1. Introduction
Paper products-related industries are still heavily polluting industries. If the paper used in our lives can be reused, it will greatly reduce the demand for paper, which will also reduce the pollution brought by the paper industry [
1,
2]. The recyclable paper based on photochromic materials [
3,
4,
5,
6], which use light source printing, not only reduces the pollution in the paper manufacture process, but also reduces the pollution caused by the use of ink, which can be said to be multiple birds. Therefore, in order to control the pollution generated by the paper product-related industries from the source, to realize ink-free printing and paper recycling, ink-free printing technology has become the primary way to solve this problem [
7,
8,
9]. Inspired by this, the UV photochromic material was loaded onto the paper and a printing device was made using the UV laser as the light source [
10]. According to the characteristics that the UV photochromic material can change color under ultraviolet light irradiated and the fading in the air, the paper can be reused. Compared with ultraviolet light, which is 7% of the sunlight, visible light accounts for nearly 40% of the sunlight, and the energy of visible light is lower than the energy of ultraviolet light, so the excitation energy barrier required for photochromic materials that can respond to visible light must be lower than that of ultraviolet photochromic materials [
10].Therefore, the study of visible light photochromic materials is of great significance for the wider application of ink-free printing technology in the future. At the same time, compared with ultraviolet light, visible light can obviously reduce the cost of inkless printing.
At present, the researches on photochromic materials mainly focus on Keggin-type heteropoly acids [
11,
12,
13], especially phosphomolybdic acid and phosphotungstic acid. Scholars have used different preparation methods to compound heteropoly acids into different organic/inorganic materials [
14,
15] to construct heteropoly acid-based composite photochromic materials. Through the interface interaction between the two, the charge transfer ability of heteropoly compounds is improved, and the excitation energy required for the photochromic reaction of the composite materials is reduced. Zhang Wei [
16] prepared a series of heteropoly acid/ ethyl cellulose (PMoA/EC), phosphomolybdic acid/ polyvinylpyrrolidone (PMoA/ PVP), phosphomolybdic acid/ silica/ ZnFe2O4 (PMoA/SiO2/ZnFe2O4) and other heteropoly acid photochromic nanocomposite films which can respond to visible light by compounding heteropoly acid with organic polymer or inorganic semiconductor nanomaterial excited by visible light. It is pointed out that the photochromic reaction of the thin film system is carried out according to the transfer mechanism of photogenerated electrons. Due to the advantages of rapid carrier mobility and overwhelming production capacity of active protons under bandgap excitation, ZnO is considered as a potential candidate for high efficiency photocatalyst materials [
17,
18]. Wang Xuan et al. [
18] prepared inorganic nanomaterials ZnO by calcination method, hydrothermal synthesis method and electrospinning method, and then combined it with heteropoly acid to prepare ZnO/PMoA composite film system with excellent visible photochromic properties, and pointed out that Zn-O-Mo bond charge transfer bridge was formed at the interface between ZnO prepared by hydrothermal synthesis and PMoA.
In this paper, the ZnO nanomaterials, phosphomolybdic acid and phosphotungstic acid were combined in the organic polymer polyethylpyrrolidone ethanol system with different concentration ratios by ultrasonic dispersion compounding method, and the heteropoly-acid-based multi-component visible light photochromic hybrid film system was constructed by drip coating. The PMoA-PWA/ZnO/PVP hybrid thin film with best visible light response and good reversibility was selected through the phototropism test, and the photochromic mechanism of visible photochromic composite films was studied according to the energy level transition characteristics of ZnO and the charge transfer mechanism of the charge transfer bridge of heteropolyacid. Finally, PMoA-PWA/ZnO/PVP hybrid thin film with the best photochromic performance was selected and loaded on ordinary A4 paper to prepare visible light photochromic paper, and its application was tested and the stability and reversibility of photochromic properties were investigated.
2. Experiment
2.1. Preparation
2.1.1. Preparation of PMoA-PWA/ZnO/PVP hybrid film
Zinc acetate dihydrate (Zn(OOCCH3)2‧2H2O, Sinopharm Chemical Reagent Co., Ltd.) was used as the Zn precursor. Absolute alcohol was used as the solvent and ethanolamine (MEA, HOCH2CH2NH2, Sinopharm Chemical Reagent Co., Ltd.) was used as the stabilizer. The final solutions were fabricated by stirring at 60℃ for 30 minutes. The solutions were all aged at room temperature for 24 hours to form solution NO.1. A 3.6866 g aliquot of H3PMo12O40 (PMoA, analytical reagent, Sinopharm Chemical Reagent Co., Ltd.) was mixed with 50 mL absolute alcohol ethanol (analytical reagent, Sinopharm Chemical Reagent Co., Ltd.) to form solution No. 2 with a density of 0.4 mol/mL. A 2.3042 g aliquot of H3O40PW12(PWA, analytical reagent, Sinopharm Chemical Reagent Co., Ltd.) was mixed with 20 mL absolute alcohol ethanol (analytical reagent, Sinopharm Chemical ReagentCo.,Ltd) to form solution No. 3 with a density of 0.4 mol/mL. A 1.00 g aliquot of Polyvinylpyrrolidone (PVPd, Mw=50000) was mixed with 100 mL absolute alcohol ethanol (analytical reagent, Sinopharm Chemical ReagentCo., Ltd.) to form solution No. 4 with a density of 0.01 g/mL. Solution No.1 and solution No. 2 were mixed in a ratio of 7:3. Then, under the condition of continuous stirring, we dripped an appropriate amount of the above mixed solution into the solution No.3 to prepare the PMoA:PWA concentration ratio of 1:1, 2:1, 3:1 and 4:1 respectively to form 10 mL mixed solution. A pipetting gun was used to drop this mixed solution into solution NO.4 with constantly stirring. Then we dropped this mixed solution onto a quartz substrate to form a PMoA-PWA/ZnO/PVP hybrid thin film with an amount of 100 µL. We recorded sample number as Ai(i=1,2,3,4).
2.1.2. Preparation of visible light photochromic paper
Use a pipette to measure 2 mL 21PMoA-7PWA/9ZnO mixed solution A3, and slowly add dropwise to 100 mL of PVP ethanol solution under constant stirring. After fully stirring for 30 minutes, ultrasonic compounding was performed for 1 hour. And then stirring fully for 1 hour, the transparent light yellow compound solution was obtained. The whole process was carried out under dark conditions. Then, the A4 paper was completely soaked in the above solution for 2 hours, then it was taken out and hung in the air. The paper was put in a dark box when it was completely dried. The preparation process of the visible light photochromic paper was carried out under dark conditions, because the composite system had excellent visible light photochromic properties.
2.2. Characterization
TEM images of the sample were obtained on a JEM-200CX (JEOL, Tokyo, Japan) transmission electron microscopy by dropping complex solution onto the copper grids. Photochromic property measurements were performed on a V-500 UV-optical spectrophotometer (JASCO, Tokyo, Japan) in the range of 350–900 nm with an optical detection resolution of 1 nm. XPS measurements were performed on an ESCALAB250 photoelectron spectrometer (Thermo, Waltham, Massachusetts, USA) to obtain the information of chemical binding energy of the hybrid film.
2.3. Photochromic Experiments
Photochromic properties experiments were performed with a 75 W xenon light source (PLS-SXE, Beijing Perfectlight Technology Co. Ltd., Beijing, China) filtered by a UV filter (pass above 422 nm wavelength) as the optical-light source. The straight-line distance between the light and the hybrid thin film was 150 mm. The hybrid film was exposed to air during the process of visible light irradiation. Under visible light irradiation, the hybrid thin film responded to visible light and generated a photochromic reaction, which turned blue from colorless. The color response of the film to visible light can be expressed by the change of the absorption peak on the absorbance curve of the film. After irradiation for a certain time, in situ absorbance curve of the film was obtained. The irradiation time was recorded until the curve was just as the same as the former one. Then the film was sheltered from light under air conditions and the absorption spectra was measured at regular intervals to monitor the bleaching process. At the same time, we put the colored film in a drying oven at 100°C for 30 minutes to test the absorption curve of the film. Repeatedly irradiate the film with visible light, and then faded under heating conditions. Record the absorption curve after coloring and fading, and drew the film coloring-fading cycle experiment diagram. We repeated the process of irradiating the film with visible light and fading under heating conditions and recorded the absorption curves after staining and fading. Finally, the experimental diagram of the film coloring-fading cycle was obtained.
2.4. Application test of the visible light photochromic paper
The visible light photochromic paper was put in an opaque box and sent to Changchun New Industry Optoelectronic Technology Co., Ltd. for application test. The test contents were as follows: The company used an inkless printing device prepared with a visible light source laser as the light source to print simple patterns, complex patterns and words on the prepared visible light photochromic paper, and took photos for the printed visible light photochromic paper.
3. Results and Discussion
3.1. TEM Analysis
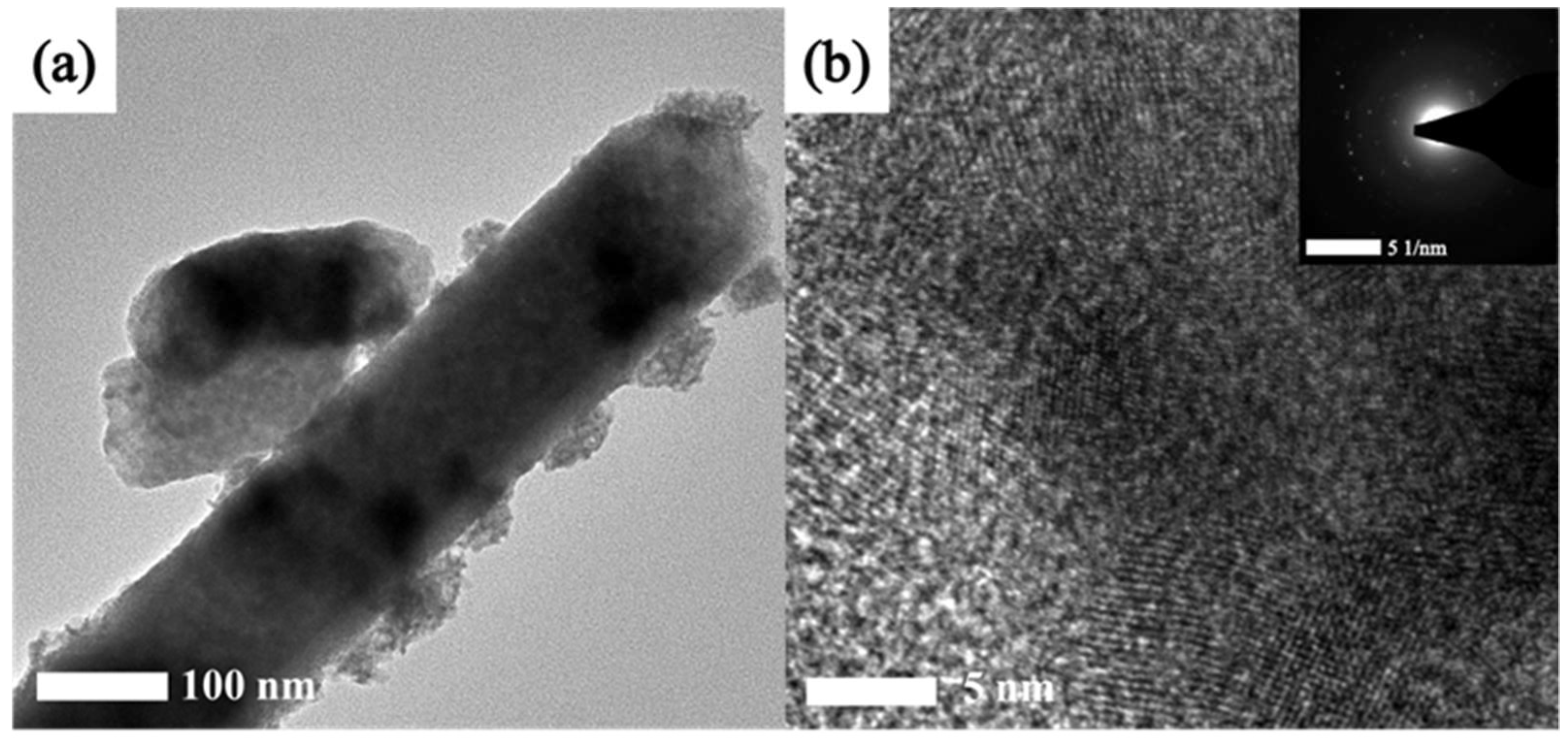
The microstructure of the PMoA-PWA/ZnO/PVP hybrid film was measured by transmission electron microscopy. The TEM images and HRTEM of this hybrid film are shown in
Figure 1 (a and b). It can be seen that the ZnO nanoparticles exhibit rod-like structure with an average diameter of 100-160 nm from the
Figure 1 (a). PMoA particles exhibited sphere structure with an average diameter of 50 nm and PWA particles exhibited sphere structure with an average diameter of 50 nm and a length of 120 nm. The structure of ZnO can be clearly seen in
Figure 1 (b). The lattice fringe spacing of ZnO particle was measured at 0.248 nm, and the pattern of concentric circles appeared in the optical diffraction image of HRTEM. It indicated that the ZnO particles was not a single crystal structure.
3.2. Photochromic Behavior
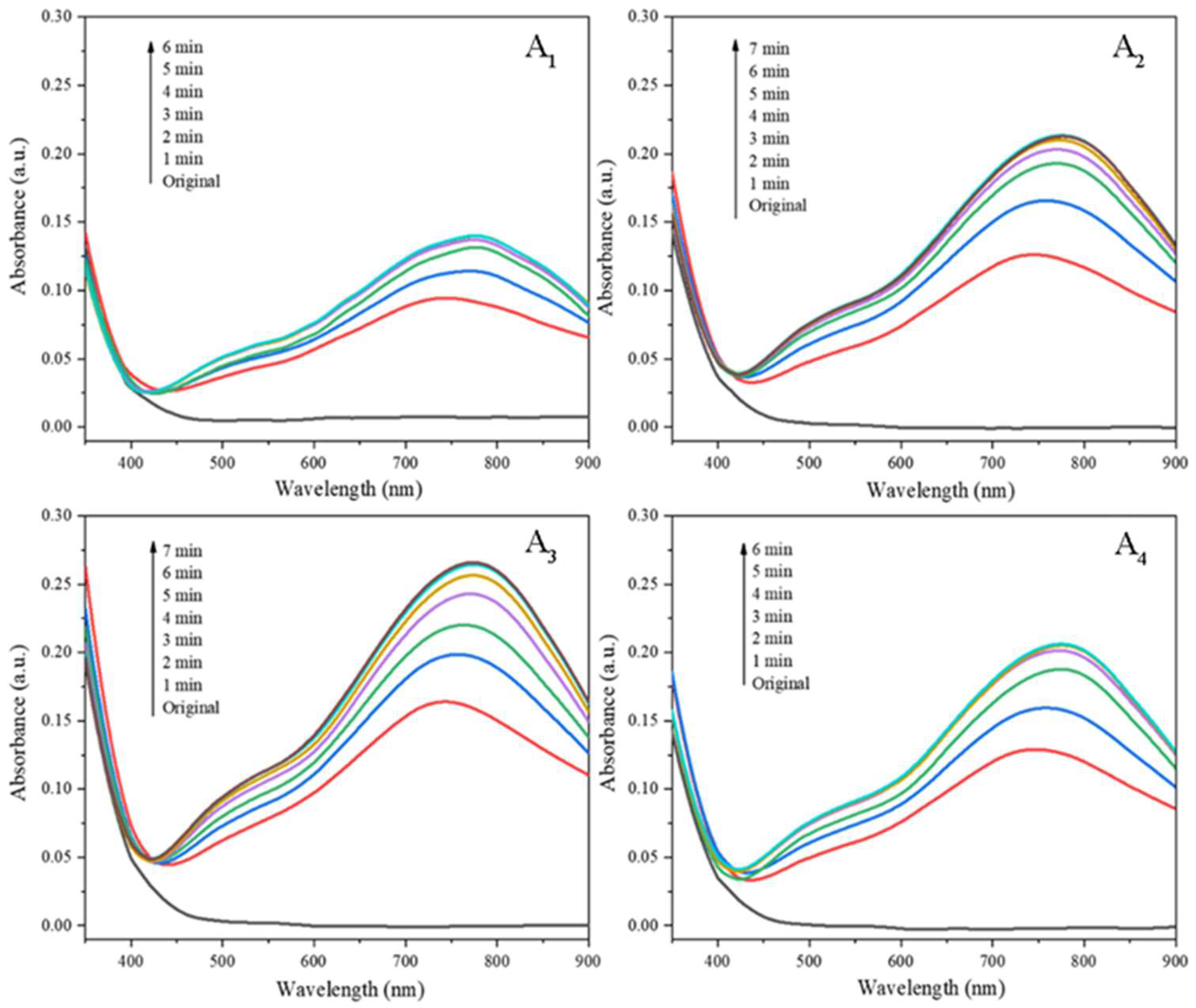
The UV-Vis absorption spectra of PMoA-PWA/ZnO/PVP hybrid films Ai(i=1,2,3,4) in the coloration process are illustrated in . There was no significant absorption peak in the range of 350 nm~900 nm before irradiation. After the irradiation of visible light, the color of the four hybrid films turned from light yellow to blue and the broad absorption bands appeared at 775nm in the UV-Vis absorption spectra of the four hybrid films, which indicated that PMoA-PWA/ZnO/PVP hybrid films can respond to visible light.
Comparing the UV-vis absorption spectra of A
1-A
4 in
Figure 2, it is found that after 6 min, 7 min, 7 min, and 6 min visible light irradiation respectively, the UV-vis absorption spectra of the hybrid films A
i (i=1,2,3,4) all had new features at 775 nm. At the same time, the absorption peak intensity of the hybrid films A
i (i=1,2,3,4) reached the maximum, which saturated absorbance reached 0.141, 0.213, 0.266, 0.206, respectively. The above result showed that the PMoA-PWA/ZnO/ PVP hybrid film system prepared in this experiment had excellent photochromic properties of visible light. When the ratio of PMoA:PWA was 3:1, was the best, and the saturated absorbance of the characteristic absorption peak of the hybrid film was as high as 0.266. This was because under the premise of ensuring the PMoA:ZnO ratio of 7:3 in the PMoA-PWA/ZnO/PVP hybrid film system, adding PWA components will increase the specific gravity of the chromophore groups (PMoA, PWA) and reduce the proportion of ZnO in the system. In the hybrid film system, when PMoA:PWA is 3:1, the saturated absorbance was the highest, indicating that the ratio of the chromophore to the photosensitizer ZnO was the best; when PMoA:PWA is greater than 3:1, the proportion of the PWA component decreased, the chromophore group decreased, and the photosensitizer ZnO was excessive, so the saturated absorbance of the hybrid film decreased; when PMoA:PWA is less than 3: 1, the specific gravity of PWA increased, the chromogenic group was excessive, the photosensitizer ZnO was insufficient, so the saturated absorbance of the composite film also decreased. Therefore, when preparing PMoA-PWA/ZnO/PVP hybrid film, the best ratio of PMoA:PWA is 3:1, and the ratio of PMoA:PWA:ZnO in the system is 21:7:9. Therefore, the hybrid film prepared when the ratio of PMoA to PWA was 3 (A
3) was used in subsequent studies.
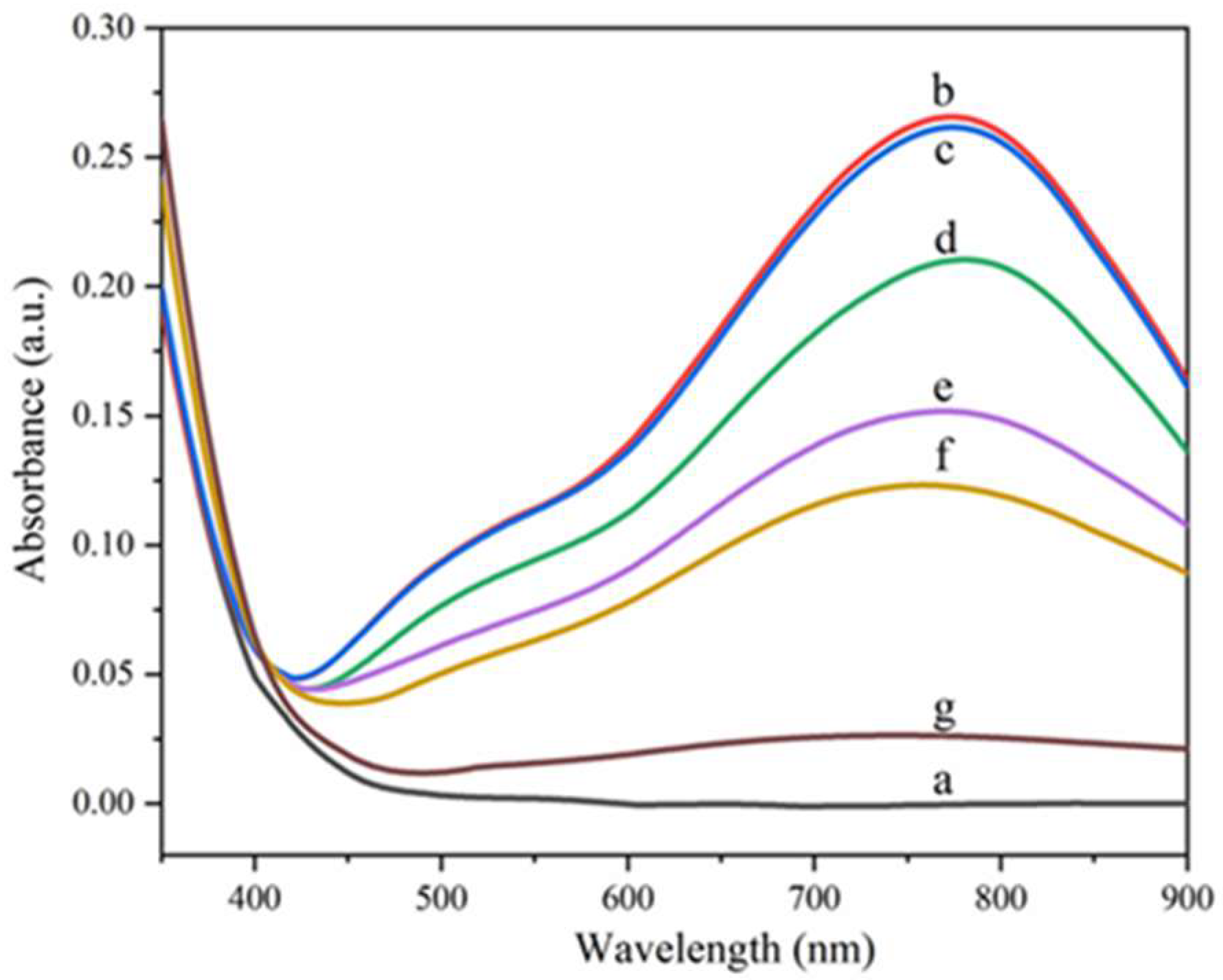
The fading process of the colored hybrid film A
3 is shown in
Figure 3. As shown in
Figure 3, the absorbance of the colored hybrid film sample A
3 was almost unchanged after being stored in vacuum for three days. When exposed to air, the hybrid film began to fade slowly, which indicated that oxygen played a key role in the bleaching process of the hybrid film. When the colored film faded in the air for three days, the absorbance of the hybrid film decreased to 74%, 55% and 46% of the saturated absorbance, respectively, which indicated that the fading rate of the hybrid film gradually decreased with the prolong of time. When the colored hybrid film was heated at 100 ℃ for 30 minutes, the absorbance of the hybrid film would be rapidly reduced to 10% of the saturated absorbance, which indicated that heating accelerated the discoloration rate of the hybrid film.
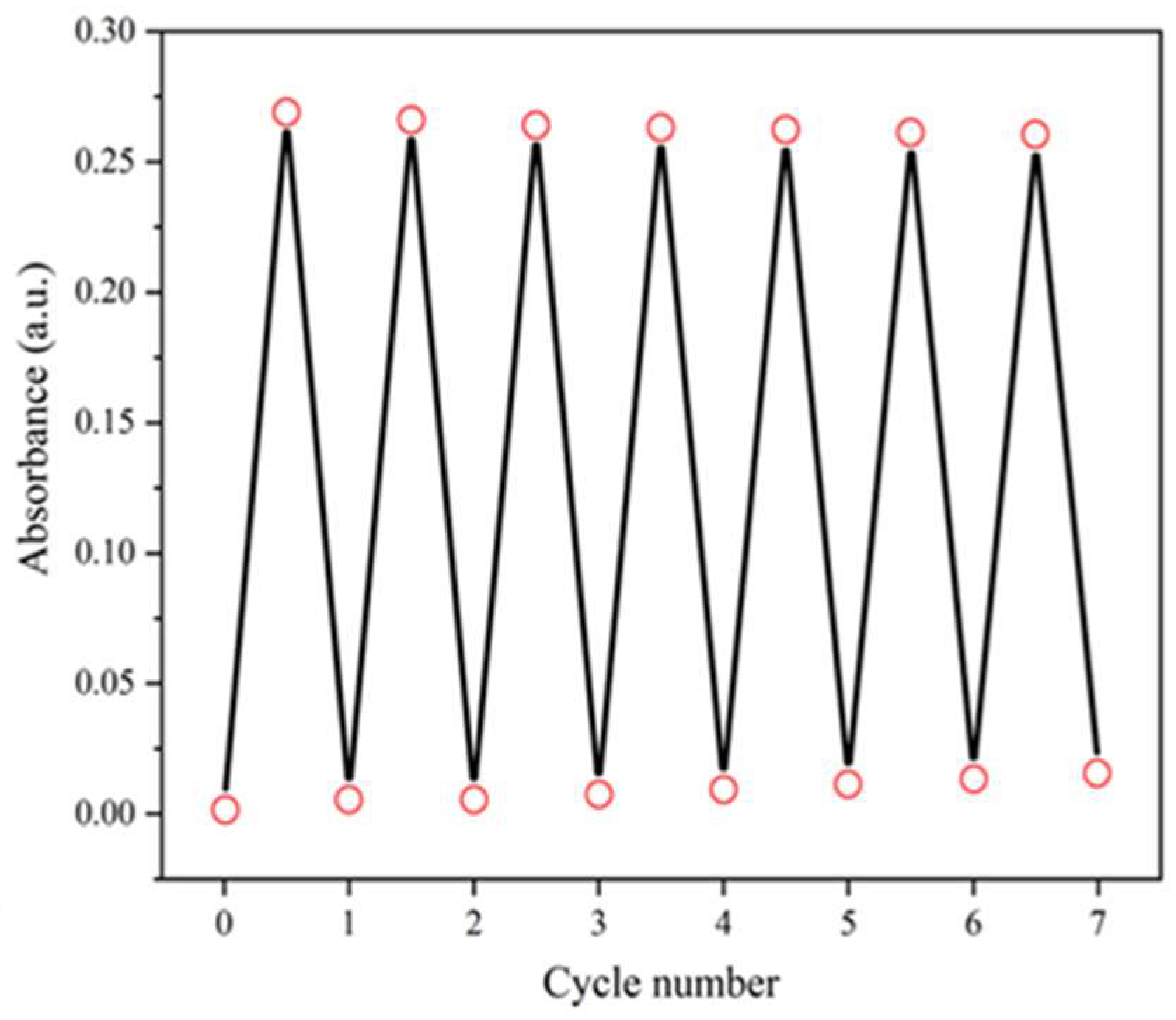
The reversibility of the coloration-decoloration cycles of the hybrid film is shown in
Figure 4. As shown in
Figure 4, the saturated absorbance of the hybrid film in 7 coloration-discoloration cycles was almost unchanged, and the original absorbance increased slightly, but almost unchanged. It showed that the hybrid film prepared in this experiment had high-stability and good reversibility. After 7 cycles of experiments, the hybrid film was still firmly adhered to the substrate, which indicated that the film had good adhesion and stable physical properties.
3.3. Photochromic Mechanism
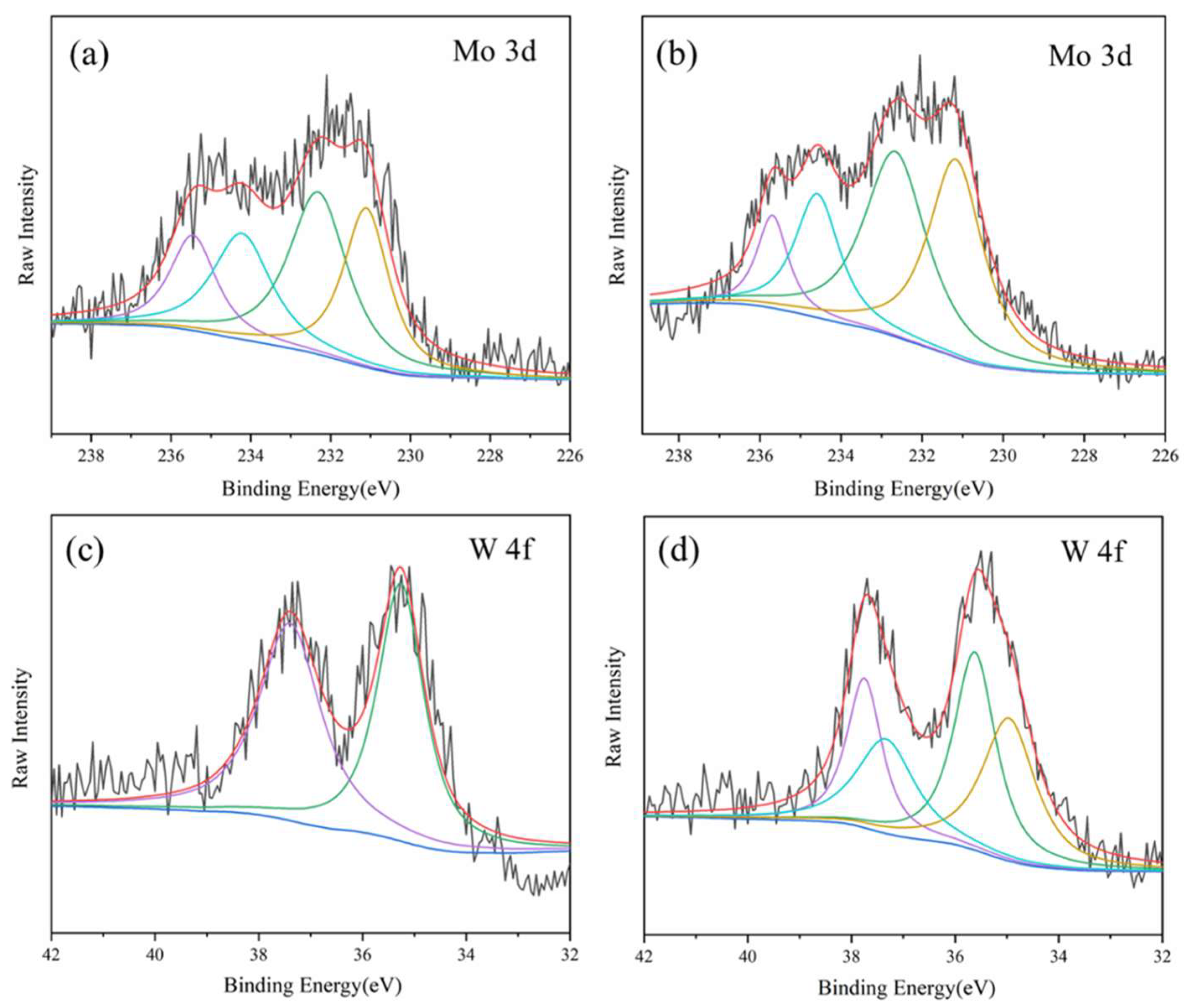
In order to exploring the photochromic mechanism of PMoA-PWA/ZnO/PVP hybrid film under visible light, we studied the electronic structure changes of Mo 3d and W 4f in the hybrid film before and after irradiation by XPS spectroscopy in
Figure 5.
The electronic structure of Mo 3d before and after irradiation are shown in
Figure 5(a)-(b). The binding energies (BE) of Mo 3d had two spin-orbit configurations, Mo3d
5/2 and Mo3d
3/2 respectively, which are obtained by Gaussian spectral decomposition. As shown in Figure (b), the characteristic signal of Mo
5+ was detected in the PMoA-PWA/ZnO/PVP hybrid film after visible light irradiation, which indicated that the PMoA in the hybrid film undergo a photoreduction reaction under visible light irradiation. In
Figure 5(a), the characteristic signal of Mo
5+ was also observed, which was caused by the X-ray excitation of the hybrid film before irradiation.
The binding energy of Mo3d
5/2 and Mo3d
3/2 in the hybrid film before and after irradiation are shown in
Table 1. The binding energies of Mo3d
5/2 and Mo3d
3/2 were reduced after irradiation, which showed that the chemical microenvironment of atom Mo had changed after irradiation. The proportion of atoms Mo occupied by Mo
5+ before and after irradiation is calculated as shown in
Table 1. It can be seen that the Mo
5+/Mo ratio was 0.40 before irradiation, and the ratio of Mo
5+/Mo reached 0.53 after irradiation, which can be attributed to the intervalence charge transfer and Mo
6+ in the hybrid film underwent a photoreduction reaction.
The electronic structure of W 4f before and after irradiation is shown in
Figure 5(c)-(d). The binding energy of W 4f can be divided into two configurations of spin-orbit merger, W4f
5/2 and W4f
3/2, which were obtained by Gaussian spectral decomposition. The binding energy of W4f
5/2 and W4f
3/2 before and after irradiation is shown in
Table 2. It can be seen from Fig.5(d) that after visible light irradiation, the characteristic signal of W
5+ is detected in the PMoA-PWA/ZnO/PVP hybrid film, which indicated that the PWA in the hybrid film underwent a photoreduction reaction under visible light irradiation.
It can be seen from
Table 2 that the BE values of W4f
5/2 and W4f
3/2 after illumination were both reduced, which indicated that the chemical microenvironment of W atoms had changed after irradiation. The ratio of W
5+/W reaches 0.49 after the irradiation, which indicated that during the irradiation process, W
6+ in the hybrid film underwent a photoreduction reaction to generate W
5+.
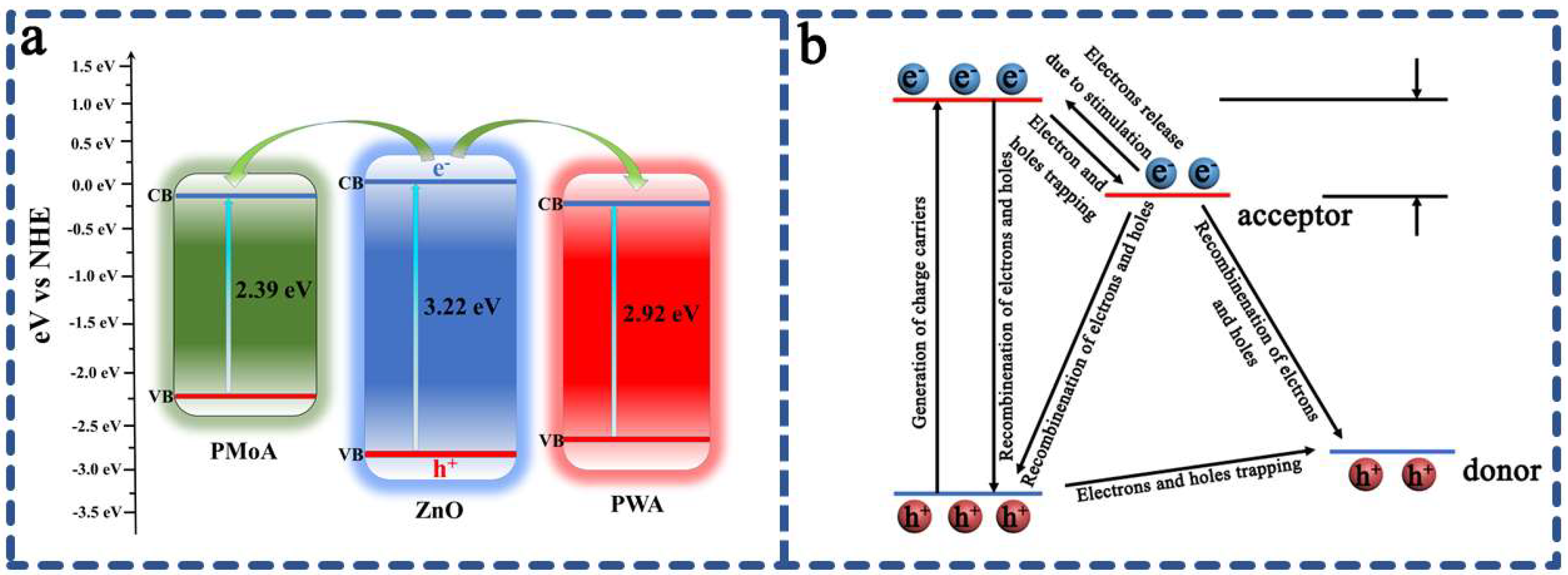
The photochromic energy level of PMoA-PWA/ ZnO/ PVP hybrid film system is shown in
Figure 6. The valence band bottom of ZnO was significantly lower than that of PMoA, which indicated that the electrons in ZnO can be more easily to enter PMoA. As a visible light photosensitizer, ZnO can generate photogenerated electrons under the excitation of visible light. The conduction bands of PMoA and PWA were both lower than the conduction band of ZnO, which facilitated the injection of excited electrons into the conduction band of PMoA or PWA. In this reaction, ZnO acted as a visible light photosensitizer and electron donor to generate photogenerated electrons under the excitation of visible light, and transfered electrons to the conduction bands of PMoA and PWA through the relative energy level difference with the conduction bands of PMoA and PWA. PMoA and PWA obtained electrons to undergo a photoreduction reaction, Mo
6+ is reduced to Mo
5+, W
6+ is reduced to W
5+, and heteropoly blue was formed. It was consistent with the conclusion drawn by XPS analysis. Therefore, the photochromic mechanism of PMoA-PWA/ ZnO/ PVP hybrid film was based on the photogenerated electron transfer mechanism.
3.4. Research on printing performance of visible light photochromic paper
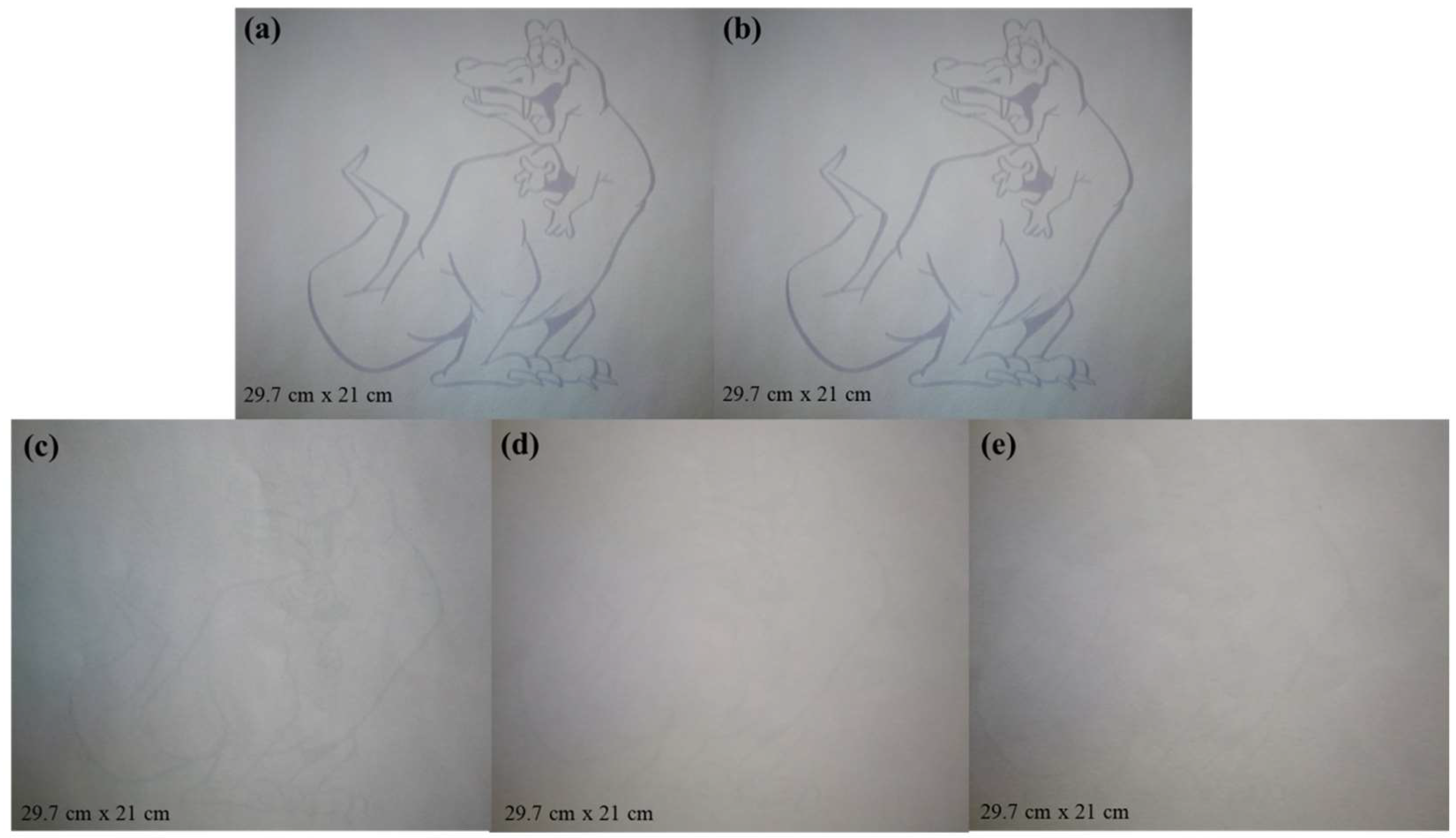
Figure 7 shows photos of the paper with visible photochromic after printing on an ink-free printer. It can be seen that the prepared visible light photochromic paper was directly used for printing. Whether it was used for printing simple patterns, complex patterns or words, the printed contents can be clearly presented on the paper. There was not much difference between inkless printing and normal ink printing, which preliminarily showed that it was feasible to replace ordinary paper with visible light photochromic paper.
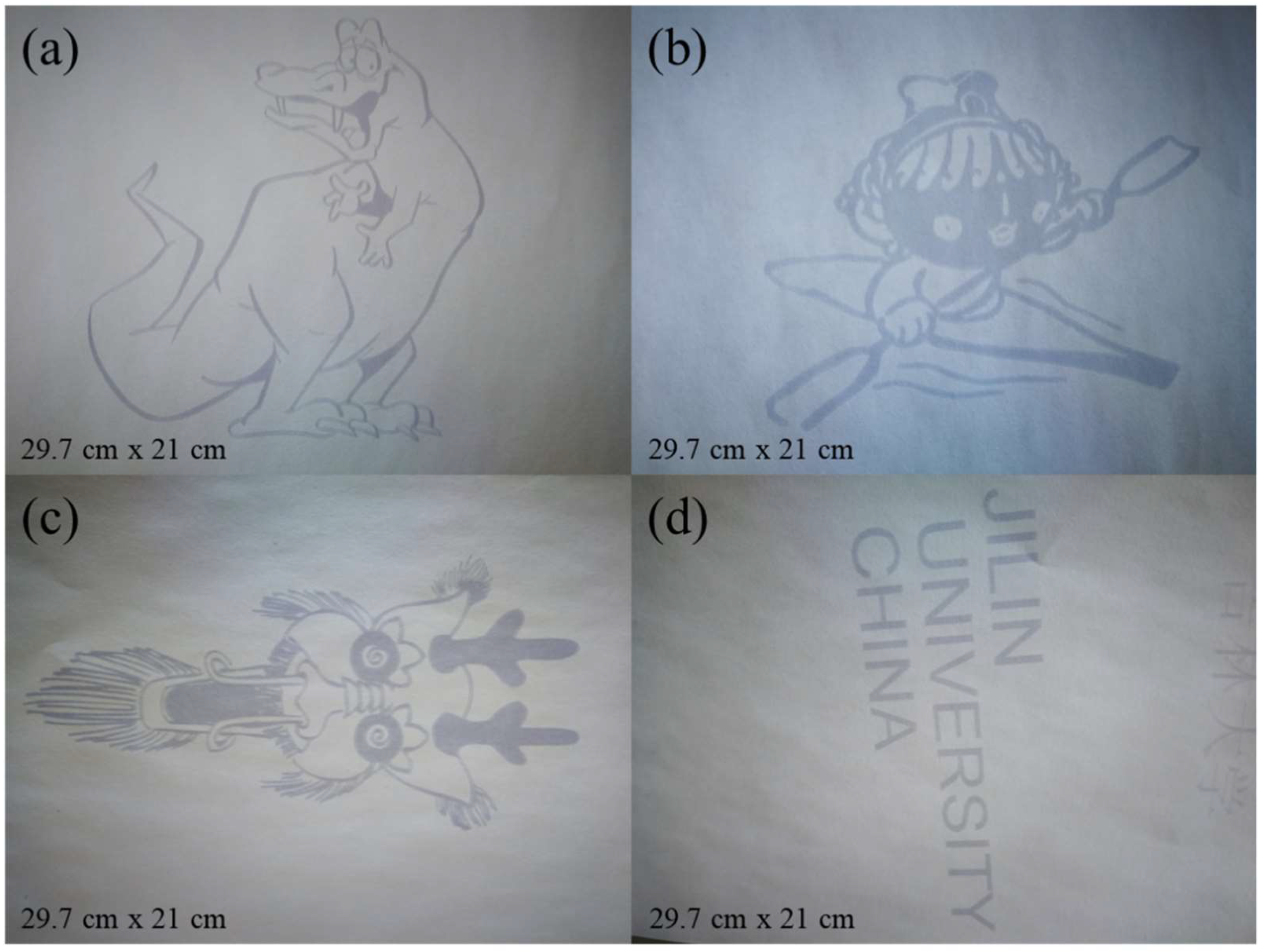
The photos of visible light photochromic paper printed with photos are shown in Fig.8. It can be seen that the pattern printed on the visible light photochromic paper was very clear in
Figure 8a. After the patterned visible light photochromic paper was put in a vacuum bag for three days, the pattern was hardly changed (
Figure 8b). It indicated that the pattern on the paper can be preserved intact under vacuum condition. The patterned visible light photochromic paper was naturally faded in the air for three days, and the pattern on the visible light photochromic paper was a little fuzzy, but the outline of the pattern is still left (
Figure 8c). It indicated that the patterned paper had a fading reaction in the air. The fading reaction is consistent with the fading rule of the photochromic hybrid film described above, and the paper cannot be directly used for printing at this time. The visible light photochromic paper printed with pattern was heated at 100°C for 30 minutes, and the content of the pattern is almost invisible(
Figure 8d). It indicated that the heat treatment accelerated the fading rate of the visible light photochromic paper, which is the same as the fading rule of the hybrid film described above. From Fig.8e, it can be clearly seen that the visible light photochromic paper is still intact after repeated use for 7 times, which showed that the visible light photochromic paper had excellent, stable and reversible performance when it was applied to print pattern.
In summary, the visible light photochromic paper prepared in this experiment had extremely stable, excellent and reversible visible light photochromic properties, whether it is printing patterns or words. It further showed that the visible light photochromic paper could replace the ordinary paper to realize the reuse of paper. The success of the application test of visible light photochromic paper and the analysis results of printing performance in this experiment laid a practical foundation for the daily use of the visible light photochromic paper.
4. Conclusions
In this paper, the PMoA-PWA/ZnO/PVP hybrid film was prepared by the ultrasonic composite method. Under the excitation of visible light, the hybrid film changes from colorless to dark blue. Under the excitation of visible light, ZnO can generate photogenerated electrons. Because of the relative energy level difference between ZnO and the conduction band of PMoA and PWA, the photogenerated electrons generated by ZnO can be injected into PMoA and PWA, which underwent photoreduction reaction after obtaining photogenerated electrons, Mo6+ is reduced to Mo5+, W6+ is reduced to W5+, and heteropoly acid produced heteropoly blue. In order to study the application performance of PMoA-PWA/ZnO/PVP hybrid film in paper recycling, visible light photochromic paper was prepared by loading hybrid film on paper. Under the excitation of visible light, the visible light photochromic paper presented stable and reversible excellent visible light photochromic properties, which provided a reliable practical basis for visible light photochromic paper to replace ordinary paper and to realize the reuse of paper. Because the excitation light source of the visible light photochromic paper was visible light, and the cost was low, so the application of visible light photochromic paper in the real life in the future was very promising. Therefore, the successful research and development of visible photochromic paper in this experiment was of great guiding significance for the realization of paper reuse.
References
- White paper: Environmental issues associated with toner and ink Usage (Preton Ltd., 2010).
- Sarantis, H. Business Guide to Paper Reduction, 2002.
- Ge J, Goebl, J, He, L, et al. Rewritable photonic paper with hygroscopic salt solution as ink. Adv. Mater. 2009, 21, 4259–4264. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic materials: more than meets the eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Zayat, M.; Levy, D. Photochromic organic-inorganic hybrid materials. Chem. Soc. Rev. 2011, 40, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Sun H, Gao N, Ren J, et al. Polyoxometalate-Based Rewritable Paper. Chem. Mater. 2015, 27, 7573–7576. [Google Scholar] [CrossRef]
- Garai, B.; Mallick, A.; Banerjee, R. Photochromic metal-organic frameworks for inkless and erasable printing. Chem. Sci. 2016, 7, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Jeong W, Khazi MI, Park D-H et al. Full color light responsive diarylethene inks for reusable paper. Adv. Funct. Mater. 2016, 26, 5230–5238. [Google Scholar] [CrossRef]
- Li D, Wei J, Dong S et al. Novel PVP/HTA hybrids for multifunctional rewritable paper. Acs Appl Mater Inter. 2018, 10, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Preparation and photochromic properties of heteropolyacid based composite films by visible light [D]. Jilin University, 2016.
- Duan Y, Waerenborgh JC, Clemente-Juan JM, et al. Light-induced decarboxylation in a photo-responsive iron-containing complex based on polyoxometalate and oxalato ligands. Chem. Sci. 2017, 8, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Parrot A, Bernard A, Jacquart A, et al. Photochromism and dual-color fluorescence in a polyoxometalate-benzospiropyran molecular switch. Chem. Int. Ed. 2017, 56, 4872–4876. [Google Scholar] [CrossRef] [PubMed]
- Liao JZ, Wu C, Wu XY. et al. Exceptional photosensitivity of a polyoxometalate-based charge-transfer hybrid material. Chem. Commun. 2016, 52, 7394–7397. [Google Scholar] [CrossRef] [PubMed]
- Qin LJ, Chen QJ, Lan RJ, et al. Effect of anodization parameters on morphology and photocatalysis properties of TiO2 nanotube arrays. J. Mater. Sci. Technol. 2015, 31, 1059–1064. [Google Scholar] [CrossRef]
- Sun Y, Wang X, Lu Y, et al. Preparation and visible-light photochromism of phosphomolybdic acid/polyvinylpyrrolidone hybrid film. Chem. Res. Chin. Univ. 2014, 30, 703–708. [Google Scholar] [CrossRef]
- Zhang, W. Preparation and properties of visible light photochromic composite films based on heteropoly metal compounds [D]. Jilin University, 2013.
- Sun W, Si Y, Jing H, et al. Visible-light photochromism of phosphomolybdic acid/ZnO composite. Chem. Res. Chin. Univ. 2018, 34, 464–469. [Google Scholar] [CrossRef]
- Wang Xuan. Preparation and photochromism of ZnO-based semiconductor composites with visible light [D]. Jilin Jianzhu University, 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).