostain positive | IM Benzathine PCN weekly X 3 doses; JHR = No; Resolved with hyperpigmented macules |
|
26 |
16/F/ [31] |
HIV negative/ JRA, on Immunosupp./ VDRL 1:32 |
Necrotic ulcers/face, trunk, arms, legs, hands, palms, oral mucosa/ duration not stated | Superficial and deep vacuolar interface dermatitis with many plasmocytes/W-S stain negative | IM Benzathine PCN weekly x 3 wks; JHR = No; Complete resolution |
| 27 | 56/M/[32] | HIV-negative/ Malnutrition/ RPR 1:16 |
Ulcerated and necrotic papules and nodules with black crusts/face, trunk, extremities, genitals/8 wks/ neurosyphilis | Obliterative vasculitis; dermal infiltrate of lymphocytes and plasma cells/Immunostain positive | IV PCN x 11-d; JHR = No (corticosteroids); then benzathine PCN weekly X 3-weeks; Resolved w/ hyperpigmented scarring |
| 28 | 29/F/ [33] |
HIV-negative Crohn's disease, on adalimumab/ VDRL 1:16 |
Ulcerated papules and nodules with crusts that became rupioid/face, neck, trunk, arms/ duration not stated | Epidermal ulcers, basal cell degeneration, dermal lymphohistiocytosis; vessels w/ reactive endothelial changes/ Immunostain not done | IM Benzathine PCN weekly X 3 doses; JHR = Yes; Resolved with hypopigmented scarring |
| 29 | 22/M/[34] | HIV-positive/ CD4 236/ VL uncontrolled/ RPR 1:256 |
Palpable purpura on legs followed by nodules and ulcers w/ thick crusts/ face, trunk, and arms/3 wks/leukocytoclastic vasculitis | Superficial and perivascular infiltrate of plasma cells, lymphocytes; central ulcer w/ psoriasiform hyperplasia. Dermal, perivascular plasma cells, lymphocytes, and histiocytes/Immunostain not done | IM Benzathine PCN weekly X 3 doses; JHR = No (corticosteroids); Resolved with hyperpigmented macules |
| 30 | 30/F/ [4] |
HIV-positive/ CD4 444/ VL untreated/ Cachexia/ VDRL 1:32 |
Ulcers with ecthymatous crusts/back, abdomen, limbs, genitals/20 wks/ neurosyphilis, splenic abscesses, osteitis/ vaginal candidiasis | Acanthosis, spongiosis; dermal edema with peri-vascular and peri-appendageal infiltrate of plasma cells, lymphocytes/ Immunostain not done | IV PCN X 15-days; JHR not specified; resolved over 2 weeks with hypopigmented scarring |
| Characteristic | Wibisono Series (total N=45) [7] | This Series (total N=30) (Table 1) |
|---|---|---|
| Age Range, yrs; mean age, yrs | 20-86; 44.4 | 16-61; 35.7 |
| Male, % | 84 | 63.3 |
| HIV Infection, % | 33/45 (73%) | 17/30 (57%) |
| CD4 Range for HIV Patients | (N=28); 57-1294 (21 with counts above 200 (75%)) | (N=17); 23-504 (10 with counts above 200 (59%)) |
| Mean CD4 Count, cells/µL | 397 (N=28) | 291 (N=16) |
| % HIV with Uncontrolled VL | 22/27 (81.5%) | 15/17 (88.2%) |
| Time to Presentation (weeks): Range; Mean; Mode | N=42; 1-36; mean = 7.1; mode = 4 | N=27; 2-20; mean = 6; mode = 4 |
| Other Syphilis Manifestations (number of cases) | neurosyphilis (2), uveitis (1), vitritis (2), keratitis (1), mucus patch (1), condyloma lata (1), osteitis (2), orchitis (1), pulmonary nodules (1) | neurosyphilis (4), uveitis (3), papillitis (1), mucus patch (1), condyloma lata (2), osteitis (1), splenitis (1) |
| Positive Jarisch-Herxheimer Reactiona | 9/38 (24%) | 5/18 (28%) |
| Spirochete Visualization, no. of cases (W-S = Warthin-Starry (silver)) |
20: no visualization specified; 12/15: immunostain positive (80%); 2/5: W-S or Steiner stains positive (40%) |
13: no visualization specified; 10/12: immunostain positive (83%); 2/4: W-S positive (50%); 1 positive by darkfield microscopy |
| Rx w/ Benzathine PCN weekly X 1 dose versus 3 doses; number of cases, %, outcome (improved = improved or resolved) |
26 received benzathine PCN only; 5/26 received 1 wk (19%); all improved; 21/26 received 3 wks (81%): 18 improved, 3 lost to follow-up |
18 received benzathine PCN only; 5/18 received 1 wk (28%); 3 improved, 2 lost to follow-up 13/18 received 3 wks (72%): all Improved |
| Neisser [43] | Fisher et al. [6] |
|---|---|
| (1) Relatively short incubation period (2) Constitutional symptoms are pronounced (3) The skin and often mucous membranes of mouth and nose present multiple lesions consisting of large pustules, ulcers, and rupioid ecthymatous lesions (4) May have milder forms of the disease such as mucous patches, etc. (5) Round or oval pleomorphic skin lesions: papulopustules, ulcerations, ulcers with brown-black rupioid crusts, and healing lesions |
(1) compatible gross and microscopic morphology (2) a high titer serologic test for syphilis (3) Jarisch-Herxheimer Reaction (JHR) (4) dramatic response to antibiotic therapy Gross morphology: similar to Neisser Microscopic morphology: not defined |
| Wibisono et al. [7] | Our Series (Table 1) | Zhu et al. [55] | |
|---|---|---|---|
| RPR | (N = 21) | (N = 21) | (N = 26) |
| Range | 4-1024 | 16-512 | 32-256 |
| Mean | 245 | 161 | 140 |
| Median | 128 | 128 | 128 |
| Mode | 256 | 128 | 128 |
| Comment | 17/21 (81%) had 1:64 or higher | 16/21 (76%) had 1:64 or higher | 24/26 (92%) had 1:64 or higher |
| VDRL | (N = 14) | (N = 11) | (N = 0) |
| Range | 8-512 | 16-512 | |
| Mean | 179 | 175 | |
| Median | 128 | 128 | |
| Mode | 128 | 32 | |
| Comment | 13/14 (93%) had 1:32 or higher | 10/11 (91%) had 1:32 or higher |
| Histopathologic Findings |
Wibosono et al. (35 cases) [7] |
Our Series (28 cases) |
Both Series (63 cases) |
|---|---|---|---|
| Plasma cell infiltrate | 29/35 (82.9%) | 23/28 (82.1%) | 52/63 (82.5%) |
| Lymphohistiocytic infiltrate | 23/35 (65.7%) | 22/28 (78.6%) | 45/63 (71.4%) |
| Vascular involvement | 13/35 (37.1%) | 12/28 (42.9%) | 25/63(39.7%) |
| Granulomas | 9/35 (25.7%) | 8/28 (28.6%) | 17/63 (27.0%) |
| Neutrophilic infiltrate | 5/35 (14.2%) | 5/28 (17.9%) | 10/63 (15.9%) |
| Giant Cells | 3/35 (8.6%) | 1/28 (3.6%) | 4/63 (6.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





