1. Introduction
B-cell lymphomas (BCL) are the most frequent hematological cancer in dogs [
1]. Despite ongoing research, the exact cause remains unknown, with environmental factors and genetic predispositions implicated [
1]. These tumors present in dogs with heterogenous clinical symptoms and are classified into histological subtypes with varying molecular, epigenetic, and genetic origins [
1,
2]. Nonetheless, generalized lymphadenopathy is a common feature, often accompanied by varying degrees of blood and bone marrow infiltration [
1]. Among BCL histotypes, diffuse large B-cell lymphoma (DLBCL) is predominant, followed by marginal zone lymphoma and follicular lymphoma [
3]. Diagnosis primarily relies on histological examination, focusing on recognizing growth pattern and assessing cells size [
3]. Immunohistochemical markers such as Pax5, CD79a, and CD20 are routinely employed to confirm the B-cell origin [
2,
3]. Treatment typically consists of chemotherapy, primarily using a CHOP regimen (cyclophosphamide, doxorubicin, vincristine, prednisone) [
4]. However, a notable number of dogs experience tumor relapse, treatment-related toxicity, and short overall survival, necessitating exploration of novel therapeutic strategies with a targeted approach [
4,
5].
The
MYC proto-oncogene encodes the Myc transcription factor that is crucial for regulating cellular processes such as proliferation, differentiation, and apoptosis [
6]. Dysregulation of Myc is implicated in numerous malignancies in humans, including BCLs [
6,
7,
8]. In canine BCL (cBCL),
MYC aberrations are also common, with point mutations potentially stabilizing the protein and evading degradation mediated by FBXW7 [
9]. Additionally, chromosomal gains affecting whole Chromosome 13 (CFA 13), where
MYC is located, influence prognosis in canine DLBCL [
9,
10].
Directly targeting Myc poses significant challenges due to its role as a general transcription factor lacking well-defined drug-binding pockets and its predominantly nuclear localization [
11]. Alternative strategies that have been proposed are the following: 1. inhibiting
MYC transcription, 2. targeting
MYC mRNA translation, 3. modulating its stability, 4. disrupting Myc–Max interaction, and 5. influencing its downstream gene accessibility [
11].
Proteolysis targeting chimeras (PROTAC) are heterobifunctional molecules that degrade specific endogenous proteins, such as oncoproteins, through the E3 ubiquitin ligase pathway [
12,
13]. PROTAC consist of a target binding unit, a linker, and an E3 ligase binding moiety in which the ubiquitin proteasome system is responsible for degrading the protein of interest [
14]. The E3 ligase ligand can hijack the E3 ligase and label the protein with ubiquitin [
14]. In this process, PROTAC itself are not degraded, instead are recycled to promote ubiquitination and degradation of other target proteins [
14]. In recent years, novel PROTAC agents have been engineered to target proteins more specifically [
14]. Of particular interest is the BET degrader MZ1. BET proteins recruit transcription factors involved in elongation step (P-TEFb) to acetylated chromatin to induce gene transcription and carcinogenesis [
15]. MZ1 derived from JQ1, which exhibits the capacity to degrade BRD2, BRD3, and BRD4 [
16]. It demonstrated more potent protein degradation activities compared to JQ1 and shows significant cytotoxicity against colorectal cancer, triple-negative breast cancer, and lymphoma in humans [
16,
17,
18,
19].
Another potential candidate drug for targeting Myc is BI2536 [
20]. Initially identified as an inhibitor of PLK1, recent studies have demonstrated that BI2536 also interacts with bromodomain functions, potentially resulting in an anti-human lymphoma drug through Myc inactivation [
21]. Our hypothesis is that the dual targeting of BI2536 may similarly result in strong antiproliferative effects in canine lymphoma [
21]. More important, simultaneous blockade of two targets may reduce the risk of therapy resistance, as the probability of clonal adaptation to targeted therapy is lower for combination therapies [
21].
Given these premises, this study aims to evaluate the efficacy of indirectly inhibiting Myc in canine BCL using MZ1 and BI2536 compounds in two in vitro models, namely CLBL-1 and KLR-1201. In our experiment, we used the opnMe platform from Boehringer Ingelheim (
https://opnme.com) to obtain the two drugs and investigate their anti-proliferative effects
in vitro individually and in combination, along with their impacts on c-Myc protein expression and whole transcriptome.
2. Materials and Methods
2.1. Cell Lines and Molecules
CLBL-1 cells were kindly provided by Dr Barbara Rütgen (University of Wien, Austria). KLR-1201 cell line was developed in the Drs. Angela McCleary-Wheeler and Kristy Richards’ laboratories and was provided by Dr Wilfred Leung (Cornell University, NY, USA). Both cell lines were cultured in IMDM supplemented with fetal bovine serum (10% for CLBL-1; 20% for KLR-1201), 1% glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin. BI2536 and MZ1 compounds were purchased from Boehringer Ingelheim (Ingelheim, Germany) and were dissolved in dimethyl sulfoxide (DMSO) at 10 µM stock solution.
2.2. Cell Proliferation Assay
To evaluate the anti-proliferative effects of the compounds on cBCL cell lines, 15 × 10
4 cells/well were seeded in 96-wells cell culture plates and incubated with BI2536 at 2.5, 5, 7.5, 10, 12.5 and 15 nM for 12, 24 and 48 hours and with MZ1 at 10, 15, 25, 50, 75, 100 nM for 24, 48, 72 hours, as previously described [
22,
23]. The effect of the combinations of both drugs was also tested (
Table 1). Cells treated with DMSO were used as control. After the treatment, CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was performed following the manufacturer’s instructions.
2.3. Western Blotting
The expression of PLK1, BRD4 and c-Myc proteins was analyzed by Western Blot (WB) in treated and untreated cells. Proteins were extracted in lysis buffer (1% Triton X-100, 10% glycerol, 50 mM Tris, 150 mM sodium chloride, 2 mM EDTA, pH 8.0 and 2mM magnesium chloride) containing protease inhibitor cocktail (Sigma Aldrich, St Louis, MO). Twenty micrograms of total proteins were separated by SDS-PAGE (10% or 15%) and transferred onto a nitrocellulose membrane (Thermo Fisher Scientific, Waltham, MA). After washing, membranes were incubated in TBS/BSA 10% (bovine serum albumin) at room temperature for 1 h and incubated overnight at 4°C with PLK1 (PA5-95265, Invitrogen, Waltham, MA, diluted 1:1000), BRD4 (E2A7X, Cell Signaling Technology, Danvers, MA, diluted 1:1000) and c-Myc antibodies (5605T, Cell Signaling Technology, Danvers, MA, diluted 1:1000). β-tubulin (T5201, Sigma-Aldrich, St Louis, MO, diluted 1:10000) was used as an internal control. After incubation with horseradish peroxidase (HRP)-linked secondary antibody diluted 1:15000 in TBS-Tween, membranes were washed 6 times in TBS-Tween and incubated with Clarity Western ECL Substrate (Biorad Laboratories, Hercules, CA). The proteins were visualized by briefly exposing the membrane to an autoradiographic CL-XPosure Film (Thermo Fisher Scientific, Waltham, MA). WB results were then acquired with an Epson scanner.
2.4. Transcriptome Profiling
Total RNA was extracted from treated and untreated cells using Maxwell RSC simplyRNA Cells Kit (Promega, Madison, WI) according to the manufacturer’s instructions. RNA concentration and integrity were measured by Nanodrop 2000 Spectrophotometer (ThermoFisher Scientific, Waltham, MA) and assessed through the Bioanalyzer 2010 instrument (Agilent Technologies, Palo Alto, CA, USA). Multiple libraries were prepared for all the conditions described above with the SureSelect Strand Specific RNA-Seq Library Preparation kit (Agilent Technologies, Palo Alto, CA, USA) and a single end sequencing (50SE) was carried out on an Illumina HiSeq2500 (Illumina Inc., San Diego, CA, USA).
All Illumina reads were firstly analyzed with FastQC software to assess sequence quality and the resulting quality reports were grouped in a unified report using the MultiQC program. Afterward the raw data underwent a trimming procedure using Trimmomatic to remove primers and low-quality reads. Briefly the first and last 20 bp were removed, using a sliding window of 4 bp with a required quality score of 20. Additionally reads shorter than 50 bp were excluded from further analysis. The quality metrics of the data were accessed again after trimming as described above. Finally, the FASTQ files were aligned to the reference genome (canFam3, UCSC) and ENSCAF gene names were annotated with GTF file (UCSC) using STAR aligner. Annotated data were analyzed in R to conduct differential expression analysis using Limma and edgeR packages. Differentially expressed transcripts were defined as those with an average normalized log expression [counts per million reads mapped (cpm)] of at least 2, presenting an absolute |logFC| ≥ 1 and Benjamini-Hochberg (BH) multiple tests corrected p value [false discovery rate (FDR)] ≤ 0.05. Functional annotation was done using Gene Set Enrichment Analysis (GSEA) on fold-change pre-ranked lists of genes considered differentially expressed ranked by the respective logFC weighted by corresponding adjusted pvalue. Genesets from the MSigDB collection (hallmark, c4, c6, c7) were used, applying as threshold FDR values ≤ 0.05.
2.5. Statistical Analysis
Statistical analyses were performed in R environment. Two-way ANOVA was used to analyze data from proliferation assays to investigate the effects of BI2536 and MZ1 treatment and their combination and results were considered significant when p-value was ≤ 0.05.
3. Results
3.1. BI2536 and MZ1 Exhibit Anti-Tumor Activity in Canine B-Cell Lymphoma Cell Lines as Single Agents and in Combination
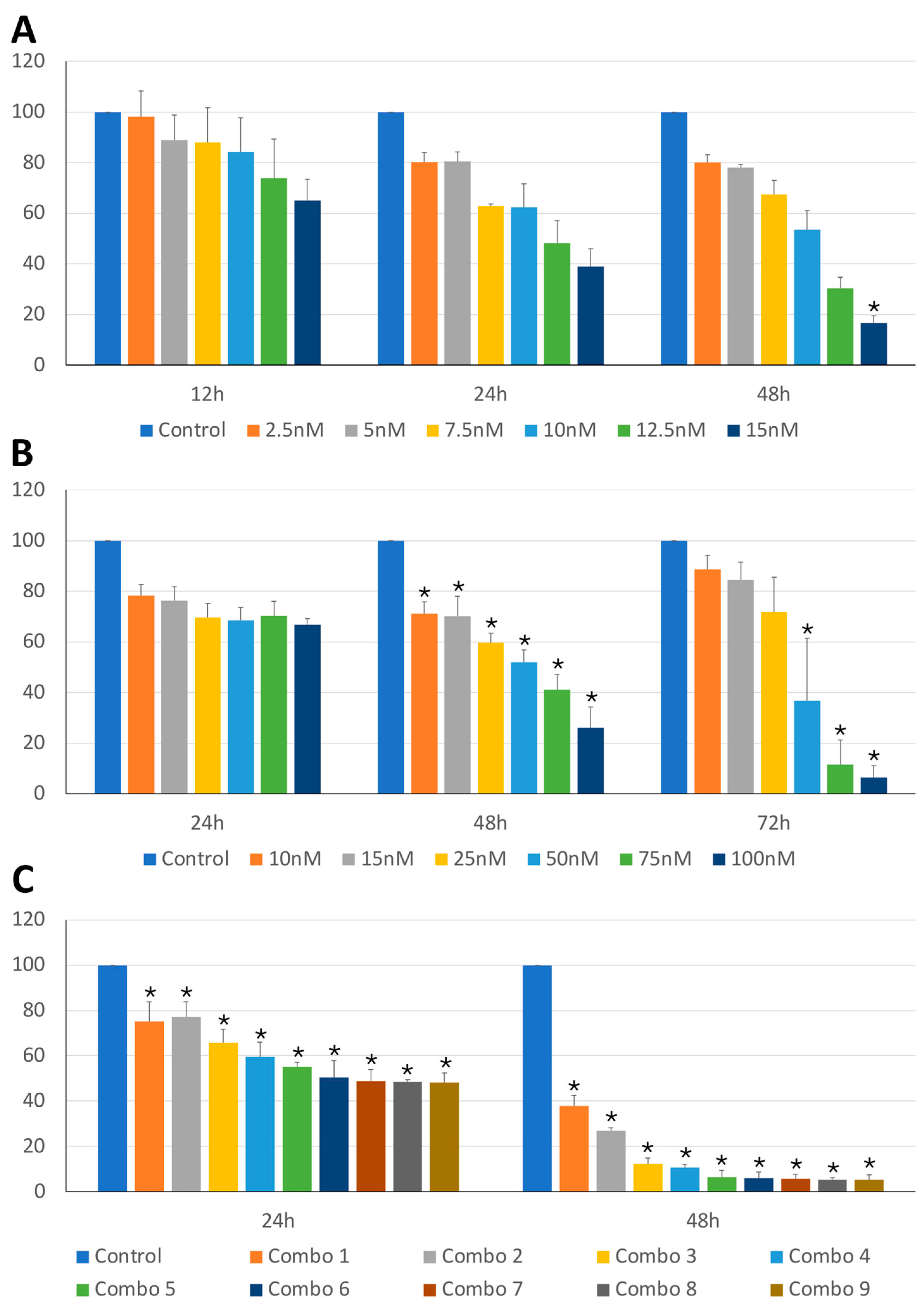
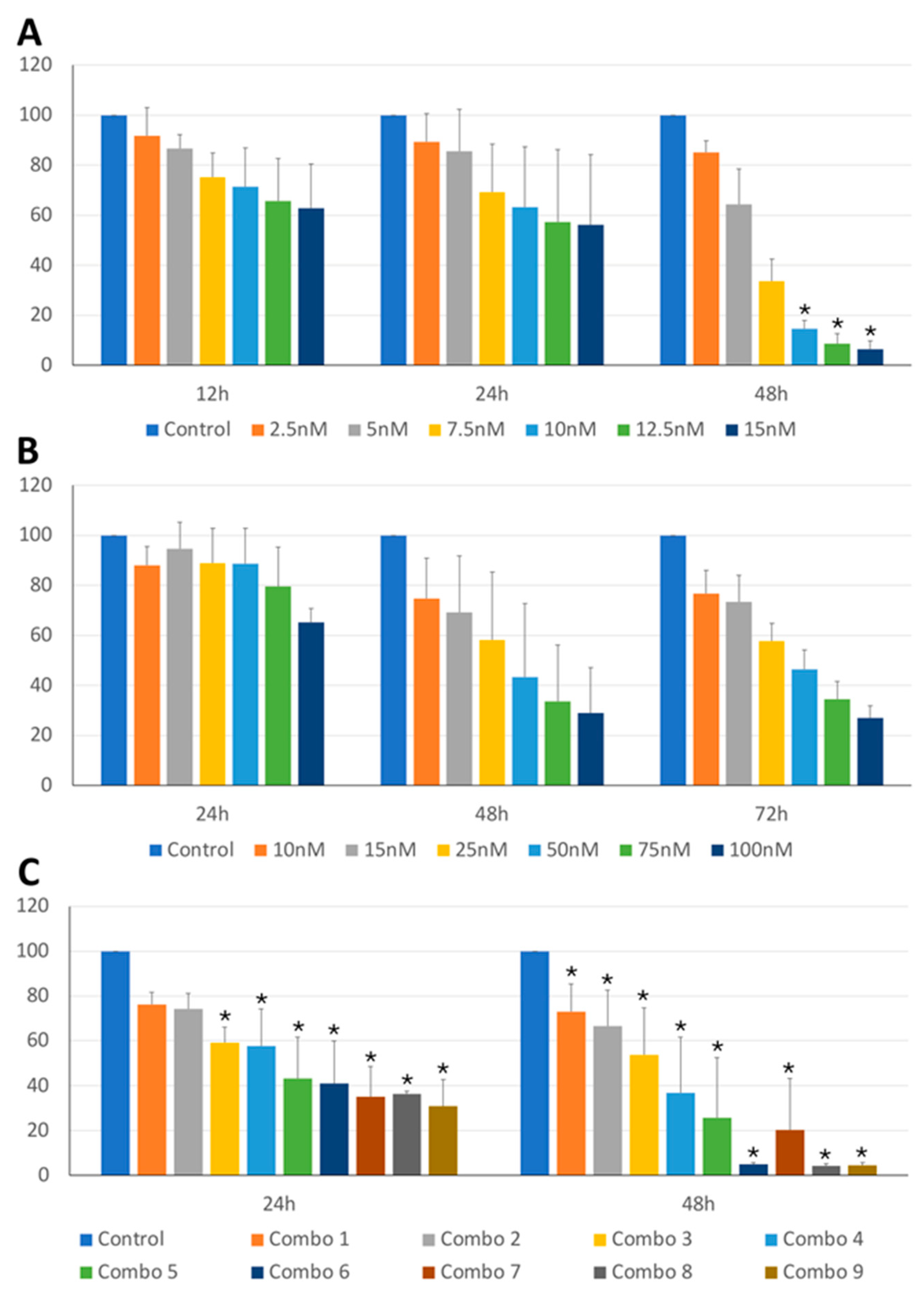
The anti-tumor activity of BI2536 and MZ1 on cBCL cell lines was assessed. As shown in
Figure 1A,B and
Figure 2A,B, BI2536 and MZ1 affected the cell viability of CLBL-1 and KLR-1201 cells in a significant concentration (p-value < 0.001 and p-value < 0.001, respectively) and time-dependent manner (p-value < 0.001 and p-value < 0.001, respectively).
Subsequently, the effects of the combination of the two drugs were also evaluated. The combination led to a concentration-dependent viability reduction in both CLBL-1 and KLR-1201 (p-value < 0.001 and p-value < 0.001, respectively) with a significant time-related effect observed only in KLR-1201 cells (p-value < 0.001) (
Figure 1C and
Figure 2C). CLBL-1 cells showed a reduction in cell viability already at low MZ1 doses, but the most dramatic consequences in both cell lines were obtained by combining BI2536 15nM and MZ1 100nM (
Figure 1C and
Figure 2C).
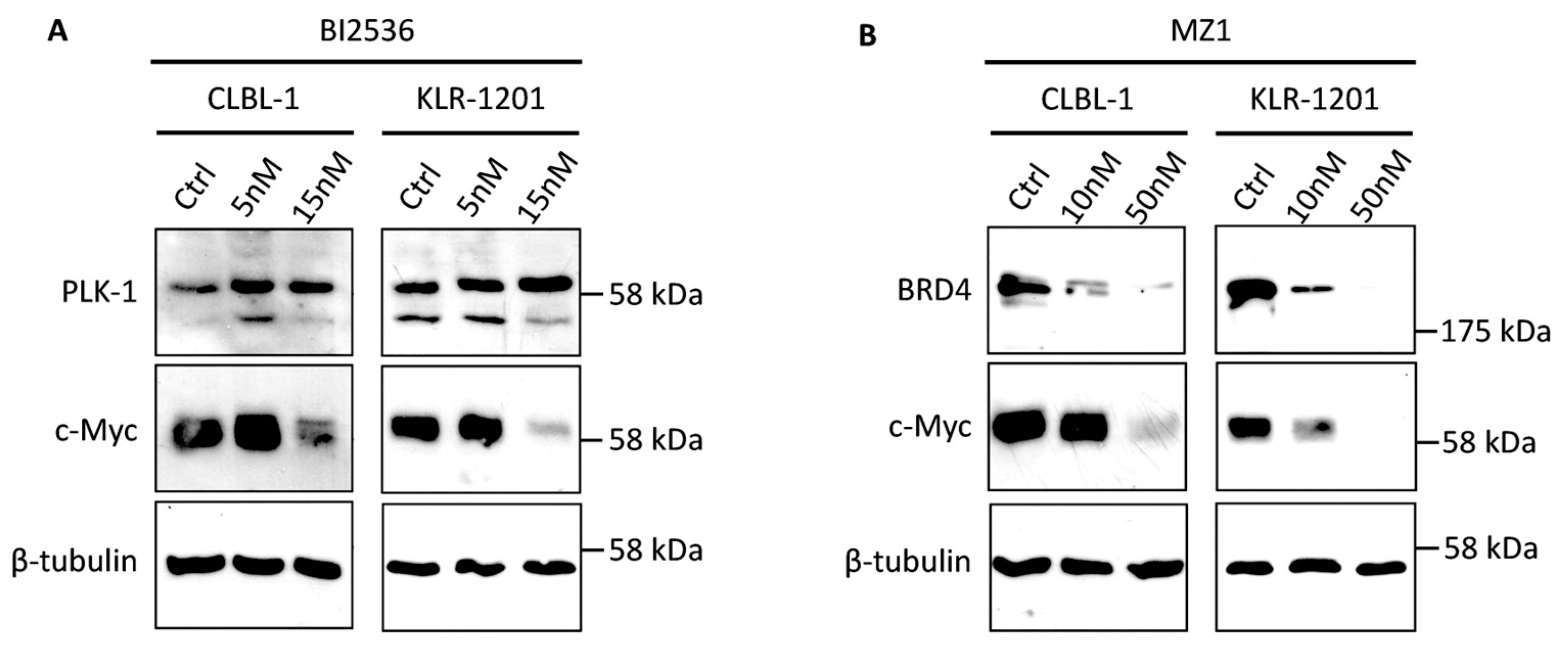
3.2. BI2536 and MZ1 Affect C-Myc Protein Expression in Canine B-Cell Lymphoma Cell Lines
To evaluate the specificity of BI2536 and MZ1 for their respective targets and their indirect impact on c-Myc expression, WB was conducted on CLBL-1 and KLR-1201 cell lines following a 24-hour treatment with concentrations of 5 and 15nM for BI2536 and 10 and 50nM for MZ1, respectively. Remarkably, both cell lines exhibited an upregulation of PLK1 expression when treated with BI2536, in association with a concentration-dependent reduction in c-Myc protein levels, as illustrated in
Figure 3A. Conversely, MZ1 demonstrated a conspicuous concentration-dependent decrease in its primary target, BRD4, along with a corresponding reduction in c-Myc protein expression in CLBL-1 and KLR-1201 cells (
Figure 3B). Furthermore, the combination of the two drugs induced a downregulation of cMyc protein expression (data not shown).
3.3. BI2536 Alone and in Combination with MZ1 Induces Transcriptomic Changes in Canine B-Cell Lymphoma Cell Lines
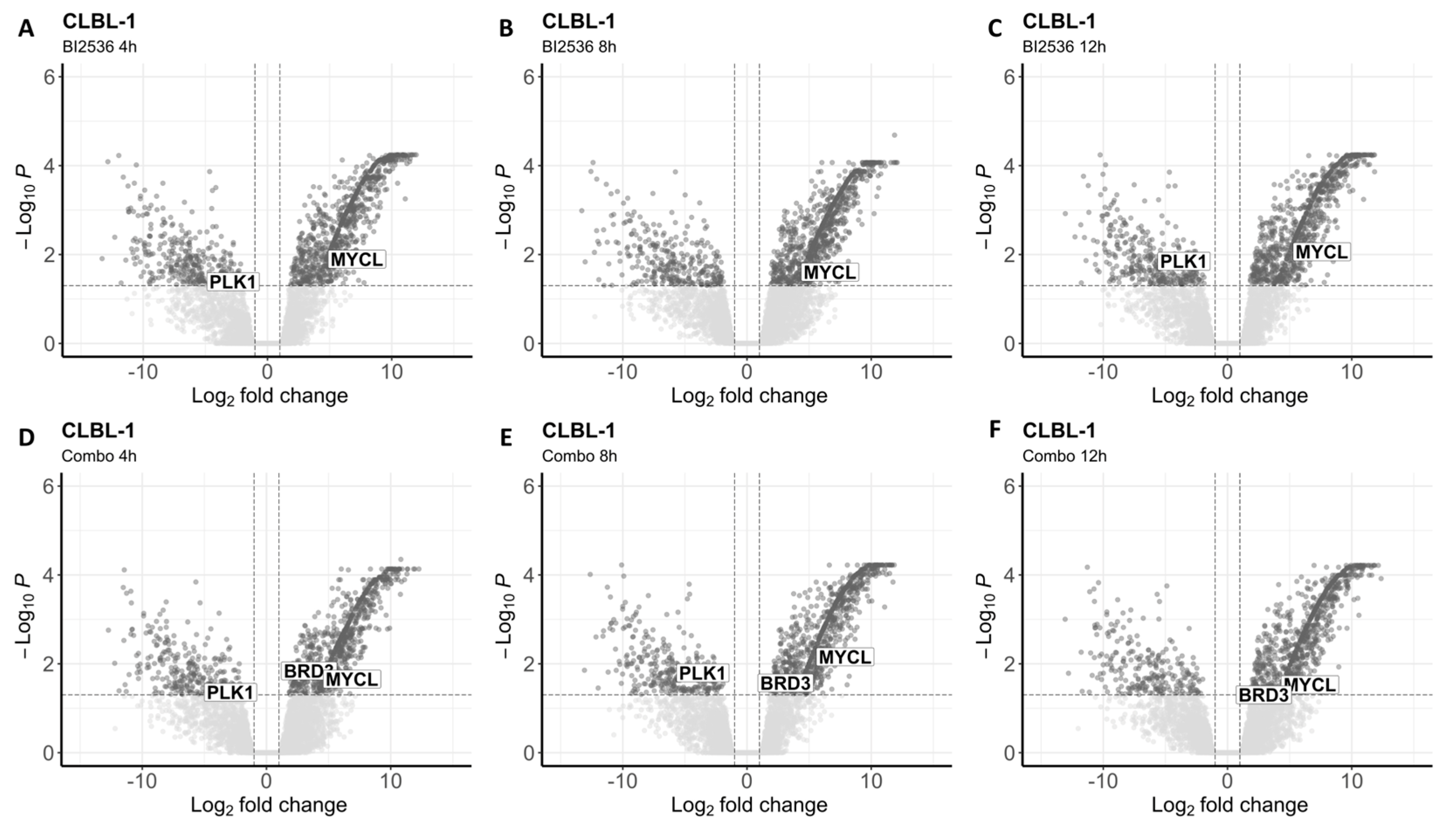
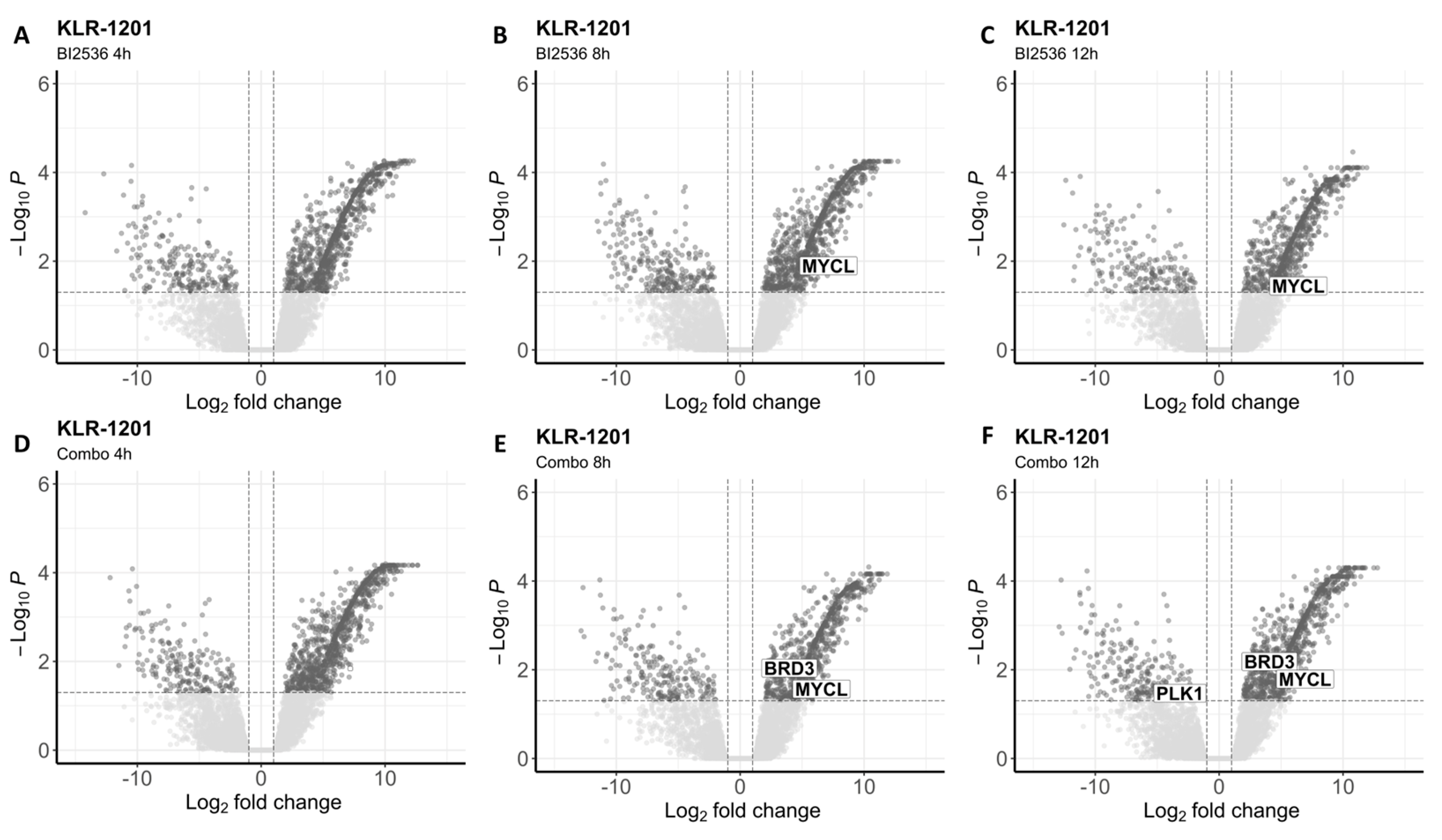
To investigate the impact of both drugs on the whole transcriptome, we conducted RNA-seq analysis after exposing the two BCL cell lines to BI2536 (20nM) and MZ1 (80nM), individually and in combination (BI2536 15nM + MZ1 100nM) for durations of 4, 8, and 12 hours. We found significant differences in gene expression only in cells treated with BI2536 and BI2536 and MZ1 combination, but not in cells treated with MZ1 alone (
Table S1 and
Table S2). The number of differentially expressed transcripts (FDR ≤ 0.05) was similar in CLBL-1 and KLR-1201 when comparing BI2536 to control cell lines (mean= 1231 and 1134, respectively). Across the experiments, an average of 330 and 337 genes was found downregulated while 902 and 868 were upregulated in CLBL-1 and KLR-1201, respectively (
Figure 4A-C and
Figure 5A-C). However, the combination of compounds induced a larger change in the transcriptome of the CLBL-1 cell line (mean=1148) compared to the KLR-1201 cell line (mean=766). On average, 276 genes were found downregulated and 872 were upregulated in CLBL-1 (
Figure 4D-F). Similarly, 174 and 596 genes were down- and upregulated in KLR-1201, respectively (
Figure 5D-F).
In CLBL-1, BI2536 and MZ1, both alone and in combination, induced a downregulation of PCNA and Ki67-related signatures at each timepoint of exposure (
Table S3). Additionally, BI2536 affected the expression of genes regulated by
MYC and
E2F as well as those involved in the G2/M checkpoint after 8 and 12 hours of incubation (
Table S3). Early treatment with MZ1 downregulated
MYC target genes and mTORC1 signatures, and subsequently JAK/STAT3 and NF-kB signaling (
Table S3). After 8 hours of incubation, genes related to
H2AFX also resulted downregulated (
Table S3). The combination recapitulated the effects observed with the single treatments on the transcriptome (
Table S3).
In KLR-1201 cells, BI2536 as single agent or combined with MZ1 exhibited similar effects as observed in CLBL-1, affecting the same gene signatures (
Table S4). Differences were noted in cells treated with MZ1 alone, which induced a downregulation of p53 pathway after 8 hours of incubation (
Table S4).
Interestingly, we observed downregulation of
PLK1 in CLBL-1 cells after exposure to BI2536 for 4 or 12 hours, and to the combination for 4 or 8 hours (
Table S1). In the KLR-1201 cell line, downregulation of
PLK1 was observed only after treatment with the drug combination for 12 hours (
Table S2). In CLBL-1 cells at each time point of exposure and in KLR-1201 cells after 8 and 12 hours, the combination of the two drugs did not affect
BRD4 expression but led to the upregulation of
BRD3, for which MZ1 is known to have a milder affinity (
Table S1 and
Table S2). Among the
MYC family genes,
MYCL resulted upregulated in both cell lines treated with BI2536 and in combination (
Table S1 and
Table S2).
4. Discussion
In this study, we have developed a dual approach aimed at indirectly targeting Myc using two well-known drugs in human oncology setting [
11]. In the first approach we were intrigued by the effects of the dual kinase–bromodomain inhibitor BI2536 in canine lymphoma and we tested the two cBCL cell lines. BI2536 was discovered and developed as a PLK1 kinase inhibitor but was later found to potently inhibit BRD4 [
21]. PLK1 interacts with and phosphorylates FBXW7, leading to its autopolyubiquitination and degradation, thereby promoting Myc stabilization [
24]. In a feedforward loop, PLK1 transcription is promoted by stabilized Myc [
24]. Therefore, PLK1/FBXW7/Myc axis creates a positive auto-regulatory signal crucial in promoting tumorigenesis and underscores PLK1 as potential therapeutic target in Myc-overexpressing tumors, as validated in our previous study on osteosarcoma cell lines [
23,
25]. Here, PLK1 inhibition strongly affected the viability of cBCL cells and resulted in a reduction of c-Myc stabilization, indicated by the decrease in c-Myc protein expression observed in treated cells, and consequently, in the downregulation of
PLK1 gene. Conversely, PLK1 exhibited an increase in protein expression similarly to osteosarcoma cell line models [
23]. One plausible explanation for this inconsistency might stem from the interference of Myc-induced
PLK1 transcription due to the intricate interplay of signaling pathways that regulate the cell cycle [
23].
The indirect effect exerted by PLK1 inhibition on Myc activity was confirmed by the downregulation of
MYC target genes. However, the expression of
MYC genes was not significantly affected by the treatment with BI2536, except for
MYCL, whose upregulation suggests a rebound effect that warrants further investigations. BI2536 strongly affected the cell cycle regulation, compromising
E2F target genes and genes involved in G2/M checkpoint. The E2F transcription factor family is involved in transactivation of target genes crucial for the G1/S transition, and loss of E2F results in cell cycle arrest [
26]. The G2/M checkpoint plays a crucial role in preventing cells with DNA damage from undergoing mitosis. When the G2/M checkpoint fails, cells may undergo erroneous replication and, consequently, apoptosis or cell death [
27]. Additionally, two other cell cycle-related signatures downregulated in cBCL cell lines after BI2536 treatment were associated with PCNA, whose expression is under the control of E2F transcription factors, and Ki67, which is involved in active phases of the cell cycle [
28,
29].
In the second approach, we tested MZ1, a novel PROTAC-based BRD4 degrader. PROTAC technology has emerged as an effective tool for endogenous protein degradation [
14]. MZ1 and other BET-degraders are bifunctional PROTAC molecules consisting of ligands for proteins of interest and covalently linked for E3 ubiquitin ligases, which can suppress tumor growth through ubiquitinate target proteins via the ubiquitin-proteasome system [
14]. In our assays, MZ1 specifically degraded BRD4 protein, consequently affecting c-Myc expression and providing a specific target for cBCL. In addition, MZ1 affected the cell viability of CLBL-1 and KLR-1201 cells in a significant concentration- and time-dependent manner. This finding aligns with results obtained with BET degraders in human lymphomas, where BRD4 is selectively degraded by drugs with mechanisms of action involving E3 ligase recruitment to the proteasome [
19].
In terms of transcriptome effects, MZ1 exhibited different behavior in the two cBCL cell lines utilized in the study. Specifically, the effects of the drug on CLBL-1 were largely attributed to the interference on
MYC target genes expression and several signaling pathways (JAK/STAT3, mTORC1, NF-kB) involved in cellular processes such as cell survival and proliferation, which are known to be dysregulated in cancer. Additionally, along with the downregulation of proliferation markers signatures (PCNA and Ki67) as observed after treatment with BI2536, MZ1 also downregulated the signature associated with
H2AFX. This gene codes for the H2A histone family member X, which undergoes phosphorylation in response to DNA double-strand breaks [
30]. Therefore, it appears that the DNA damage response may not be primarily responsible for the cellular modifications induced by MZ1, as observed in KLR-1201 cells, that displayed a downregulation of p53 pathway signature.
Despite these intriguing GSEA results, we did not identify significantly differentially expressed genes in either of the cell lines treated with MZ1. We have ruled out technical issues that could have been responsible. Nevertheless, it is important to acknowledge that the significance thresholds we used in the RNA-seq data analysis were highly stringent.
Combining BI2536 and MZ1 led to a greater decrease in cell viability compared to their individual use. These results imply that the anti-tumor efficacy of BI2536 and MZ1 might be partially attributed to their impact on c-Myc expression and subsequent effects on the cell cycle, as suggested by RNA-seq data [
11].
cBCL, especially focusing on cDLBCL, represents an excellent animal model mirroring its human counterpart, exhibiting numerous pathological, molecular, and clinical resemblances [
31,
32]. Incorporating a canine model into the drug development process is increasingly recognized as a promising strategy to address various challenges associated with in vitro and murine models [
33]. Our findings indicate promising therapeutic implications for human BCL as well.
5. Conclusions
In conclusion, our study offers valuable insights into the mechanisms of action of BI2536 and MZ1 in canine B-cell lymphoma and highlights their potential as targeted therapies for this cancer subtype. Further investigation is warranted to elucidate the precise molecular mechanisms underlying their anti-tumor activity and to evaluate their effectiveness and safety in both preclinical and clinical settings.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Table S1: Differential expression analysis in CLBL-1; Table S2: Differential expression analysis in KLR-1201; Table S3: GSEA in CLBL-1; Table S4: GSEA in KLR-1201.
Author Contributions
Conceptualization, L.L., L.A.; methodology, L.L., E.M., P.A., A.R., S.D., W.L., S.W., R.D.M.; software, E.M.; validation, C.T., L.A.; formal analysis, L.L., L.A.; investigation, C.T, L.A.; resources, W.L., S.W., L.A.; data curation, L.L., L.A; writing—original draft preparation, L.L., L.A.; writing—review and editing, L.L., C.T., W.L., L.A.; visualization, L.L., P.A.; supervision, R.D.M., L.A.; project administration, L.A.; funding acquisition, L.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Raw Illumina sequencing data are deposited in the SRA database (GenBank) under accession number PRJNA1099474.
Acknowledgments
The authors express their gratitude to Drs. Angela McCleary-Wheeler and Kristy Richards for the kind gift of KLR-1201 cells, developed in their laboratories (College of Veterinary Medicine, Cornell University, NY, USA).
Conflicts of Interest
Dr Wilfred Leung is employed and owner of equity at Genentech Inc/Hoffman-La Roche Ltd. The other authors have nothing to disclose.
References
- Avery, A.C. The Genetic and Molecular Basis for Canine Models of Human Leukemia and Lymphoma. Front Oncol 2020, 10, 23. [Google Scholar] [CrossRef]
- Valli, V.E.; San Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011, 48, 198–211. [Google Scholar] [CrossRef]
- Aresu, L. Canine Lymphoma, More Than a Morphological Diagnosis: What We Have Learned about Diffuse Large B-Cell Lymphoma. Front Vet Sci 2016, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Childress, M.O.; Ramos-Vara, J.A.; Ruple, A. Retrospective analysis of factors affecting clinical outcome following CHOP-based chemotherapy in dogs with primary nodal diffuse large B-cell lymphoma. Vet Comp Oncol 2018, 16, E159–E168. [Google Scholar] [CrossRef]
- Marconato, L.; Stefanello, D.; Sabattini, S.; Comazzi, S.; Riondato, F.; Laganga, P.; Frayssinet, P.; Pizzoni, S.; Rouquet, N.; Aresu, L. Enhanced therapeutic effect of APAVAC immunotherapy in combination with dose-intense chemotherapy in dogs with advanced indolent B-cell lymphoma. Vaccine 2015, 33, 5080–5086. [Google Scholar] [CrossRef] [PubMed]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; Rosenwald, A.; Campo, E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013, 122, 3884–3891. [Google Scholar] [CrossRef]
- Nguyen, L.; Papenhausen, P.; Shao, H. The Role of c-MYC in B-Cell Lymphomas: Diagnostic and Molecular Aspects. Genes (Basel) 2017, 8. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Marconato, L.; Fanelli, A.; Licenziato, L.; De Maria, R.; Rinaldi, A.; Rotta, L.; Rouquet, N.; Birolo, G.; Fariselli, P.; et al. The genomic landscape of canine diffuse large B-cell lymphoma identifies distinct subtypes with clinical and therapeutic implications. Lab Anim (NY) 2022, 51, 191–202. [Google Scholar] [CrossRef]
- Aricò, A.; Ferraresso, S.; Bresolin, S.; Marconato, L.; Comazzi, S.; Te Kronnie, G.; Aresu, L. Array-based comparative genomic hybridization analysis reveals chromosomal copy number aberrations associated with clinical outcome in canine diffuse large B-cell lymphoma. PLoS One 2014, 9, e111817. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Yin, J.; Gan, Y.; Xu, S.; Gu, Y.; Huang, W. Alternative approaches to target Myc for cancer treatment. Signal Transduct Target Ther 2021, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Cheng, M.; Park, K.S.; Kaniskan, H.; Xiong, Y.; Jin, J. Advancing targeted protein degradation for cancer therapy. Nat Rev Cancer 2021, 21, 638–654. [Google Scholar] [CrossRef]
- Raina, K.; Crews, C.M. Chemical inducers of targeted protein degradation. J Biol Chem 2010, 285, 11057–11060. [Google Scholar] [CrossRef]
- Troup, R.I.; Fallan, C.; Baud, M.G.J. Current strategies for the design of PROTAC linkers: a critical review. Explor Target Antitumor Ther 2020, 1, 273–312. [Google Scholar] [CrossRef]
- Spriano, F.; Stathis, A.; Bertoni, F. Targeting BET bromodomain proteins in cancer: The example of lymphomas. Pharmacol Ther 2020, 215, 107631. [Google Scholar] [CrossRef]
- Zengerle, M.; Chan, K.H.; Ciulli, A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem Biol 2015, 10, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Noblejas-López, M.D.M.; Nieto-Jiménez, C.; Galán-Moya, E.M.; Tebar-García, D.; Montero, J.C.; Pandiella, A.; Burgos, M.; Ocaña, A. MZ1 co-operates with trastuzumab in HER2 positive breast cancer. J Exp Clin Cancer Res 2021, 40, 106. [Google Scholar] [CrossRef]
- Otto, C.; Schmidt, S.; Kastner, C.; Denk, S.; Kettler, J.; Müller, N.; Germer, C.T.; Wolf, E.; Gallant, P.; Wiegering, A. Targeting bromodomain-containing protein 4 (BRD4) inhibits MYC expression in colorectal cancer cells. Neoplasia 2019, 21, 1110–1120. [Google Scholar] [CrossRef]
- Tarantelli, C.; Cannas, E.; Ekeh, H.; Moscatello, C.; Gaudio, E.; Cascione, L.; Napoli, S.; Rech, C.; Testa, A.; Maniaci, C.; et al. The bromodomain and extra-terminal domain degrader MZ1 exhibits preclinical anti-tumoral activity in diffuse large B-cell lymphoma of the activated B cell-like type. Explor Target Antitumor Ther 2021, 2, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Steegmaier, M.; Hoffmann, M.; Baum, A.; Lénárt, P.; Petronczki, M.; Krssák, M.; Gürtler, U.; Garin-Chesa, P.; Lieb, S.; Quant, J.; et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol 2007, 17, 316–322. [Google Scholar] [CrossRef]
- Li, Z.; Yang, C.; Li, X.; Du, X.; Tao, Y.; Ren, J.; Fang, F.; Xie, Y.; Li, M.; Qian, G.; et al. The dual role of BI 2536, a small-molecule inhibitor that targets PLK1, in induction of apoptosis and attenuation of autophagy in neuroblastoma cells. J Cancer 2020, 11, 3274–3287. [Google Scholar] [CrossRef] [PubMed]
- Aresu, L.; Ferraresso, S.; Marconato, L.; Cascione, L.; Napoli, S.; Gaudio, E.; Kwee, I.; Tarantelli, C.; Testa, A.; Maniaci, C.; et al. New molecular and therapeutic insights into canine diffuse large B-cell lymphoma elucidates the role of the dog as a model for human disease. Haematologica 2019, 104, e256–e259. [Google Scholar] [CrossRef] [PubMed]
- Gola, C.; Licenziato, L.; Accornero, P.; Iussich, S.; Morello, E.; Buracco, P.; Modesto, P.; Aresu, L.; De Maria, R. The mitotic regulator polo-like kinase 1 as a potential therapeutic target for c-Myc-overexpressing canine osteosarcomas. Vet Comp Oncol 2022, 20, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Yue, M.; Su, H.; Ren, P.; Jiang, J.; Li, F.; Hu, Y.; Du, H.; Liu, H.; Qing, G. Polo-like Kinase-1 Regulates Myc Stabilization and Activates a Feedforward Circuit Promoting Tumor Cell Survival. Mol Cell 2016, 64, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pierce, A.; Veo, B.; Fosmire, S.; Danis, E.; Donson, A.; Venkataraman, S.; Vibhakar, R. A Regulatory Loop of FBXW7-MYC-PLK1 Controls Tumorigenesis of MYC-Driven Medulloblastoma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Löbrich, M.; Jeggo, P.A. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer 2007, 7, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an Effective Strategy to Inhibit Tumor Cell Proliferation. Curr Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: more than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef]
- Coyle, K.M.; Hillman, T.; Cheung, M.; Grande, B.M.; Bushell, K.R.; Arthur, S.E.; Alcaide, M.; Thomas, N.; Dreval, K.; Wong, S.; et al. Shared and distinct genetic features in human and canine B-cell lymphomas. Blood Adv 2022, 6, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Gelain, M.E.; Comazzi, S. The dog as a possible animal model for human non-Hodgkin lymphoma: a review. Hematol Oncol 2013, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, N.; Mohin, S.A.; Bruce, S. Canine models of human cancer: Bridging the gap to improve precision medicine. In Progress in Molecular Biology and Traslational Science; Elsevier, 2022; Volume 189, pp. 67–90. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).