Submitted:
27 April 2024
Posted:
28 April 2024
You are already at the latest version
Abstract
Keywords:
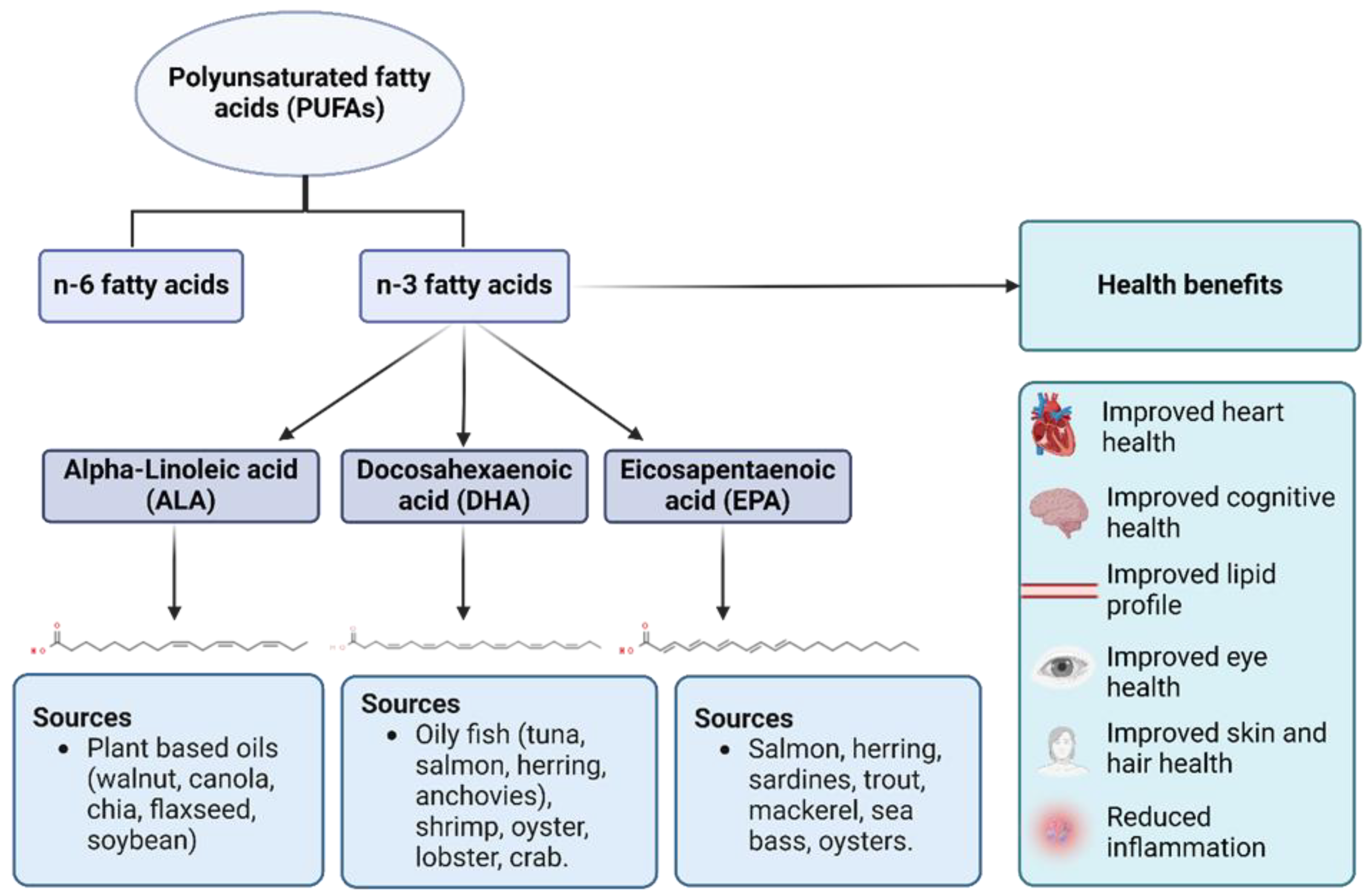
1. Introduction
1.1. Preclinical Studies
1.2. Clinical Studies
1.3. Epidemiological Studies
1.4. Case-Control Studies
2. Discussion
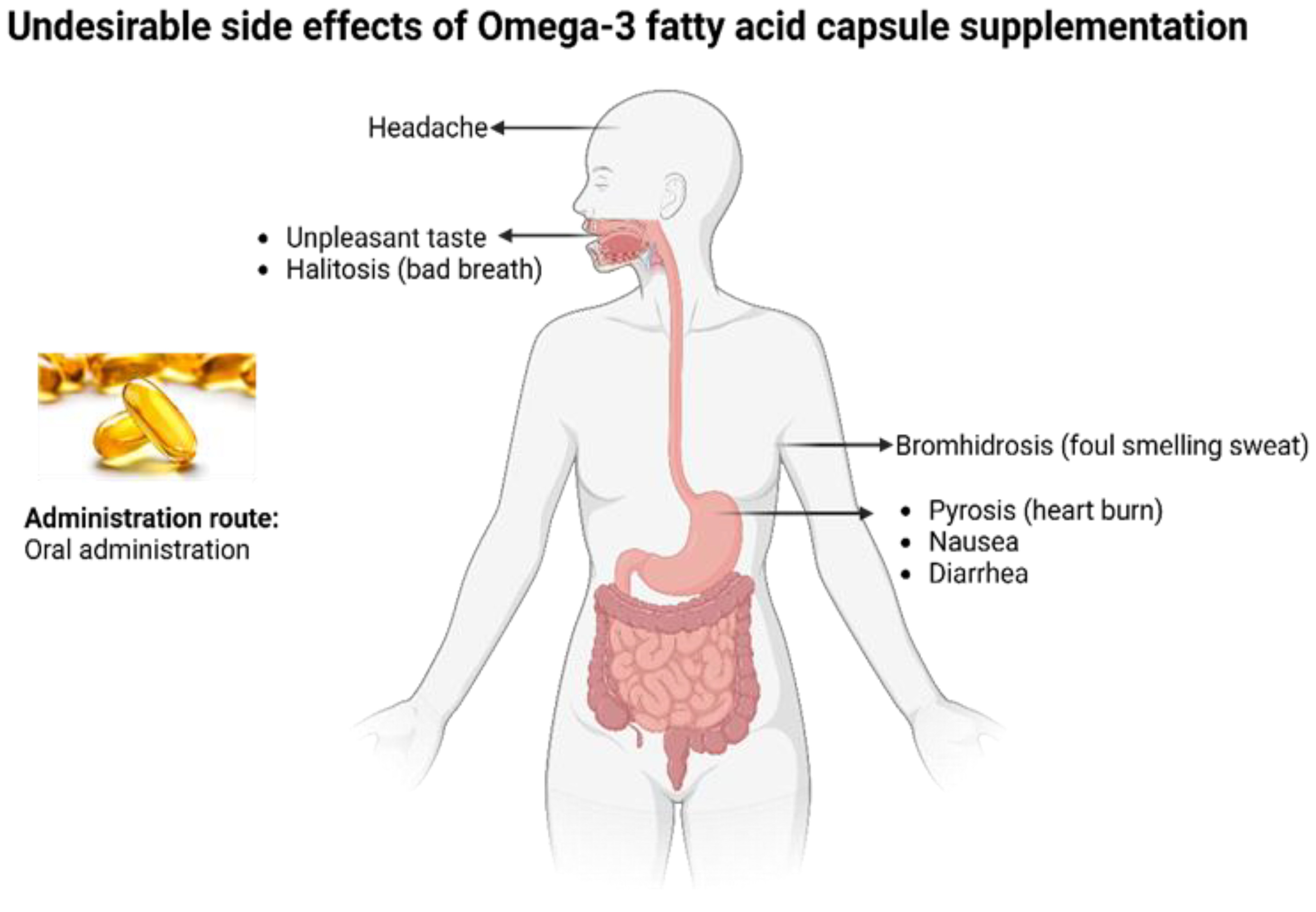
2.1. Obstacles in the Administration of Omega-3 PUFA Effectively
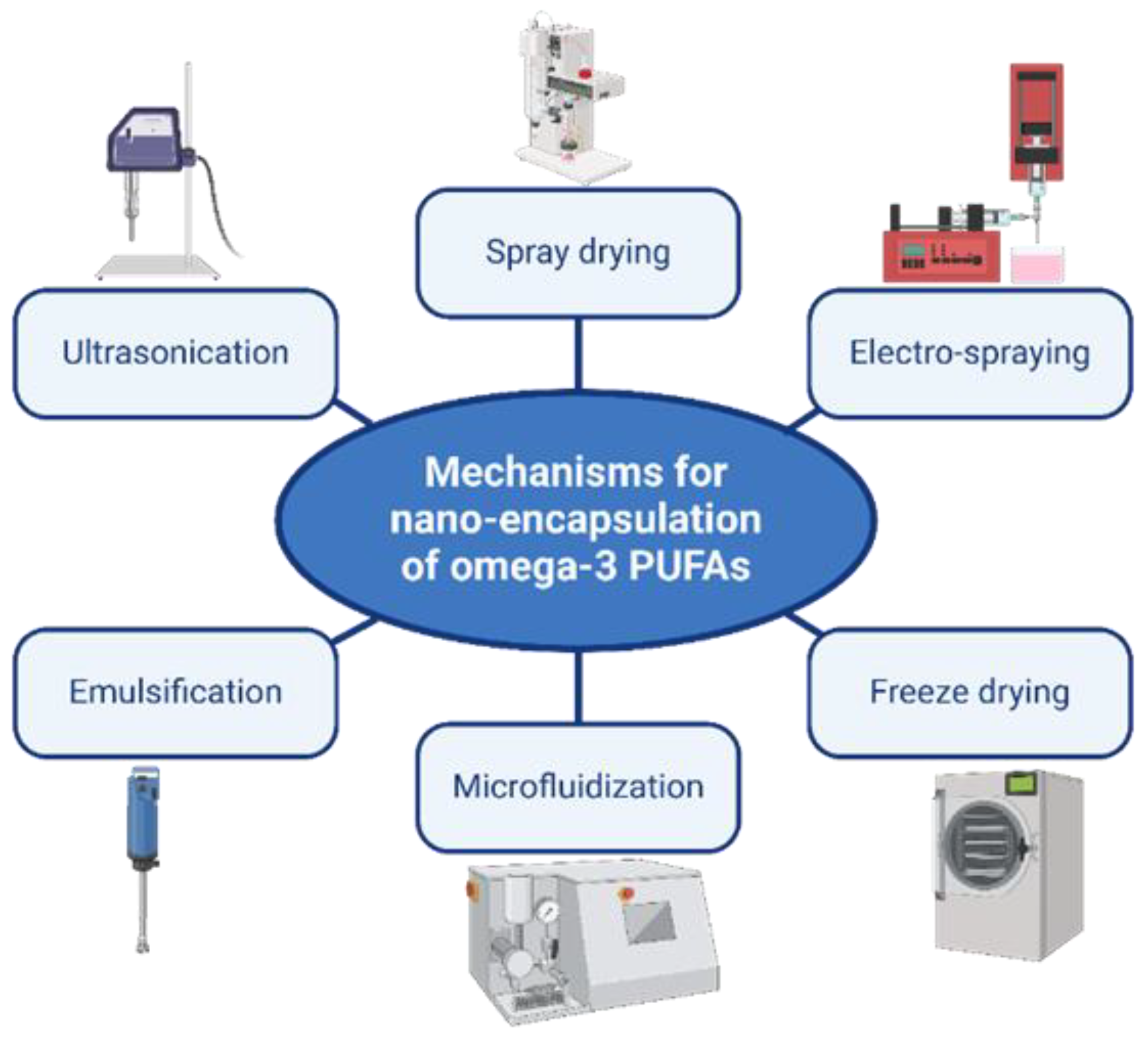
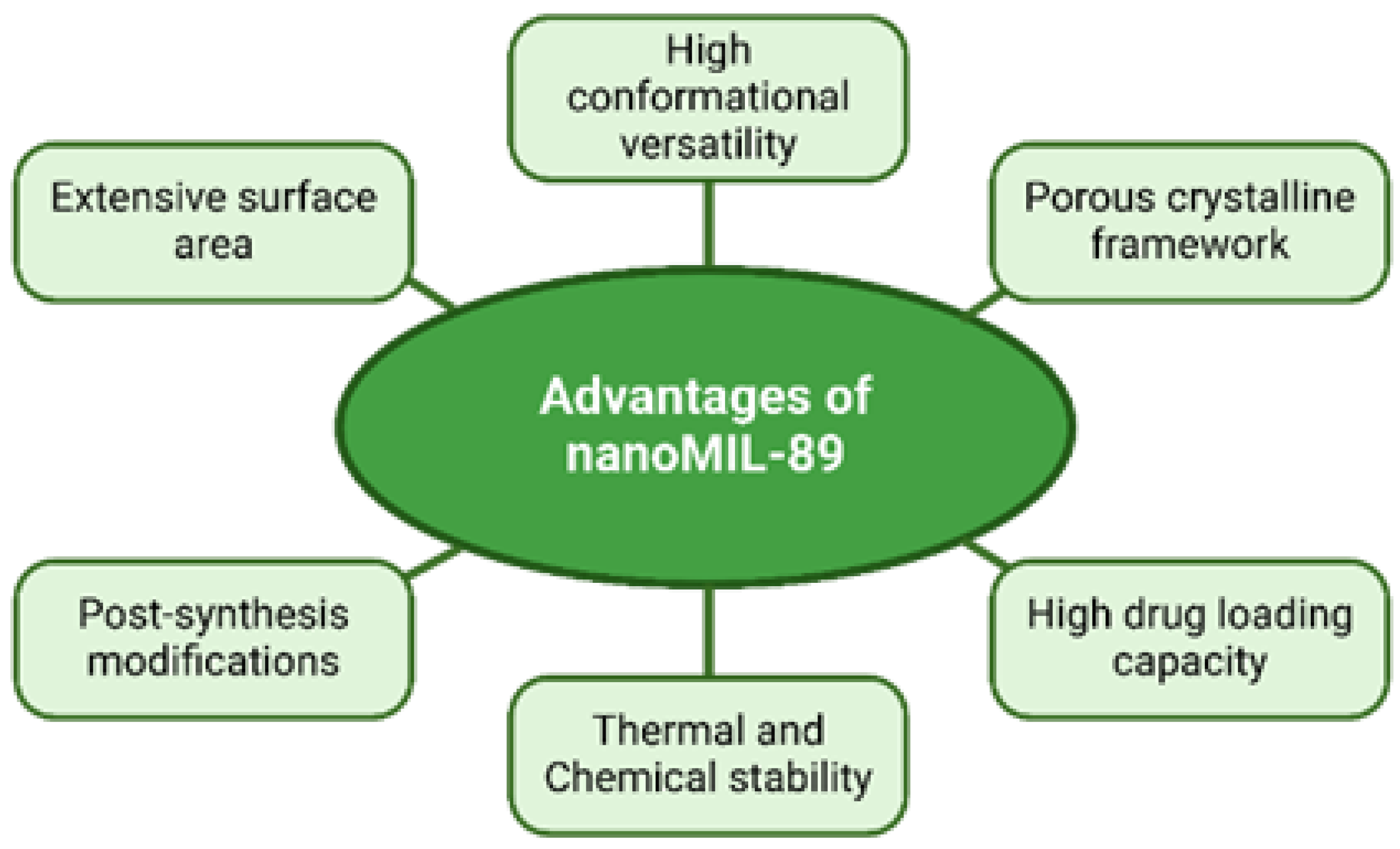
2.2. Nano-Encapsulation of Omega-3 PUFA
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation. 2023 Jan 25;147(8). [CrossRef]
- National Center for Health Statistics. Multiple Cause of Death Data on CDC WONDER. wonder.cdc.gov. 2023. Available from: https://wonder.cdc.gov/mcd.html (Accessed on January 2, 2024).
- World Health Organization. Cardiovascular diseases (CVDs). World Health Organization. World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed on January 2, 2024).
- Centers for Disease Control and Prevention. Heart disease and stroke. Centers for Disease Control and Prevention. 2020. Available from: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm (Accessed on January 2, 2024).
- American Heart Association. Consuming about 3 grams of omega-3 fatty acids a day may lower blood pressure. www.heart.org. Available from: https://www.heart.org/en/news/2022/06/01/consuming-about-3-grams-of-omega-3-fatty-acids-a-day-may-lower-blood-pressure (Accessed on January 2, 2024).
- Vonschacky C, Harris W. Cardiovascular benefits of omega-3 fatty acids. Cardiovascular Research. 2007 Jan 15;73(2):310–5. [CrossRef]
- Higdon J. Essential Fatty Acids, Micronutrient Information Center 179. 2019. Available online: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids (Accessed on January 2, 2024).
- Ulven SM, Kirkhus B, Lamglait A, Basu S, Elind E, Haider T, et al. Metabolic Effects of Krill Oil are Essentially Similar to Those of Fish Oil but at Lower Dose of EPA and DHA, in Healthy Volunteers. Lipids. 2010 Nov 2;46(1):37–46. [CrossRef]
- Gammone M, Riccioni G, Parrinello G, D’Orazio N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients. 2018 Dec 27 ;11(1):46. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6357022/. [CrossRef]
- Østbye TKK, Oddrun Anita Gudbrandsen, Aslaug Drotningsvik, Ruyter B, Gerd Marit Berge, Vogt G, et al. Different Dietary Ratios of Camelina Oil to Sandeel Oil Influence the Capacity to Synthesise and Deposit EPA and DHA in Zucker Fa/Fa Rats. Nutrients. 2023 May 17;15(10):2344–4. [CrossRef]
- Halade GV, Md. Mizanur Rahman, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic Acid-Enriched Fish Oil Attenuates Kidney Disease and Prolongs Median and Maximal Life Span of Autoimmune Lupus-Prone Mice. 2010 May 1;184(9):5280–6. [CrossRef]
- Fernandes G, Bhattacharya A, Rahman M, Zaman K, Banu J. Effects of n-3 fatty acids on autoimmunity and osteoporosis. Frontiers in Bioscience. 2008; Volume (13):4015. [CrossRef]
- Bentsen H. Dietary polyunsaturated fatty acids, brain function and mental health. Microbial Ecology in Health and Disease. 2017 Feb 6;28(sup1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5445635/. [CrossRef]
- Rennie KL, Hughes J, Lang R, Jebb SA. Nutritional management of rheumatoid arthritis: a review of the evidence. Journal of Human Nutrition and Dietetics: The Official Journal of the British Dietetic Association. 2003 Apr 1 [cited 2021 Mar 7];16(2):97–109. Available from: https://pubmed.ncbi.nlm.nih.gov/12662368/. [CrossRef]
- Bhattacharya A, Md. Mizanur Rahman, Banu J, Lawrence R, H. Stan McGuff, I. Ross Garrett, et al. Inhibition of Osteoporosis in Autoimmune Disease Prone MRL/Mpj-FaslprMice by N-3 Fatty Acids. Journal of The American College of Nutrition. 2005 Jun 1;24(3):200–9. [CrossRef]
- Abou-Saleh H, Ouhtit A, Halade GV, Rahman MM. Bone Benefits of Fish Oil Supplementation Depend on its EPA and DHA Content. Nutrients. 2019 Nov 8;11(11):2701. [CrossRef]
- Freeman M. Omega-3 Fatty Acids in Psychiatry: A Review. Annals of Clinical Psychiatry. 2000;12(3):159–65. [CrossRef]
- Holm T. Omega-3 fatty acids improve blood pressure control and preserve renal function in hypertensive heart transplant recipients. European Heart Journal. 2001 Mar 1;22(5):428–36. [CrossRef]
- Anan Yaghmur, Ghayas S, Jan H, Gökçe Dicle Kalaycıoğlu, S. Moein Moghimi. Omega-3 fatty acid nanocarriers: Characterization and potential applications. Current Opinion in Colloid and Interface Science. 2023 Oct 1; 67:101728–8. [CrossRef]
- Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. The Journal of Nutritional Biochemistry. 2007 Jan;18(1):23–30. [CrossRef]
- L. Kesavalu, V. Bakthavatchalu, Rahman MM, Su J, Raghu B, Dawson D, et al. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiology and Immunology. 2007 Jun 28;22(4):232–9. [CrossRef]
- Rahman MM, Veigas JM, Williams PJ, Fernandes G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Research and Treatment. 2013 Sep 24;141(3):341–52. [CrossRef]
- Crovella S, Ouhtit A, Rahman SM, Rahman MM. Docosahexaenoic Acid, a Key Compound for Enhancing Sensitization to Drug in Doxorubicin-Resistant MCF-7 Cell Line. Nutrients [Internet]. 2023 Jan 1;15(7):1658. Available from: https://www.mdpi.com/2072-6643/15/7/1658. [CrossRef]
- McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. American Heart Journal. 1992 Jun;123(6):1555–61. [CrossRef]
- Billman GE, Kang JX, Leaf A. Prevention of Sudden Cardiac Death by Dietary Pure ω-3 Polyunsaturated Fatty Acids in Dogs. Circulation. 1999 May 11;99(18):2452–7. [CrossRef]
- Baum JR, Dolmatova E, Tan A, Duffy HS. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Frontiers in Physiology. 2012;3. [CrossRef]
- Kalish BT, Matte A, Andolfo I, Iolascon A, Weinberg O, Ghigo A, et al. Dietary ɷ-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica. 2015 May 1;100(7):870–80. [CrossRef]
- Angelotti A, Snoke DB, Ormiston K, Cole RM, Borkowski K, Newman JW, et al. Potential Cardioprotective Effects and Lipid Mediator Differences in Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplemented Mice Given Chemotherapy. Metabolites. 2022 Aug 24;12(9):782. [CrossRef]
- Burr ML, Gilbert JF, Holliday RM, Elwood PC, Fehily AM, Rogers S, et al. Effects of changes in fat, fish, and fiber intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). The Lancet. 1989 Sep;334(8666):757–61. [CrossRef]
- Jialal I, Devaraj S, Huet B, Traber M. GISSI-Prevenzione trial. The Lancet. 1999 Oct;354(9189):1554. [CrossRef]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. The Lancet. 2007 Mar;369(9567):1090–8. [CrossRef]
- Einvik G, Ole Klemsdal T, Sandvik L, Hjerkinn EM. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. European Journal of Cardiovascular Prevention & Rehabilitation. 2010 Oct;17(5):588–92. [CrossRef]
- Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy After Myocardial Infarction. Circulation. 2010 Nov 23;122(21):2152–9. [CrossRef]
- Kromhout D, Giltay EJ, Geleijnse JM. n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. New England Journal of Medicine. 2010 Nov 18;363(21):2015–26. [CrossRef]
- Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo-controlled trial. BMJ [Internet]. 2010 Nov 29;341(nov29 1):c6273–3. Available from: https://www.bmj.com/content/341/bmj.c6273. [CrossRef]
- Bowman L, Mafham M, Stevens W, Haynes R, Aung T, Chen F, et al. ASCEND: A Study of Cardiovascular Events in Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. American heart journal. 2018; 198:135–44. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29653635. [CrossRef]
- Manson JE, Cook NR, Lee I-Min, Christen W, Bassuk SS, Mora S, et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. New England Journal of Medicine. 2019 Jan 3;380(1):23–32. [CrossRef]
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. New England Journal of Medicine. 2019 Jan 3;380(1):11–22. [CrossRef]
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA. 2020 Dec 8;324(22):2268. [CrossRef]
- Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction. Circulation. 2021 Feb 9;143(6):528–39. [CrossRef]
- Welty F, Bistrian B, Driscoll D. Omega-3 Fatty Acids Effect on Major Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA. 2021 Apr 6;325(13):1333. [CrossRef]
- Gillum RF, Mussolino M, Madans JH. The relation between fish consumption, death from all causes, and incidence of coronary heart disease. Journal of Clinical Epidemiology. 2000 Mar;53(3):237–44. [CrossRef]
- Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, et al. Intake of Fish and n3 Fatty Acids and Risk of Coronary Heart Disease Among Japanese. Circulation. 2006 Jan 17;113(2):195–202. [CrossRef]
- Kühn T, Teucher B, Kaaks R, Boeing H, Weikert C, Buijsse B. Fish consumption and the risk of myocardial infarction and stroke in the German arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Germany). British Journal of Nutrition. 2013 Feb 15;110(6):1118–25. [CrossRef]
- Nahab F, Pearson K, Frankel MR, Ard J, Safford MM, Kleindorfer D, et al. Dietary fried fish intake increases risk of CVD: the reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Public Health Nutrition. 2016 Jun 24;19(18):3327–36. [CrossRef]
- Bonaccio M, Ruggiero E, Di Castelnuovo A, Costanzo S, Persichillo M, De Curtis A, et al. Fish intake is associated with lower cardiovascular risk in a Mediterranean population: Prospective results from the Moli-sani study. Nutrition, Metabolism and Cardiovascular Diseases. 2017 Oct;27(10):865–73. [CrossRef]
- Hengeveld LM, Praagman J, Beulens JWJ, Brouwer IA, van der Schouw YT, Sluijs I. Fish consumption and risk of stroke, coronary heart disease, and cardiovascular mortality in a Dutch population with low fish intake. European Journal of Clinical Nutrition. 2018 May 22;72(7):942–50. Available from: https://www.nature.com/articles/s41430-018-0190-2. [CrossRef]
- Ward RE, Cho K, Nguyen XMT, Vassy JL, Ho YL, Quaden RM, et al. Omega-3 supplement use, fish intake, and risk of non-fatal coronary artery disease and ischemic stroke in the Million Veteran Program. Clinical Nutrition. 2020 Feb;39(2):574–9. [CrossRef]
- Pertiwi K, Küpers LK, de Goede J, Zock PL, Kromhout D, Geleijnse JM. Dietary and Circulating Long-Chain Omega-3 Polyunsaturated Fatty Acids and Mortality Risk After Myocardial Infarction: A Long-Term Follow-Up of the Alpha Omega Cohort. Journal of the American Heart Association. 2021 Dec 7;10(23). [CrossRef]
- Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive Medicine. 2004 Jul;39(1):212–20. Available from: https://www.sciencedirect.com/science/article/pii/S0091743504000878. [CrossRef]
- Harris W. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacological Research. 2007 Mar;55(3):217–23. [CrossRef]
- Harris WS, Thomas RM. Biological variability of blood omega-3 biomarkers. Clinical Biochemistry. 2010 Feb;43(3):338–40. [CrossRef]
- Gramenzi A, Gentile A, Fasoli M, Negri E, Parazzini F, La Vecchia C. Association between certain foods and risk of acute myocardial infarction in women. BMJ. 1990 Mar 24;300(6727):771–3. [CrossRef]
- Siscovick DS. Dietary Intake and Cell Membrane Levels of Long-Chain n-3 Polyunsaturated Fatty Acids and the Risk of Primary Cardiac Arrest. JAMA: The Journal of the American Medical Association. 1995 Nov 1;274(17):1363. [CrossRef]
- Hallgren CG, Göran Hallmans, Jansson JO, Marklund SL, F. Huhtasaari, Andrejs Schütz, et al. Markers of high fish intake are associated with decreased risk of a first myocardial infarction. British Journal of Nutrition. 2001 Sep 1;86(3):397–404. [CrossRef]
- Panagiotakos DB, Pitsavos C, Zampelas A, Chrysohoou C, Griffin BA, Stefanadis C, et al. Fish consumption and the risk of developing acute coronary syndromes: the CARDIO2000 study. International Journal of Cardiology. 2005 Jul;102(3):403–9. [CrossRef]
- Amani R, Noorizadeh M, Rahmanian S, Afzali N, Haghighizadeh MH. Nutritional related cardiovascular risk factors in patients with coronary artery disease in IRAN: A case-control study. Nutrition Journal. 2010 Dec;9(1). [CrossRef]
- Mónica Venegas-Calerón, Napier JA. New alternative sources of omega-3 fish oil. Advances in food and nutrition research. 2023 Jan 1;343–98.
- Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: public health risks and benefits. British Journal of Nutrition. 2012 May 17;107(S2): S23–52. [CrossRef]
- Cholewski M, Tomczykowa M, Tomczyk M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients [Internet]. 2018 Nov 4;10(11):1662. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6267444/. [CrossRef]
- McClements DJ. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Progress in Lipid Research. 2021 Jan; 81:101081. [CrossRef]
- Schuchardt JP, Hahn A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2013 Jul;89(1):1–8. [CrossRef]
- Li J, Pora BLR, Dong K, Hasjim J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Science & Nutrition. 2021 Jul 23;9(9):5229–43. [CrossRef]
- Snigdha Homroy, Chopra RN, Priyanka Kumari Singh, Dhiman A, Rama S, Talwar B. Role of encapsulation on the bioavailability of omega-3 fatty acids. Comprehensive Reviews in Food Science and Food Safety. 2023 Dec 13;23(1):1–35. [CrossRef]
- C.D. Dacaranhe, Terao J. Effect of Phosphatidic Acid and Phosphatidylserine on Lipid Oxidation in Beef Homogenate During Storage and in Emulsified Sardine Oil. Journal of Food Science. 2001 Apr 1;66(3):422–7. [CrossRef]
- Arab-Tehrany E, Jacquot M, Gaiani C, Imran M, Desobry S, Linder M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends in Food Science & Technology. 2012 May;25(1):24–33. [CrossRef]
- Nair V, Cooper CS, Vietti DE, Turner GA. The chemistry of lipid peroxidation metabolites: Crosslinking reactions of malondialdehyde. Lipids. 1986 Jan;21(1):6–10. [CrossRef]
- Jacobsen C, Hartvigsen K, Lund P, Meyer AS, Jens Adler-Nissen, J. Holstborg, et al. Oxidation in fish-oil-enriched mayonnaise. European Food Research and Technology. 1999 Nov 3;210(1):13–30. [CrossRef]
- Jacobsen C, Hartvigsen K, Lund P, Thomsen M, Skibsted LH, Gunhild Hølmer, et al. Oxidation in fish oil-enriched mayonnaise: 4. Effect of tocopherol concentration on oxidative deterioration. European Food Research and Technology. 2001 Feb 16;212(3):308–18. [CrossRef]
- Omer AM, Ziora ZM, Tamer TM, Khalifa RE, Hassan MA, Mohy-Eldin MS, et al. Formulation of Quaternized Aminated Chitosan Nanoparticles for Efficient Encapsulation and Slow Release of Curcumin. Molecules. 2021 Jan 16;26(2):449. [CrossRef]
- Mohamed NA, H. Abou Saleh, Y. Kameno, I. Marei, G. de Nucci, B. Ahmetaj-Shala, et al. Metal-organic framework (MOF) nanomedicine preparations of sildenafil designed for the future treatment of pulmonary arterial hypertension. bioRxiv (Cold Spring Harbor Laboratory). 2019 Jul 30. [CrossRef]
- Mohamed H, Mohamed N, Macasa S, Basha H, Adan A, Isra Marei, et al. Managing diabetes with nanomedicine: nanoMIL-89 as a promising drug delivery system for metformin. Research Square (Research Square). 2024 Feb 6. [CrossRef]
- Mohamed NA, Marei I, Crovella S, Abou-Saleh H. Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases. International Journal of Molecular Sciences. 2022 Jan 26;23(3):1404. [CrossRef]
- de Jong. Drug delivery and nanoparticles: Applications and hazards. International Journal of Nanomedicine [Internet]. 2008 Jun;3(2):133. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2527668/. [CrossRef]
- R. Preethi, Dutta S, Moses JA, C. Anandharamakrishnan. Green nanomaterials and nanotechnology for the food industry. Elsevier eBooks. 2022 Jan 1;215–56. [CrossRef]
- M. El-Say K, S. El-Sawy H. Polymeric nanoparticles: Promising platform for drug delivery. International Journal of Pharmaceutics. 2017 Aug 7;528(1-2):675–91. Available from: https://www.sciencedirect.com/science/article/pii/S0378517317305604?via=ihub. [CrossRef]
- Kahraman E, Güngör S, Özsoy Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Therapeutic Delivery. 2017 Nov;8(11):967–85. [CrossRef]
- Klinkova A, Thérien-Aubin H. Chapter 6 - Polymer nanoparticles. Klinkova A, Thérien-Aubin H, editors. ScienceDirect. Elsevier ; 2024. p. 167–215. Available from: https://www.sciencedirect.com/science/article/abs/pii/B9780443214479000023.
- Perumal S, Atchudan R, Lee W. A Review of Polymeric Micelles and Their Applications. Polymers [Internet]. 2022 Jun 20;14(12):2510. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9230755/#:~:text=Micelles%20are%20prepared%20by%20various. [CrossRef]
- Wakaskar RR. Polymeric Micelles and their Properties. Journal of Nanomedicine & Nanotechnology. 2017;08(02). [CrossRef]
- Mittal P, Saharan A, Verma R, Altalbawy FMA, Alfaidi MA, Batiha GES, et al. Dendrimers: A New Race of Pharmaceutical Nanocarriers. BioMed Research International. 2021 Feb 16;2021: e8844030. Available from: https://www.hindawi.com/journals/bmri/2021/8844030/. [CrossRef]
- Chis AA, Dobrea C, Morgovan C, Arseniu AM, Rus LL, Butuca A, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020 Sep 1;25(17):3982. [CrossRef]
- Santos A, Veiga F, Figueiras A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials. 2019 Dec 21;13(1):65. [CrossRef]
- Cavalli R, Caputo O, Gasco MR. Solid lipospheres of doxorubicin and idarubicin. International Journal of Pharmaceutics. 1993 Jan;89(1): R9–12. [CrossRef]
- Nguyen TTL, Duong VA. Solid Lipid Nanoparticles. Encyclopedia. 2022 May 18;2(2):952–73. [CrossRef]
- Jha S, K. Sharma P, Malviya R. Liposomal Drug Delivery System for Cancer Therapy: Advancement and Patents. Recent Patents on Drug Delivery & Formulation. 2016 Dec 7;10(3):177–83. [CrossRef]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013 Feb 22;8(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3599573/. [CrossRef]
- Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter [Internet]. 2016 [cited 2019 Nov 3];12(11):2826–41. Available from: https://doylegroup.mit.edu/sites/default/files/documents/Gupta_164_v2.pdf. [CrossRef]
- Bhosale RR, Osmani RA, Ghodake PP, Shaikh SM, Chavan SR. Nanoemulsion: A Review on Novel Profusion in Advanced Drug Delivery. Indian Journal of Pharmaceutical and Biological Research. 2014 Mar 20;2(01). [CrossRef]
- Yeh YC, Creran B, Rotello VM. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale. 2012 Mar 21;4(6):1871–80. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4101904/. [CrossRef]
- Prashant Kesharwani, Ma R, Liang S, Fatima M, Sheikh A, Mohammed, et al. gold nanoparticles and gold nanorods in the landscape of cancer therapy. Molecular Cancer. 2023 Jun 21;22(1). [CrossRef]
- Almatroudi A. Silver nanoparticles: synthesis, characterization and biomedical applications. Open Life Sciences. 2020 Nov 19;15(1):819–39. [CrossRef]
- Ferdous Z, Nemmar A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. International Journal of Molecular Sciences [Internet]. 2020 Mar 30;21(7). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7177798/. [CrossRef]
- Ali A, Zafar H, Zia M, ul Haq I, Phull AR, Ali JS, et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnology, Science and Applications. 2016 Aug;Volume 9:49–67. [CrossRef]
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, et al. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chemical Reviews. 2008 Jun;108(6):2064–110. [CrossRef]
- Attia NF, El-Monaem EMA, El-Aqapa HG, Elashery SEA, Eltaweil AS, El Kady M, et al. Iron oxide nanoparticles and their pharmaceutical applications. Applied Surface Science Advances. 2022 Oct 1; 11:100284. Available from: https://www.sciencedirect.com/science/article/pii/S2666523922000745. [CrossRef]
- Jitendra Kumar Sahoo, Shraban Kumar Sahoo. Applications of magnetic nanocomposites in wastewater treatment. Elsevier eBooks. 2023 Jan 1;47–63. [CrossRef]
- Kok Hoong Leong, Yik Heng Chin, Lan Ching Sim, Tan B, Dai C, Pichiah Saravanan. Physical properties of quantum dots. Elsevier eBooks. 2022 Jan 1;687–709. [CrossRef]
- Drbohlavova J, Adam V, Kizek R, Hubalek J. Quantum Dots — Characterization, Preparation and Usage in Biological Systems. International Journal of Molecular Sciences. 2009 Feb 20;10(2):656–73. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2660652/. [CrossRef]
- Pednekar PP, Godiyal SC, Jadhav KR, Kadam VJ. Chapter 23 - Mesoporous silica nanoparticles: a promising multifunctional drug delivery system [Internet]. Ficai A, Grumezescu AM, editors. ScienceDirect. Elsevier; 2017. p. 593–621. Available from: https://www.sciencedirect.com/science/article/abs/pii/B9780323461443000234.
- Frickenstein AN, Hagood JM, Britten CN, Abbott BS, McNally MW, Vopat CA, et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics. 2021 Apr 17;13(4):570. [CrossRef]
- Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. International Journal of Pharmaceutical Investigation. 2015;5(3):124–33. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4522861/#:~:text=In%20particular%2C%20mesoporous%20silica%20nanoparticles. [CrossRef]
- Serini S, Cassano R, Trombino S, Calviello G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: potential application in cardiovascular and neoplastic diseases. International Journal of Nanomedicine. 2019 Apr;Volume 14:2809–28. [CrossRef]
- Deshpande D, Janero DR, Amiji M. Engineering of an ω-3 polyunsaturated fatty acid-containing nanoemulsion system for combination C6-ceramide and 17β-estradiol delivery and bioactivity in human vascular endothelial and smooth muscle cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2013 Oct;9(7):885–94. [CrossRef]
- Deshpande D, Kethireddy S, Janero DR, Amiji MM. Therapeutic Efficacy of an ω-3-Fatty Acid-Containing 17-β Estradiol Nano-Delivery System against Experimental Atherosclerosis. Zhu X, editor. PLOS ONE. 2016 Feb 3;11(2): e0147337. [CrossRef]
- Sreedhar R, Kumar VS, Bhaskaran Pillai AK, Mangalathillam S. Omega-3 Fatty Acid Based Nanolipid Formulation of Atorvastatin for Treating Hyperlipidemia. Advanced Pharmaceutical Bulletin. 2019 Jun 1;9(2):271–80. [CrossRef]
- Wakil A, Mackenzie G, Diego-Taboada A, Bell JG, Atkin SL. Enhanced Bioavailability of Eicosapentaenoic Acid from Fish Oil After Encapsulation Within Plant Spore Exines as Microcapsules. Lipids. 2010 May 22;45(7):645–9. [CrossRef]
- Sanguansri L, Shen Z, Weerakkody R, Barnes M, Lockett T, Augustin MA. Omega-3 fatty acids in ileal effluent after consuming different foods containing microencapsulated fish oil powder – an ileostomy study. Food Funct. 2013;4(1):74–82. [CrossRef]
- Busolo MA, Torres-Giner S, Prieto C, Lagaron JM. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innovative Food Science & Emerging Technologies. 2019 Jan; 51:12–9. [CrossRef]
- Wang GS, Chen H, Feng G, Yuan Y, Wan Z, Guo J, et al. Polyphenol-Enriched Protein Oleogels as Potential Delivery Systems of Omega-3 Fatty Acids. Journal of Agricultural and Food Chemistry. 2022 Dec 19;71(1):749–59. [CrossRef]
- Yoon G, Park JW, Yoon IS. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. Journal of Pharmaceutical Investigation. 2013 Aug 11;43(5):353–62. [CrossRef]
- Makwana V, Jain R, Patel K, Manish Nivsarkar, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. International Journal of Pharmaceutics. 2015 Nov 10;495(1):439–46. [CrossRef]
- Tran TH, Ramasamy T, Truong DH, Choi HG, Yong CS, Kim JO. Preparation and Characterization of Fenofibrate-Loaded Nanostructured Lipid Carriers for Oral Bioavailability Enhancement. AAPS PharmSciTech. 2014 Jul 18;15(6):1509–15. Available from: https://link.springer.com/article/10.1208/s12249-014-0175-y. [CrossRef]
- Mohamed NA, Abou-Saleh H, Kameno Y, Marei I, de Nucci G, Ahmetaj-Shala B, et al. Studies on metal–organic framework (MOF) nanomedicine preparations of sildenafil for the future treatment of pulmonary arterial hypertension. Scientific Reports. 2021 Feb 22;11(1):4336. Available from: https://www.nature.com/articles/s41598-021-83423-6. [CrossRef]
- Feng Q, Li D, Li Q, Cao X, Dong H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioactive Materials. 2022 Mar 1; 9:105–19. Available from: https://www.sciencedirect.com/science/article/pii/S2452199X21003534. [CrossRef]
- El-Seedi HR, Said NS, Nermeen Yosri, Hamada B.I. Hawash, El-Sherif DM, Abouzid M, et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. 2023 May 1;9(5): e16228–8. [CrossRef]
- Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Reticular synthesis and the design of new materials. Nature. 2003 Jun;423(6941):705–14. [CrossRef]
- Baumann AE, Burns DA, Liu B, Thoi VS. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Communications Chemistry. 2019 Jul 26;2(1):1–14. Available from: https://www.nature.com/articles/s42004-019-0184-6. [CrossRef]
- Hirschle P, Preiß T, Auras F, Pick A, Völkner J, Valdepérez D, et al. Exploration of MOF nanoparticle sizes using various physical characterization methods – is what you measure what you get? CrystEngComm. 2016;18(23):4359–68. [CrossRef]
- Liu L, Zhou Y, Liu S, Xu M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem. 2018 Jan 2;5(1):6–19. [CrossRef]
- Al-Ansari DE, Mohamed NA, Marei I, Zekri A, Kameno Y, Davies RP, et al. Internalization of Metal–Organic Framework Nanoparticles in Human Vascular Cells: Implications for Cardiovascular Disease Therapy. Nanomaterials. 2020 Jun 1;10(6):1028. Available from: https://www.mdpi.com/2079-4991/10/6/1028. [CrossRef]
- Mohamed NA. Metal Organic Frameworks, Their Properties and Future Promises in the Medical Field. qspace.qu.edu.qa. Nova Science Publishers, Inc.; 2021. Available from: https://qspace.qu.edu.qa/handle/10576/48948.
- Xiong F, Chen Y, Chen J, Yang B, Zhang Y, Gao H, et al. Rubik-like magnetic nanoassemblies as an efficient drug multifunctional carrier for cancer theranostics. Journal of Controlled Release. 2013 Dec 1;172(3):993–1001. [CrossRef]
- Chen Y, Feng X. Gold nanoparticles for skin drug delivery. International Journal of Pharmaceutics. 2022 Sep 25; 625:122122. Available from: https://www.sciencedirect.com/science/article/pii/S0378517322006767?via%3Dihub. [CrossRef]
- Gao W, Sun Y, Cai M, Zhao Y, Cao W, Liu Z, et al. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nature Communications. 2018 Jan 15;9(1). [CrossRef]
- Mykola Ya Spivak, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ul'berg Zr. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: a theranostic potential for PPP cardiology. The EPMA Journal. 2013 Jul 29;4(1). [CrossRef]
- Li H, Yan J, Meng D, Cai R, Gao X, Ji Y, et al. Gold Nanorod-Based Nanoplatform Catalyzes Constant NO Generation and Protects from Cardiovascular Injury. ACS Nano. 2020 Sep 21;14(10):12854–65. [CrossRef]
- Bejarano J, Rojas A, Ramírez-Sagredo A, Riveros AL, Morales-Zavala F, Flores Y, et al. Light-induced release of the cardioprotective peptide angiotensin-(1–9) from thermosensitive liposomes with gold nanoclusters. Journal of Controlled Release. 2020 Dec; 328:859–72. [CrossRef]
- Ahmed SM, Abdelrahman SA, Salama AE. Efficacy of gold nanoparticles against isoproterenol induced acute myocardial infarction in adult male albino rats. Ultrastructural Pathology. 2017 Mar;41(2):168–85. [CrossRef]
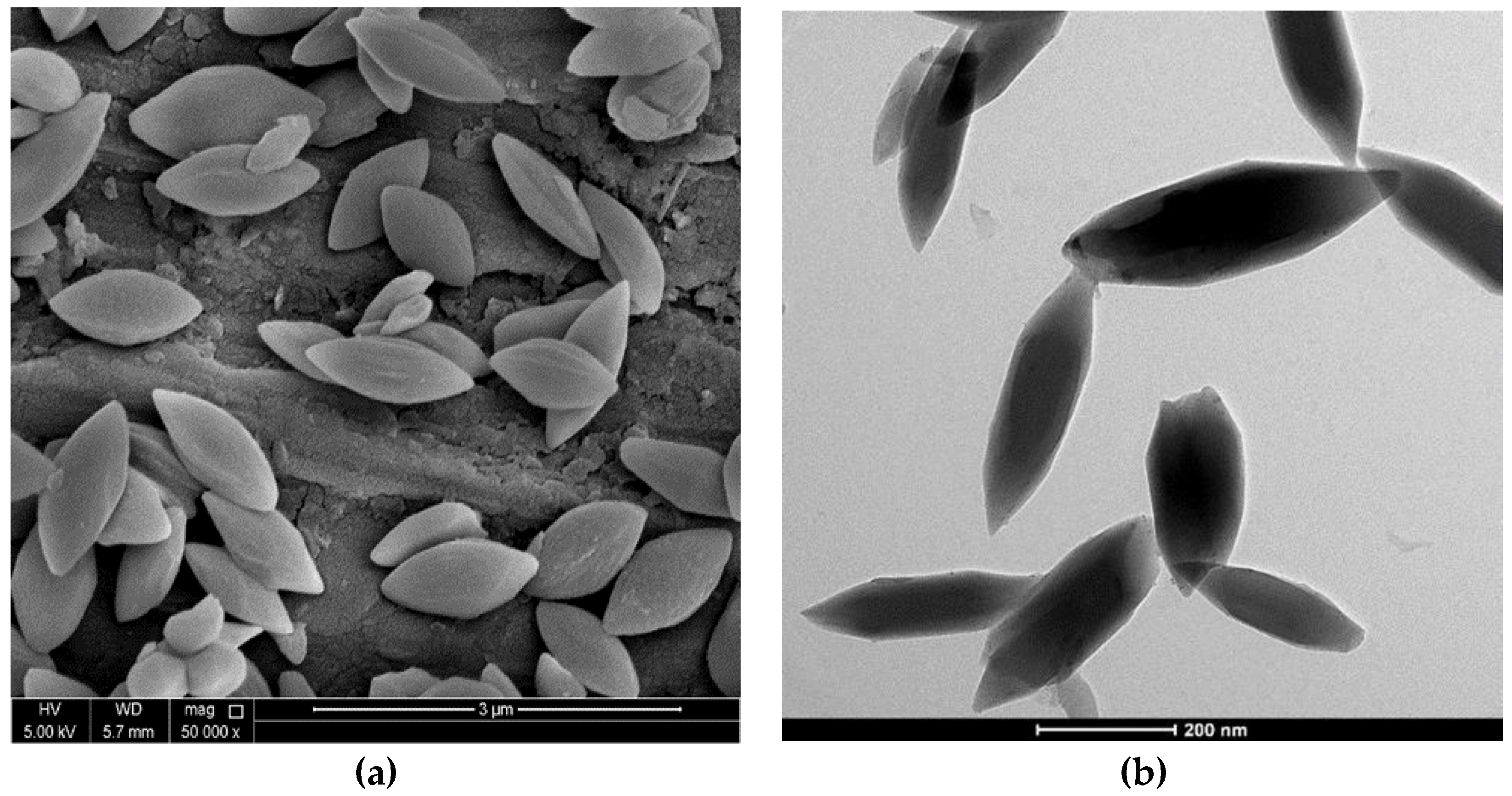
| Experimental unit |
Sample size (n) | Omega-3 FA diet/dose | Treatment period | Outcomes | References |
|---|---|---|---|---|---|
| Marmoset monkey | 29 | 22.8% | 30 months | ↓ in the threshold for fibrillation | [24] |
| Mongrel dogs | 17 | 5 mL (Intravenous) |
1 week | ↓ in ventricular flutter-fibrillation | [25] |
| Mice | 3 | 3% | 6 weeks | ↓ in loss of Connexin43 (Cx43) | [26] |
| Mice | 14 | 2.1% | 6 weeks | ↓ in Endothelin-1 (ET-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) | [27] |
| Mice | 36 | 8% | 2 weeks | ↓ in the expression of the Myosin Heavy Chain 7 (Myh7) gene and Collagen type III alpha 1 chain (Col3a1) | [28] |
| Study name | Sample size (n) | Omega-3 FA dose/day | Follow up period | Outcomes | References |
|---|---|---|---|---|---|
| DART Clinical Trials | 2,033 | 900mg EPA and DHA | 2 years | 32% ↓ reinfarction, 29% ↓ in mortality from all causes. | [29] |
| GISSI-Prevenzione Trials | 11,232 | 850 mg of DHA or EPA | 3.5 years | 28% ↓ in mortality from all causes, and 45% ↓ in sudden cardiac death. | [30] |
| JELIS Clinical Trials | 18,645 | Statin + 1.8 g of EPA | 5 years | 19% ↓ in mortality, revascularization, MI, and angina. | [31] |
| DOIT Clinical Trials | 563 | 2.4 g of ω-3 PUFA | 3 years | ↓ in mortality from all causes. | [32] |
| OMEGA Trial | 3,851 | 460 mg EPA and 380 mg DHA | 1 year | No ↓ in mortality due to unexpected cardiac events. | [33] |
| Alpha Omega Trial | 4837 | EPA (400mg), DHA (400mg), ALA(2g) | 3.3 years | No ↓ in incidence of serious cardiac events. | [34] |
| SU.FOL.OM3 Trial | 2501 | 600mg of ω-3 FAs | 5 years | No ↓ in incidence of serious cardiovascular events. | [35] |
| ASCEND Trial | 15,480 | 380 mg DHA and 460 mg EPA | 2.5 years | No ↓ in major vascular incidents | [36] |
| VITAL Trial | 25,871 | 380 mg DHA and 460 mg EPA | 5.3 years | No ↓ in risk of cardiovascular diseases | [37] |
| REDUCE-IT Trial | 8179 | 4g of icosapent ethyl (EPA ethyl ester) | 4.9 years | ↓ in the incidence of ischemic events. | [38] |
| STRENGTH Trial | 13,078 | 4g omega-3 carboxylic acid formulation (EPA+DHA) per day | 2.6 years | No ↓ in cardiovascular events | [39] |
| OMEMI Trial | 1,027 | 1.8 g ɷ-3 FAs (930 mg EPA + 660 mg DHA) | 2 years | No ↓ in the frequency of cardiovascular events or deaths from all causes | [40] |
| Sample size (n) | Diet (consisting of ɷ-3 FA) | Follow up period | Outcomes | References |
|---|---|---|---|---|
| 8,825 | 1 serving of fish/week | 19 years | No ↓ in risk of (CVDs) cardiovascular disease | [42] |
| 41,578 | 40-60 g ɷ-3 FA /day | 9 years | ↓ incidence of coronary heart disease (CHDs), ↓ in fatal cardiac events | [43] |
| 48,315 | 31.3 g ɷ-3 FA /day | 4 years | No ↓ risk of myocardial infarction (MI) or stroke | [44] |
| 16,479 | > 2 servings of fried fish/week | 4 years | ↑ in the chance of cardiovascular events | [45] |
| 20,969 | > 4 servings of fish/week | 4 years | 40% ↓ in the likelihood of CHD and stroke | [46] |
| 34,033 | 1 serving of fish/week | 18 years | ↓ in risk of ischemic stroke | [47] |
| 197,761 | 1 serving of fish/week | 3.3 years | ↓ in risk of non-lethal ischemic stroke | [48] |
| 4,067 | 200 mg ɷ-3 FA /day | 12 years | 30% ↓ in the possibility of deadly CHD | [49] |
| Sample size (n) | ɷ-3 FA in fish consumption | Study period | Outcomes | References |
|---|---|---|---|---|
| 287 | 1 serving of fish/weekly | 5 years | ↓ the likelihood of cardiovascular diseases (CVDs) | [53] |
| 334 | 5.5g/monthly | 6 years | 50% ↓ in the possibility of first cardiac arrest. | [54] |
| 78 | >1 fish serving/weekly | 18 months | ↓ in risk of myocardial infarction (MI) | [55] |
| 848 | <150 g fish/weekly | 12 months | 38% ↓ in likelihood of developing acute coronary syndrome (ACS) | [56] |
| 108 | 2 servings of fish/weekly | 2 years | ↓ in risk of coronary events | [57] |
| Nanoparticle type | Characteristics | Advantages | Disadvantages | Application/Use | References |
|---|---|---|---|---|---|
 Polymeric NPs (organic) |
Solid particles (colloidal) that range in size from 10 to 1000 nm. Two main types; nanocapsules and nanospheres. | Controlled and sustained drug release, stable, and efficient. biodegradable, good biocompatibility. | Difficult to scale up, lack of toxicological evaluation, can be an environmental hazard and can pose an occupational hazard during production. | Vaccine delivery, cancer treatment, antibiotic delivery, purification of biomolecules and bioimaging | [75,76,77]. |
 Polymeric micelles (organic) |
Spherical, 10 – 100 nm in diameter. Amphiphilic block copolymers produce nanoscopic core/shell structures via covalent bonding. Hydrophobic core, hydrophilic shell. | Highly stable, high loading efficiency, selective and controlled drug release, and kinetically stable. | Low solubility, low loading capacity, low stability in vivo, and can dissociate in vivo. | Cancer treatment, food-based technology, drug delivery, photodynamic therapy, and gene delivery. | [78,79,80]. |
 Dendrimer NPs (organic) |
Spherical, compact, 1 – 15 nm in diameter. Comprises a central core atom, followed by repeating branching subunits and terminal groups. | High loading capacity, high bioavailability, high penetrability, high symmetry, and surface groups can be customized easily. | Low water solubility, high nonspecific toxicity, challenging to separate the NPs from the reactants, and time consuming. | Biomedical applications, targeted delivery, cancer treatment, cancer diagnosis, antibacterial therapy. | [81,82,83]. |
 Solid lipid NPs (organic) |
Spherical shape, diameter ranging from 50nm - 1μm, big surface area, substantial drug loading capacity, and surfactant on the outer layer. | Controlled and/or targeted medication release, optimized drug stability, higher and improved drug content, and non-toxic. | Drug ejection upon polymeric transformation during storage and high moisture content of the dispersions. | Gene vector transporter, topical drug application, cosmetics, agricultural usage, anticancer medication carrier. | [84,85]. |
 Liposomes (organic) |
Spherical lipid vesicles 50-500 nm in diameter comprised of several lipid bilayers formed by the emulsification of real or artificial lipids in water-based solutions | Improved effectiveness, improved therapeutic value of drugs, improved stability by encapsulation, not toxic, adaptive, and biocompatible. | Poor solubility, transient half-life, oxidation, hydrolysis, leak, coagulation of enclosed molecules and expensive manufacturing. | Anticancer drug delivery, antifungal drug delivery, analgesic delivery, COVID–19 mRNA vaccines, photodynamic therapy. | [86,87]. |
 Nanoemulsion (organic) |
Spherical, 20-500 nm diameter, 10-20% polydispersity, unstable thermodynamically, stable kinetically. | Large surface area, high free energy, manufactured in an array of formulations, not toxic, and non-irritant. | Stabilization requires a high concentration of surfactant, and stability is regulated by pH and temperature. | Cosmetics, food, pharmaceuticals, drug delivery, vaccine delivery, material synthesis, and encapsulation of natural food preservatives | [88,89]. |
 Gold NP (inorganic) |
Spherical, 10-100 nm in diameter, colored orange, brown, red, or purple, and absorbs between 500-550 nm. | High surface area to volume proportion, very stable, good biocompatibility, customizable, steady size and shape. | Gold NPs can be toxic at large doses. Gold NPs entrapped in the liver might impair its function and costly manufacturing. | Imaging, electronic gadgets, material production, colorimetric and electrochemical sensing, drug delivery, and cancer diagnosis. | [90,91]. |
 Silver NP (organic) |
Various shapes (spherical, triangular, hexagonal, octagonal, etc.), 1-100 nm diameter, small size crystalline, high heat conductivity, and high electric conductivity | High surface area, bactericidal, catalytic features, fungicidal, not toxic, anticancer properties, very stable, and high solubility | Limited resolution, numerous light scatterings, sedimentation, and high energy required in preparation | Disease diagnosis, agriculture, cosmetics biotechnology, wound dressing, textile industry, and antiseptic reagents. | [92,93]. |
 Iron oxide NP (inorganic) |
Various shapes (spherical, cubes, hexagonal, rods, etc.), superparamagnetic, 10-20 nm in diameter or less, different forms such as hematite, magnetite, maghemite | High surface area to volume proportion, inexpensive, low toxicity, high binding capability, substantial dispersibility, not toxic | Highly reactive, agglomerate, surface oxidation, absence of functional groups, reduced capacity to adsorb molecules, slow kinetics, leach in low pH | Biomedical, magnetic resonance imaging diagnosis, drug delivery, antibody and vaccine manufacture, gene therapy, cancer therapy, sensory probes | [94,95,96,97]. |
 Quantum dots (inorganic) |
Various shapes (spherical, cuboidal, conical, etc.), 2-20 nm in diameter, metallic or semi-conductors, can be zero, one, two, or three dimensional, nanocrystals, and have 100 – 10000 atoms and <100 electrons. | Customizable morphology, great biocompatibility, high ability to disperse, magnetic, and great optical features. | Toxic, lacks significant polarization, water insolubility, and needs strong polymer casing. | Photocatalysis, biosensing, bioimaging in vivo and in vitro, optoelectrical gadgets, and microscopy. | [98,99]. |
 Mesoporous NP (inorganic) |
Spherical or rod-shaped, 30-300 nm in diameter, majorly made up of silicone, highly structured pores, stable porous matrix, 5 different types of nanocomposites | Low toxicity, high biocompatibility, large surface area, big pore volume, heat stable, chemically stable, customizable pore size | Mild toxicity, silanol moieties on the surface can interact with the outermost layer of red blood cell membrane phospholipids causing hemolysis and induction of metabolic alterations promoting melanoma | Cancer treatment, biosensing, bioimaging, targeted illness treatment, radiotherapy, chemotherapy, dynamic therapy, thermal therapy, Immune therapy, gene therapy, | [100,101,102]. |
| Omega-3 dose and source | Nanoparticle type | Production Technique | Physiochemical characteristics | Effect | References |
|---|---|---|---|---|---|
| Flax seed oil 20%, w/v | Nanoemulsion | Microfluidization | Average diameter =146 nm Surface charge =34 mV Encapsulation efficiency = 93% and 99%% |
Strong anti-proliferative impact on vascular smooth muscle cells | [104] |
| Flaxseed oil 20%, w/v | Nanoemulsion | Microfluidization | Average diameter = 187 ± 7.5 nm and 176 ± 4.8 nm Surface charge = (-54.6 ± 4.1 mV and -56.4 ± 5.1 mV Encapsulation efficiency = 94.6% |
Improved acute vascular damage with only 30% arterial stenosis | [105] |
| Omega-3 FA | Atorvastatin-loaded nano lipid carrier | Melt emulsification and ultrasonication method | Particle size = 87.29 ± 6.68 nm Surface charge = -36.03 ± 1.50 mV Encapsulation efficiency = 86.70 % ± 0.15 |
Improving Omega-3 FA bioavailability and antihyperlipidemic action | [106] |
| 85 wt.% Docosahexaenoic acid-supplemented fish oil | Nanofiber | Electrospraying assisted by pressurized gas technology (EAPG). | Average particle size = 3.7 ± 1.8 μm Encapsulation efficiency = 84 % |
Supplemented reconstituted milk with zein/DHA-enriched fish oil microcapsules showed no signs of oxidation even after 45 days. | [109] |
| 85 wt % DHA enriched algal oil | Oleogel based microgel | Ball milling | Whey protein microgel particle size = 250 nm Polydispersity index = 0.29 Diameter = 380 nm |
Protein microgels addressed various obstacles in the development of omega-3 polyunsaturated fatty acid oils, such as long-term oxidative resistance and better sensory and textural qualities. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





