Submitted:
27 April 2024
Posted:
28 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of LFP Sample
2.3. Identification of Constituents in LFP
2.4. Tyrosinase Inhibitory Activity and Kinetic Type Assays
2.5. Determination of Copper Ion Chelating Ability of LFP
2.6. Analysis of the Secondary Structure of Tyrosinase
2.7. Analysis of the Conformation Change of Tyrosinase
2.8. Cell Viability Assay
2.9. Determination of Tyrosinase Activity and Melanin Content in Cells
2.10. Western Blotting Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Constituents of LFP
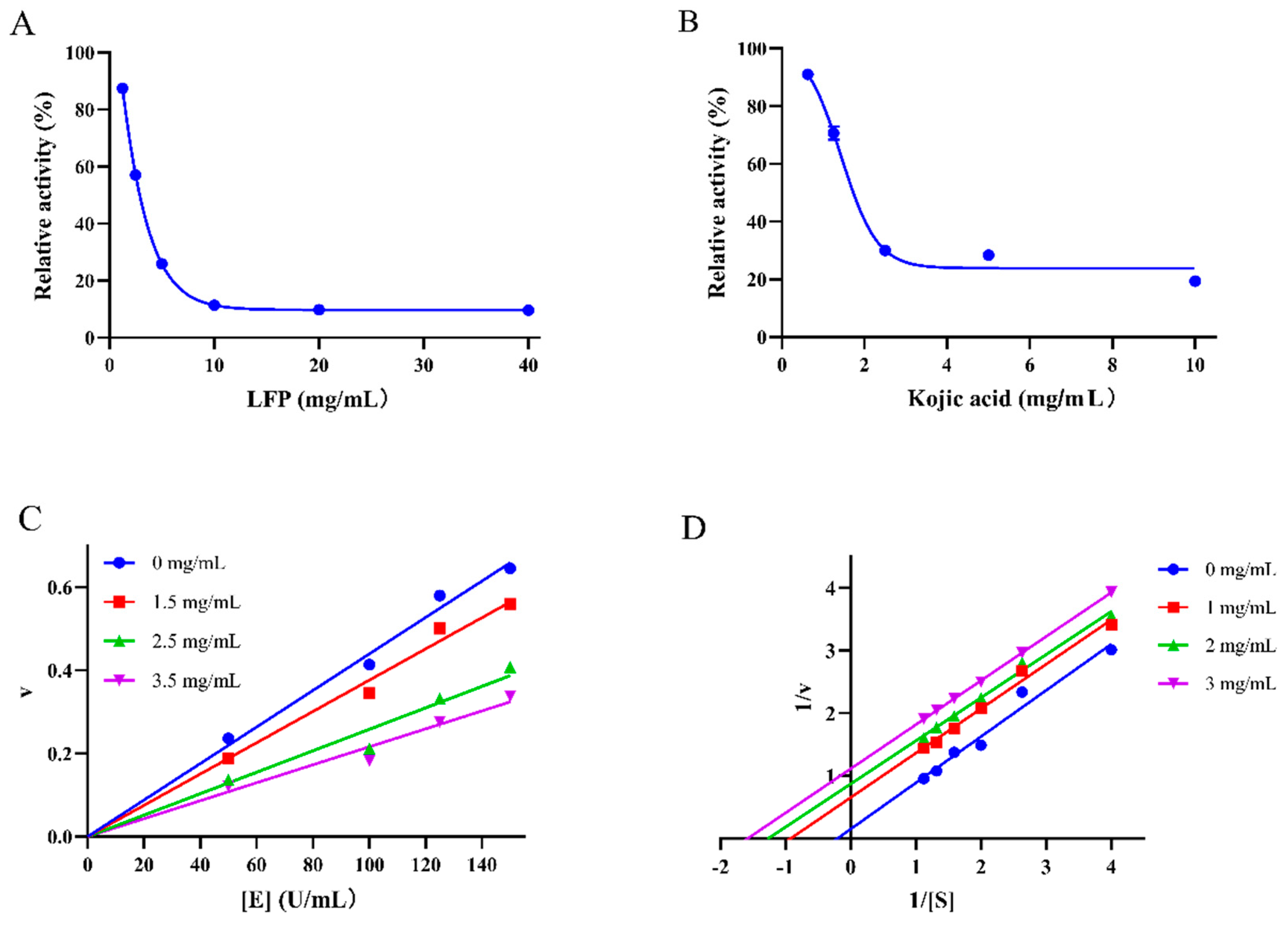
3.2. Inhibition Type of LFP on Tyrosinase Activity
3.3. Copper-Ion Chelating Ability of LFP
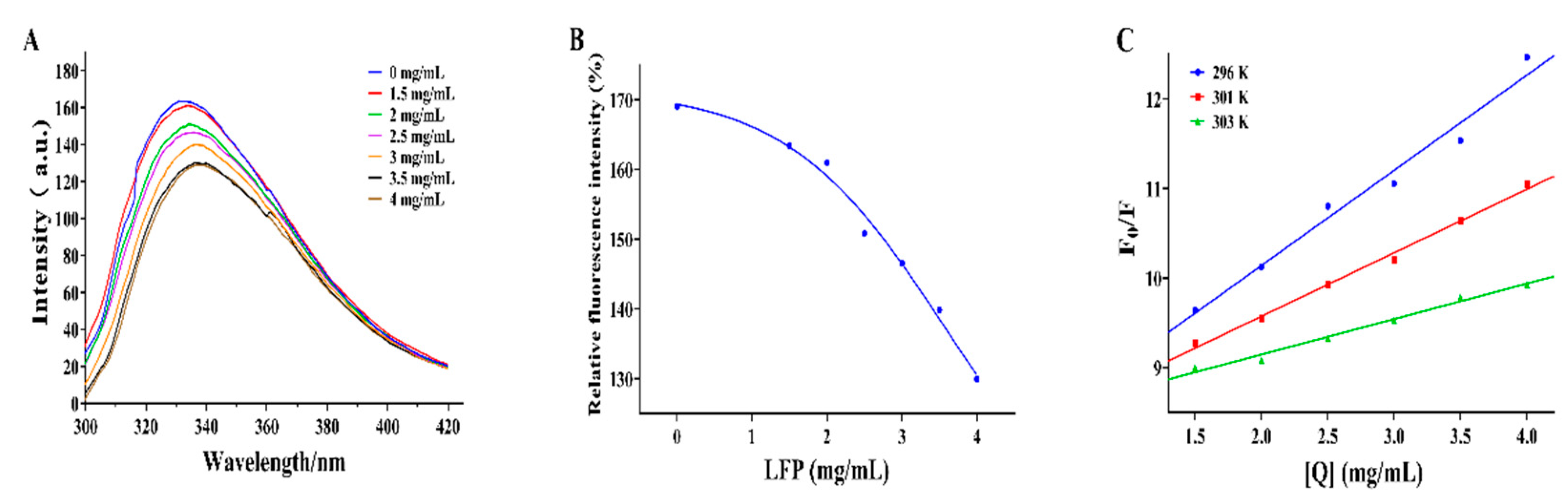
3.4. Effect of LFP on Secondary Structure of Tyrosinase
3.5. Effect of LFP on Conformation of Tyrosinase
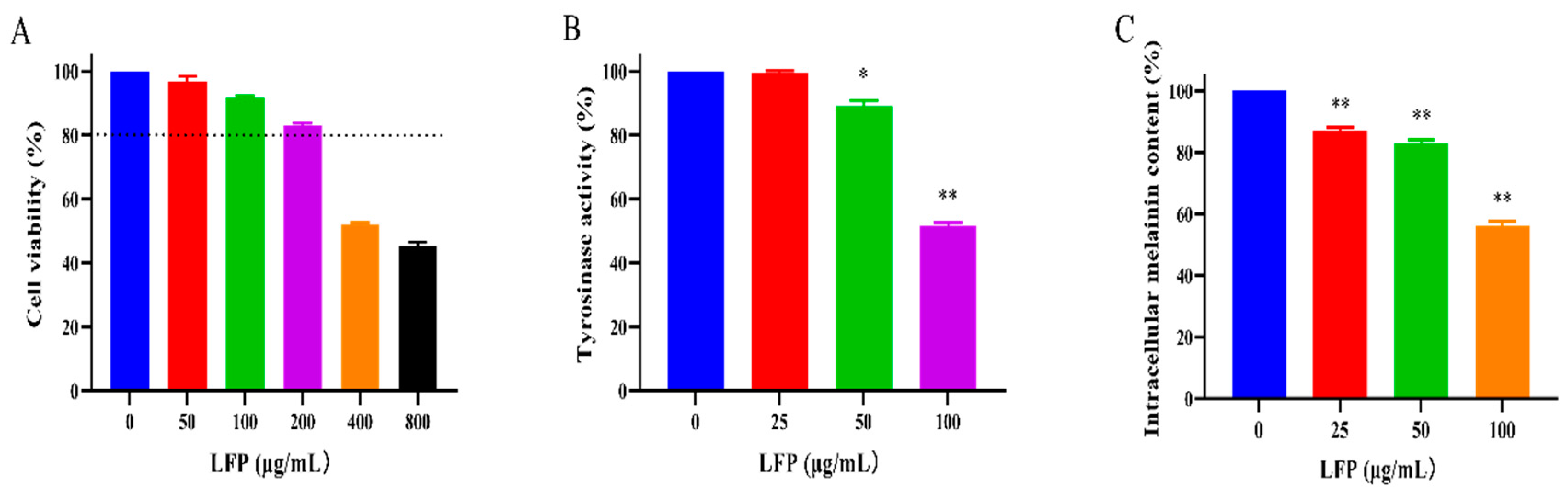
3.6. Effect of LFP on the Viability of Mouse Melanoma B16 Cells
3.7. Effect of LFP on Tyrosinase Activity and Melanin Synthesis in B16 Cells
3.8. Western Blotting Results
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Baltacioglu, H.; Bayindirli, A.; Severcan, M.; Severcan, F. Effect of thermal treatment on secondary structure and conformational change of mushroom polyphenol oxidase (PPO) as food quality related enzyme: A FTIR study. Food Chemistry 2015, 4, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, E.; Faure, C.; Carotti, M.; Bergantino, E.; Hardre, R.; Maresca, M.; Philouze, C.; Vanthuyne, N.; Boumendjel, A.; Bubacco, L.; du Moulinet d'Hardemare, A.; Jamet, H.; Reglier, M.; Belle, C. Exploiting HOPNO-dicopper center interaction to development of inhibitors for human tyrosinase. Eur J Med Chem 2023, 4, 115090. [Google Scholar] [CrossRef]
- Chen, Q.; Shang, C.; Han, M.; Chen, C.; Tang, W.; Liu, W. Inhibitory mechanism of scutellarein on tyrosinase by kinetics, spectroscopy and molecular simulation. Spectrochim Acta A Mol Biomol Spectrosc 2023, 4, 122644. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, X.; Xu, W.; McClements, D.J.; Liu, X.; Liu, F. Effects of different polyphenols on the structure and properties of sodium caseinate films mediated by tyrosinase. Journal of Agriculture and Food Research 2022, 4, 100395. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, J.; He, M.; Tian, S. Curcumin treatment enhances bioactive metabolite accumulation and reduces enzymatic browning in soybean sprouts during storage. Food Chem X 2023, 4, 100607. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine 2022, 4, 154449. [Google Scholar] [CrossRef] [PubMed]
- Gasowska-Bajger, B.; Wojtasek, H. Oxidation of baicalein by tyrosinase and by o-quinones. Int J Biol Macromol 2023, 4, 123317. [Google Scholar] [CrossRef] [PubMed]
- Ghalla, H.; Issaoui, N.; Bardak, F.; Atac, A. Intermolecular interactions and molecular docking investigations on 4-methoxybenzaldehyde. Computational Materials Science 2018, 4, 291–300. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomedicine Pharmacotherapy 2020, 128, 110301. [Google Scholar] [CrossRef]
- Ha, A.C.; Le, T.M. Improvement of anti-tyrosinase activity in potential skin whitening products by combining herbal extracts and reducing their tannin content by collagen fibre adsorption. South African Journal of Botany 2023, 4, 118–126. [Google Scholar] [CrossRef]
- Haas, B.; Hild, M.B.; Kussicke, A.; Kurre, A.; Lindinger, A. Fluorescence contrast improvement by polarization shaped laser pulses for autofluorescent biomolecules. Optik 2020, 4, 163777. [Google Scholar] [CrossRef]
- Imen, M.B.; Chaabane, F.; Nadia, M.; Soumaya, K.J.; Kamel, G.; Leila, C.G. Anti-melanogenesis and antigenotoxic activities of eriodictyol in murine melanoma (B16-F10) and primary human keratinocyte cells. Life Sciences 2015, 4, 173–178. [Google Scholar] [CrossRef]
- Jenkins, B.R.; Vitousek, M.N.; Safran, R.J. Signaling stress? An analysis of phaeomelanin-based plumage color and individual corticosterone levels at two temporal scales in North American barn swallows, Hirundo rustica erythrogaster. Horm Behav 2013, 4, 665–672. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, X.; Li, X.; Zhang, S.; Huang, Y.; Zhang, D.; Li, Y.; Qi, B. Soy protein-phlorizin conjugate prepared by tyrosinase catalysis: Identification of covalent binding sites and alterations in protein structure and functionality. Food Chem 2023, 4 (Part A), 134610. [Google Scholar] [CrossRef]
- Ju, X.; Cheng, S.; Li, H.; Xu, X.; Wang, Z.; Du, M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem 2022, 4, 133146. [Google Scholar] [CrossRef] [PubMed]
- Khouya, T.; Ramchoun, M.; Elbouny, H.; Hmidani, A.; Bouhlali ED, T.; Alem, C. Loquat (Eriobotrya japonica (Thunb) Lindl.): Evaluation of nutritional value, polyphenol composition, antidiabetic effect, and toxicity of leaf aqueous extract. J Ethnopharmacol 2022, 4, 115473. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhang, G.; Deng, S.; Huang, Z.; Peng, J.; Zhang, G.; Su, L.; He, W.; Wu, Y.; Ding, N.; Zhang, Z.; Lai, W. Synergistic dual-mechanism fluorescence quenching immunochromatographic assay based on Fe-polydopamine submicrobeads for sensitive detection of enrofloxacin. Chemical Engineering Journal 2023, 4, 140444. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, Y.; Jin Jung, H.; Ullah, S.; Ko, J.; Young Kim, G.; Yoon, D.; Hong, S.; Kang, D.; Park, Y.; Chun, P.; Young Chung, H.; Ryong Moon, H. Anti-tyrosinase flavone derivatives and their anti-melanogenic activities: Importance of the beta-phenyl-alpha,beta-unsaturated carbonyl scaffold. Bioorg Chem 2023, 4, 106504. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qi, Z.; Liu, C. Inhibition mechanisms of humic acid and protein on the degradation of sulfamethazine by horseradish peroxidase. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 4, 127473. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, L.; Wei, S.; Qin, Z.; Liang, D.; Ding, B.; Chen, H.; Song, W. Conformational changes of tyrosinase caused by pentagalloylglucose binding: Implications for inhibitory effect and underlying mechanism. Food Res Int 2022, 4, 111312. [Google Scholar] [CrossRef]
- Mokhtari, I.; Moumou, M.; Harnafi, M.; Milenkovic, D.; Amrani, S.; Harnafi, H. Loquat fruit peel extract regulates lipid metabolism and liver oxidative stress in mice: In vivo and in silico approaches. J Ethnopharmacol 2023, 4, 116376. [Google Scholar] [CrossRef]
- Satish, L.; Santra, S.; Tsurkan, M.V.; Werner, C.; Jana, M.; Sahoo, H. Conformational changes of GDNF-derived peptide induced by heparin, heparan sulfate, and sulfated hyaluronic acid - Analysis by circular dichroism spectroscopy and molecular dynamics simulation. Int J Biol Macromol 2021, 4, 2144–2150. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, J.; Zhu, J.; Yu, H. Changes of bioactive composition and concentration in loquat flower extracted with water/Chinese Baijiu. Heliyon 2023, 4, e14701. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Q.X.; Wang, Q.; Song, K.K.; Qiu, L. Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase. Food Chemistry 2005, 4, 707–712. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, Y.; Li, X.; Luo, X.; Qiu, Y.; Liu, G. A tyrosinase fluorescent probe with large Stokes shift and high fluorescence enhancement for effective identification of liver cancer cells. Spectrochim Acta A Mol Biomol Spectrosc 2023, 4, 121831. [Google Scholar] [CrossRef]
- Suzuki, T.; Hoshino, M.; Nishimura, M.; Ide, F.; Kusama, K.; Sakashita, H.; Kikuchi, K. A rare case of melanin-pigmented dentinogenic ghost cell tumor. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology 2023, 4, 335–340. [Google Scholar] [CrossRef]
- Varela, M.T.; Ferrarini, M.; Mercaldi, V.G.; Sufi B da, S.; Padovani, G.; Nazato LI, S.; Fernandes JP, S. Coumaric acid derivatives as tyrosinase inhibitors: Efficacy studies through in silico, in vitro and ex vivo approaches. Bioorganic Chemistry 2020, 4, 104108. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Zhang, N.; Shen, X.R.; Mei, W.W.; He, Y.; Ge, W.H. Preparation of total flavonoids from loquat flower and its protective effect on acute alcohol-induced liver injury in mice. J Food Drug Anal 2015, 4, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fan, L. Understanding the combined effect and inhibition mechanism of 4-hydroxycinnamic acid and ferulic acid as tyrosinase inhibitors. Food Chemistry 2021, 4, 129369. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Ding, Z. The inhibition mechanisms between asparagus polyphenols after hydrothermal treatment and tyrosinase: A circular dichroism spectrum, fluorescence, and molecular docking study. Food Bioscience 2022, 4, 101790. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chemistry 2019, 4, 124910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Y.; Xu, M.; Hu, X. UV–Vis spectroscopy combined with chemometric study on the interactions of three dietary flavonoids with copper ions. Food Chemistry 2018, 4, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, M.; Zhu, X.; Liu, R.; Lu, Q. Metabolomics reveals that phenolamides are the main chemical components contributing to the anti-tyrosinase activity of bee pollen. Food Chemistry 2022, 4, 133071. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Zengin, G.; Sinan, K.I.; Yıldıztugay, E.; Mahomoodally, M.F.; Ak, G.; Picot-Allain MC, N.; Gevrenova, R. Identification of bioactive compounds from Rhaponticoides iconiensis extracts and their bioactivities: An endemic plant to Turkey flora. Journal of Pharmaceutical and Biomedical Analysis 2020, 4, 113537. [Google Scholar] [CrossRef] [PubMed]


| Code | Compound Name | Molecular Formula | Molecular Weight |
CAS Number |
Retention Time (min) |
|---|---|---|---|---|---|
| Flavonoids | |||||
| 1 | Quercetin (Yu et al., 2019) | C15H10O7 | 302. 042 0 | 117-39-5 | 6.643 |
| 2 | Isorhamnetin (Gong et al., 2020) | C16H12O7 | 316. 057 7 | 480-19-3 | 7.108 |
| 3 | Eriodictyol (Imen et al., 2015) | C15H12O6 | 288. 063 0 | 209-016-4 | 6.894 |
| Cinnamic acid and its derivatives | |||||
| 4 | Tricoumaroyl spermidine (Zhang et al., 2022) | C34H37N3O6 |
583. 267 4 | NA | 9.236 |
| 5 | Dicoumaramide spermidine (Zhang et al., 2022) | C25H31N3O4 |
437. 230 7 | NA | 9.225 |
| 6 | Ethyl caffeate (Shi et al., 2005) | C11H12O4 | 208. 073 2 | 102-37-4 | 9.294 |
| 7 | 2-Hydroxycinnamic acid (Varela et al., 2020) | C9H8O3 |
164. 047 2 | 614-60-8 | 5.88 |
| 8 | Coumaric acid (Varela et al., 2020) | C9H8O3 | 164. 047 2 | 614-60-8 | 10.271 |
| 9 | trans-4-Methoxycinnamic acid (Zheleva-Dimitrova et al., 2020) | C10H10O3 |
178. 062 9 | 943-89-5 | 7.181 |
| 10 | Ferulic acid (Yu & Fan, 2021) | C10H10O4 | 194. 057 7 | 1 135-24-6 | 6.894 |
| Benzene and its derivatives | |||||
| 11 | 4-Methoxybenzaldehyde (Ghalla et al., 2018) | C8 H8O2 | 136. 052 4 | 123-11-5 | 5.952 |
| 12 | p-Anisic acid (4-Methoxybenzoic acid) (Ghalla et al., 2018) | C8 H8O3 | 152. 047 2 | 100-09-4 | 10.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





