Introduction
HF following myocardial infarction (MI) – ischemic cardiomyopathy – is a major cause of death and disability worldwide [
1]. It is responsible for 1-2% total healthcare expenditure in industrialized countries, and estimated annual direct cost reaching
$70 billion in the American society impacting 6 million Americans. Globally, there are 65 million people who are affected by HF [
2,
3]. HF events have increased in the past 30 years [
4] and are expected to increase at least until 2050, with the global expectation of a “heart failure pandemic”, as it is the second most common inpatient diagnosis billed to Medicare [
5,
6]. MI is a condition of acute injury to myocardium which kills as much as 25% of cardiomyocytes (CMs) located within the chamber of left ventricle (LV) [
7]. The structural alteration will lead to more intensified mechanical stress of the ventricular wall to compensate, where contractile dysfunction worsens, leading to HF. The extent of this heart failure condition is directly correlated to the amount of cardiomyocyte loss [
8].
Ischemic cardiomyopathy accounts for ~50% of heart failure with reduced ejection fraction (HFrEF) and ~25% of all HF [
9]. While existing pharmacological and device therapies improve functional status and reduce mortality in this syndrome, many patients progress to advanced HF and death [
10]. Cardiac transplantation can be an effective treatment, but it is limited by organ donor availability [
11]. To fill this therapeutic gap, stem cell therapy has recently emerged in the treatment of HF, and numerous preclinical and clinical studies using various types of stem cells have been shown to improve cardiac function and attenuate LV remodeling. While ESCs are derived from preimplantation embryos, where some ethical considerations of blastocyst destruction at 6th – 8th day of human embryo development arise [
12], iPSCs are produced by epigenetic reprogramming of somatic cells such as skin fibroblasts or mononuclear blood cells. iPSCs have been proven to possess a potential capacity to acquire functional cardiomyocytes. Thus, the extended production of iPSCs has become more and more favorable in the current cardiac regenerative medicine industry [
13,
14].
Induced Pluripotent Stem Cell (iPSC)
In 2006, Yamanaka succeeded in accomplishing the goal of rebooting fully matured somatic cells into pluripotent stem cells (iPSCs) using four reprogramming factors that is now famously known as “Yamanaka Factors”. These four factors are: Octamer-binding Transcription Factor 4 (OCT4), Krüppel-like Factor 4 (KLF4), Sex-determining Region Y-box 2 (SOX2), and c-Myc protein [
15,
16]. The resultant stem cells from this reprogramming done by Yamanaka are what is now called popularly as iPSCs, which are by definition reprogrammed somatic cells with cell differentiation capacity to all the three embryonic germ layers [
17]. The main idea behind the finding of iPSCs is the sufficiency of genes with pluripotency capability, if overexpressed, to reprogram a somatic cell into an embryonic stem cell-like state [
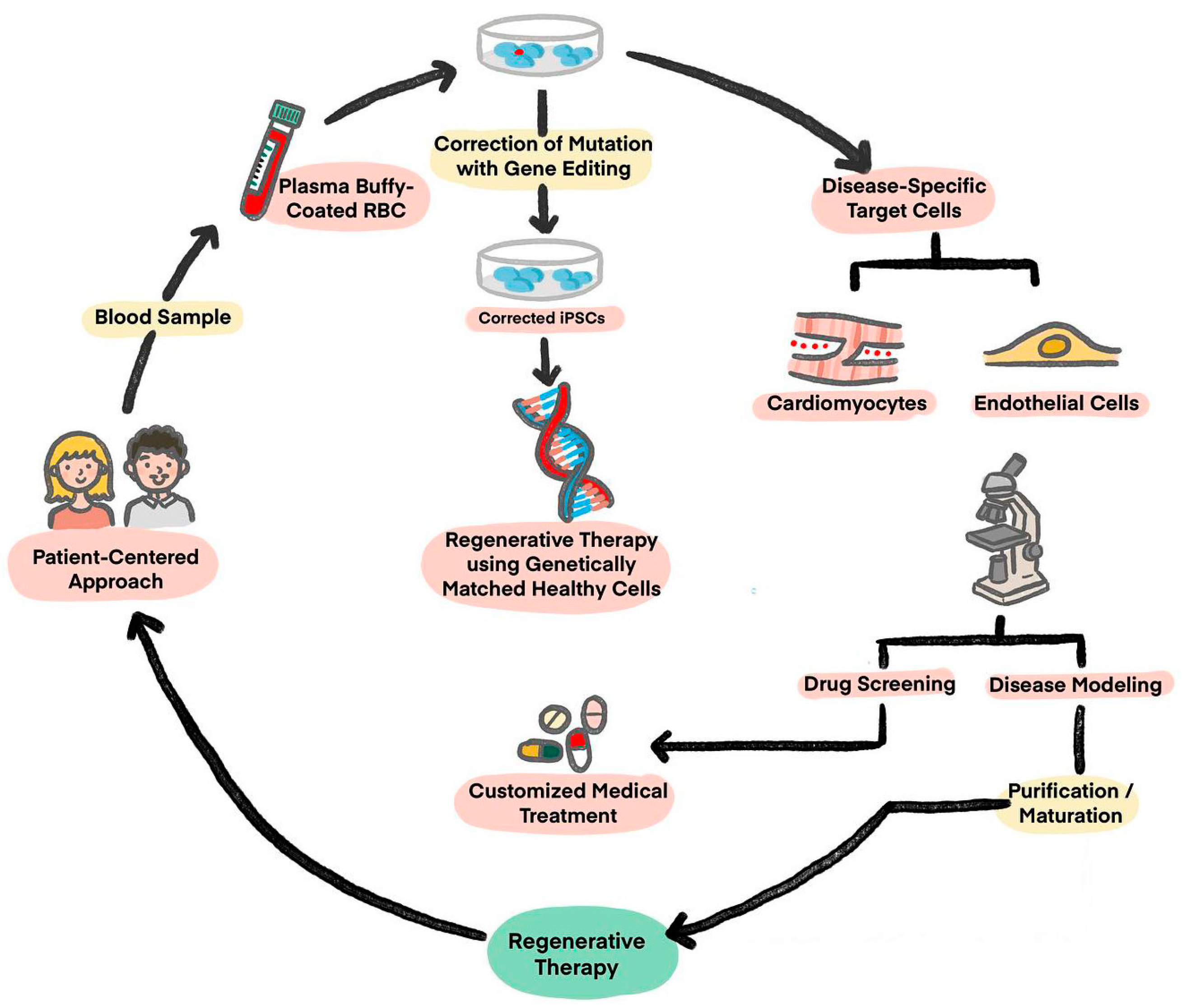
15]. These cells can then be differentiated into almost any cell of interest for use in regenerative therapies (
Figure 1) [
18]. The character properties of these cells are similar in ways that they offer long-term proliferation, with capacity to expand into iPSC-CMs. Then, these cells differ into specific engineered differences using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR associated protein 9 (Cas9) technology, which in the future can provide more mechanical profile insights into genetic disease. The fact will help enable the further development of precision medicine for drugs, biomarkers, and diagnostic purposes, with a patient-centered approach.
Advantages of an iPSC-based approach include an unlimited source of replacement cells and avoidance of potential controversy concerning the use of fetal tissue [
19]. Another advantage of undifferentiated iPSCs is that they can be cryo-banked in large numbers, which enables genetically identical seed stocks to be differentiated into CMs. This overcomes issues regarding genetic variation between different individuals and also makes it possible to create prescribed, large-scale banks of iPSCs with desired genotypes such as disease classes & genetic groups. Moreover, microarray research has demonstrated that hundreds of genes as well as DNA methylation patterns, are differently expressed between iPSCs and ESCs [
20,
21,
22], showing the cruciality of cell derivation being used for the success of cell regeneration. Measurement of a range of properties of iPSC and ESCs, such as gene expression, DNA methylation, microRNA expression, differentiation propensity, and complement activities which have been found in embryos suggest that the properties do vary [
23]. Additionally, with different kinds of Cas9/CRISPR configurations, recent science makes it possible to create iPSCs derived CMs (iPSC-CMs) carrying targeted gene knockout or knock-ins, and polymorphic substitutions, whilst activation of certain genes, its repression of expression, and epigenetic regulation are also feasible [
24]. This enables certain polymorphisms’ effects on cell function to be assessed in an otherwise constant genome (isogenic), which is crucial as the genetic background of phenotypes can exceed the mutations.
Since its advent, iPSC technology has expanded with three major applications: disease modeling, drug screening, and regenerative therapies. The current findings of iPSC profile have brought a new light to research advancement for the utilization of it in CMs regeneration purpose, that can potentially affect human lifespan’s extension and productivity. The prospect of changing plasticity of terminally differentiated cells toward pluripotency has completely altered the outlook for biomedical research. iPSCs became a landmark development in regenerative medicine, tested on animal models [
25], and has gotten to the start of its clinical trials.
Limitations and Solutions for Translation of iPSC-CMs Practically
Therapy using iPSC-CMs has drawn global attention as a potential therapy for treating different kinds of heart disease. However, the idea of bringing the invention into mass production of iPSC-CMs remains challenging, thus limiting the potential in regenerative medicine. There are still some obstacles in the production of iPSC-CMs into the public market. Based on the stage of process, these obstacles are: (1) Immaturity of iPSC-CMs, (2) Delivery routes, (3) Poor engraftment of implanted cells, (4) Immunogenicity and immune rejection, (5) Arrhythmogenicity, and (6) Possible tumorigenesis.
Overcoming iPSC-CMs Immaturity
iPSC-CMs still exhibit immature morphology and are similar to fetal CMs. The morphology is rounded, smaller than adult CMs, and mostly mononuclear. In contrast, adult CMs tend to be rod-shaped, anisotropic, and have a high aspect ratio, with 25-30% binucleated. As sarcomere is the essential contractile unit of CMs necessary for cardiac function, iPSC-CMs lack of clear T-tubes and show a disordered sarcomere stripes, which are shorter by length (1.6 µm), contain irregular myofibril and most cells only have immature high-density Z-band and I-band [
26].
Mature iPSC-CMs are characterized by mitochondrial maturation, increased oxidative capacity, and enhanced fatty acid use for energy production [
27]. As the physiological hypertrophy caused by organization of sarcomere structures contributes to the process of iPSC-CMs maturation, so is the development of T-tubules’, which associates with the transition to mature CMs pattern of excitation-contraction coupling, synchronously increasing Ca
2+ across Cells. Thus, the iPSC-CM maturation process must involve more efficient calcium handling as well as improved electrophysiological properties causing higher force of contractility. This shows that CMs with properties that more closely resemble adult myocardium would reduce arrhythmia risk and have improved contractile properties when transplanted. Investigation to achieve such a goal (iPSC-CM maturation) has been done with several methods. Trials include long period culture (expected to last 80 - 120 days) [
28], alteration to the culture’s substrate stiffness [
29], electrical stimuli [
30], and biochemical properties [
31]. Mechanical loading has also been used to stimulate maturation[
32]. Additionally, tissue engineering methods and electrical training of iPSC-CMs in a three-dimensional culture system has also contributed to the advancement of morphologically matured iPSCs [
33]. Lastly, a 3D culture containing multiple cell types has also been investigated to be promotional to more developed iPSC-CM phenotypes [
34].
Delivery Routes
Previous studies used isolated floating CMs for transplantation. The method involves trypsin, where it helps with cell degradation, cell-adhesion molecule on cell-surface, and many growth factor (GF) receptors and ion channels. Moreover, there is a method found to also help with improving transplantation success rate, which is by freezing CMs. This method is found to lower cell engraftment rate after transplantation by 5% [
35]. This low rate leads to inflammatory cell infiltration that works by removing necrotic cells that fail to conduct angiogenesis. If the transplanted tissue on the epicardial surface fails to connect to host CMs, its beating will be unsynchronized with that of host CMs. To solve this problem, treatment without trypsin was needed, one of them is by using CM Spheroids [
35]. A CM spheroid is a 1,000-CM aggregate with some advantages: cells harvesting without trypsinization, protects cell-surface proteins from being damaged, retained extracellular matrix, and intact humoral factors (such as GF). Hence, CM Spheroid increases cell-survival rate after transplantation remarkably.
Cardiomyocyte sheet, patch, or spheroid engraftment need a transplantation device that would create a hospitable environment for regenerating myocardium to sustain in a stable condition for a long period of time. If the implantation process does not achieve this, most of the cells were not retained in myocardium. Since the blade tip of a normal injection needle would cause bleeding that interferes with the process of transplantation, recent research has found that an injection needle with a cone-shaped blind end without a blade at the tip would reduce the bleeding interference that otherwise caused by conservative blade [
36]. It has six holes on the side for injecting CM Spheroids into heart tissue. Engraftment would be easier to achieve success with this innovation. Still, there are rooms for improvements so the transplanted cells can enlarge physiologically and begin to resemble adult CMs by longitudinal elongation morphologically [
37].
Prevention of Immunogenicity
Immunological barrier is one of major hurdles in stem cell therapy, including iPSC. Transplantation from allogeneic cells or tissues often elicits immune responses that eventually leads to graft rejection. In reality, immune rejection is a tandem reaction produced by immune cells attacking the graft through cytokine release, inflammation process, cytotoxic mechanism, and phagocytosis [
38]. The process of immune rejection is divided in two stages: sensitization and effector stage. The first stage, sensitization, of the lymphocyte of the recipient identifies the antigen of the graft, then it activates and proliferates. In the effector stage, the graft is entailed to destruction. In the process, it might have harmful consequences for the transplant recipient. The rejection depends on how far organs are involved, which is initiated by T cells (that also plays the role of adaptor to secondary lymphocytes). Acute rejection starts within the first weeks after the transplant procedure is done, typically caused by syngeneic grafts and approximately happens within 10 days after the transplantation process, resulting into tissue destruction by macrophages & lymphocyte infiltration. In human, T-cell mediated hypersensitivity and cytotoxicity play important roles in graft rejection. Solutions to the issue usually comprises major histocompatibility (MHC)-matching and the production of iPSC banks for options, autologous iPSCs that offer potential immune privilege, to the idea of a knockout to human leukocyte antigens (HLA) that may generate ready-to-use immunocompatible cells.
The idea for carrying knockout mutations relies on two key components (β2 microglobulin and class II MHC class transactivator) of MHC I and II (i.e. HLA I/II knockout iPSCs) were generated using CRISPR/Cas9 gene editing system and differentiated in CMs. It is still in study progress but gives promise to its suitability for allogeneic transplantation inducing little to no activity in human immune cells [
39].
In MHC matching, Shiba performed an investigation on a cynomolgus macaque with MI, in which allogeneic non-human primate iPSC-CMs were transplanted 14 days after injury [
40]. Improved cardiac function, electrical coupling synergized with host’s myocardium, and no evidence of immune rejection in MHC-matched iPSC-CM trial group are found, only to suggest MHC-matched donor-derived iPSC-CMs transplantation would potentially promise safety if tested in humans. However, with autologous iPSCs, theoretically it should be able to avoid immune rejection. However, genetic and epigenetic alterations may also occur during reprogramming, so there is still a fair risk of immune rejection with autologous iPSCs transplantation [
38]. Moreover, it takes several months to make autologous iPSCs as it requires the step of collecting patient’s cells and generating CMs at a large scale. As it is highly costly and takes a long duration, it may not be feasible in current clinical practice.
Solutions for Arrhythmogenesis
Previous investigations of iPSC-CM transplantation showed that intramyocardial engraftment into non-human primates and porcine models was associated with the events of transient ventricular arrhythmia. Conductive scaffolds are then proposed to aid signal propagation [
19]. Furthermore, engrafted iPSC-CMs tend to have immature phenotypes associated with spontaneous beatings affecting cardiac electrical signaling. To reduce arrhythmia events, protocols on subtype-specific ventricular differentiation combined with iPSC-CMs maturation methods would be efficient to eliminate pacemaker-like activity elicited by engrafted cells [
35]. Although epicardial transplantation of iPSC-CM patches has not been shown to provoke arrhythmia conduction in guinea pig and porcine [
19], this should be investigated further as mechanism allegedly causing the absence is due to graft’s fibrotic isolation and the electromechanical coupling lacking (with host myocardium).
Solutions to Poor Engraftment
Although significant advancement has been made over the past decade in improving the purity and yield of iPSC-CMs and scaling the differentiation process from 2D to 3D, efforts to induce phenotypic maturation during manufacturing has been very slow. The truncated rate of cell survival and its livelihood retention after transplantation has so far been the central barrier in the advancement of effective iPSC-CM-based cardiac regenerative therapy. To alleviate this issue to improve intramyocardial injected iPSC-CM survival rate, thus addressing the common cause of graft death, injection is supported by a developed pro-survival cocktail [
41]. In epicardial patches, pre-vascularization strategies have been explored to promote anastomosis of the patches with host vasculature, to promote the idea of cell survival [
42].
In intramyocardial injection of iPSC-CMs, fibrosis develops around transplanted iPSC-CMs as a wound healing response from MI condition and the injection itself. This is the second problem aside from the host immune response. It has been reported that less than 10% of injected cells successfully engraft and survive after its cardiac delivery [
43]. Given how transplanted CMs die from ischemia in the first few days following transplantation procedure, it is important to create a favorable environment by enhancing local vascularization to promote the cell’s survival rate [
44]. If not, this poor engraftment causing ischemia of the cell would affect graft’s signal propagation and proper electromechanical integration leading to arrhythmia. Thus, the inventions to solve this issue includes use of overexpression of certain proteins, such as Cyclin D2 (which is involved in cell cycle regulation) [
45], N-cadherin (protein responsible for cell adhesion) [
46], and co-transplant of readily made microvessels obtained from adipose tissue [
19]. All results in increased graft perfusion, adhesion, and cell survival rate, leading to improved functional recovery following myocardial infarction [
47].
Prevention of Tumorigenesis
Although iPSCs have a high growth rate and pluripotency suitable for regenerative medicine material, not all cells cultured from iPSCs can differentiate into targeted cells. The remainings, nontarget cells and the undifferentiated cells, would configure into a tumor called teratoma. This formation would impede clinical application. Previous studies have indicated that the iPSC tumorigenic potential is related to the C-Myc oncogene expression and p53 genotype status of the cells [
48,
49]. As mentioned ealier, C-Myc is one of The Yamanaka Factors, origin of somatic tissues from which pluripotent stem cells are induced. However, the thorough molecular mechanism behind iPSC tumor potentiality has to be further elucidated.
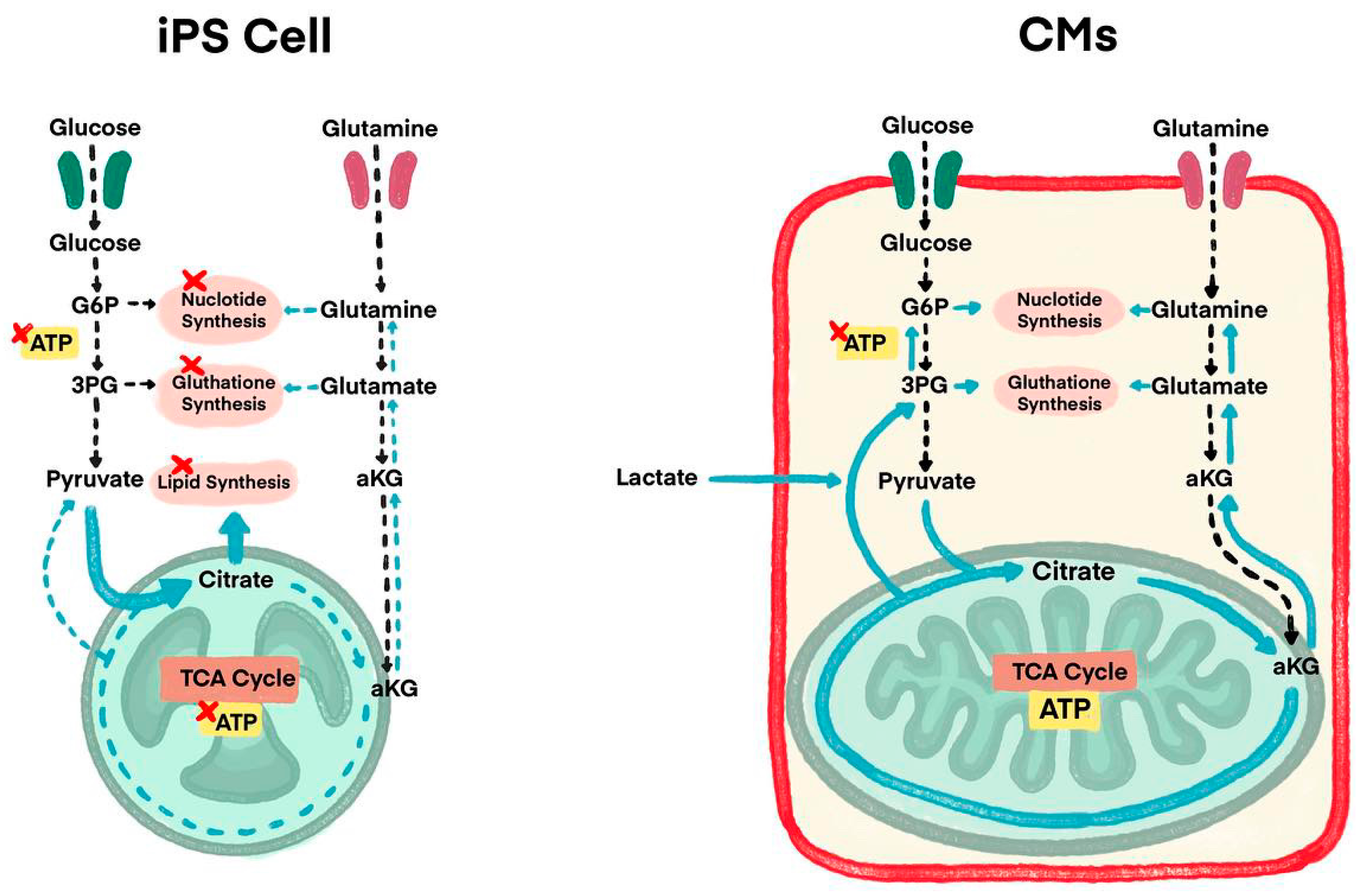
Some studies use purification stages to eliminate redundant non-CM cells as another method to prevent tumorigenesis. To solve this teratogenic tendency, studies have performed detailed analysis of energy metabolism-related pathways of CMs and iPSCs, which lands researchers on some solutions where lactate (which is the substance used for uptakes by CMs alongside glucose and glutamine) is added in the culture medium of iPSCs (
Figure 2) [
35]. CMs additionally use lactate as an energy source, where iPSCs use both glucose and glutamine. This is why, it is only CMs that can survive in a culture medium containing lactic acid without glucose and glutamine as buffers. This purification process by adding lactate is in recent findings of iPSCs exploration. This new method results in CMs remaining active (continuing to beat) with undifferentiated iPSCs and non-CMs count reduced to 0.001% in detection sensitivity, hence, teratoma formation did not occur.
Current Therapeutic Clinical Trials
Various clinical trials using iPSC-CMs are ongoing worldwide, but majority is nontherapeutic studies, such as disease modeling, drug screening, or cell banks [
50]. We introduce 4 therapeutic clinical trials of cardiac regenerative therapy using iPSC-CMs currently in progress.
iPSC-Derived Cardiomyocyte Patch
Cuorips Inc (Osaka, Japan) and a surgical group in Japan have explored a new strategy of myocardial regeneration therapy using iPSC-CMs and cell-sheet technique. Cell-sheet technology involves utilizing poly(N-isopropylacrylamide) PNIPAAm (a thermo-responsive polymer) as a coat for culture dishes. This method accelerates the release of cells, produces cell-sheets following temperature fluctuation [
51] and is useful for scaffoldless cardiomyocyte patch fabrication for iPSCs. Preclinical studies with swine myocardial infarction models have demonstrated their therapeutic potential [
52,
53,
54]. The group has been conducting a clinical trial implanting an iPSC-CM patch in patients with end-stage ischemic cardiomyopathy (Trial ID: NCT04696328) and recently reported one-year outcomes [
55]. The inclusion criteria were a left ventricular ejection fraction (LVEF) of 35% or less and New York Heart Association (NYHA) class III or higher heart failure symptoms. Three iPSC-CM patches, with 3.3 × 10
7 cells per patch, about 3.5 cm in diameter, were transplanted on the LV anterior and lateral wall by left thoracotomy. In the first three cases of the trial, no transplanted cell-related adverse events were observed during the 1-year observation period. Improvements in LV contractility by echocardiogram, electrocardiogram-gated cardiac CT, and an improvement in myocardial blood flow neovascularization by NH3-PET/CT were observed in two of the three patients. There was no evidence of tumor formation detected by whole-body FDG-PET scan or in surveillance blood testing of four biomarkers (alpha-fetoprotein, carbohydrate antigen 19-9, carcinoembryonic antigen, human chorionic gonadotropin) after transplantation. In this clinical trial, no immunosuppressive agents were administered three months after transplantation of the allogeneic iPSC-CM patches without matching HLA typing since the transplanted cells were expected to have been lost by immune rejection within the first three months. Paracrine effect is considered the primary mechanism of improvement in cardiac function, which leads to the improvement in myocardial blood flow by angiogenesis and functional improvement of residual cardiomyocytes.
Cardiac Spheroid
Heartseed Inc. (Tokyo, Japan) has been conducting a phase I/II clinical study (LAPiS Study: jRCTa032200189) with a cardiac spheroid which is an aggregation of allogeneic iPSC-derived highly purified ventricular CMs used on patients with ischemic cardiomyopathy. Highly purified (claimed to be >99%) ventricular CMs decrease the presence of arrhythmia after transplantation of iPSC-CMs [
56,
57]. Fukuda who leads the Heartseed stated to have developed methods to safely and efficiently generate iPSCs from peripheral blood T cells, and then generate it into type of ventricle-specific CMs and distinguish the high quality iPSCs from its residual iPSCs using “metabolic selection method” and get the iPSCs purified from its derivatives. The team also claimed to have developed a technology in which they can mass-produce engraftment of CMs efficiently using cardiac spheroids. By forming micro-tissue-like spheroids, retention rate and viability of transplanted cells are improved. One cardiac spheroid contains approximately 1,000 CMs, and its size is about 200µm. The LAPiS Study is a 52-week, phase I/II, dose-escalation clinical study in patients with ischemic cardiomyopathy which is being conducted in Japan. The primary endpoint of the study is safety at 26-weeks post-transplantation, and secondary efficacy endpoints include LVEF and myocardial wall motion. In this study, the cardiac spheroids are transplanted using a special administration needle into the LV myocardium in combination with coronary artery bypass grafting (CABG) surgery. The expected mechanism of action is that the transplanted CMs electrically couple with the patient’s myocardium to improve cardiac output by remuscularization and secretion of angiogenic factors (angiogenesis). They have recently reported 6-months outcomes of the first two patients in this study. They reported that LVEF improved and a decreased left ventricular end-diastolic volume (LVEDV) by echocardiogram and cardiac MRI in both patients. There was also improvement in the NYHA functional class by one or two class reductions and a decrease in NT-proBNP in both patients. The study will enroll ten patients in two dose cohorts of 50 million and 150 million CMs.
Biological Ventricular Assist Tissue (BioVAT)
Repairon (Goettingen, Germany) has been conducting a Phase I/II trial (NCT04396899) in Germany evaluating the safety and efficacy of iPSC-derived Engineered Human Myocardium (EHM) as Biological Ventricular Assist Tissue (BioVAT) in end-stage heart failure [
58]. The EHM were constructed from iPSC- CMs and stromal cells suspended in a bovine collagen type I hydrogel. EHM are produced from a comprehensively characterized iPS-cell line for off-the-shelf administration as allografts under concomitant immune suppression. EHM allografts can be produced on-stock and delivered as off-the-shelf products for individual assembly to meet clinical dosing and size demands. EHM is administered by minimally invasive surgery onto the beating heart with an elective left-lateral mini thoracotomy. Zimmermann recently reported the latest outcomes of the BioVAT. The study demonstrated:1) Safe maximal dose of EHM grafts constructed from 800 million iPSC-CMs and stromal cells with 12 months follow-up. 2) Proof of principle for vascularized remuscularization of the heart. 3) Evidence for sustainable thickening of the target heart wall by EHM grafts. 4) Evidence for improved EF and symptoms in NYHA and the Kansas City Cardiomyopathy Questionnaire (KCCQ).
Epicardial Injection of iPSC-CMs
Wang et al reported that two patients with ischemic cardiomyopathy underwent epicardial injection of allogeneic iPSC-CMs at the time of CABG in China under a clinical trial (NCT03763136) [
59]. This study is a dose-escalation, placebo-controlled, single-center phase I/IIa clinical trial, in which the dose escalation is for three doses (1×10
8, 2×10
8 and 4×10
8 cells) [
60]. Patients with advanced heart failure are randomly allocated to receive epicardial injection of iPSC-CMs during CABG surgery or CABG surgery alone, followed by a 12-month follow-up investigation. The primary endpoint is to assess the safety of iPSC-CMs transplantation, including hemodynamic compromised ventricular arrhythmias and newly formed tumors during the initial six months postoperatively. The secondary endpoint is to evaluate the efficacy of the epicardial injection of iPSC-CMs and CABG surgery combination in comparison to isolated CABG surgery. The outcomes have not been reported yet.
Summary
Transplantation of iPSC-CMs represents an emerging therapeutic option for patients with end-stage HF and is keenly anticipated. Although clinical translation of iPSC-CMs therapy still has several limitations, where future investigation of these barriers to translation should be conducted to push findings forward. The transplantation of iPSC-CMs offers hope for cardiac regenerative therapy, is being studied in various clinical trials globally, and could potentially escort a long-awaited answer for severely intractable HFrEF by supplementing with freshly matured ventricular muscles.
References
- Shah, A.M.; Mann, D.L. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 2011, 378, 704–712. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Fonarow, G.C.; et al. Economic issues in heat failure in the United States. Journal of Cardiac Failure. 2022, 28, 453–66. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Dorsey, H.; et al. The global burden of heart failure: a systematic analysis for The Global Burden of Disease Study 2021. European Heart Journal 2023, 44. [Google Scholar] [CrossRef]
- Bristow, M.R. Management of heart failure. Braunwald’s Heart Disease; 2005, 603–624. [Google Scholar]
- Starling, R.C. Epidemiology of heart failure: progression to pandemic? Heart failure: A combined medical and surgical approach. 2007. Jan 8:1-8.
- Urbich, M.; Globe, G.; et al. A systematic review of medical costs associated with heart failure in USA (2014-2020). Pharmacoeconomics (38), p 1219-36.
- Murry, C.E.; Reinecke, H.; et al. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006, 47, 1777–85. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Murry, C.E. Regenerating the heart. Nature biotechnology Vol 23 [7]. Nature Publishing Group. 2005.; p. 845. [CrossRef]
- Hunt, S.A.; Abraham, W.T.; et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005, 112, e154–e235. [Google Scholar]
- Azevedo, P.S.; Polegato, B.F.; et al. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq Bras Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; et al. Heart disease and stroke statistics– update: a report from the American Heart Association. Circulation. 2014, 129, e28–e292. [Google Scholar]
- Joseph, A.; Kaplan, S. After criticism from scientists, congress eases its pursuit of faster stem cell therapies. Stat News. 2016. Available on: https://www.statnews.com/2016/11/30/stem-cells-cures-act/.
- Romito, A.; Cobellis, G. Pluripotent stem cells: current understanding and future directions. Stem Cells Int. 2016, 9451492. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; et al. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed Pharmacother. 2019, 109, 304–13. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yuasa, S.; Fukuda, K. Cardiac regenerative medicine. Circ J. 2008, 72, A49–A55. [Google Scholar] [CrossRef]
- Sayed, N.; Liu, C.; et al. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016, 67, 2161–2176. [Google Scholar] [CrossRef]
- Silver, S.E.; Barrs, R.W.; et al. Transplantation of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Regenerative Therapy. Front Cardiovasc Med. 2021, 8, 707890. [Google Scholar] [CrossRef]
- Shanak, S.; Helms, V. DNA methylation and the core pluripotency network. Developmental Biology. 2020, 464, 145–60. [Google Scholar] [CrossRef]
- Chin, M.H.; Mason, M.J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Newman, A.M.; Cooper, J.B. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell. 2010, 7, 258–62. [Google Scholar] [CrossRef]
- De Korte, T.; Katili, P.A.; et al. Unlocking personalized biomedicine and drug discovery with human induced pluripotent stem cell-derived cardiomyocytes: fit for purpose or forever elusive? Annual review of pharmacology and toxicology. 2020, 6, 529–51. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Macadangdang, J.; et al. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef]
- Vo, Q.D.; Saito, Y.; et al. Induced pluripotent stem cell-derived cardiomyocytes therapy for ischemic heart disease in animal model: A meta-analysis. International Journal of Molecular Sciences. 2024, 25, 987. [Google Scholar] [CrossRef]
- Wu, P.; Deng, G.; et al. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Bioscience Reports. 2021. [CrossRef]
- Vuckovic, S.; Dinani, R.; et al. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: lessons for maturation and disease modeling. Stem Cell Research & Therapy. 2022, 13, 332. [Google Scholar]
- Lundy, S.; Zhu, W.; et al. Structural and functional maturation of cardiomyocytes derived from stem cells. Stem Cells Dev. 22. 2013.
- Körner, A.; Mosqueira, M.; et al. Substrate stiffness influences structural and functional remodeling in induced pluripotent stem cell-derived cardiomyocytes. Frontiers in Physiology. 2021, 12, 710619. [Google Scholar] [CrossRef]
- Crestani, T.; Steichen, C.; et al. Electrical stimulation applied during differentiation drives the hiPSC-CMs towards a mature cardiac conduction-like cells. Biochemical and biophysical research communications. 2020, 533, 376–82. [Google Scholar] [CrossRef]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, Ó.; et al. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR protocols. 2022, 3, 101560. [Google Scholar] [CrossRef]
- Dou, W.; Wang, L.; et al. A microdevice platform for characterizing the effect of mechanical strain magnitudes on the maturation of iPSC-Cardiomyocytes. Biosensors and Bioelectronics. 2021, 175, 112875. [Google Scholar] [CrossRef]
- Huang, C.Y.; Maia-Joca, R.P.; et al. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. Journal of molecular and cellular cardiology. 2020, 138, 1–1. [Google Scholar] [CrossRef]
- Ziang, Y.; Miller, K.; et al. 3d Bioprinting of complex tissues in vitro state-of-the-art and future perspectives. Archives of toxicology. 2022, 96, 691–710. [Google Scholar]
- Fukuda, K. Establishment and Industrialization of a New Treatment Method Using Regenerative Cardiomyocyte Transplantation for Refractory Severe Heart Failure-Secondary Publication. JMA journal. 2023, 6, 388–92. [Google Scholar] [PubMed]
- Kishino, Y.; Fukuda, K. Unlocking the Pragmatic Potential of Regenerative Therapies in Heart Failure with Next-Generation Treatments. Biomedicines. 2023, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Tadevosyan, K.; Igesias-Garcia, O.; et al. Engineering and assessing cardiac tissue complexity. International Journal of Molecular Sciences. 2021, 22, 1479. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.H.; Wang, S.P.; et al. Immunogenicity in stem cell therapy for cardiac regeneration. Acta Cardiologica Sinica. 2020, 36, 588. [Google Scholar] [PubMed]
- Mattapally, S.; Pawlik, K.M.; et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell0derived cells: universal donor for cell therapy. J Am Heart Assoc. 2018, 7. [Google Scholar]
- Shiba, Y.; Gomibuchi, T.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016, 538, 388–91. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Okano, S.; et al. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Scientific reports. 2017, 7, 8630. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Murry, C.E. Human pluripotent stem cell-derived engineered tissues: clinical considerations. Stem Cell. 2018, 22, 294–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hu, Q.; et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2017, 115, 1866–75. [Google Scholar] [CrossRef]
- Robey, T.E.; Saiget, M.K.; et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008, 45, 567–81. [Google Scholar] [CrossRef]
- Zhao, M.; Nakada, Y.; et al. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation. 2021, 144, 210–28. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, M.; et al. N-cadherin overexpression enhances the reparative potency of human induced pluripotent stem cell-derived cardiomyocytes in infarcted mouse hearts. Cardiovasc Res. 2020, 116, 671–85. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; et al. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci Transl Med. 2020, 12. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, Y.; et al. Correlation between genetic abnormalities in induced pluripotent stem cell-derivatives and abnormal tissue formation in tumorigencity tests. Stem Cells Translational Medicine. Vol 2022, 11, 527–38. [Google Scholar]
- Prieto Gonzalez, E.A. A multilevel approach to the causes of genetic instability in stem cells. in Handbook of Stem Cell Therapy 2022, 1-55. Singapore: Springer Singapore.
- Kim, J.Y.; Nam, Y.; et al. Review of the Current Trends in Clinical Trials Involving Induced Pluripotent Stem Cells. Stem Cell Rev Rep. 2022, 18, 142–154. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; et al. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012, 126, S29–S37. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; et al. Enhanced therapeutic effects of human iPS cell derived cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci Rep. 2017, 7, 8824. [Google Scholar] [CrossRef]
- Ishida, M.; Miyagawa, S.; et al. Transplantation of human-induced pluripotent stem cell derived cardiomyocytes is superior to somatic stem cell therapy for restoring cardiac function and oxygen consumption in a porcine model of myocardial infarction. Transplantation. 2019, 103, 291–298. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, Y.; et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: first three case reports. Front Cardiovasc Med. 2023, 10, 1182209. [Google Scholar] [CrossRef]
- Oikonomopoulos, A.; Kitani, T.; et al. Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol Ther. 2018, 26, 1624–1634. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; et al. Erratum: Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018, 36, 899. [Google Scholar] [CrossRef]
- Ensminger, S.; Fujita, B.; et al. Safety and efficacy of induced pluripotent stem cell-derived engineered human myocardium as biological ventricular assist tissue in terminal heart failure (BioVAT-DZHK20): Trial design and experience at UKSH Lubeck. The Thoracic and Cardiovascular Surgeon. 2024, 72, DGTHG–V045. [Google Scholar]
- Mallapaty, S. Revealed: two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature. 2020, 581, 249–250. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; et al. Epicardial injection of allogeneic human-induced-pluripotent stem cell-derived cardiomyocytes in patients with advanced heart failure: protocol for a phase I/IIa dose-escalation clinical trial. BMJ Open. 2022, 12, e056264. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).