Submitted:
27 April 2024
Posted:
28 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
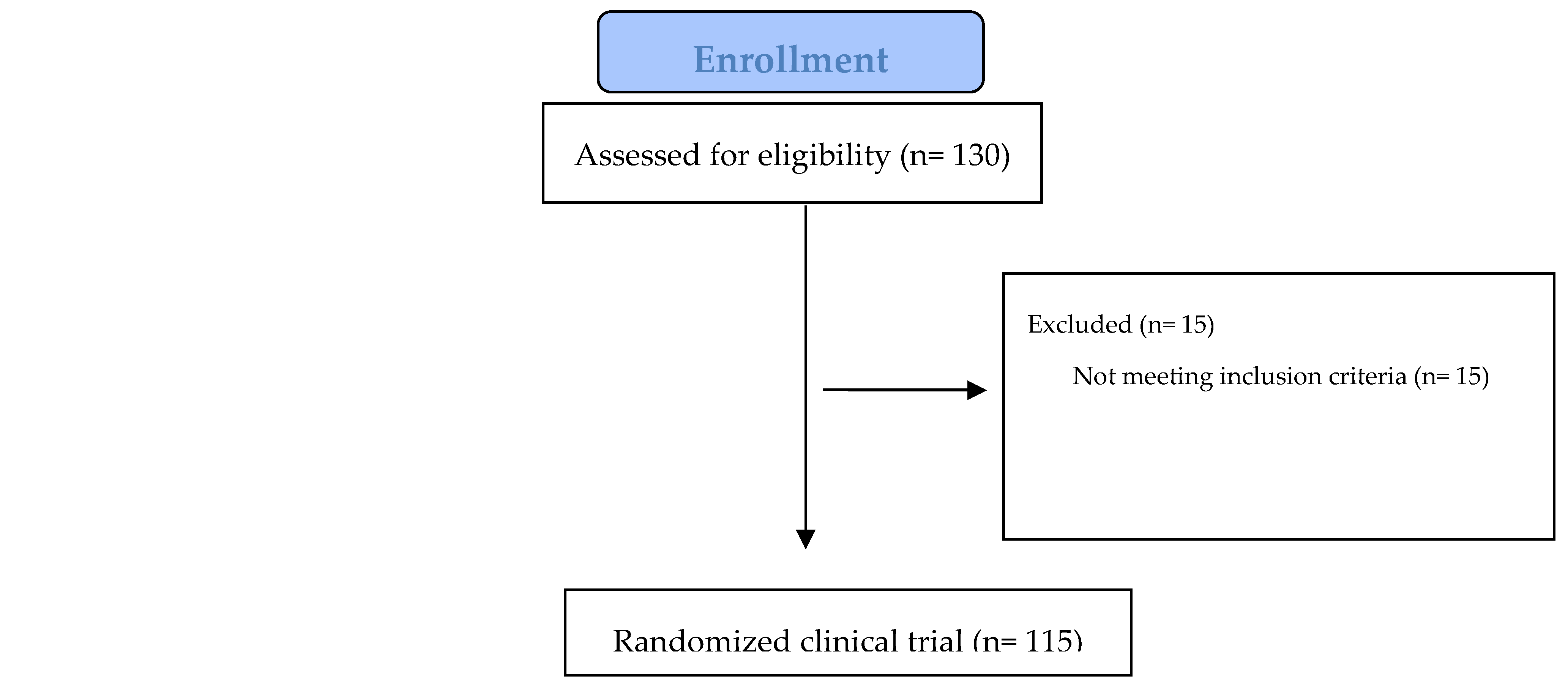
2.1. Recruitment Process
2.2. Intervention Study Design
2.2.1. Questionnaires
2.2.2. Anthropometric measurements
2.3. Biomarkers
2.4. Olive paste enriched with mountain tea
| Nutritional Composition (per 100 g) | |
|---|---|
| Energy (kcal) | 157 |
| Carbohydrates (g) | 6.6 |
| Fat, total (g) | 12.4 |
| Protein (g/kg) | 2.7 |
| Saturated fat (g) | 1.1 |
| Sugar , total (g) | 2.7 |
| Total phenolic ingredients (μg Gallic Acid) | 728±311 |
| Total Antioxidant activity (μmol FeSO4) | 956±33 |
2.5. Statistical Analysis
2.5.1. Sample size calculation
2.5.2. Data analysis
3. Results
| Physical activity levels | Total bone density (rho) | P-value |
|---|---|---|
| moderate | ||
| Group I | - | - |
| Group II | - | - |
| Group III | - | - |
| Group IV | 3.20 ± 0.28 | 0.726 |
| P-value | - | - |
| intense | ||
| Group I | 2.65 ± 0.48 | 0.032 |
| Group II | 2.83 ± 0.38 | 0.032 |
| Group III | 2.61 ± 0.50 | 0.032 |
| Group IV | - | - |
| P-value | - | - |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makras, P.; Athanasakis, K.; Boubouchairopoulou N.; Rizou, S.; Anastasilakis, A. D.; Kyriopoulos, J.; Lyritis, G. P.; Cost-effective osteoporosis treatment thresholds in Greece. Intern Osteoporosis Found, 2015 7, 1949-57. [CrossRef]
- Kanis, J.A. et al.; A reference standard for the description of osteoporosis. Bone 2008, 42, 467-75. [CrossRef]
- World Health Organization. (2019). Decade of Healthy Ageing 2020-2030 Proposal [PDF]. Retrieved from https://www.who.int/docs/default-source/documents/decade-of-health-ageing/decade-ageing-proposal-en.pdf?Status=Temp&sfvrsn=b0a7b5b1_12.
- Vaisi-Raygani, A.; Mohammadi, M.; Jalali, R.; Ghobadi, A.; Salari, N.; The prevalence of obesity in older adults in Iran: a systematic review and metaanalysis. BMC Geriatr 2019, 19(1), 1–9. [CrossRef]
- Khoddam, H.; Eshkevarlaji, S.; Nomali, M.; Modanloo, M.; Keshtkar, A.A.; Prevalence of malnutrition among elderly people in Iran: protocol for a systematic review and meta-analysis. JMIR Res Protoc 2019, 8(11), e15334. [CrossRef]
- Cheraghi P.; Cheraghi, Z.; Bozorgmehr, S.; The Prevalence and risk factors of osteoporosis among the elderly in Hamadan province: a cross sectional study. Med J Islam Repub Iran 2018, 32, 111. [CrossRef]
- Cauley, J.A.; Public health impact of osteoporosis. J Gerontol Ser A Biomed Sci Med Sci 2013, 68(10), 1243–51.
- Bryant, L.; Osteoporosis and Osteopenia – Vitamin Therapy for stronger bones. e-book, 1st Edition; Chicago, 2019; pp.16.
- Liu, H.F.; Yang, L.; He, H.C.; Zhou, J.; Liu, Y; Wang, C.Y.; Wu, Y. C.; and He, C.Q. Pulsed electromagnetic fields on postmenopausal osteoporosis in southwest China: a randomized, active-controlled clinical trial. Bioelectromagnetics, 2013, 34, 323–332. [CrossRef]
- Yi-Chou, H.; Wu, C.C.; Liao, M. T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K.C.; Role of Nutritional Vitamin D in Osteoporosis Treatment. ClinicaChimicaActa, 2018, 84, 179-191. [CrossRef]
- Garnero, P.; and Cremers, S. Bone turnover markers. Principles of Bone Biology, 2020; pp. 1801-1832.
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 1999, 69, 727–736. [CrossRef]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: the Framingham Osteoporosis Study. Am J Clin Nutr 2009, 89, 416–424. [CrossRef]
- Booth, S.L.; Tucker, K.L; Chen, H.; Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr 2000, 71,1201–1208. [CrossRef]
- Rondanelli, M.; Peroni, G.; Fossari F.; Vecchio; Faliva, M.A.; Naso, M.; Perna, S.; Di Paolo, E.; Riva, A.; Petrangolini G, Nichetti, M.; Tartara A.; Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density. Nutrients 2021, 13 (3), 1012. [CrossRef] [PubMed]
- Clarke, M.; Ward, M.; Strain, JJ.; Hoey, L.; Dickey, W.; McNulty H.; B-vitamins and bone in health and disease: the current evidence. Proc Nutr Soc 2014, 73(2), 330-9. Epub 2014 Feb 26. [CrossRef] [PubMed]
- Position Statement Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause: The Journal of The North American Menopause Society, 2010, 17(1), 25-54.
- Smith, J. (2021). The effects of polyphenols on bone metabolism. In Johnson, A. (Ed.), Advances in Nutrition Research (pp. 45-62). Elsevier. [CrossRef]
- Konstantinidi M, Lydatakis A, Olympiou A, et al. Study of the relationship among the consumption of bio-functional foods ingredients with bone metabolism indices in middle-aged and elderly people with osteoporosis risk. Public Health and Toxicology. 2022;2(Supplement 1):A127. [CrossRef]
- Weaver CM, Liebman M. Biomarkers of bone health appropriate for evaluating functional foods designed to reduce risk of osteoporosis. Br J Nutr. 2002 Nov;88 Suppl 2:S225-32. [CrossRef] [PubMed]
- . Arnold M, Rajagukguk YV, Gramza-Michałowska A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis-A Narrative Review. Foods. 2021 Mar 19;10(3):656. [CrossRef] [PubMed]
- Dimakopoulos, I.; Magriplis, E.; Mitsopoulou, A.V.; Karageorgou, D.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; Argyri, K.; Panagiotakos, D.B.; Zampelas, A. Association of serum vitamin D status with dietary intake and sun exposure in adults. Clin Nutr ESPEN, 2019, 34:23-31. [CrossRef]
- Sun, L.L.; Li, B.L.; Xie, H.L. Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: a case-control study. Br J Nutr 2014, 112, 1706–1714. [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J Trace Elem Med Biol; 2015; 32:86-106. 8: 32. [CrossRef]
- . Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: dairy intake and bone health. A viewpoint from the state of the art. J Dairy Sci 2011, 94:5249-62. [CrossRef]
- Tabatabaei-Malazy, O.; Salari, P.; Khashayar, P.; Larijani, B. New horizons in treatment of osteoporosis Daru 2017 25(1):2.
- Albani, E.; Petrou, P. A systematic review and meta-analysis of vitamin D and calcium in preventing osteoporotic fractures. Clinical Rheumatology, 2020, 3571-3579.
- Brown, L.; Caligiuri, S.; Brown, D.; Pierce, G. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [CrossRef]
- Martirosyan, D.; Singh, J. A new definition of functional food by FFC: What makes a new definition unique? Functional Foods in Health and Disease. FFHD 2015, 5, 209–223.
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional Foods for Dyslipidaemia and Cardiovascular Risk Prevention. Nutr. Res. Rev 2009, 22, 244–261. [CrossRef]
- Koutelidakis, A.; Dimou, C. The effects of functional food and bioactive compounds on biomarkers of cardiovascular diseases. In Functional Foods Text Book, 1st ed.; Martirosyan, D., Ed.; Functional Food Center: Dallas, TX, USA, 2016; pp. 89–117. Functional Food Center: Dallas, TX, USA.
- Kyritsakis, A. Olive Oil – Conventionla and Organic, Edible Olive – Olive Paste, 4th edition, Thessaloniki, 2007; p.328-336.
- Franke, A.A.; Cooney, R.V.; Henning, S.M.; & Custer, L. J. Bioavailability and antioxidant effects of orange juice components in humans. Journal of Agricultural and Food Chemistry 2005, 53(13), 5170–5178. [CrossRef]
- Dontas IA, Lelovas PP, Kourkoulis SK, Aligiannis N, Paliogianni A, Mitakou S, Galanos A, Kassi E, Mitousoudis A, Xanthos TT, Papaioannou N, Lyritis GP. Protective effect of Sideritis euboea extract on bone mineral density and strength of ovariectomized rats. Menopause. 2011 Aug;18(8):915-22. [CrossRef] [PubMed]
- NHANES. NHANES Food Questionnaire. Atlanta, 2019; Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/tq_fpq_c.pdf (accessed on 10 December 2019).
- Ntrigios, I.; Ntrigiou, V.; Dimou, C.; Rigopoulos, N.; Koutelidakis, A. Correlation of specific functional foods consumption with anthropometric characteristics and body composition on a sample of 18–65 years old aged adults from Greece. In Proceedings of the 22nd International Conference Functional Foods and Chronic Diseases: Science and Practice, Boston, MA, USA, 22 September 2017.
- Alissa, E.M.; and Gordon, A.F. “Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases”. Journal of Nutrition and Metabolism 2012, Volume 2012, p.p. 16.
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 2006, 16(8), 559-568.
- Papagianni, O.; Moulas, I.; Loukas, T.; Magkoutis, A.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.Μ.; Karantonis, H. C.; Koutelidakis, A. E. Trends in Food Innovation: An Interventional Study on the Benefits of Consuming Novel Functional Cookies Enriched with Olive Paste. Sustainability, 2021 13(20).
- Shedd, K.M.; Hanson, K.B.; Alekel, D.L.; Schiferl, D.J.; Hanson, LN.; Van Loan MD.; Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc 2007 39(12), 2189-98. [CrossRef] [PubMed]
- WHO. Obesity and Overweight Fact Sheet. 2021; Available online: https://www.who.int/news-room/fact-sheets/obesity-andoverweight (accessed on 10 June 2021).
- Myint, PK.;Kwok, CS.; Luben,; RN.; Wareham, NJ.; Khaw KT.; Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart 2014 100(20), 1613-9. [CrossRef] [PubMed]
- Mukhopadhyay P, Ghosh S, Bhattacharjee K, Chowdhury S. Inverse Relationship Between 25 Hydroxy Vitamin D and Parathormone: Are there Two Inflection Points? Indian J Endocrinol Metab. 2019 Jul-Aug;23(4):422-427. [CrossRef] [PubMed]
- Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011 Jan 20;364(3):248-54. PMID: 21247315. [CrossRef] [PubMed]
- Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M, Yeh J. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab. 2010 Jul;95(7):3216-24. Epub 2010 May 12. [CrossRef] [PubMed]
- Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open-label prospective trial. J Bone Miner Res. 2004 Aug;19(8):1221-30. Epub 2004 May 24. [CrossRef] [PubMed]
- Weisman SM, Matkovic V. Potential use of biochemical markers of bone turnover for assessing the effect of calcium supplementation and predicting fracture risk. Clin Ther. 2005 Mar;27(3):299-308. [CrossRef] [PubMed]
- Méndez-Sánchez L, Clark P, Winzenberg TM, Tugwell P, Correa-Burrows P, Costello R. Calcium and vitamin D for increasing bone mineral density in premenopausal women. Cochrane Database Syst Rev. 2023 Jan 27;1(1):CD012664. [CrossRef] [PubMed]
- Kemmler W, Engelke K, Lauber D, Weineck J, Hensen J, Kalender WA. Exercise effects on fitness and bone mineral density in early postmenopausal women: 1-year EFOPS results. Med Sci Sports Exerc. 2002 Dec;34(12):2115-23. [CrossRef] [PubMed]
- Sahni S, Tucker KL, Kiel DP, Quach L, Casey VA, Hannan MT. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: the Framingham Offspring Study. Arch Osteoporos. 2013;8(0):119. Epub 2013 Feb 1. Erratum in: Arch Osteoporos. 2013 Dec;8(1-2):132. [CrossRef] [PubMed]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D; Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669-83. Erratum in: N Engl J Med. 2006 Mar 9;354(10):1102. [CrossRef] [PubMed]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.J.; Coxam, V. Twelve-Month consumption of a polyphenol extract from olive (OLEA EUROPAEA) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging, 2015; Volume 19, Number 1, (1):77-86.
- Kim YA, Kim KM, Lim S, Choi SH, Moon JH, Kim JH, Kim SW, Jang HC, Shin CS. Favorable effect of dietary vitamin C on bone mineral density in postmenopausal women (KNHANES IV, 2009): discrepancies regarding skeletal sites, age, and vitamin D status. Osteoporos Int. 2015 Sep;26(9):2329-37. Epub 2015 Apr 24. [CrossRef] [PubMed]
- . Bolland M, Barber P, Doughty R, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ (Clin Res Ed) 2008;336(7638):262e6. [CrossRef]
- Kim, M.H.; Lee, H.J.; Osteoporosis, vitamin C intake, and physical activity in Korean adults aged 50 years and over. J Phys Ther Sci 2016, 28, 725–730. [CrossRef]
- Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015 Mar;239(1):260-7. Epub 2015 Jan 20. [CrossRef] [PubMed]
- Morvaridzadeh, M.; Agah S, Alibakhshi P, Heydari H, Hoseini AS, Palmowski A, Toupchian O, Abdollahi S, Rezamand G, Heshmati J.; Effects of Calcium and Vitamin D Co-supplementation on the Lipid Profile: A Systematic Review and Meta-analysis. Clin Ther. 2021, 43(9), 274-296. Epub 2021 Aug 27. [CrossRef] [PubMed]
- Kim, K. C., Shin, D. H., Lee, S. Y., Im, J. A., Lee, D. C., & Lee, H. R. (2012). Relationship between serum total cholesterol level and osteoporotic status in elderly Korean men and women. The Korean Journal of Internal Medicine, 27(2), 176-182.
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessmentof the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep., 2018; 5, 251–257.
- Ulewicz-Magulska, B.; Wesolowski, M. Total Phenolic Contents and Antioxidant Potential of Herbs Used for Medical and Culinary Purposes. Plant Foods Hum. Nutr., 2018; 74, 61–67.
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines, 2019; 6, 94. [CrossRef]
- Annunziata, A.; Mariani, A. Do consumers care about nutrition and health claims? Some evidence from Italy. Nutrients, 2019; 11, 2735. [CrossRef]
- Stanghelle B, Bentzen H, Giangregorio L, Pripp AH, Skelton DA, Bergland A. Effects of a resistance and balance exercise programme on physical fitness, health-related quality of life and fear of falling in older women with osteoporosis and vertebral fracture: a randomized controlled trial. Osteoporos Int. 2020 Jun;31(6):1069-1078. Epub 2020 Jan 10. Erratum in: Osteoporos Int. 2020 Apr 27. [CrossRef] [PubMed]
- Gibbs JC, MacIntyre NJ, Ponzano M, Templeton JA, Thabane L, Papaioannou A, Giangregorio LM (2019) Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev 7:Cd008618.
- Filipović TN, Lazović MP, Backović AN, Filipović AN, Ignjatović AM, Dimitrijević SS, et al. A 12-week exercise program improves functional status in postmenopausal osteoporotic women: randomized controlled study. Eur J Phys Rehabil Med 2021;57:120-30. [CrossRef]

| Characteristics | Group I (n=40) | Group II (n=42) | Group III (n=18) | Group IV (n=15) | P-value |
|---|---|---|---|---|---|
| Age (years) | 45 ± 8 | 56 ± 8 | 56 ± 8 | 45 ± 7 | 0.839 |
| Height (cm) | 154 ± 36 | 162 ± 9 | 159 ± 6.5 | 162 ± 8.4 | 0.39 |
| Weight (kg) | 74 ± 15.4 | 76 ± 17.4 | 73 ± 13 | 69 ± 10 | 0.628 |
| BMI (kg/m2) | 28.34 ± 5.95 | 29.11 ± 6.45 | 28.79 ± 3.86 | 26.7 ± 0.82 | 0.385 |
| BMI overweight category (kg/m2) | 28.34 ± 5.95 | 29.11 ± 6.45 | 28.79 ± 3.86 | 26.7 ± 0.82 | 0.385 |
| Body fat (Kg) | 36.23 ± 10.87 | 35.91 ± 8.2 | 39.17 ± 13.32 | 35.0 ± 10.74 | 0.61 |
| Muscle mass (Kg) | 44.88 ± 7.22 | 44.21 ± 8.05 | 43.16 ± 6.40 | 43.17 ± 7.01 | 0.4 |
| Total body water (Kg) | 47.01 ± 5.48 | 44 ± 6.13 | 44.86 ± 5 | 44.84 ± 5.20 | 0.25 |
| Beginning of study | End of study | % change | P-value* | |
|---|---|---|---|---|
| 25(OH)D3 (ng/ml) | ||||
| Group I | 27.42 ± 12.12 | 26.48 ± 7.96 | - 3.43 % | 0.2 |
| Group II | 23.15 ± 8.37 | 24.01 ± 8.68 | 3.71 % | 0.2 |
| Group III | 28.21 ± 8.84 | 28.62 ± 7.78 | 1.45 % | 0.2 |
| Group IV | 26.69 ± 6.83 | 28.19 ± 6.44 | 5.62 % | 0.81 |
| PTH (pg/ml) | ||||
| Group I | 62.63 ± 27.00 | 76.44 ± 36.45 | 22.05 % | 0.77 |
| Group II | 58.95 ± 23.96 | 56.71 ± 23.85 | - 3.80 % | 0.77 |
| Group III | 69.01 ± 17.82 | 52.21 ± 17.87 | - 24.34 % | 0.77 |
| Group IV | 48.84 ± 19.49 | 55.6 ± 19.63 | 13.84 % | 0.11 |
| Beginning of study | End of study | % change | P-value* | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | ||||
| Group I | 210.82 ± 30.17 | 207 ± 29.32 | - 1.81 % | 0.54 |
| Group II | 200.1 ± 33.14 | 197.85 ± 36.96 | - 1.12 % | 0.54 |
| Group III | 192.89 ± 29.46 | 197.17 ± 13.72 | 2.22 % | 0.034 |
| Group IV | 185.14 ± 34.17 | 181.31 ± 32.21 | - 2.07 % | 0.034 |
| P-value† | 0 | 0 | -0.70% | 0.39 |
| Glucose (mg/dl) | ||||
| Group I | 91.82 ± 8.93 | 93.33 ± 4.62 | 1.64 % | 0.37 |
| Group II | 96.15 ± 15.27 | 99.56 ± 17.68 | 3.55 % | 0.37 |
| Group III | 108 ± 16.97 | 109.5 ± 14.85 | 1.40 % | 0.37 |
| Group IV | 93.81 ± 8.98 | 96 ± 11.74 | 2.33 % | 0.048 |
| P-value† | 0.136 | 0.29 | 2.23 % | 0.29 |
| HbA1c (%) | ||||
| Group I | 5.64 ± 0.39 | 5.77 ± 0.42 | 2.30 % | 0.27 |
| Group II | 5.74 ± 0.53 | 5.72 ± 0.55 | - 0.35 % | 0.27 |
| Group III | 5.71 ± 0.67 | 5.76 ± 0.8 | 0.88 % | 0.27 |
| Group IV | 5.76 ± 0.81 | 5.85 ± 0.83 | 1.56 % | 0.027 |
| Beginning of study | End of study | % change | P-value* | |
|---|---|---|---|---|
| Whole-body BMD (g/cm2) | ||||
| Group I | 1.38 ± 0.49 | 1.85 ± 0.5 | 3.46 % | 0.027 |
| Group II | 1.29 ± 0.45 | 1.31 ± 0.47 | 1.55 % | 0.036 |
| Group III | 1.39 ± 0.5 | 1.56 ± 0.51 | 12.23 % | 0.043 |
| Group IV | 1.67 ± 0.48 | 1.87 ± 0.35 | 11.98 % | 0.003 |
| P-value | 0.298 | 0.187 | 14.96 % | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





