Submitted:
28 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
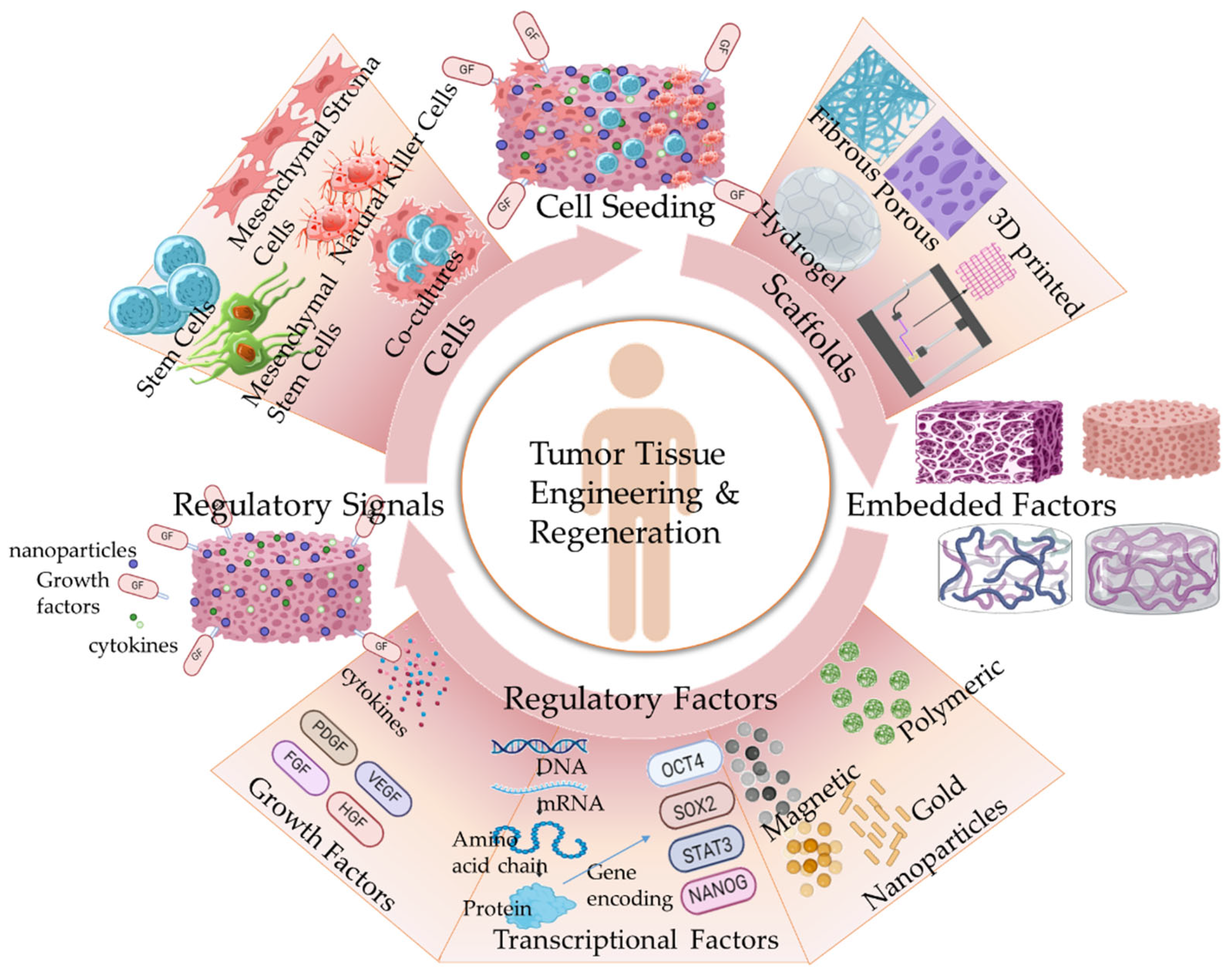
1. Introduction
2. Tissue Engineering in Cancer Therapeutics: Regenerative Medicine
2.1. Breast Cancer
2.2. Bone Cancer
2.3. Other Cancer Types
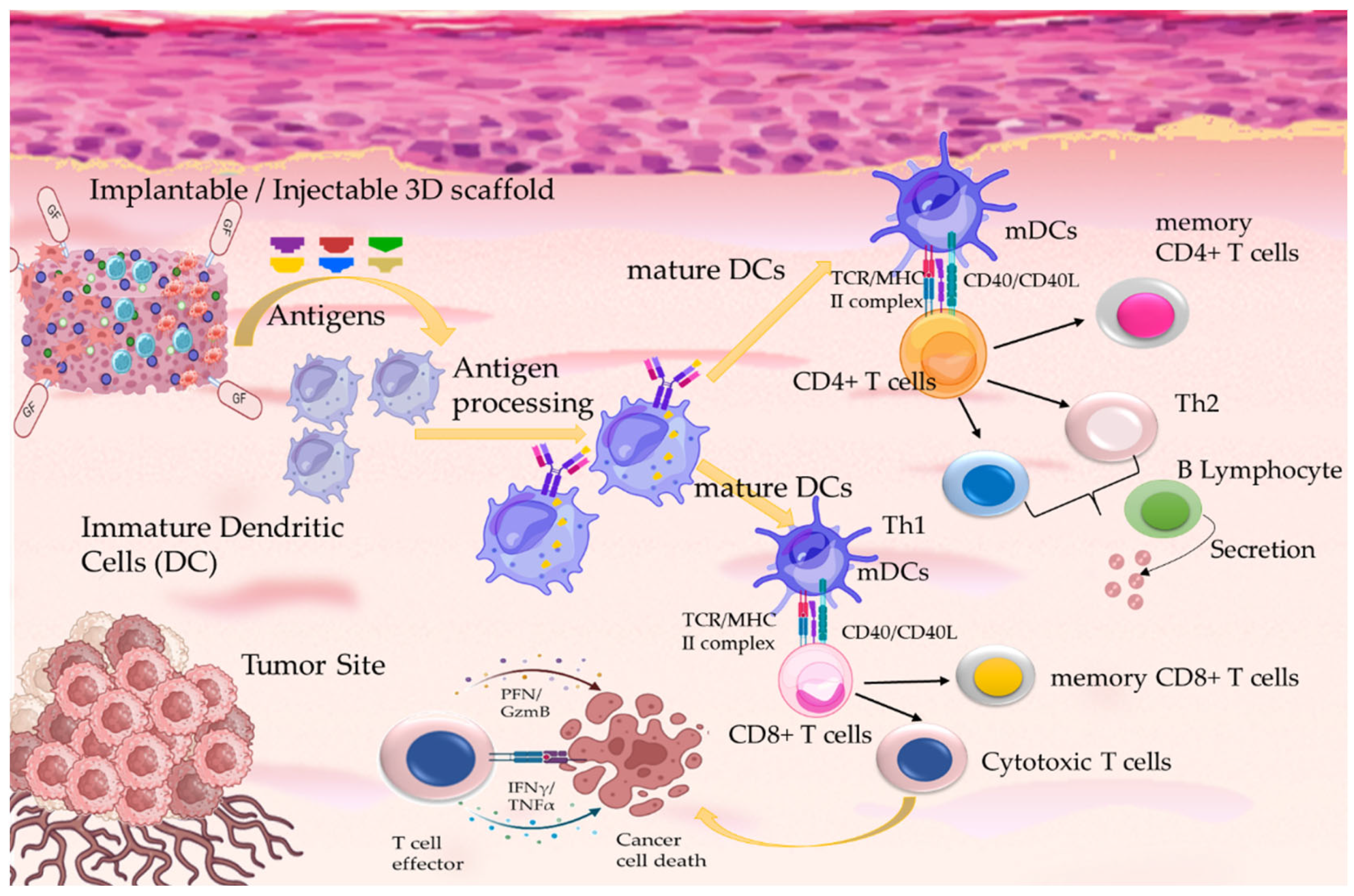
3. Tissue Engineering in Cancer Therapeutics: Immunotherapy
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res Let 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajączkowsk, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch Med Sci 2018, 14, 910. [Google Scholar] [CrossRef]
- Raja, I.S.; Kang, M.S.; Kim, K.S.; Jung, Y.J.; Han, D.-W. Two-Dimensional Theranostic Nanomaterials in Cancer Treatment: State of the Art and Perspectives. Cancers 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front Cell Dev Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends in Immunology. 2020, 41, 652. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics. 2020, 12, 630. [Google Scholar] [CrossRef]
- Singh, A.K.; Malviya, R.; Prajapati, B.; Singh, S.; Goyal, P. Utilization of Stimuli-Responsive Biomaterials in the Formulation of Cancer Vaccines. J Funct Biomater. 2023, 15, 247. [Google Scholar] [CrossRef]
- Ruan, S.; Huang, Y.; He, M.; Gao, H. Advanced Biomaterials for Cell-Specific Modulation and Restore of Cancer Immunotherapy. Adv. Sci. 2022, 9, 2200027. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: a review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front Bioeng Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Claridge, S.E.; Cavallo, J.-A.; Hopkins, B.D. Patient-Derived In Vitro and In Vivo Models of Cancer. Adv Exp Med Biol. 2022, 1361, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, Q.; Ju, Y.; Song, G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020, 17, 282. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Mechanobiology of solid tumors. BBA—Molecular Basis of Disease. 2022, 1868, 166555. [Google Scholar] [CrossRef] [PubMed]
- Avgoustakis, K.; Angelopoulou, A. Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics 2024, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Katti, A.; Diaz, B.J.; Caragine, C.M.; Sanjana, N.E.; Dow, L.E. CRISPR in cancer biology and therapy. Nature Reviews Cancer. 2022, 22, 259. [Google Scholar] [CrossRef] [PubMed]
- Kinker, G.S.; Greenwald, A.C.; Tal, R.; et al. Pan-cancer single-cell RNA-seq identifies recurring programs of cellular heterogeneity. Nat Genet 2020, 52, 1208. [Google Scholar] [CrossRef]
- Girard, Y.K.; Wang, C.; Ravi, S.; Howell, M.C.; Mallela, J.; Alibrahim, M.; Green, R.; Hellermann, G.; Mohapatra, S.S.; Mohapatra, S. A 3D fibrous scaffold inducing tumoroids: a platform for anticancer drug development. PLoS One. 2013, 8, e75345. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Yang, X.; Xiao, Z.; Tong, X.; Tuerdi, A.; Li, S.; Lei, L. Flourishing tumor organoids: History, emerging technology, and application. Bioeng Transl Med. 2023, 8, e10559. [Google Scholar] [CrossRef]
- Unnikrishnan, Κ.; Velutheril Thomas, L.; Kumar, R.M.R. Advancement of Scaffold-Based 3D Cellular Models in Cancer Tissue Engineering: An Update. Front Oncol. 2021, 11, 733652. [Google Scholar] [CrossRef]
- Lv, J.; Du, X.; Wang, M.; Su, J.; Wei, Y.; Xu, C. Construction of tumor organoids and their application to cancer research and therapy. Theranostics. 2024, 14, 1101. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.-C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Marques, J.R.O.F.; Gonzalez-Alva, P.; Lin, R.Y.-T.; Fernandes, B.F.; Chaurasia, A.; Dubey, N. Advances in tissue engineering of cancer microenvironment-from three-dimensional culture to three-dimensional printing. SLAS Technology. 2023, 28, 152. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Vunjak-Novakovic, G. Tissue-engineered models of human tumors for cancer research. Expert Opin Drug Discov. 2015, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Banobre-Lopez, M.; Minas, G.; Teixeira, S.F.C.F.; Lima, R.; Rodrigues, R.O. The integration of spheroids and organoids into organ-on-a-chip platforms for tumour research: A review. Bioprinting. 2022, 27, e00224. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Mariano, C.; Shen, K. Engineered in vitro tumor models for cell-based immunotherapy. Acta Biomater. 2021, 132, 345. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achacheloue, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthes, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Le, D.; Movsas, R.; Gonsalves, A.; Menon, J.U. Porous Polymeric Microspheres With Controllable Pore Diameters for Tissue Engineered Lung Tumor Model Development. Front. Bioeng. Biotechnol. 2020, 8, 799. [Google Scholar] [CrossRef]
- Eslami, H.; Azimi Lisar, H.; Kashi, T.S.J.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Abbas, F.M.; Tayebi, L. Poly(lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals. 2018, 53, 51. [Google Scholar] [CrossRef]
- Mansouri, V.; Beheshtizadeh, N.; Gharibshahian, M.; Sabouri, L.; Varzandeh, M.; Rezaei, N. Recent advances in regenerative medicine strategies for cancer treatment. Biomed. Pharmacother. 2021, 141, 111875. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural killer cells in cancer biology and therapy. Molecular Cancer. 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Katti, P.D.; Jasuja, H. Current Advances in the Use of Tissue Engineering for Cancer Metastasis Therapeutics. Polymers 2024, 16, 617. [Google Scholar] [CrossRef] [PubMed]
- Calamak, S.; Ermis, M.; Sun, H.; Islam, S.; Sikora, M.; Nguyen, M.; Hasiri, V.; Steinmetz, L.M.; Demirci, U. A Circulating Bioreactor Reprograms Cancer Cells Toward a More Mesenchymal Niche. Adv. Biosys. 2020, 4, 1900139. [Google Scholar] [CrossRef] [PubMed]
- Guller, A.E.; Grebenyuk, P.N.; Shekhter, A.B.; Zvyagin, A.V.; Deyev, S.M. Bioreactor-Based Tumor Tissue Engineering. Acta Naturae. 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Khaboushan, A.S.; Neishabouri, A.; Rezaei, N. Tissue Engineering and Regenerative Medicine in Cancer Therapy. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer: Cham, 2023. [Google Scholar] [CrossRef]
- Westwood, L.; Nixon, I.J.; Emmerson, E.; Callanan, A. The road after cancer: biomaterials and tissue engineering approaches to mediate the tumor microenvironment postcancer treatment. Front. Front. Biomater. Sci. 2024, 3, 1347324. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthes, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 2, 92. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics. 2022, 14, 1127. [Google Scholar] [CrossRef]
- Aldhaher, A.; Shahabipour, F.; Shaito, A.; Al-Assaf, S.; Elnour, A.A.M.; Sallam, E.B.; Teimourtash, S.; Elfadil, A.A. 3D hydrogel/ bioactive glass scaffolds in bone tissue engineering: Status and future opportunities. Heliyon. 2023, 9, e17050. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Bahadur, A.; Iqbal, S.; Al-Anazy, M.M.; LAref, A.; Tahir, M.A.; Channar, P.A.; Noreen, S.; YAsir, M.; Iqbal, A.; Ali, K.W. Magnesium doped mesoporous bioactive glass nanoparticles: A promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells. J. Alloys Comp. 2021, 866, 159013. [Google Scholar] [CrossRef]
- Zhu, H.; Monavari, M.; Zheng, K.; Dostler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small. 2022, 18, 2104996. [Google Scholar] [CrossRef] [PubMed]
- Matic, A.; Sher, E.K.; Farhat, E.K.; Sher, F. Nanostructured Materials for Drug Delivery and Tissue Engineering Applications. Mol Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Praglowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matysek, D. 3D Hierarchical, Nanostructured Chitosan/PLA/HA Scaffolds Doped with TiO2/Au/Pt NPs with Tunable Properties for Guided Bone Tissue Engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Kawazoe, N.; Chen, G. Composite scaffolds of gelatin and gold nanoparticles with tunable size and shape for photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Tencomnao, T.; Apijaraskul, A.; Rakkhithawatthana, V.; Chaleawlert-umpon, S.; Pimpa, N.; Sajomsang, W.; Saengkrit, N. Gold/cationic polymer nano-scaffolds mediated transfection for non-viral gene delivery system. Carbohyd. Pol. 2011, 84, 216. [Google Scholar] [CrossRef]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010, 132, 1517. [Google Scholar] [CrossRef]
- del Mar Encabo-Berzosa, M.; Sancho-Albero, M.; Crespo, A.; Andreu, V.; Sebastian, V.; Irusta, S.; Arruebo, M.; Martin-Duque, P.; Santamaria, J. The effect of PEGylated hollow gold nanoparticles on stem cell migration: potential application in tissue regeneration. Nanoscale. 2017, 9, 9848. [Google Scholar] [CrossRef]
- Raghav, P.K.; Mann, Z.; Ahlawat, S.; Mohanty, S. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Pharmacology. 2022, 918, 174657. [Google Scholar] [CrossRef]
- Brown, P.T.; Handorf, A.M.; Jeon, W.B.; Li, W.-J. Stem Cell-based Tissue Engineering Approaches for Musculoskeletal Regeneration. Curr Pharm Des. 2013, 19, 3429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cheng, Y.; Wang, J.; Chen, H.; Wang, X.; Li, X.; Tan, W.; Tan, Z. 3D printed intelligent scaffold prevents recurrence and distal metastasis of breast cancer. Theranostics. 2020, 10, 10652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Kuang, G.; Yu, Y.; Zhao, Y. Photopolymerized 3D Printing Scaffolds with Pt(IV) Prodrug Initiator for Postsurgical Tumor Treatment. Research, 2022; 2022, 1–13. [Google Scholar] [CrossRef]
- Guiro, K.; Patel, S.A.; Greco, S.J.; Rameshwar, P.; Arinzeh, T.L. Investigating Breast Cancer Cell Behavior Using Tissue Engineering Scaffolds. PLoS ONE 2015, 10, e0118724. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Ferdowsi, S.; Ebrahimi-Barough, S.; Kamian, S.; Ai, J.J. Med. Eng. Technol. 2020, 44, 162. [CrossRef] [PubMed]
- Rad, R.A.; Naghdi, Y.; Jamalabadi, M.M.; Masoumi, S.; Rezakhani, L.; Alizadeh, M. Breast Cancer: Basic Clin. Res. 2024, 18, 1. [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; Minami, H. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, B.L.; Gagliardi, M.; Iles, L.; Evans, K.; Ivan, C.; Liu, X.; Liu, C.-G.; Souza, G.; Rao, A.; Meric-Bernstam, F.; Ueno, N.T.; Bartholomeusz, G.A. Clinically relevant inflammatory breast cancer patient-derived xenograft–derived ex vivo model for evaluation of tumor-specific therapies. PLoS ONE. 2018, 13, e0195932. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016, 7, 45745. [Google Scholar] [CrossRef]
- Gangadhara, S.; Smith, C.; Barrett-Lee, P.; Hiscox, S. 3D culture of Her2+ breast cancer cells promotes AKT to MAPK switching and a loss of therapeutic response. BMC Cancer. 2016, 16, 345. [Google Scholar] [CrossRef]
- Uematsu, N.; Zhao, Y.; Kiyomi, A.; O Yuan, B.; Onda, K.; Tanaka, S.; Sugiyama, K.; Sugiura, M.; Takagi, N.; Hayakawa, A.; Hirano, T. Chemo-sensitivity of Two-dimensional Monolayer and Three-dimensional Spheroid of Breast Cancer MCF-7 Cells to Daunorubicin, Docetaxel, and Arsenic Disulfide. Anticancer Res. 2018, 38, 2101. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Com. 2020, 533, 268. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.; Liu, Y.; Cui, Z.; Zhang, T.; Li, Z.; Ma, W. Cancer cells growing on perfused 3D collagen model produced higher reactive oxygen species level and were more resistant to cisplatin compared to the 2D model. J. Applied Biomat. Funct. Mat. 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.P.; Gopsill, J.A.; Gomm, J.J.; Jones, J.L.; Grose, R.P. A 3D in vitro model of the human breast duct: a method to unravel myoepithelial-luminal in- teractions in the progression of breast cancer. Breast Cancer Res 2017, 19, 50. [Google Scholar] [CrossRef]
- Fisher, S.A.; Tam, R.Y.; Fokina, A.; Mohsen Mahmoodi, M.; Distefano, M.D.; Shoichet, M.S. Photo-immobilized EGF chemical gradients differentially impact breast cancer cell invasion and drug response in defined 3D hydrogels. Biomaterials. 2018, 178, 751. [Google Scholar] [CrossRef]
- Xiong, G.; Luo, H.; Zhu, Y.; Raman, S.; Wan, Y. Creation of macropores in three-dimensional bacterial cellulose scaffold for potential cancer cell culture. Carbohydr. Polym. 2014, 114, 553. [Google Scholar] [CrossRef]
- Yue, X.; Nguyen, T.D.; Zellmer, V.; Zhang, S.; Zorlutuna, P. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions. Biomaterials. 2018, 170, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Qiao, X.; Lin, T.; Wang, Y.; Wang, M. Biomaterial-based strategy for bone tumor therapy and bone defect regeneration: An innovative application option. Front. Mater. 2022, 9, 990931. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomat. 2018, 80, 1. [Google Scholar] [CrossRef] [PubMed]
- Marew, T.; Birhanu, G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regener. Ther. 2021, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16. [Google Scholar] [CrossRef]
- Awad, H.A.; O’Keefe, R.J.; Lee, C.H.; Mao, J.J. Chapter 83—Bone Tissue Engineering: Clinical Challenges and Emergent Advances in Orthopedic and Craniofacial Surgery. In Principles of Tissue Engineering, 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Publisher: Academic Press, 2014; pp. 1733–1743. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.; Zhang, T.; Zhang, Q.; Chen, Y.; Xie, Y.; Lei, J.; Cai, L. Biphasic Injectable Bone Cement with Fe3O4/GO Nanocomposites for the Minimally Invasive Treatment of Tumor-Induced Bone Destruction. ACS Biomater. Sci. Eng. 2019, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Huang, K.; Li, Y.; Liu, Y.; Yu, H.; Iv, Z.; Zou, R.; Yao, Q. Mesoporous porphyrinic metal-organic framework nanoparticles/3D nanofibrous scaffold as a versatile platform for bone tumor therapy. Mater. Today Chem. 2022, 24, 100829. [Google Scholar] [CrossRef]
- Yuan, J.; Ye, Z.; Zeng, Y.; Pan, Z.; Feng, Z.Z.; Bao, Y.; Li, Y.; Liu, X.; He, Y.; Feng, Q. Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials. Mater. Today Bio. 2022, 15, 100318. [Google Scholar] [CrossRef]
- Blackburn, G.; Scott, T.G.; Bayer, I.S.; Chosh, A.; Biris, A.S.; Biswas, A. Bionanomaterials for bone tumor engineering and tumor destruction. J. Mater. Chem. B. 2013, 1, 1519. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Mohammadi-Aghdam, M.; Saber-Samandari, S. A novel magnetic bifunctional nanocomposite scaffold for photothermal therapy and tissue engineering. Int. J. Biol. Macromol. 2019, 138, 810. [Google Scholar] [CrossRef]
- Jasemi, A.; Moghadas, B.K.; Khandan, A.; Saber-Samanandari, S. A porous calcium-zirconia scaffolds composed of magnetic nanoparticles for bone cancer treatment: Fabrication, characterization and FEM analysis. Ceram. Int. 2022, 48, 1314. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Func. Mater. 2019, 30, 1907071. [Google Scholar] [CrossRef]
- Lu, J.-W.; Yang, F.; Ke, Q.-F.; Xie, X.-T.; Guo, Y.-P. Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomed.: Nanotech. Biol. Med. (NBM) 2018, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; MA, B.; Huan, Z.; Lin, R.; Wang, X.; Li, T.; Wu, J.; Ma, N.; Zhu, H.; Chang, J.; Wu, C. LaB6 surface chemistry-reinforced scaffolds for treating bone tumors and bone defects. Applied Mater. Today. 2019, 16, 42. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioactive Mater. 2021, 6, 2221. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; Liu, X.; Wu, S.; Yang, C. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today. 2020, 36, 48. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Zhao, Y.; Bai, L.; He, Z.; Tong, Q.; Xie, X.; Zhu, H.; Cai, D.; Zhou, Y.; Lu, B.; Wei, Y.; Mei, L.; Xie, D.; Wang, M. Biofabrication, 2020; 12, 035004. [CrossRef]
- Safarulla, S.; Khillar, P.S.; Kini, S.; Jaiswal, A.M. Tissue engineered scaffolds as 3D models for prostate cancer metastasis to bone. Mater. Today Com. 2021, 28, 102641. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Guo, J.; Tierney, E.G.; Curtin, C.M.; Malhotra, M.; Darcy, R.; O’Brien, F.J.; O’Driscoll, C.M. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomater. 2015, 66, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Florczyk, S.J.; Stephen, Z.R.; Zhang, M. 3D Porous Chitosan−Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules. 2015, 16, 3362. [Google Scholar] [CrossRef]
- Dozzo, A.; Chullipalliyalil, K.; McAuliffe, M.; O’Driscoll, C.M.; Ryan, K.B. Nano-Hydroxyapatite/PLGA Mixed Scaffolds as a Tool for Drug Development and to Study Metastatic Prostate Cancer in the Bone. Pharmaceutics 2023, 15, 242. [Google Scholar] [CrossRef]
- Ditto, M.; Jacho, D.; Eisenmann, K.M.; Yildirim-Ayan, E. Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix. Bioengineering 2023, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Copland, J.A.; Chaakrabarti, R.; Florczyk, S.J. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials. 2019, 217, 119311. [Google Scholar] [CrossRef]
- Long, T.J.; Spenger, C.C.; Plymate, S.R.; Ratner, B.D. Prostate cancer xenografts engineered from 3D precision-porous poly(2-hydroxyethyl methacrylate) hydrogels as models for tumorigenesis and dormancy escape. Biomaterials. 2014, 35, 8164. [Google Scholar] [CrossRef]
- Qiao, T.; Yang, W.; He, X.; Song, P.; Chen, X.; Liu, R.; Xiao, J.; Yang, X.; Li, M.; Gao, Y.; Chen, G.; Lu, Y.; Zhang, J.; Leng, J.; Ren, H. Dynamic differentiation of F4/80+ tumor-associated macrophage and its role in tumor vascularization in a syngeneic mouse model of colorectal liver metastasis. Cell Death Dis. 2023, 14, 117. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Lister, N.L.; Hashimoto, K.; Teng, L.; Flandes-Iparraguirre, M.; Eder, A.; Sanchez-Herrero, A.; Niranjan, B.; et al. Tissue engineered human prostate microtissues reveal key role of mast cell derived tryptase in potentiating cancer-associated fibroblast (CAF)-induced morphometric transition in vitro. Biomaterials 2019, 197, 72. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, Q.; Kuang, G.; Fan, Q.; Ye, F. Construction and application of liver cancer models in vitro. Engineered Regeneration. 2022, 3, 310. [Google Scholar] [CrossRef]
- Sharifi, F.; Yesil-Celiktas, O.; Kazan, A.; Maharjan, S.; Saghazaddeh, S.; Firoozbakhsh, K.; Firoozabadi, B.; Zhang, Y.S. A hepatocellular carcinoma–bone metastasis-on-a-chip model for studying thymoquinone-loaded anticancer nanoparticles. Bio-Design and Manufacturing. 2020, 3, 189. [Google Scholar] [CrossRef]
- Gupta, P.; Perez-Mancera, P.A.; Kocher, H.; Nisbet, A.; Schettino, G.; Velliou, E.G. A Novel Scaffold-Based Hybrid Multicellular Model for Pancreatic Ductal Adenocarcinoma—Toward a Better Mimicry of the in vivo Tumor Microenvironment. Front. Bioeng. Biotechnol. 2020, 8, 290. [Google Scholar] [CrossRef]
- de la Pena, D.O.; Trabulo, S.M.D.; Collin, E.; Liu, Y.; Sharma, S.; Tatari, M.; Bahrens, D.; Erkan, M.; Lawlor, R.T.; Scarpa, A.; Heeschen, C.; Mata, A.; Loessner, D. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nature Communications. 2021, 12, 5623. [Google Scholar] [CrossRef]
- Sensi, F.; D’Angelo, E.; D’Aronco, S.; Molinaro, R.; Agostini, M. Preclinical three-dimensional colorectal cancer model: The next generation of in vitro drug efficacy evaluation. J. Cell Physiol. 2018, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, Y.; Wang, X.; Wang, J.; Shi, X.; Li, X.; Tan, W.; Tan, Z. 3D printed in vitro tumor tissue model of colorectal cancer. Theranostics. 2020, 10, 12127. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, S.K.; DeHaan, R.K.; Milelr, P.G.; Bose, S.; Shen, X.; Shuler, M.L.; Huang, E.H. A Tissue Engineering Approach to Metastatic Colon Cancer. iScience. 2020, 19, 101719. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Natarajan, D.; Sensi, F.; Ajayi, O.; Fassan, M.; Mammano, E.; Pilati, P.; Pavan, P.; Bresolin, S.; Preziosi, M.; et al. Patient-Derived Scaffolds of Colorectal Cancer Metastases as an Organotypic 3D Model of the Liver Metastatic Microenvironment. Cancers 2020, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, L.; Park, K.S.; Li, Q.; Lowenstein, P.R.; Castro, M.G.; Schwendeman, A.; Moon, J.J. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 2019, 3, 768. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Mariano, C.; Shen, K. Engineered in vitro tumor models for cell-based immunotherapy. Acta Biomater. 2021, 132, 345. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C. Neoantigens: promising targets for cancer therapy. Sig. Transduct. Target Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Bodas, D. Knockout cancer by nano-delivered immunotherapy using perfusion-aided scaffold-based tumor-on-a-chip. Nanotheranostics 2024, 8, 380. [Google Scholar] [CrossRef]
- Han, S.; Wu, J. Three-dimensional (3D) scaffolds as powerful weapons for tumor immunotherapy. Bioactive Mater. 2022, 17, 300. [Google Scholar] [CrossRef]
- Yu, S.; Wang, C.; Yu, J.; Wang, J.; Lu, Y.; Zhang, Y.; Zhang, X.; Hu, Q.; Sun, W.; et al. Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade. Adv. Mater. 2018, 1801527, 1. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; Deutsch, B.C.; et al. Organoid modeling of the tumor immune microenvi- ronment. Cell 2018, 175, 1972. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yu, L.; Yao, H.; Chen, Y.; Hao, Y. Combinatorial Photothermal 3D-Printing Scaffold and Checkpoint Blockade Inhibits Growth/Metastasis of Breast Cancer to Bone and Accelerates Osteogenesis. Adv. Funct. Mater. 2020, 2006214, 1. [Google Scholar] [CrossRef]
- Atik, A.F.; Suryadevara, C.M.; Schweller, R,M; West, J.L.; Healy, P.; Herndon II, J.E.; Congdon, K.L.; et al. Hyaluronic acid based low viscosity hydrogel as a novel carrier for Convection Enhanced Delivery of CAR T cells. J. Clin. Neurosci.. 2018, 56, 163. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Dong, X.; Bai, Y.; Sheng, S.; Zhang, Y.; Liu, T.; Zhu, D.; Lv, F. Doxorubicin/CpG self-assembled nanoparticles prodrug and dendritic cells co-laden hydrogel for cancer chemo-assisted immunotherapy. Chem. Eng. J. 2021, 416, 129192. [Google Scholar] [CrossRef]
- Elashiry, M.; Elsayed, R.; Cutler, C.W. Exogenous and Endogenous Dendritic Cell-Derived Exosomes: Lessons Learned for Immunotherapy and Disease Pathogenesis. Cells 2022, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner. Bioactive Mater. 2021, 6, 3036. [Google Scholar] [CrossRef]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; et al. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Scientific Reports. 2020, 10, 22294. [Google Scholar] [CrossRef] [PubMed]
- Mencia Castano, I.; Raftery, R.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Duffy, G.P.; O’Brien, F.J.; Curtin, C.M. Rapid bone repair with the recruitment of CD206 + M2-like macrophages using non-viral scaffold-me diate d miR-133a inhibition of host cells. Acta Biomater. 2020, 109, 267. [Google Scholar] [CrossRef]
- Brock, C.K.; Hebert, K.L.; Artiles, M.; Wright, M.K.; Cheng, T.; Windsor, G.O.; Nguyen, K.; Alzoubi, M.S.; Collins-Burow, B.M.; Martin, E.C.; Lau, F.H.; Bunnell, B.A.; Burow, M.E. A Role for Adipocytes and Adipose Stem Cells in the Breast Tumor Microenvironment and Regenerative. Medicine Front. Physiol. 2021, 12, 751239. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liew, S.S.; Wang, J.; Pu, K. Bioinspired and Biomimetic Delivery Platforms for Cancer Vaccines. Adv. Mater. 2021, 34, 2103790. [Google Scholar] [CrossRef] [PubMed]
- Staros, R.; Michalak, A.; Rusinek, K.; Mucha, K.; Pojda, Z.; Zagożdżon, R. Perspectives for 3D-Bioprinting in Modeling of Tumor Immune Evasion. Cancers 2022, 14, 3126. [Google Scholar] [CrossRef] [PubMed]
| Carrier Type | Agent | Characteristics | Ref |
|---|---|---|---|
| patient-derived xenograft (PDX) | Paclitaxel, Doxorubicin, 5-Fluorouracil | Drug screening in multicellular spheroids, expressed drug resistance, hypoxia, elevated cleaved-PARP expression levels, protection of the tumor cells from drug-induced apoptosis, similar expression levels to in vivo tumor models | [59] |
| Ex vivo 3D bioprinted PDX and mouse PDX models | eribulin, TAK228, doxorubicin, carboplatin, talazoparib, paclitaxel, and gemcitabine | Drug screening, common inter- and intra-cellular interactions and responses with the in vivo tumor models, spheroids Ki67 positive, active proliferation rate | [60] |
| 3D models of HER2-positive breast cancer | Neratinib, docetaxel | Increased innate drug resistance, expression of Akt, ERK proteins and EGFR, pEGFR, HER3 receptors, increased activity of drug metabolizing enzymes | [61] |
| 3D models of ER-positive/HER2-positive breast cancer | endocrine agents (tamoxifen, fulvestrant) and trastuzumab | ECM induced pathway switch from AKT to MAPK signaling, suppression of PI3K/AKT pathway, reduced drug sensitivity, MAPK/MEK signaling | [62] |
| 3D MCF7 breast cancer models | Daunorubicin, Docetaxel and Arsenic Disulfide | increased drug resistance, P-glycoprotein function | [63] |
| 3D models of thirteen triple negative breast cancer cell lines | epirubicin, cisplatin, and docetaxel | significant drug resistance | [64] |
| 3D collagen scaffolds with MCF7 breast cancer and glioblastoma U118-MG cells | cisplatin | elevated levels of reactive oxygen species, reduced drug sensitivity, reduced drug uptake by the spheroids | [65] |
| 3D collagen-based gels reforming a ring/hollow bilayer | doxycycline and trastuzumab | expressed similar levels of P-cadherin, vimentin, CK8, EpCAM biomarkers with human sections, destabilization of the bilayer and luminal filling of the center | [66] |
| 3D hyaluronic acid hydrogel scaffold / MMP peptide / nitrodibenzofuran | EGF inhibitor cetuximab | varied EGFR expression levels on the invasive capacity of MDA-MB-231, MDA-MB-468, and MCF-7 breast cancer cell lines, differential cellular responses, different invasion capacity | [67] |
| bacterial cellulose 3D scaffolds | No agent | effective cell adhesion, proliferation, good viability and penetration rate, effectively mimic the tumor ECM | [68] |
| 3D scaffolds of methacrylated gelatin / adipocyte stromal cells | No agent | human triple negative breast cancer cell lines HCC1806 cells and MDA-MB-231 spheroids, stiffness dependent cellular differentiation and maturation, suppressed adipogenesis | [69] |
| Carrier Type | Agent | Characteristics | Ref |
|---|---|---|---|
| α-tricalcium phosphate /calcium sulfate cements/ Fe3O4/GO | Hyperthermia | promoted bone regeneration in rat bone-marrow-derived mesenchymal stem cells and in cranial defect models of rats, effective tumor inhibition in osteosarcoma and lung metastasis tumor-bearing mice | [79] |
| 3D scaffolds of gelatin nanofibrous/ ZIF8 nanoparticles | Phenamil, photothermal effect | promoted bone morphogenic protein 2, induced osteogenic differentiation under NIR, alkaline phosphatase activity, increased expression of Col, RUNX2, BSP bone-related genes and C2C12 myoblast cells, osteosarcoma cell death, inhibited tumor growth | [80] |
| 3D scaffolds gelatin/akermanite/multiwalled carbon nanotubes/magnetic nanoparticles | No agent | adequate biodegradation rate, increased protein adsorption rate, and good biocompatibility in osteoblast G292 cells | [83] |
| porous calcium-zirconia scaffolds/ magnetic nanoparticles/chitosan | No agent | biocompatible with satisfactory mechanical and physical properties, no toxicity effects were reported upon treatment with bone marrow stem cells, increased proliferation rate and bone regeneration ability | [84] |
| 3D akermanite (AKT) scaffold/ CaO2 and Fe3O4 nanoparticles | Hyperthermia/Fenton-like reaction | catalyzed ROS formation, promoted antitumor effects, elevated protein adsorption, biodegradability, stimulating osteogenesis in rBMSCs, increased alkaline phosphatase and osteogenic genes (BMP2, OCN, RUNX2, and COL1) expression, enhanced bone-regeneration | [85] |
| 3D scaffolds of mesoporous bioglass/chitosan/ strontium hexaferrite | Photothermal effect/NIR laser | promoted attachment, proliferation, osteogenic differentiation of human bone marrow stem cells, increased expression levels of osteogenic-related genes (OCN, COL1, Runx2 and ALP), bone regeneration through the BMP-2/Smad/Runx2 signaling pathway, inhibited tumor growth and triggered apoptosis and necrosis | [86] |
| inhibited tumor growth and triggered tumor apoptosis and necrosis/ LaB6 micro-nanoparticles | NIR photothermal effect | enhanced mechanical strength, excellent photothermal effect, osteogenesis of rBMSCs rabbit bone marrow stromal cells, increased expression levels of BMP2, RUNX2 and COL 1 osteogenic genes, inhibition of bone tumor and bone regeneration effects | [87] |
| 3D hydrogels methacrylated gelatin/ chondroitin sulfate/gold nanorods/ nHA | Photothermal effect | promotion of proliferation and osteogenic differentiation of mesenchymal stem cells, prevention of tumor recurrence and in bone regeneration | [88] |
| 3D bioceramic chitosan scaffold/nHA/GO | Photothermal effect/NIR laser | promoted human osteosarcoma cell death, upregulated the BMP2/Smad signaling, promoted osteogenic differentiation, enhanced bone regeneration | [89] |
| 3D printed scaffold/ β-tricalcium phosphate/ 2D black phosphorus/ osteogenic peptide | Doxorubicin | biomimetic and mechanical properties comparable to the human cancellous bone, bone regeneration ability, increased osteogenic gene expression levels (RUNX2, ALP and COL I), increased bone mineral density an capillary vessels formation | [90] |
| Carrier Type | Agent | Characteristics | Ref |
|---|---|---|---|
| 3D collagen-based bioceramic scaffolds/ PC3, LNCaP prostate cells | Docetaxel and siRNA | secreting matrix metalloproteinase and prostate-specific antigen (PSA), expressed reduced levels of MMP1 and MMP9, LNCaP cells secreted increased levels of PSA, suppressed endogenous GAPDH gene expression | [92] |
| chitosan–alginate 3D porous scaffolds/prostate cells | plasmid DNA gene / chlorotoxin | increased expression levels of ECM related (COL1A1, LAMININ A5) and EMT related (SNAIL, SLUG, TWIST, SIP1) genes, upregulation of mRNA epithelial marker E-cadherin | [93] |
| 3D scaffolds of nano-hydroxyapatite (nHA)/PLGA/prostate cells | Docetaxel | degradation behavior promoting cell viability, increased proliferation rate, significant reduction cell viability | [94] |
| 3D collagen scaffold/ metastatic prostatic adenocarcinoma | No agent | stimulation of cytoskeletal reorganization associated with increased metastatic ability and invasion | [95] |
| 3D scaffolds of chitosan-alginate/ prostate metastasis cancer | No agent | osteolytic (PC-3) and osteoblastic (C4-2B, 22Rv1) phenotypes observed in human patients, formation of multicellular spheroids, expression of pEGFR, AR, and cytokeratin 8 biomarkers, mineralization | [96] |
| 3D polymeric hydrogel/ M12 tumorigenic cells/ prostate cells | No agent | tumorigenic response with significant F4/80+ macrophage infiltration | [97] |
| 3D poly(ε-caprolactone) scaffolds/prostate microtissue models | No agent | spatial support for CAFs growth, morphological transition of benign epithelia, epithelial-stroma interactions and promote tumorigenesis | [100] |
| 3D hepatoma spheroids and organoids | Drug screening | mimic the parental tumor microenvironment and HCC heterogeneity | [101] |
| polyurethane (PU)-based 3D scaffold/ fibronectin /collagen/PDAC | No agent | optimal growth conditions, stiffness similar to PDAC ex vivo tissues, differential cellular interactions and growth depending on the cell types, activated stellate cells from αSMA expression levels, CD31+ HMEC endothelial cells | [103] |
| 3D scaffolds/ peptide amphiphiles/ Patient-derived pancreatic stellate cells | No agent | duct-like organoid colonies with extensive stroma mimicking the topology of PDAC, SOX2 and KLF4 transcriptional factors, cancer stem cells functionality (CD133 + /CXCR4+ PDAC cells) | [104] |
| 3D printed polycaprolactone scaffolds/collagen/stromal cells | No agent | expression of MMP2 and Ki67 indicative of ECM remodeling, reprogramming of stromal cells, increased expression levels of CAF markers (αSMA, FAP, FSP-1), upregulated metabolic signals related to hypoxia, stress and anti-apoptosis (HIF-1, MAPK ErbB) | [106] |
| patient-derived decellularized ECM scaffolds/colon HT-29 cells | No agent | reduced E-cadherin expression, overexpression of vimentin, recapitulation of metastatic microenvironment, demethylation, deacetylation, metabolic stress and hypoxia genes expression profiling | [108] |
| Carrier Type | Agent | Characteristics | Ref |
|---|---|---|---|
| 3D hydrogel scaffold polypeptides/ L methionine | dextro-1-methyl tryptophan (D-1MT)/ anti-programmed cell death-ligand 1 (anti-PD-L1) antibody | sustained release of anti-PD-L1 antibody and D-1MT, effective T cell mediated immune responses, tumor-infiltrating CD45+ and CD8+ T cells | [115] |
| patient-derived organoids | anti-programmed cell death-ligand 1 (anti-PD-L1) antibody | diverse immune cell populations and expressed M2 macrophage phenotype, inhibition of PD-1 and PD-L1, activated tumor antigen-specific tumor infiltrating lymphocytes (TILs) | [116] |
| 3D printed biodegradable bioglass scaffolds/ 2D niobium carbide | Photothermal effect/ immune adjuvant R837 | profound photothermal-conversion capacity, stimulated vaccine-like effect of R837, recruitment and maturation of dendritic cells (CD11c+, CD80+, CD86+), increased cytokine secretion levels (IL-6, IL-17, TNFα), profound inhibitory anti-PD-L1 effect, increased immune activity, bone regeneration | [117] |
| low viscosity hyaluronic acid and gelatin 3D hydrogels | CAR T-cells | prevented CAR T-cells sedimentation, maintained cellular viability, and promoted elevated cellular delivery, tumor specific cytotoxicity in U87MG and U87MG.DEGFR glioma cells | [118] |
| 3D α-cyclodextrine hydrogel scaffold | doxorubicin and CpG immune adjuvant | promote immunogenic cell death, increased levels of high mobility group protein B1, promoted immunostimulatory effects, expression levels of CD86 molecules on CD11c+ cells, upregulation of cytotoxic T-lymphocytes | [119] |
| polymeric hydrogel 3D scaffolds | cyclophosphamide (CTX) | immunogenic cell death, maturation and activation of DCs (CD11c+, CD11c+CD86+, CD11c+MHC-I+), cytotoxic T-lymphocyte anti-tumor immune effects (CD3+, CD3+CD4+, and CD3+CD8+), increased stimulating the transition of T cells to Th1 phenotype | [121] |
| biomimetic hybrid hydroxyapatite-based 3D scaffolds | Cancer stem cells (CSCs) enrichment | sarcospheres formation, osteogenic and mineralization properties for bone regeneration, effective cell adhesion, migration and colonization, increased mRNA expression level of stem-related genes (OCT-4, SOX-2, NANOG), upregulated the genes expression levels (NOTC-1, HIF-1α, IL-6), signaling cascade between CSCs and tumor stem niche | [122] |
| scaffolds of collagen-nanohydroxyapatite | miRNA inhibitor (antago-miR-133a) | localized in vivo antago-miR-133a transfection, increased genes expression levels of ALP, BMP-2, OCN, increased bone defect healing, increased presence of CD206+ cells, pro-remodeling M2-like macrophage population | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





