1. Introduction
Pulmonary arteriovenous malformations (PAVMs) structurally are the consequence of an incorrect connection between pulmonary veins and arteries, leading to dilated anomalous vessels and the disappearance of the capillary network. This generates a right-to-left shunt, which increases pressure in the pulmonary circulatory system, as well as impairs regular gas exchange.
PAVMs are typically asymptomatic and undiagnosed. Approximately 80% of PAVMs are congenital in origin, but they are not commonly diagnosed in children because clinical manifestations are rarely seen before adulthood. However, patients who remain undiagnosed may develop serious complications later in life. The severity of the clinical symptoms can vary from dyspnea to cyanosis, heart palpitations and chest pain [
1,
2].
The majority of patients with congenital PAVMs have underlying Rendu-Osler-Weber syndrome, also known as Hereditary Hemorrhagic Telangiectasia (HHT). HHT is a rare multisystemic vascular disease with an autosomal dominant pattern of inheritance and a prevalence of 1 in 5,000/8000 in the general population, which increases in certain regions due to the founder effect and geographical isolation [
3,
4]. Patients with HHT often present mucocutaneous telangiectasias, the rupture of which causes recurrent and spontaneous nose hemorrhages (epistaxis), which is usually the most frequent and the first symptom to appear in HHT. Bleeding usually increases throughout the patient's life, and in some of the most severe cases can even lead to anemia and iron deficiency. Another clinical manifestation is the presence of arteriovenous malformations (AVMs) in internal organs, such as the lungs and brain, more frequent in HHT1 type, while in HHT2 is more frequent in liver. Clinical diagnosis of HHT is possible following Curaçao criteria, which are epistasis, telangiectasia, arteriovenous malformation in internal organs and a familiar pattern of autosomal dominant inheritance [
5]. Nevertheless, the Curaçao criteria are not accurate for infants, due to the late development of certain symptoms and to the intra-familial phenotypic variability.
In addition to a symptomatic perspective, HHT can be diagnosed genetically. All mutations leading to HHT are found in genes belonging to the BMP9/TGF-β signaling pathway. Mutations in
ENG (HHT1) and
ALK1/ACRVRL1(HHT2) genes are responsible for 90% of HHT cases [
6,
7]. Less common mutations, such as those found in
MADH4/SMAD4 and
GDF2 genes, have been described in less than 2% of the HHT population and result in HHT-like syndromes. Mutations in
MADH4/SMAD4 cause juvenile polyposis/HHT overlap syndrome (JPHT) that generates additionally to HHT symptoms, colon polyps and thoracic aneurysms [
8]. In the case of
GDF2 mutations generate an HHT-like syndrome classified as HHT5 [
9]. Recent research suggests that mutations in
EPHB4 or
RASA1 may be responsible for the previously known as HHT3 and HHT4 types, as there is no evidence for the existence of two independent genes linked to chromosomes 5 and 7 [
10].
EPHB4 and
RASA1 variants cause capillary malformations-arteriovenous malformation (CM)/AVM syndrome. This syndrome is characterized by the appearance of multiple small, red, randomly distributed spots with white halo known as capillary malformations. It can be difficult to distinguish CM/AVM from HHT [
11].
BMPR2 gene is also involved in the BMP9/TGF-β signaling pathway. Its expression is particularly high in pulmonary vascular endothelium. Pathological changes in this gene can lead to pulmonary arterial hypertension (PAH) [
12].
However, 15% of clinically diagnosed with HHT lack a genetic diagnosis. Furthermore, we can add the difficult diagnosis in pediatric cases and the existence of sporadic (non-inherited) cases of HHT. In this context, we have studied a clinical case with multiple diffuse pulmonary fistulas in pediatric age who underwent a bilateral lung transplantation. Previous genetic analysis did not show any genetic alterations associated with HHT or any of the other related syndromes above mentioned. Moreover, the patient does not have any family history of HHT. Given the severity of the clinical manifestations and their similarity to HHT, the aim of the present study is to establish the molecular parameters and differential gene expression profile of this symptomatology, aiming to a similarity to HHT.
Results
The Index case of this study is a 8-years-old child with clinical diagnosis of probable HHT without a familiar history of HHT. The case shows symptoms related to this syndrome since he was 3 years old, including strong cyanosis, hypoxemia (abnormal low oxygen in blood), epistaxis and multiple small diffuse pulmonary arteriovenous fistulas in both lungs. This phenotype was becoming more severe and non-compatible with life, due to the low oxygen saturation. In 2020, he was put on top of an emergency list for pediatric lung transplantation. In spite of all the genetic analysis carried out in the known genes related to HHT and HHT-like syndromes, the genetic origin of the disease remains unknown.
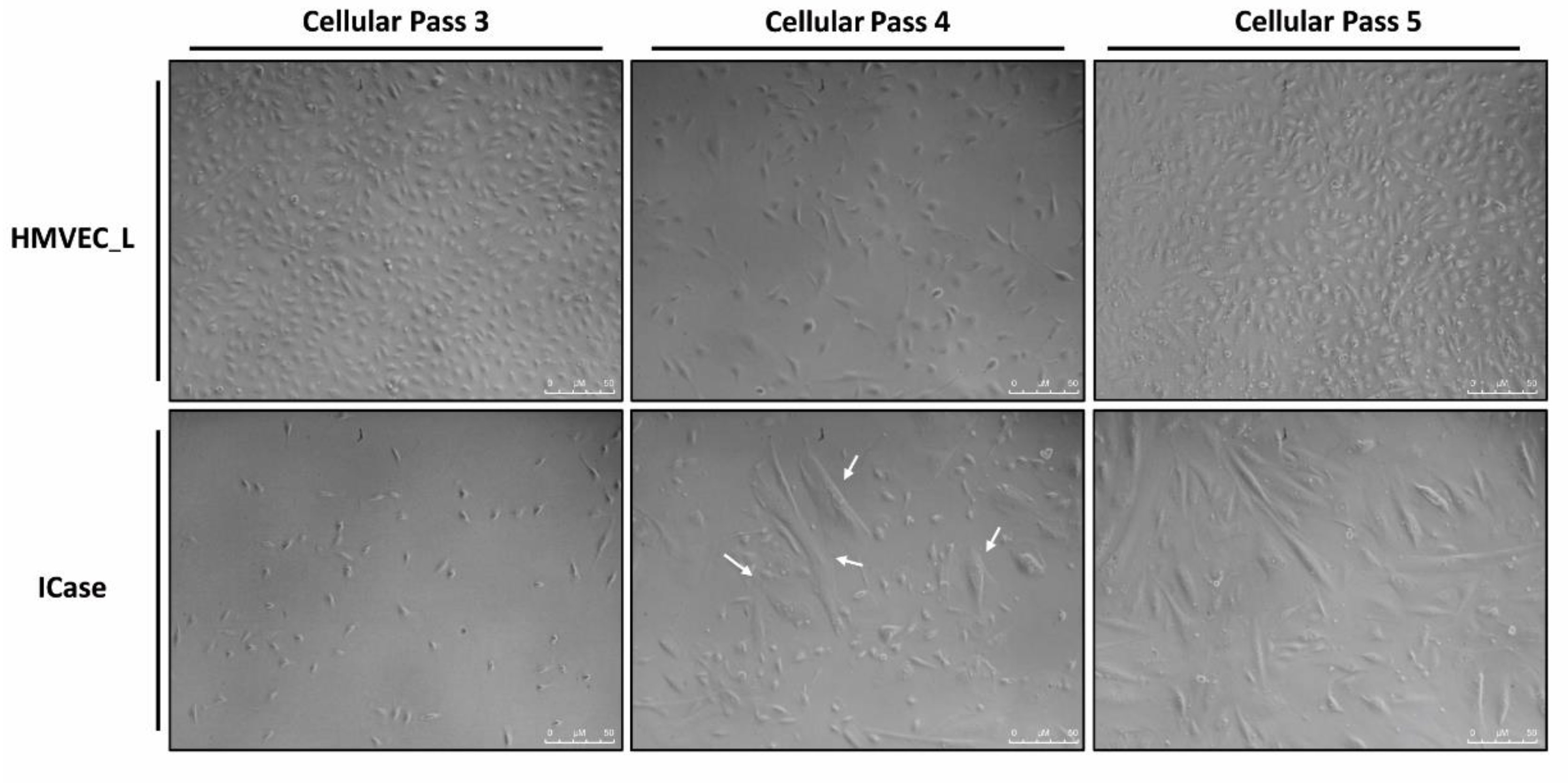
2.1. Visual Analysis of Primary Endothelial Cell Culture
Figure 1 displays photographs of two cultures of human pulmonary microvasculature: the Index case and the HMVEC_L as control of lung microvasculature. As a routinary way to confirm the identity of our established endothelial cell culture, we performed CD31/PECAM-1 and vWF staining (data not shown). The cultures appear similar at first, but over time, the cells from the case study changed in size and morphology. In the primary culture of the patient, two cell populations can be identified. One population maintains an endothelial appearance, while the other acquires a mesenchymal-like morphology and a clear increase in size is apparent. Ultimately, mesenchymal-like morphology became the dominant population in the culture.
Overall, the appearance of this mesenchymal-like cell population over time suggests the presence of an endothelial-mesenchymal transition (EndMT). Due to the prematurity and severity of the symptoms in this child, as well as the observed phenotypic in vitro changes in cells, we investigated the possibility of molecular-level alterations. As a result, experiments were designed to test the EndMT hypothesis, by analyzing the expression and functional profile of the ECs from the explanted lung versus the control HMVEC_L.
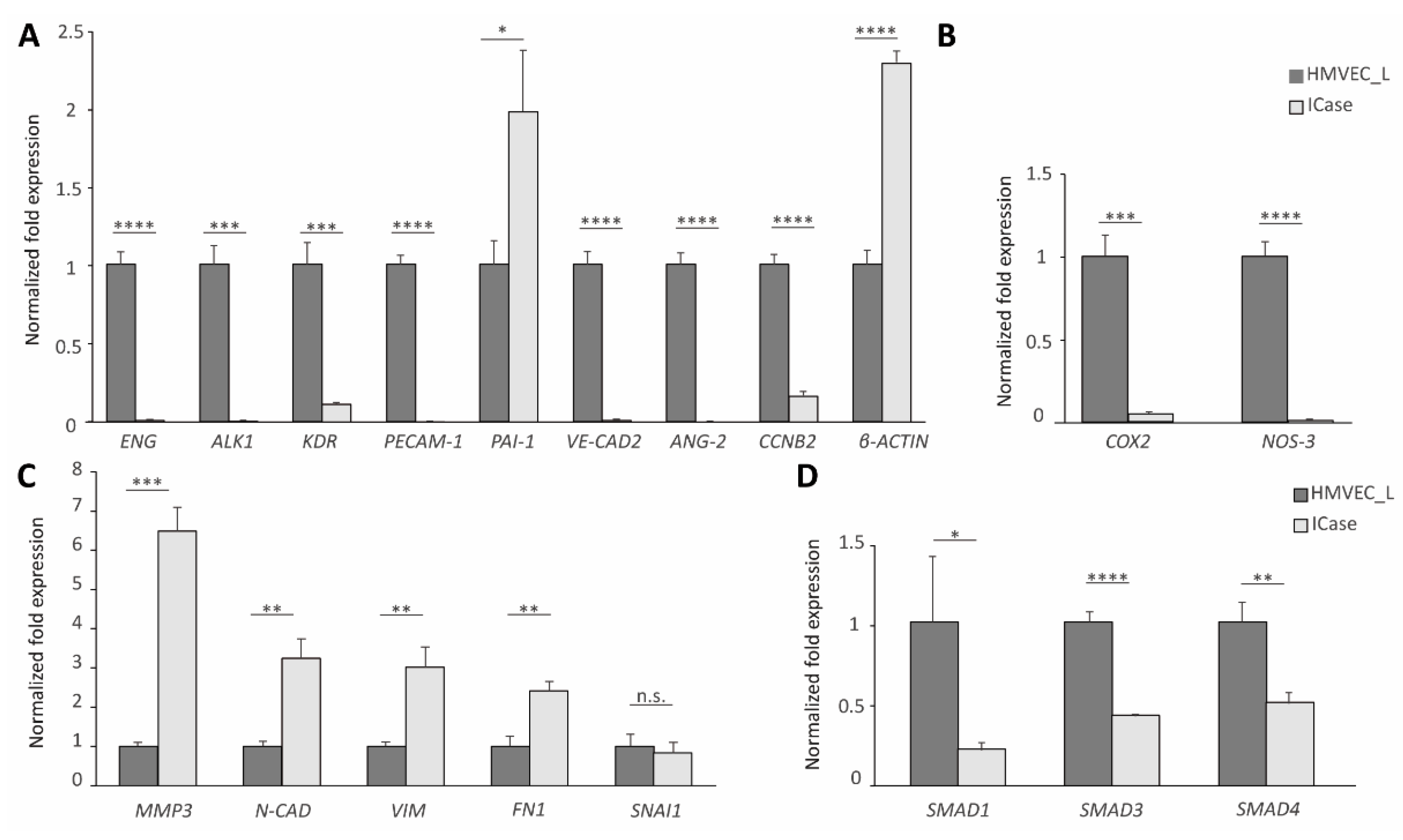
2.2. mRNA Expression Profile
In the present case, in which there was a vascular involvement, we decided to focus our gene expression analysis on endothelial and mesenchymal expression genes. The amount of RNA was normalized to the amount of 18S RNA as a housekeeping gene.
Figure 2 provides additional details about the mRNA profile.
The gene expression was compared between primary cell culture of ECs from a healthy donor and the Index case. RT-qPCR analysis showed that all of the endothelial markers were downregulated in the case under study (
Figure 2A). Although these results are to be expected in HHT, as seen in the literature [
13], the expression levels were extremely low. In HHT patients, endoglin haploinsufficiency leads to increased COX2 expression and decreased NO synthesis, which is a compensatory mechanism [
14] However, in this case, the compensatory system is not fulfilled (
Figure 2B). On the other hand, the Index case expressed high mRNA levels of mesenchymal markers, such as
MMP3,
FN1 and
Vimentin (
Figure 2C). We did not find significant differences in
SNAI1, whose functions are associated with development processes, neural differentiations and epithelium-mesenchymal transition [
15]. The replacement of usual epithelial markers with mesenchymal markers, such as Vimentin and Fibronectin, determines these changes [
15,
16].
Figure 2A and 2C confirm a decrease in endothelial markers and an increase in mesenchymal markers, supporting our initial idea that an EndMT is occurring. EndMT happens during the embryonic development, but this process has been also previously described in adults [
17,
18].
Also, we examined the expression of
SMAD genes since they play a significant role in the TGF-β pathway affected by the HHT syndrome. A downregulation of
SMAD genes is observed (
Figure 2D).
2.3. Protein Expression
To corroborate the previous expression profile comparing HMVEC_L against the Index case we performed protein analysis, by immunofluorescent assay and/or western blot.
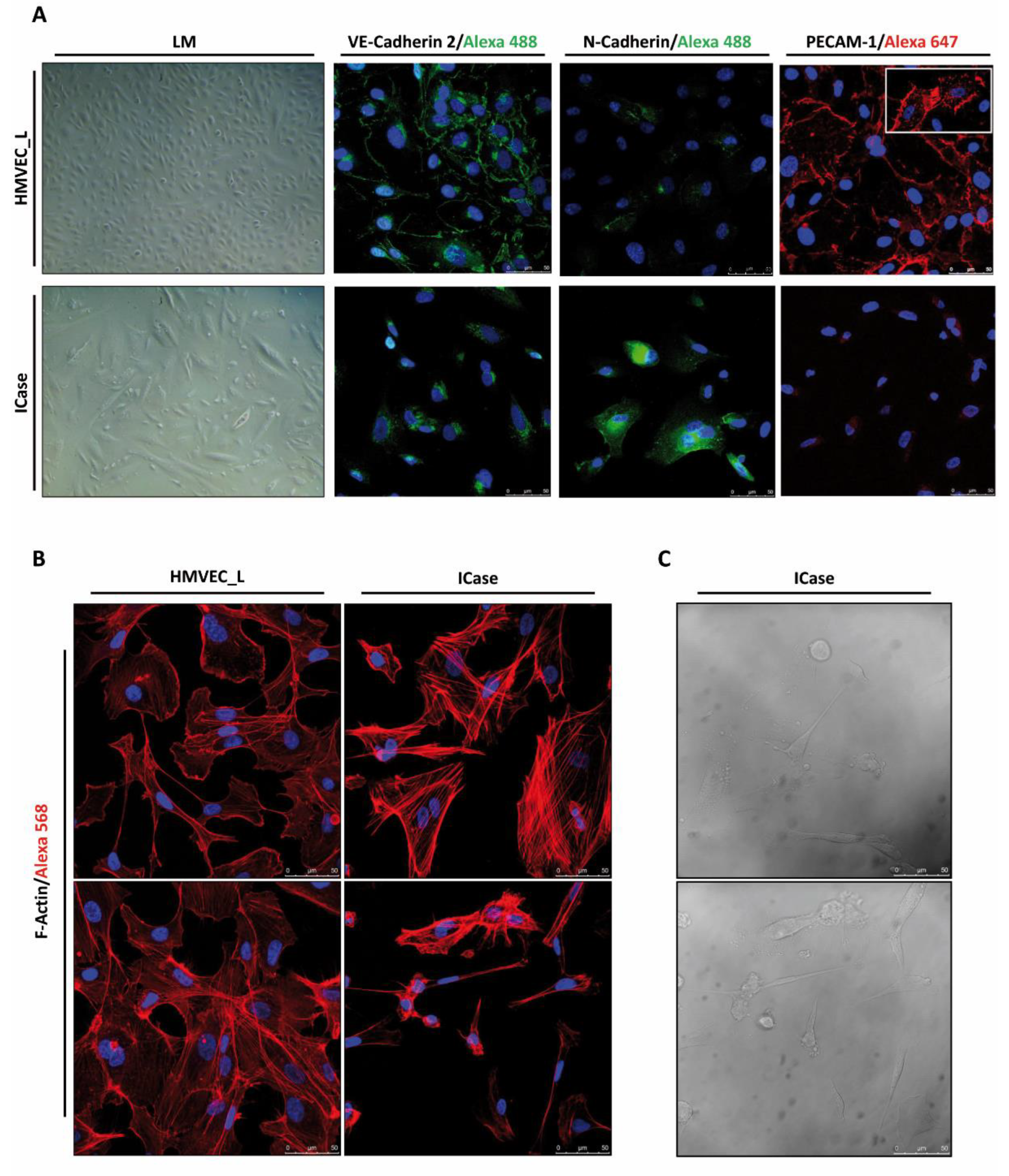
An immunofluorescent assay was conducted to corroborate the gene expression levels of endothelial cell-specific genes (VE-Cadherin 2, PECAM-1) and a specific marker of dedifferentiated cells (N-Cadherin) at the cellular level (
Figure 3A). The images confirm the presence of VE-Cadherin 2 on the cell surface of HMVEC_L control culture cells, observing strong accumulations of this protein observed in the areas of cell-cell contacts, forming adherent junctions. In contrast, VE-Cadherin 2 is less prominent in the patient's cells, and there is no accumulation of it on the cell surface. This suggests that cell-cell contacts have been lost. Since VE-Cadherin 2 plays a crucial role in maintaining endothelial integrity and leukocyte extravasation, a decrease in VE-Cadherin 2 would result in reduced cell adhesion and less cohesion among cells. To confirm endothelial identity of the cell culture CD31/PECAM-1 labelling was used as marker. This protein is almost exclusively expressed in ECs. The patient cells exhibited significantly lower levels of PECAM-1 compared to the control cells, which supports the results obtained from qPCR and confirms our initial hypothesis.
Furthermore, an examination of F-Actin in both cultures was conducted to determine if the mesenchymal shaped cells showed a different cytoskeleton distribution than healthy ECs, which might facilitate proper migration and contraction.
Figure 3B illustrates significant disparities in the quantity and configuration/organization of actin filaments. When comparing both conditions, it is evident that the labelling of the control cells enables a precise delimitation of the cell shape, which is not the case in the patient samples. In the latter, the labelling accumulates in cytoplasmic fibers, thicker and more abundant than in the control. Additionally, a significant variability of cell sizes within the same culture was observed during the experiment (see
Figure 3B). The larger, apparently mesenchymal cells showed a highly ordered network of filaments. The phase contrast allowed for the identification of smaller cells as ECs undergoing apoptosis (
Figure 3C).
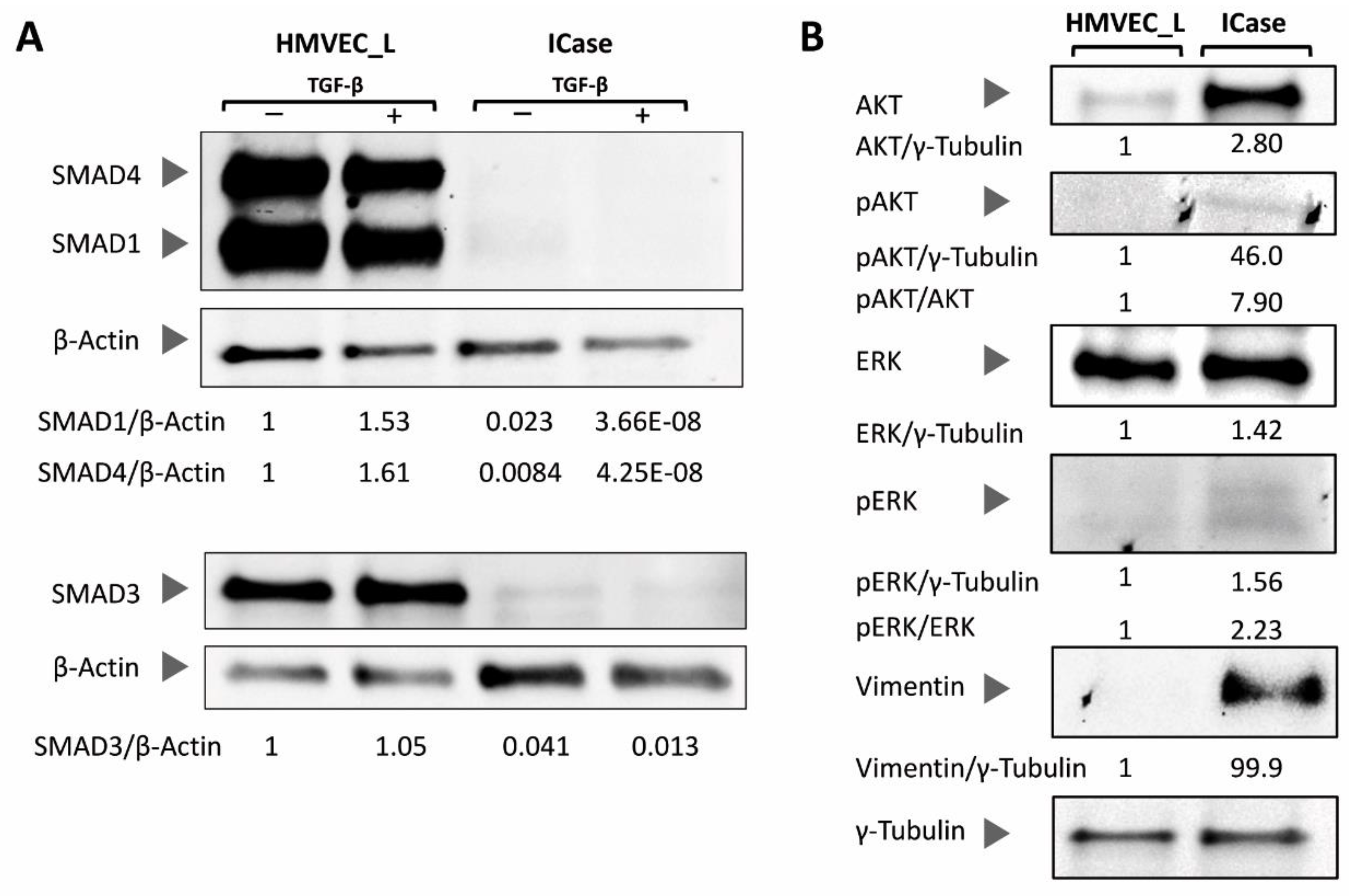
To analyze TGF-β and PI3K/MAPK pathways, we performed Western blot assays. The TGF-β pathway is closely related to the HHT syndrome and plays a crucial role in a wide range of cellular functions. The signaling status of the TGF-β pathway was analyzed through SMAD proteins (SMAD1, SMAD3 and SMAD4). We examined the levels of each of the SMAD proteins and their response to TGF-β treatment (
Figure 4A).
Figure 4A shows that the Index case has a complete inhibition of this pathway, and TGF-β stimulation has no effect.
Additionally, we aimed to investigate the status of the PI3K/MAPK pathway and its activation (phosphorylated proteins).
Figure 3B illustrates that all proteins from the Index case were overexpressed when compared with control healthy HMVEC-L cells, indicating a higher activation of both pathways through AKT/pAKT and ERK/pERK. Additionally, we aimed to examine the protein levels of the mesenchymal marker Vimentin. As previously observed in the RT-qPCR, the western blot assay revealed a higher quantity of Vimentin in the Index case, while it was undetectable in the control (
Figure 4B).
2.4. Functional Analysis
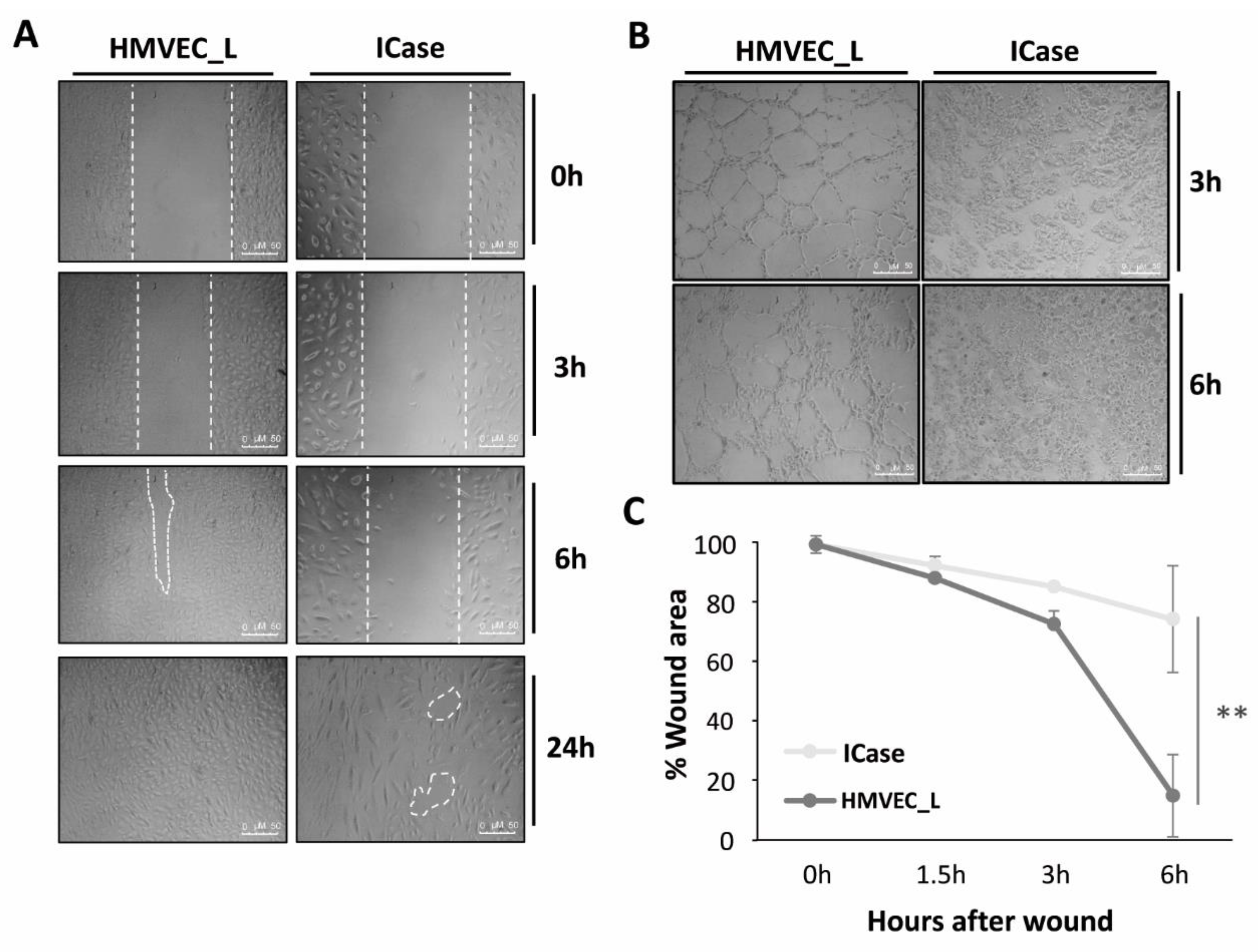
Due to the low expression levels of certain genes involved in processes such as angiogenesis (ANGPT2), migration, cell adhesion (PECAM-1 and VE-Cadherin 2) and genes involved in proper blood vessel physiology (NOS-3 and COX2), we performed functional assays of wound healing and tube formation (angiogenesis) to test how compromised patient´s cells were compared to healthy control cells.
Figure 5A shows the wound healing assay. These assays attempted to mimic a physiological condition of in vitro tissue damage and test cell function by assessing cell migration and cohesion. In both cases, the advancing front moved. However, it is evident that HMVEC_L control cells exhibited a more organized and uniform movement, while the cells of the patient under study displayed less connection with each one moving independently. It is obvious that control cells showed a greater healing capacity, as they were capable of closing almost completely the discontinuity (wound) after 6 hours. In contrast, the cells in the case under study moved towards the wound in an attempt to close it, but after 24 hours, gaps between the cells can be observed and the closure showed incomplete. Therefore, control cells have a greater capacity for repairing and forming cell cohesion, while the patient cultures experience delays and incomplete closure.
In terms of functional capacity for tubulogenesis,
Figure 5B illustrates that healthy HMVEC_L cells were able to generate completely closed and defined tubes of various sizes within 3 hours on Matrigel®. After 6 hours, the tubes formed were reinforced by several layers of cells. However, cells from the Index case were unable to build defined tubes, and a large proportion of the cells were not able to adhere to Matrigel® after 6 hours of plating.
3. Discussion
Angiogenesis processes play a dual role: they are essential for the formation of new vessels from pre-existing ones but, when impaired, are also involved in pathogenic processes. ECs undergo morphological changes and are induced to migrate during angiogenesis, enabling them to migrate and remodel the ECM. This behavior may resemble that of mesenchymal cells. However, it is not typical for ECs to detach from their neighbor cells, indicating that angiogenesis may involve a partial transition to mesenchymal cells, EndMT, followed by vessel stabilization [
19,
20]. EndMT has also been observed in processes of cardiac fibrosis and pulmonary hypertension [
17,
21].
This paper presents the first documented case of endothelial to mesenchymal transition in a sample from a pediatric patient, subjected to lung transplantation. Although this transition has been observed in adults [
17,
22], this may be the only documented case in children so far. Our hypothesis is that damaged pulmonary ECs eventually transition into mesenchymal cells as a survival mechanism when they can no longer fulfil their endothelial function. In spite of the unknown origin of this damage in, which is currently under investigation, we have studied the outcomes. The consequences are the loss of endothelial integrity, which could explain the severity of the patient's symptoms, due to a collapse of the lung vasculature and therefore the lung function. The results of the in vitro experiments described in this work support this hypothesis.
The primary lung microvascular culture from the Index case showed the progressive emergence with passages of a second population with a mesenchymal-like morphology (
Figure 1). To test this assumption, we compared the expression profile of this cell culture with a primary control culture (
Figure 2). The analyzed genes are involved in various physiological processes, including angiogenesis, cell adhesion, migration, transmigration, TGF-β signaling, cell cycle control, cytoskeleton, and vascular physiology. A decrease in the expression of vascular genes was observed, as has been described in HHT patients14. However, characteristic genes of mesenchymal cells involved in transition processes, such as
MMP3,
FN1,
Vimentin and
SNAI1 were found significantly increased in their expression, in support of our EndMT hypothesis [
20,
23].
During EndMT, ECs detach and migrate, potentially invading the underlying tissue. As part of this process, the mesenchymal phenotype overlaps with the endothelial phenotype. This is characterized by the acquisition of mesenchymal markers, such as N-Cadherin, and the complementary loss of endothelial markers, such as CD31/PECAM-1 and VE-Cadherin 2. Additionally, ECs lose their cell-cell junctions and acquire characteristics adapted to migration and invasion [
24].
To verify these changes, the expression and subcellular localization of these proteins was examined using laser confocal microscopy (
Figure 3B). Our findings confirmed that the cells in the case study had lost the cell-cell connections typical of endothelial tissue and were expressing mesenchymal markers (N-Cadherin). Significant oscillations in VE-Cadherin 2 levels can increase N-Cadherin expression in the endothelium. N-Cadherin promotes cell migration and contributes to vascular elongation, while VE-Cadherin 2 induces vascular stabilization by limiting growth factor receptor signaling [
25].
Several studies have shown that the TGF-β pathway promotes EndMT [
22,
26]. TGF-β can also activate SMAD-independent pathways such as MAPK/ERK/JNK, all of which are involved in EndMT [
27]. This is similar to the situation we observe in the Index patient, where a total inhibition of the SMAD protein-dependent TGF- β pathway is found [
28]. On the other hand, both in HHT patients, and in our case an increase in basal and functional protein levels of the PI3K/MAPK pathways is found [
29,
30]. These results are supporting the hypothesis of an EndMT present in the case under investigation in this work.
Recent studies suggest that vascular lesions in HHT may be originated by a second mutation event in
ENG or
ALK1, in angiogenic processes [
31]. Mouse models of HHT1 and HHT2 provide strong evidence supporting a two-hit mechanism as the origin of AVM. This mechanism requires complete endothelial loss of ENG or ALK1 protein for the formation of robust vascular lesions. The current mouse heterozygous model, with only one alteration, is not effective in replicating HHT symptoms [
32]. Singh et al. (2020) [
33] noted that AVMs are primarily of venous origin, where cells express higher levels of endoglin. ENG restricts endothelial proliferation, and its absence in localized regions increases the frequency of AVMs [
33]. Endoglin is also involved in cell intercalation processes, necessary for the extension and formation of new blood vessels while maintaining cell-cell contact. Therefore, its absence may be a factor for the development of these vascular lesions [
34].
HHT patients exhibit at least a 50% reduction in ENG or ALK1 functional protein levels, due to their heterozygous condition for mutations in
ENG (HHT1) or
ALK1 (HHT2). These patients experience increased and anomalous angiogenic processes, where there is no proper control on the generation and stabilization of blood vessels. The reported finding suggests that low ENG levels adversely affect both cell migration and tubule formation [
35,
36,
37]. Previous studies in human brain AVMs have found evidence that while SMAD-mediated TGF-β signaling is reduced in AVMs, non-canonical TGF-β signaling may contribute to the development of AVMs through the PI3K/MAPK pathway [
28,
38]. In HHT, due to mutations in components of the TGF-β pathway, a decrease in the canonical TGF-β pathway and an increase in the PI3K pathway is found, facilitating a partial endothelial-mesenchymal transition. In this scenario, some cells may undergo a partial EndMT transition in the AVM. However, cells are able to revert at least to some extent the EndMT transition, and HHT cells still maintain cell-cell junctions to an intermediate degree between healthy cells and the damaged cells observed in the present case [
13].
Moreover, low levels of physiological oxygen saturation as those exhibited by the patient under study, 70-80%, are sensed by cells, as hypoxia, inducing an increase in VEGF levels, and leading to a continuous pro-angiogenic state. Additionally, we observed a very strong decrease, far below 50% in the expression of both ENG and ALK1, close to the conditional knock-out mouse models of
Eng and
Acvrl1/Alk1 published in the literature [
34]. The study found a phenotype with greater aggressiveness in the knockout mouse of
Acvrl1/Alk1, resulting in a complete inhibition of the TGF-β pathway, as seen in our Index case (
Figure 4A) [
32]. Additionally, decreased levels of ENG create an adequate environment for the generation of multiple AVMs, in our case, pulmonary. A decrease in the levels of ALK1 and ENG impairs TGF-β signaling, in addiction to an hypoxic environment leads to an EndMT and finally to the generation of AVM. In the Index case, the ECs undergo the EndMT, becoming individual cells without a proper alignment with other cells, apparently unable to reverse to endothelial cell phenotype. This results in a permanent EndMT that completely compromises the integrity and function of the vasculature. Furthermore, the
Figure 3C demonstrate a significant variation in cell size, with some endothelial-like cells undergoing apoptotic processes. This suggests that ECs that are incapable of initiating an EndMT transition process may ultimately die (
Figure 5B and 5C). We identified similarities in gene expression patterns and, in the signaling related to TGF-β and PI3K/MAPK pathways between HHT patients and our case study [
13,
29,
30]. However, this case presents an extreme profile with low expression of endothelial markers and increased expression of mesenchymal cell-specific markers. This may explain a fatal compromise of vascular integrity and function.
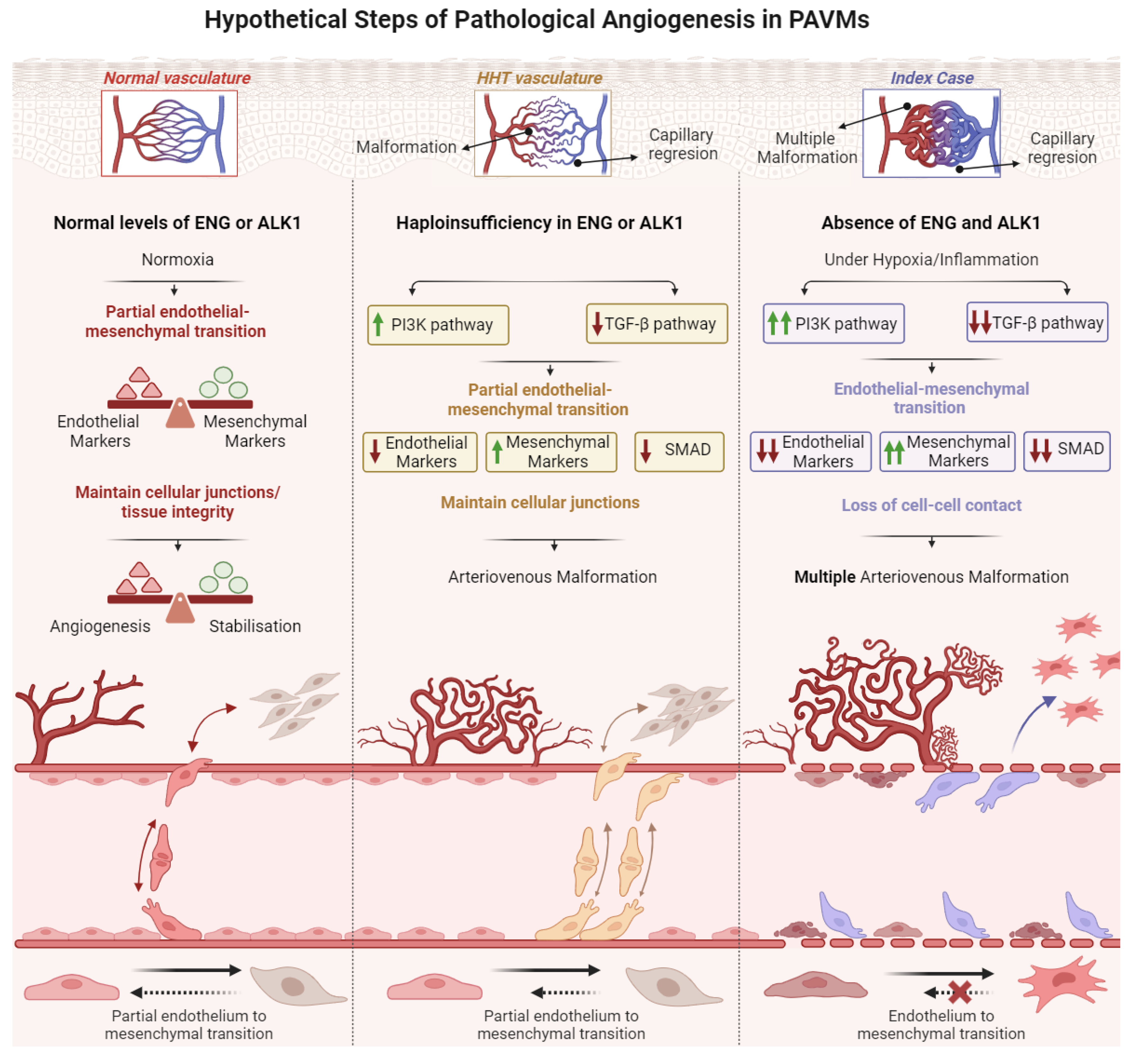
The mechanism explained above is depicted in
Figure 6 where three angiogenic situations are shown. The normal angiogenesis with transitory and reversible EndMT (
Figure 6) is undergone by healthy ECs. The transition with only partial reversion would occur in an HHT patient. Finally, the EndMT suffered by the cells presented in this case under study would lead to a loss of endothelial function in the vasculature. Although, the origin of the anomalous transition occurred in this pediatric patient remains unknown, it is likely that this could have occurred somatically, restricted mainly to the lung, without involvement of the germline.
Author Contributions
Conceptualization, V.A., A.M.C. and L.M.B.; methodology, L.R.-P., L.L.-H; software, V.A., L.L-H and .; validation, , V.A. and L.M.B.; formal analysis, V.A., and L.M.B.; investigation, L.L.-H., L.R.-P., V.A..; resources, L.L.-H and L.M.B; writing—original draft preparation, L.L.-H and L.M.B.; writing—review and editing, V.A., A.C.M., and L.M.B.; funding acquisition, L.M.B. All authors have read and agreed to the published version of the manuscript.