1. Introduction
Ménière's disease (MD) is a multifactorial inner ear disorder characterized by episodic vestibular symptoms, sensorineural hearing loss, tinnitus, and aural pressure [
1]. Despite the increasing use of magnetic resonance imaging (MRI) and computed tomography (CT) scans, MD is diagnosed clinically [
2].
Endolymphatic hydrops (EH) is a hallmark pathologic characteristic of MD, as described by Hallpike and Cairns [
3,
4]. While EH is observed in all MD patients, not all EH patients show MD symptoms (the so called “asymptomatic hydrops”) [
3,
5,
6]. The observation of EH in temporal bones of asymptomatic individuals prompted the discussion on the true role of EH - as a mere ubiquitous finding of MD instead of a causal mechanism for MD symptoms [
7]. EH is often evident in the cochlea as a distension of Reissner's membrane into the Scala vestibuli [
8]. Other membrane structures in the ear, such as those enclosing the saccule, utricle, and semicircular canal ampullae, may also be displaced to variable degrees [
9]. Membrane ruptures, herniations, and scarring have been found in certain specimens [
10]. Those who defend a causal relationship between EH and MD point to membrane´s rupture as an important contributor for MD´s periodic attacks and functional alterations [
3].
To date, it is unknown why certain individuals are more prone to develop EH. Similarly, it is uncertain which factors make EH more likely to translate into clinical MD [
3]. An interaction between genetical and environmental factors has been proposed [
11]. However, the findings of genetic research on MD are debated due to the complexity of MD´s pathophysiology. MD has been linked to a plea of different disorders such as inflammation, immunology, water and ion balance in endolymphatic fluid, viral infections, metabolism, and aberrant nerve conduction function [
12].
It is clear that no unanimously accepted model explains the pathogenesis of MD [
6,
13]. However, a recent hypothesis of microvascular dysregulation of the inner ear has been explored [
6,
13]. Some speculate that impaired endolymphatic sac's blood flow and fluid balance as a result of vascular dysfunction may lead to endolymph buildup, resulting in vertigo bouts [
14]. A decrease in blood flow to the inner ear caused by microvascular damage, oxidative stress, atherosclerotic plaque development or microthrombosis might disturb the balance of endolymphatic fluid production and absorption, raising the risk of EH and, eventually, MD [
15,
16,
17]. On the other hand, EH constitutes itself a resistor to inner ear vascular perfusion [
6]. In cases where EH is present, chronic vascular impairment of the inner ear may impose an additional irrigation challenge - resulting in lower ear perfusion pressures. This scenario of chronic ear ischemia may alter ion and fluid balance within the inner ear, favoring MD attacks [
6]. An important study revealed important degenerative changes in the capillaries of the blood-labyrinthine barrier (BLB) in MD [
18].
Small artery disease has been explicitly hypothesized to contribute to inner ear homeostasis instability and result in EH [
6,
13]. Clinical signs of inner ear´s chronic small vessel disease are hard to describe both clinically and on imaging [
19,
20]. It is known that the inner ear is irrigated by the labyrinthine artery coming from the vertebrobasilar arterial system [
20]. In parallel, small-vessel strokes are a well described clinical and imagological subtype of vertebrobasilar (posterior circulation) vascular events. In case small-vessel irrigation of the inner ear could relate to MD physiopathology, MD patients could then share similar cardiovascular risk factors to posterior circulation infarction patients (POCI), namely the ones caused by small-vessel disease.
The inner ear and brainstem/posterior cerebral regions rely on a shared arterial irrigation. Nevertheless, to date, no studies compared cardiovascular risk factors (CVRF) between MD and POCI patients. With this in mind, this study compares the prevalence of CVRF in MD to a group of individuals with POCI.
2. Materials and Methods
In order to perform a retrospective study, a sample of patients with definitive diagnosis of MD from the Otorhinolaryngology consultation were compared with a sample of patients with POCI from the Neurology consultation. Brain MRIs were assessed by a member of the Neuroradiology Department (JT) and by a Neurologist (RR). Data acquisition was made between 2019 to 2023 using non-probability sampling.
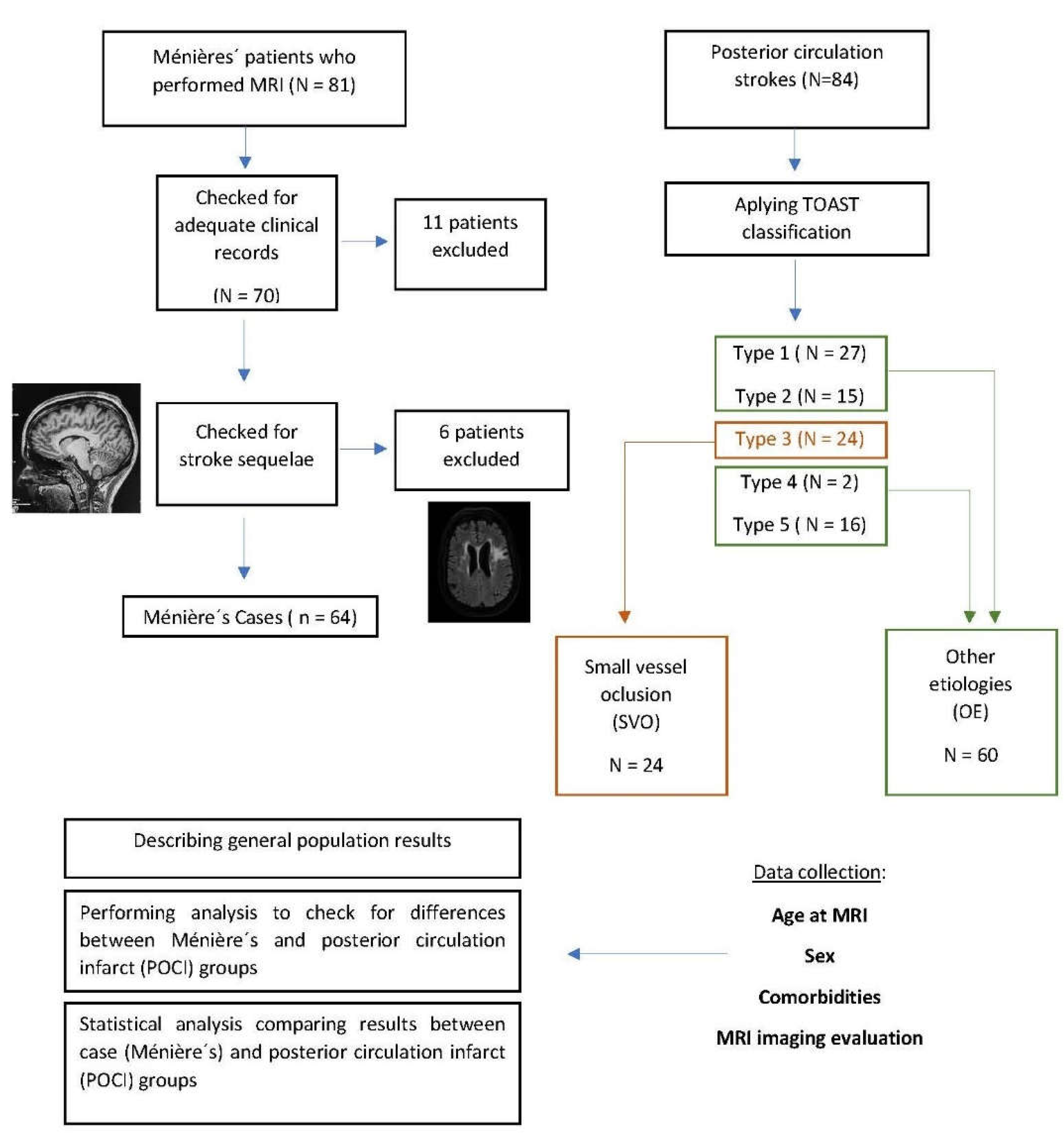
Figure 1 depicts the methodological approach.
Inclusion criteria for MD patients were: definite MD according to Bárany Society, EAONO , the AAO-HNS, the Japan Society for Equilibrium Research, and the Korean Balance Society [
21]; age ≥ 18 years; available brain MRI; absence of other cochleovestibular lesions on MRI (namely facial or vestibular Schwannoma and cerebellopontine Meningioma), adequate clinical records. Exclusion criteria: evidence of any former stroke sequelae on MRI.
For POCI, inclusion criteria were: age > 18 years, brain MRI with anatomical localization of the lesion, absence of reported hearing or vestibular impairment prior to the event, adequate clinical records.
The following CVRFs were assessed based on existent hospital and primary practice records: hypertension, diabetes mellitus, dyslipidemia, obesity, heart disease and smoking. Hypertension was considered in any patient receiving pharmacological treatment specifically aimed at addressing elevated blood pressure. Diabetes mellitus was ascertained based on documented medical history or the use of antidiabetic medications. Dyslipidemia was defined by abnormal lipid panel results or documented use of lipid-lowering agents. Obesity was determined by body mass index (BMI) measurements, with values ≥ 30 indicating obesity as per established criteria. Heart disease encompassed a history of myocardial infarction, coronary artery disease, arrhythmias or other clinically documented cardiac conditions. Smoking status was determined by self-reporting or documentation of current or recent (within the last 12 months) smoking habits in medical records.
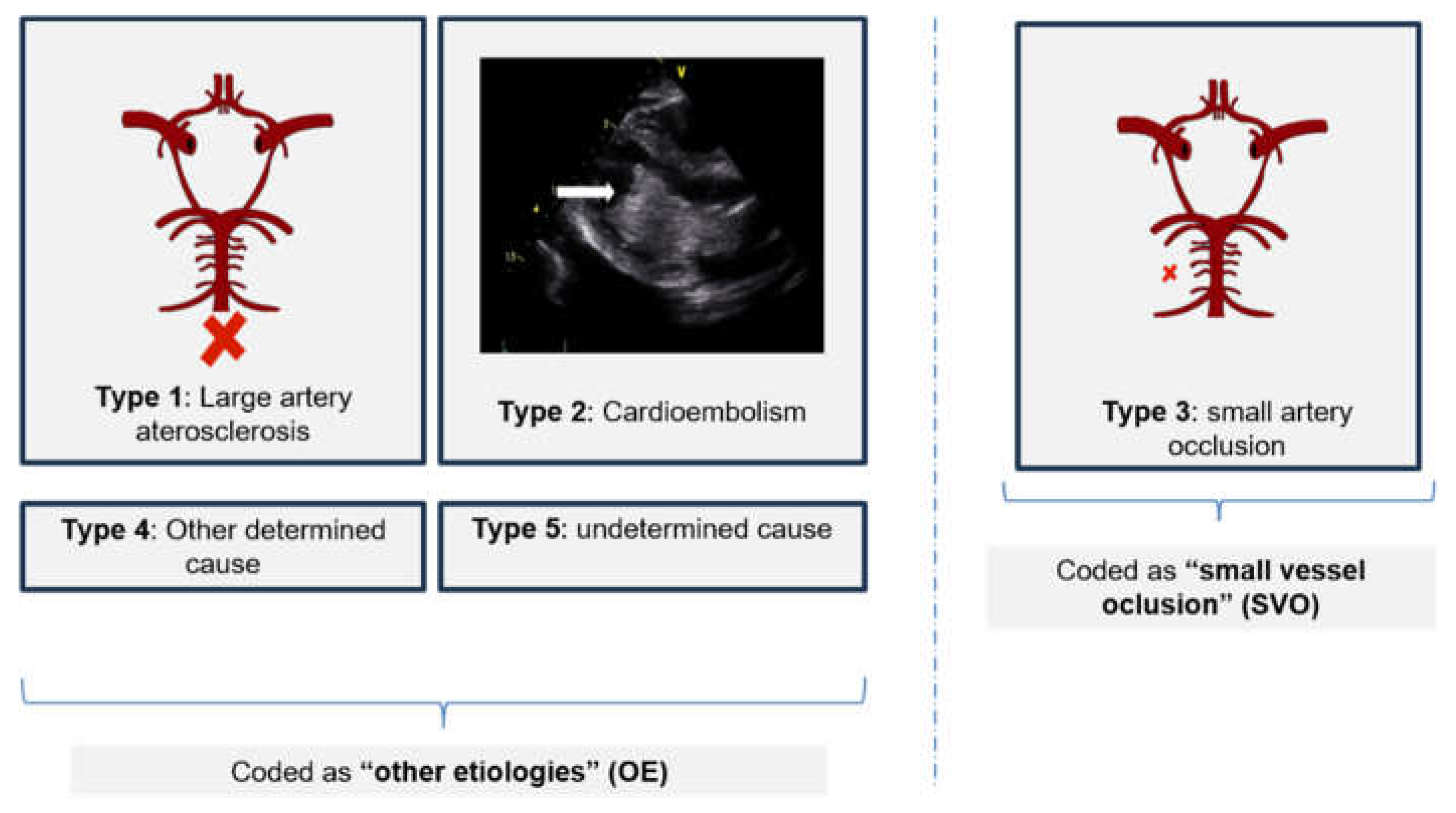
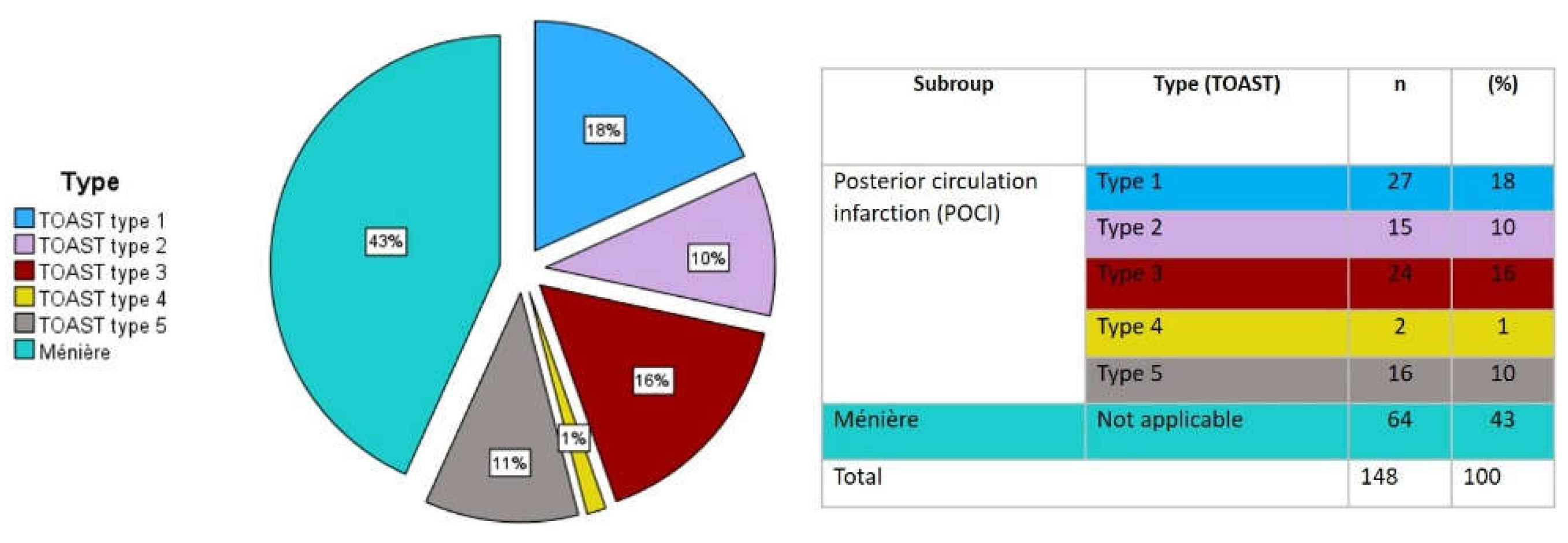
The etiology of POCI was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST). For analysis purposes, the sample was further divided into "small vessel occlusion" (SVO) - corresponding to the type 3 classification of TOAST and "other etiologies" (OE) - corresponding to Type 1,2,4 and 5 of the TOAST classification (see
Figure 1 and
Figure 2). Age categories were created to compare groups in order to minimize age bias on risk factors´ prevalence.
SPSS (IBM SPSS Statistics 29) was used for statistical analysis. Specific risk factors were coded as categorical variables and are hence reported as percentages in the descriptive analysis. Number of comorbidities in each subject were measured as an ordinal variable. Continuous variables such as age are shown as means and standard deviations. Skewness, kurtosis, and the Kolmogorov-Smirnov tests were used to ensure that the distribution was normal. The bivariate correlations were analyzed using Pearson´s chi-square test in the descriptive analysis and then inside each age category to compare the prevalence of each risk factor. A multinominal logistic regression (MLR) was employed to produce a predictive model adjusted for age, taking group (Ménière, POCI SVO or POCI OE) as the dependent variable and CVRF as covariates. MLR was preferred since the dependent outcome variable defined as “group” had 3 categories and was unordered. Additionally, proportional odds assumption proved violated in the test of Parallel lines. In the regression analysis, the decision not to include sex as an independent variable was based on the consideration of potential collider bias. All presented p-values are two-tailed, with a p-value ≤ 0.05 indicating statistical significance.
3. Results
3.1. Study Population
A total of 81 patients with MD were initially recruited, with 11 exclusions due to insufficient clinical data and 6 exclusions due to incidental cerebrovascular disease on MRI, making a total of 64 MD patients without signs of prior stroke. In parallel, a total of 84 POCI patients were included, accounting for the final 148 patients. Of those, 88 were men (56.1%) and 60 women (38.2%), with a mean age of 59 ± 14 years.
Table 1 describes the sample´s characterization within and between groups.
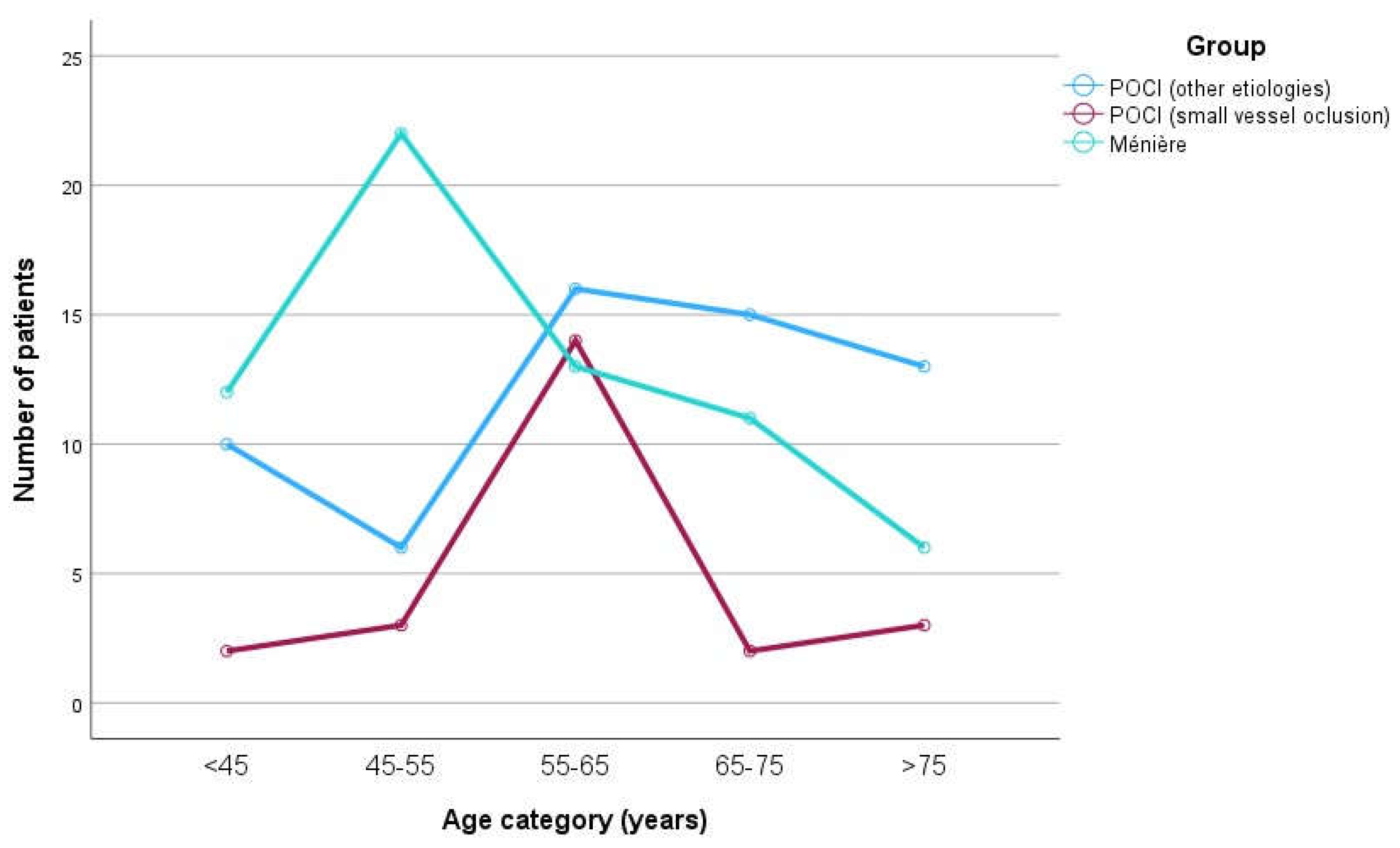
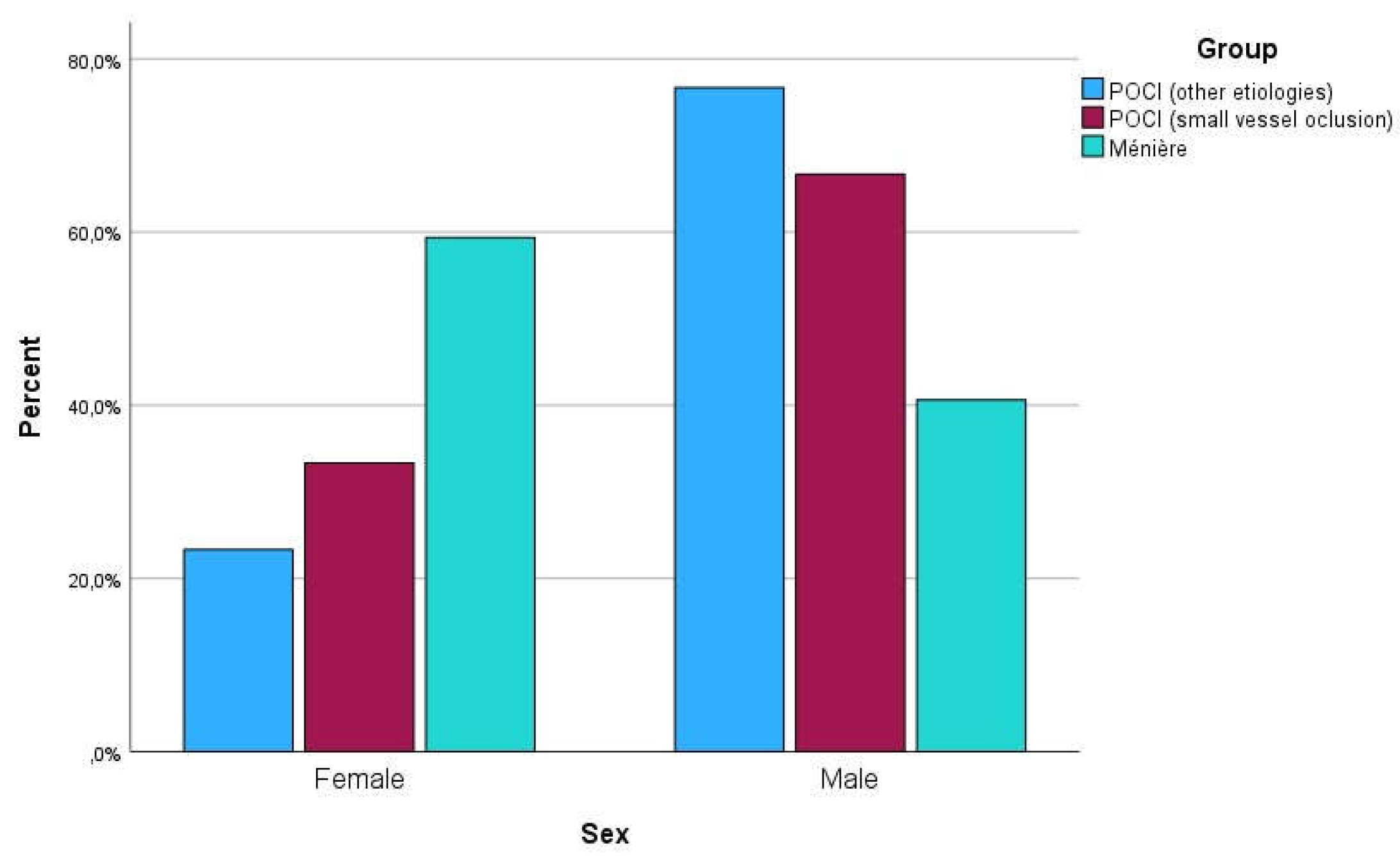
Figure 3 and
Figure 4 are graphical representations of the sample distribution within age and sex categories, respectively.
Figure 5 depicts in detail the group description within the sample.
3.2. Ménière´s versus POCI: Risk Factors
The group comparison revealed a higher prevalence of various CVRF in POCI OE patients, namely Hypertension, Dyslipidemia, Diabetes Mellitus and smoking (see
Table 2). The analysis was divided by age. Age < 45 years: MD vs patients with POCI SVO = p > 0.05 for all CVRF; MD vs patients with POCI OE: p = 0.029 for hypertension and p = 0. 011 for smoking (both higher in POCI OE); Age 45-55: MD vs POCI SVO patients = p > 0.05 for all CVRFs; MD vs POCI OE patients: p = 0.043 for diabetes mellitus, p < 0.001 for hypertension, p = 0.048 for smoking (all higher in POCI OE); Age 55-65: MD vs POCI SVO patients = p > 0. 05 for all CVRFs, with the exception of dyslipidemia (higher prevalence POCI SVO, p = 0.003); MD vs patients with POCI OE: p = 0.047 for dyslipidemia; p =0.013 for smoking (both higher in POCI OE); Age 65-75: MD vs patients with POCI SVO = p >0.05 for all CVRFs; MD vs patients with POCI OE: p =0.024 for dyslipidemia (higher in POCI OE); Age > 75 years: MD vs patients with POCI SVO and MD vs patients with POCI OE = p > 0.05 for all CVRFs.
3.3. Cardiovascular Risk Factors and Stroke Risk: A Model
A multinomial logistic regression was performed to model the relationship between predictor variables (various CVRF and age) and membership in the three groups (Ménière, POCI SVO, POCI OE). With the addition of the predictor variables, the fit between the model including only the intercept and data improved, χ2 (14, N = 148) = 76.53, Nagelkerke R2 = 0.464, p < .001. As seen in
Table 3, hypertension, dyslipidemia, and smoking each provided substantial distinct contributions. POCI OE was the reference group. As a result, each predictor contains two parameters: one for predicting Ménière group membership and one for predicting POCI SVO group membership.
Table 4 displays the parameter estimates.
When comparing the POCI OE group to the Ménière group, two of the predictors exhibited significant parameters: hyopertension and smoking. If the patient had hypertension, the odds of being in the POCI OE group rather than the Ménière group were 7 times greater (OR: 0.126, p <0.001, see
Table 4). Likewise, if the patient was a smoker, the odds of being in the POCI OE group rather than the Ménière group were more than 20 times higher (OR: 0.041, p < 0.001, see
Table 4). When comparing the POCI OE group to the POCI SVO group, only one predictor showed a significant parameter: dyslipidemia (see
Table 4). In a patient with dyslipidemia, the odds of being in the POCI SVO group rather than the POCI OE group were four times higher (OR: 4.509, p = 0.024, see
Table 4).
4. Discussion
Cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, smoking and obesity are well known causes of microvascular impairment, oxidative stress and compromise of the brain-blood and bloodlabyrinth barriers [
22]. Although there are some important studies linking MD to CVRF [
6,
13], this association is still relatively unexplored. On the other hand, it remains unclear whether there are shared risk factors between Ménière's Disease (MD) and vertebrobasilar ischemic events. The need for better understanding the etiopathogenesis of MD along with the pertinency of unravelling new lines of investigation on MD motivated the present work.
The primary research question was whether CVRF prevalence would differ between MD and POCI patients. The primary objective of the work was met. Higher prevalence of Hypertension, Diabetes Mellitus, Dyslipidemia and smoking was found in POCI OE patients compared to MD in various age categories.
Conversely, no significant differences were found regarding the prevalence of CVRF between MD and POCI SVO, with the exception of Dyslipidemia within the 45-55 age category. The multivariate model further reinforced such findings, while supporting the role of dyslipidemia in POCI SVO. These results suggest a partial overlap in CVRF prevalence between MD and POCI SVO, as opposed to non-small vessel disease (POCI OE), where CVRF prevalence was more pronounced. Importantly, the similarity in CVRF´s prevalence between MD and POCI SVO frame the possibility of microvascular dysregulation as a common contributor in these two entities.
Well-known target end-organs of cardiovascular disease such as the brain, heart, kidney or the eye have already been described [
22,
23,
24]. Nevertheless, a lot less is known concerning microvascular affection of the inner ear. Since microvascular dysfunction is pointed as a systemic disorder [
23] it seems licit to consider that the inner ear may not be an exception [
22]. There are two main potential pathways in which microvascular disease could relate to MD. One would be by increasing the risk of EH (etiopathogenic theory); the other by enhancing attacks in an already hydropic ear (attack-triggering theory) [
25].
There are some arguments in favor of the etiopathogenic pathway. Microvascular dysfunction has been associated with various markers common to both typical end-target organs (brain, heart, kidney) and the inner-ear. Aquaporins, adducins, dermatopontin and potassium voltage-gated channel subunits are some examples [
1,
11,
23,
26]. Many endolymph-bound ion transport channels have been shown to be controlled by hormonal processes such as β-adrenergic, muscarinic, and purinergic receptors. [
27,
28]. However, the relationship between ion transport and endolymph ion concentration and volume remains unknown. Only an osmotic inflow of water may cause a volume change. Aquaporins, which are expressed in the inner ear, have a function in water equilibration across endolymphatic borders and are regulated by hormones [
3]. In fact, vasopressin (V2) receptors have been found in the inner ear and may balance water flow by control of aquaporin expression, in a mechanism similar to the Kidney [
3]. Nevertheless, it is still unclear how this system is regulated and what are the exact roles of the various inner ear structures [
3]. MD etiology has already been linked to a variety of genes involved in ionic composition, water transport, and cardiovascular development [
1,
11,
26].
Endothelial dysfunction, inflammation (including reactive oxidation), immunological activation, and coagulation are all possible mechanisms driving systemic microvascular dysfunction [
23]. In fact, a recent study of the human utricle´s macula microvasculature demonstrated that vascular endothelial cells and pericytes are damaged in MD [
18,
29]. Two oxidative stress markers have been implied in such damage: inducible nitric oxide synthase (iNOS) and nitrotyrosine. These markers have been found in vascular endothelial cells of BLB from MD patients suggesting that oxidative stress is involved in BLB disruption [
30]. Recent research in BLB pathophysiology emphasizes the relevance of BLB integrity for ion and water homeostasis [
31,
32,
33], suggesting that BLB dysfunction is important in understanding the pathophysiology of EH and possibly MD.
The attack-triggering theory, on the other hand, proposes that hydrops operates as a variable starling resistor on the inner ear vasculature, capable of generating ischemic episodes in those with low ear perfusion pressure [
6]. An animal experiment showed that MD attacks are not caused by EH itself, but they can be provoked by decreased vascular flow in the inner ear [
34]. The BLB is essential for maintaining inner ear fluid ionic equilibrium [
18]. In case of hydropic ear transient ischemia, resulting BLB disruption could activate deleterious mechanisms culminating in the MD attack. Moreover, ischemia and hypoxia cause fast calcium translocation from extracellular to intracellular regions in brain tissues [
3]. Since endolymph calcium has been shown to influence transduction in hair cells, it is possible that calcium influx in the setting of inner ear hypoxia contributes to the functional losses observed in MD attacks [
3].
This study has its strengths. It is the first to compare a MD with a POCI population from a cardiovascular point of view. It includes a relatively large sample of patients (both MD and POCI) formerly submitted to brain imaging. Also, it underscores microvascular dysregulation as a potential novel mechanism for MD development and/or progression.
It is essential to acknowledge some limitations of our study. Firstly, the retrospective nature of this research may incur in selection bias. Secondly, it would be pertinent to include an age-matched “control” group without MD or POCI to further validate our results and check for asymmetries in the prevalence of CVRF between MD and the general population. Considering broader demographic variations in future studies could offer more comprehensive insights into the applicability of the findings across different populations. Also, since clinical data was derived from pre-existing records, it is possible that certain comorbidities were overlooked. Additionally, since the POCI SVO group included a relatively small number of patients, comparison with the MD population may have been statistically affected. It is important to note that the duration of risk factors such as hypertension, diabetes mellitus, smoking, and related indices, including smoking index, could not be precisely ascertained due to limitations in the available database. The absence of this information represents a constraint in our analysis and necessitates cautious interpretation of the results with regard to the temporal aspects of risk factor exposure. Therefore, the authors consider that results should be regarded thoughtfully and validated by further larger, prospective studies.
5. Conclusions
In conclusion, this study compared cardiovascular risk factors in Ménière's disease (MD) and posterior circulation infarction (POCI) patients. Similar cardiovascular risk factors' (CVRFs) prevalence was observed between MD and small vessel POCI (POCI-SVO), with the exception of dyslipidemia in the 55-65 age group. More notable disparities were evident in larger vessel POCI cases (POCI-OE), with odds of POCI OE more significantly associated with hypertension and smoking in the multivariate analysis. This work points to the potential for exploring determinants of microvascular dysregulation in Ménière's Disease. While the exact MD´s pathophysiologic mechanisms remain unknown, it is possible that MD patients share common ground with thrombotic microangiopathy entities such as POCI-SVO. Finally, further research is needed on this topic. New future findings could have substantial clinical influence, given that an early preventive action on CVRF´s or its adequate treatment would potentially improve MD´s care and prognosis. Considering the complex interplay of factors involved, exploring collaborations across disciplines like otolaryngology, neurology, and primary care could offer valuable insights.
Author Contributions
Francisco Sousa: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing-original draft. João Tarrio: Investigation, Data curation, Resources, Writing-Review & Editing. Rita Rodrigues: Investigation, Resources, Data Curation, Writing - Review & Editing, Supervision. Clara Alves: Investigation, Data curation, Writing-Review & Editing. Mariline Santos: Writing - Review & Editing, Supervision, Project administration. Ana Pinto: Writing - Review & Editing, Supervision, Project administration; Luís Meireles: Supervision, Project administration; Ângela Rego: Conceptualization, Writing - Review & Editing; Supervision, Project administration;
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics committee of Unidade Local de Saúde de Santo António (2024-048(044-DEFI/044-CE, 19th March 2024).
Informed Consent Statement
Informed consent was waived due to the retrospective nature and anonymized methodology of the study.
Data Availability Statement
No public available datasets related to this publication
Acknowledgments
In Francisco Sousa and João Tarrio significantly to the work and could be considered co-first authors. Author order was defined by primordial idealization. We extend our gratitude towards Dr. João Xavier and Dr. Bruno Moreira from the Neurroradiology Department of Unidade Local de Saúde de Santo António for their invaluable support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Espinosa-Sanchez JM, Lopez-Escamez JA: Menière’s disease. Handb Clin Neurol. 2016, 137:257-77. [CrossRef]
- Ito T, Kitahara T, Inui H, et al.: Endolymphatic space size in patients with Meniere’s disease and healthy controls. Acta Otolaryngol. 2016, 136:879-82. 1169. [CrossRef]
- Salt AN, Plontke SK: Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am. 2010, 43:971-83. [CrossRef]
- Nakashima T, Pyykkö I, Arroll MA, et al.: Meniere’s disease. Nat Rev Dis Prim. 2016, 2:16028. [CrossRef]
- Attyé A, Eliezer M, Boudiaf N, et al.: MRI of endolymphatic hydrops in patients with Meniere’s disease: a case-controlled study with a simplified classification based on saccular morphology. Eur Radiol. 2017, 27:3138-46. [CrossRef]
- Foster CA, Breeze RE: The Meniere attack: An ischemia/reperfusion disorder of inner ear sensory tissues.Med Hypotheses. 2013, 81:1108-15. [CrossRef]
- Merchant SN, Adams JC, Nadol JBJ: Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops?. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol. 2005, 26:74-81. [CrossRef]
- Kimura RS: Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1967, 76:664-87. [CrossRef]
- Kimura RS: Experimental pathogenesis of hydrops. Arch Otorhinolaryngol. 1976, 212:263-75. [CrossRef]
- Schuknecht HF.: THE PATHOPHYSIOLOGY OF MENIERE’S DISEASE. Otol Neurotol. 1984, 5:526-527.
- Chiarella G, Petrolo C, Cassandro E: The genetics of Ménière’s disease . Appl Clin Genet. 2015, 8:9-17. [CrossRef]
- Dai Q, Long L, Zhao H, Wang R, Zheng H, Duan M: Genetic advances in Meniere Disease. Mol Biol Rep. 2023, 50:2901-8. [CrossRef]
- Rego ÂR, Dias D, Pinto A, e Castro SS, Feliciano T, e Sousa CA: The cardiovascular aspects of a Ménière’s disease population - A pilot study. J Otol. 2019, 14:51-6. [CrossRef]
- Akagi N, Takumida M, Anniko M: Effect of inner ear blood flow changes on the endolymphatic sac. Acta Otolaryngol. 2008, 128:1187-95. [CrossRef]
- Friis M, Sørensen MS, Qvortrup K: The vein of the vestibular aqueduct with potential pathologic perspectives. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc [and] Eur Acad Otol Neurotol. 2008,29:73-8. [CrossRef]
- Horton WB, Barrett EJ: Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr Rev. 2021, 42:29-55. [CrossRef]
- Wada M, Takeshima T, Nakamura Y, Nagasaka S, Kamesaki T, Kajii E: Carotid plaque is a new risk factor for peripheral vestibular disorder: a retrospective cohort study. Medicine (Baltimore. 2016, 95:4510. 2016, 95. [CrossRef]
- Ishiyama G, Lopez IA, Ishiyama P, Vinters H V, Ishiyama A: The blood labyrinthine barrier in the human normal and Meniere’s disease macula utricle. Sci Rep. 2017, 7:253-10. [CrossRef]
- Tange RA: Vascular inner ear partition: a concept for some forms of sensorineural hearing loss and vertigo.ORL J Otorhinolaryngol Relat Spec. 1998, 60:78-84. [CrossRef]
- Mei X, Glueckert R, Schrott-Fischer A, et al.: Vascular Supply of the Human Spiral Ganglion: Novel ThreeDimensional Analysis Using Synchrotron Phase-Contrast Imaging and Histology. Sci Rep. 2020, 10:5877. [CrossRef]
- Lopez-Escamez JA, Carey J, Chung W-H, et al.: [Diagnostic criteria for Menière’s disease. Consensus document of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology (EAONO), the American Academy of Otolaryngology-Head and Neck Surgery (AAO-H. Acta Otorrinolaringol Esp. 2016, 67:1-7. [CrossRef]
- González-Marrero I, Castañeyra-Ruiz L, González-Toledo JM, et al.: High Blood Pressure Effects on the Blood to Cerebrospinal Fluid Barrier and Cerebrospinal Fluid Protein Composition: A Two-Dimensional Electrophoresis Study in Spontaneously Hypertensive Rats. Int J Hypertens. 2013:164653. [CrossRef]
- Nowroozpoor A, Gutterman D, Safdar B: Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys? — A scoping review. Microvasc Res. 2021, 134:104123. [CrossRef]
- Cabrera DeBuc D, Somfai GM, Koller A: Retinal microvascular network alterations: potential biomarkers of cerebrovascular and neural diseases. Am J Physiol Heart Circ Physiol. 2017, 312:201-12. [CrossRef]
- Wang Y, Diao T, Han L, Tao Y, Yu L: Association of Meniere’s disease and retinal vascular calibre: a prospective observational study in China. BMJ Open. 2018, 8:022069-10. [CrossRef]
- Martín-Sierra C, Gallego-Martinez A, Requena T, Frejo L, Batuecas-Caletrío A, Lopez-Escamez JA: Variable expressivity and genetic heterogeneity involving DPT and SEMA3D genes in autosomal dominant familial Meniere’s disease. Eur J Hum Genet. 2017, 25:200-7. [CrossRef]
- Wangemann P, Liu J, Shimozono M, Schimanski S, Scofield MA: K+ secretion in strial marginal cells is stimulated via beta 1-adrenergic receptors but not via beta 2-adrenergic or vasopressin receptors. J Membr Biol. 2000, 175:191-202. [CrossRef]
- Wangemann P: K(+) cycling and its regulation in the cochlea and the vestibular labyrinth. Audiol Neurootol. 2002, 7:199-205. [CrossRef]
- Ishiyama G, Lopez IA, Acuna D, Ishiyama A: Investigations of the Microvasculature of the Human Macula Utricle in Meniere’s Disease. Front Cell Neurosci. 2019, 13:445-10. [CrossRef]
- Lopez IA, Ishiyama G, Hosokawa S, et al.: Immunohistochemical techniques for the human inner ear. Histochem Cell Biol. 2016, 146:367-87. [CrossRef]
- Shi X: Physiopathology of the cochlear microcirculation. Hear Res. 2011, 282:10-24. [CrossRef]
- Shi X: Pathophysiology of the cochlear intrastrial fluid-blood barrier (review) . Hear Res. 2016, 338: 52-63. [CrossRef]
- Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN: Systemic lipopolysaccharide compromises the bloodlabyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014,15:707-19. [CrossRef]
- Takumida M, Akagi N, Anniko M: A new animal model for Ménière’s disease . Acta Otolaryngol. 2008,128:263-71. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).