Submitted:
27 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
1. Eligibility Criteria
2. Research Strategy
3. Data Selection
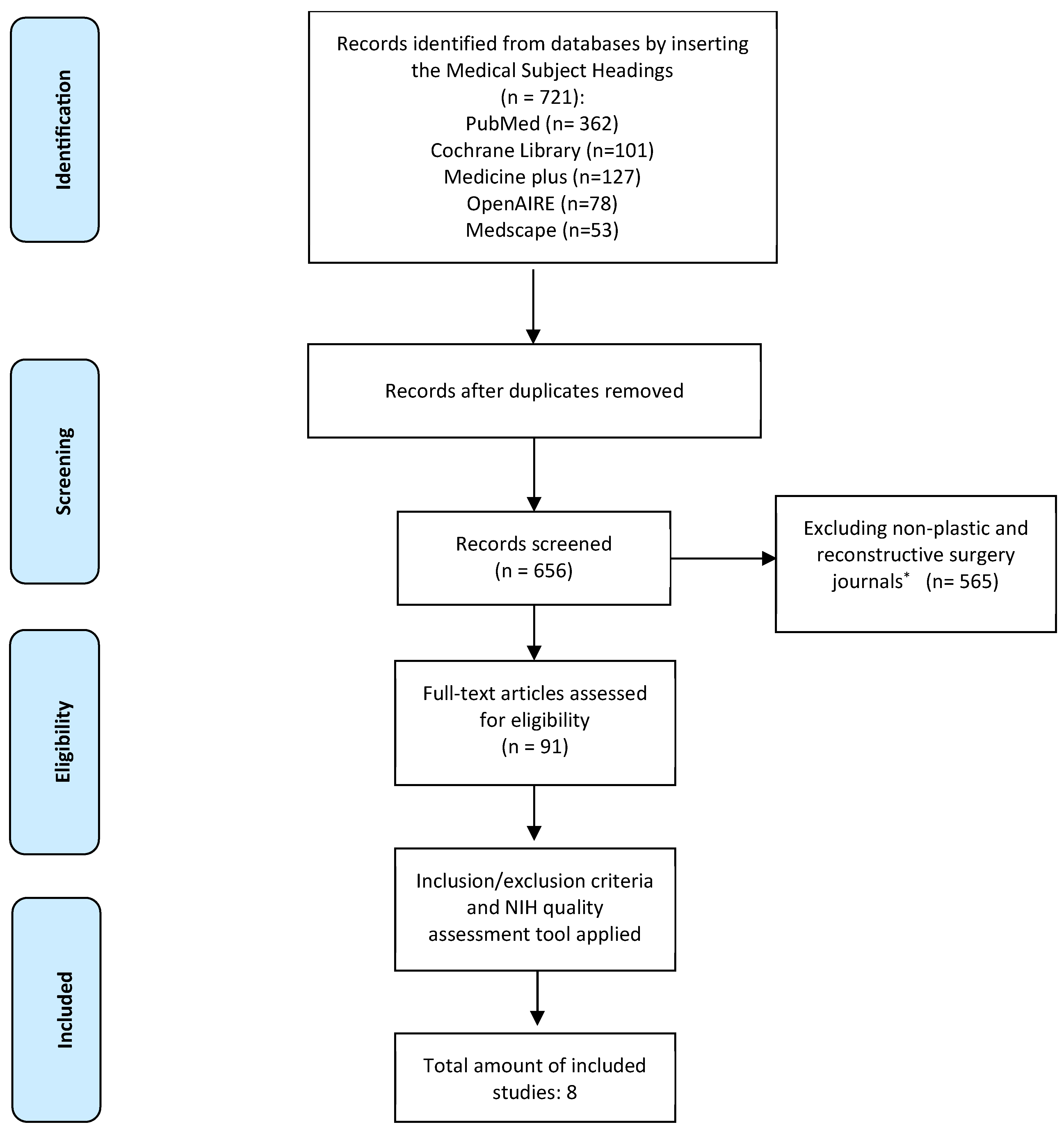
Results
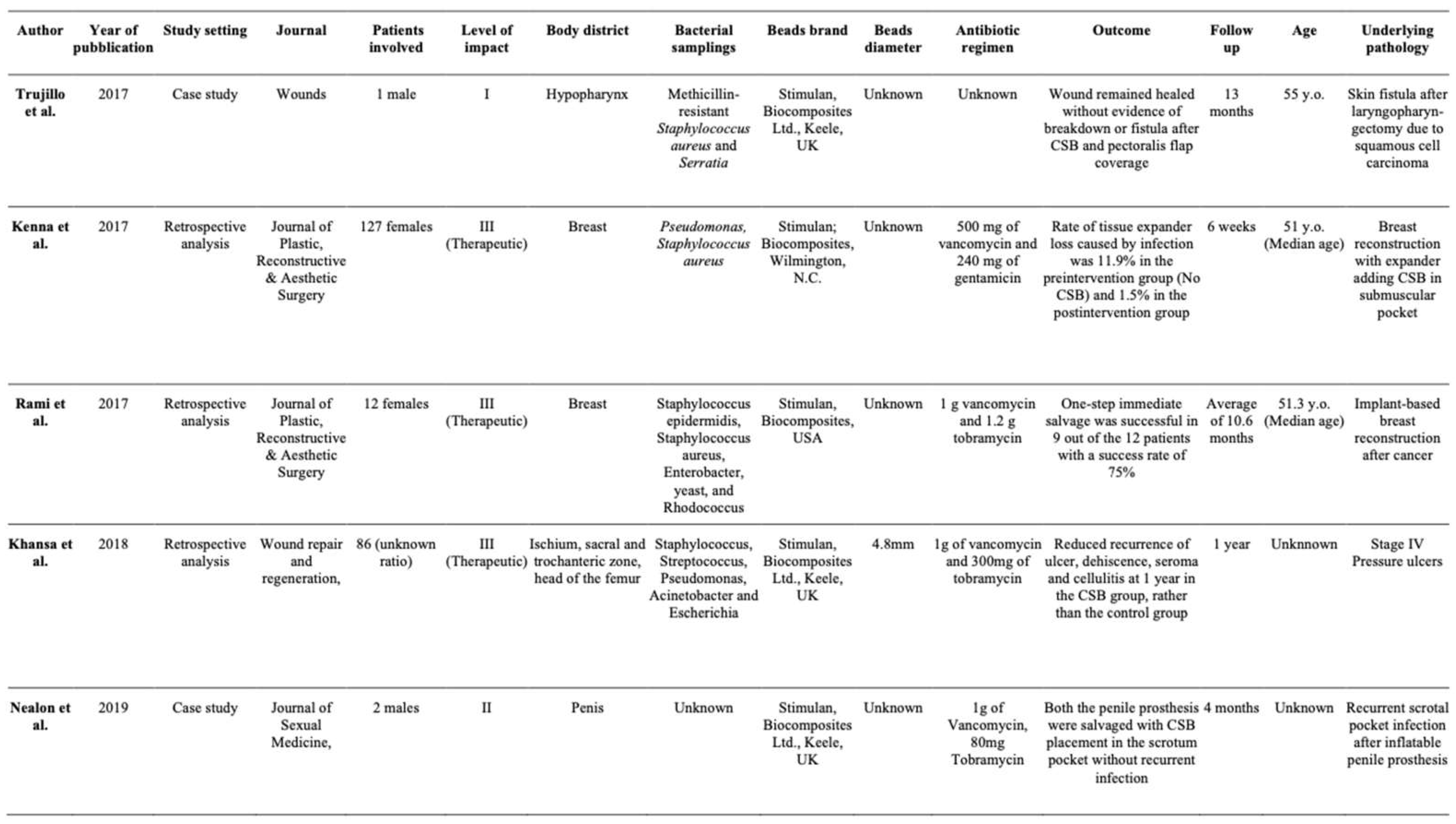
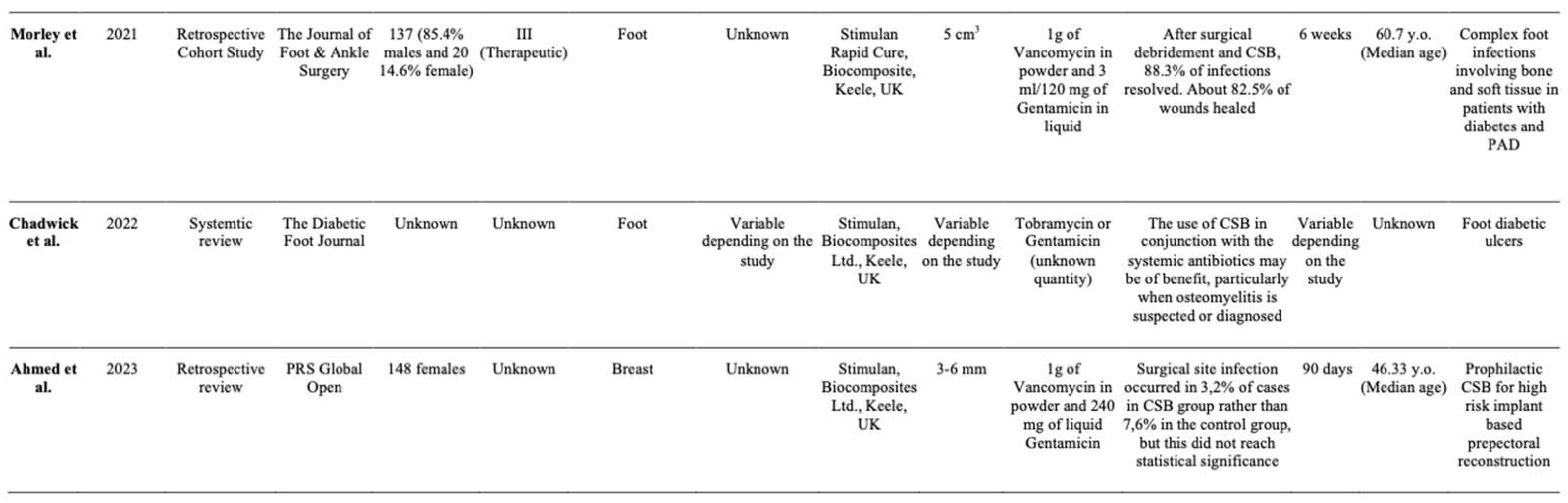
Summary of Studies
Discussion
Conclusion
Informed Consent Statement
Conflicts of interest
Financial Disclosure Statement
Ethical approval
Illustrations attached
Caption of the video attached
References
- R. Kallala, W. Edwin Harris, M. Ibrahim, M. Dipane, E. McPherson. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Joint Res 2018 and 10.1302/2046-3758.710., 7:570–579. DO.
- Jogia RM, Modha DE, Nisal K, et al. Use of highly purified synthetic calcium sulfate impregnated with antibiotics for the management of diabetic foot ulcers complicated by osteomyielitis. Diabetes Care 2015 and 38(5):79e80.
- Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016 and 98B(1_Supple_A):6–9.
- Piovan, G., Farinelli, L., Screpis, D. et al. The role of antibiotic calcium sulfate beads in acute periprosthetic knee infection: a retrospective cohort study. Arthroplasty 4, 42 (2022).
- Sheridan GA, Falk DP, Fragomen AT, Rozbruch SR. Calcium sulfate in the management of osteomyelitis: A systematic review and meta-analysis of comparative studies. Medicine 2022 and 101:45(e31364).
- McKee MD, Li-Bland EA, Wild LM, Schemitsch EH. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and in.
- Peltier LF. The use of plaster of paris to fill large defects in bone. Am J and 97:311-315.
- McPherson E, Dipane M, Sherif S. Dissolvable Antibiotic Beads in Treatment of Peripros-thetic Joint Infection and Revision Arthroplasty - The Use of Synthetic Pure Calcium Sulfate (Stimulan®) Impregnated with Vancomycin & Tobramycin. Reconstr Rev 2013.
- McConoughey, Stephen J., Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B App Biometer. 2015 May;103 (4):870-7.
- Kallala R, Haddad FS. Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. Bone Joint J 2015 and 97-B:1237-1241.
- Kelly CM, Wilkins RM, Gitelis S, et al. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001:42–50.
- Ziran BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone matrix/allograft for nonunions and posttraumatic reconstruction of the appendicular skeleton: Preliminary results and complications. J Trauma. 2007 and 63:1324–8.
- Sheridan GA, Falk DP, Fragomen AT, Rozbruch SR. Calcium sulfate in the management of osteomyelitis: A systematic review and meta-analysis of comparative studies. Medicine 2022 and 101:45(e31364).
- Gallo G, Guaitoli E, Barra F, Picciariello A, Pasculli A, Coppola A, Pertile D, Meniconi RL and Itali, SPIGC Surgical Training Working Group. Restructuring surgical training after COVID-19 pandemic: A nationwide survey on the Italian scenario on behalf of the.
- Marcasciano M, Kaciulyte J, Mori FLR, Lo Torto F, Ribuffo D, Casella D. Plastic surgery in the time of Coronavirus in Italy. Maybe we should say: “Thanks Darwin we are Plastic Sur-geons!”. J Plast Reconstr Aesthet Surg. 2021 Jul and doi:, 74(7):1633-1701.
- Lo Torto F, Turriziani G, Donato C, Marcasciano M, Redi U, Greco M, Miraldi F, Ribuffo D. Deep sternal wound infection following cardiac surgery: A comparison of the monolateral with the bilateral pectoralis major flaps. Int Wound J. 2020 Jun and 17(3):683-69.
- Greco M, Vitagliano T, Fiorillo MA, Greto Ciriaco A. A new technique of upper eyelid blepharoplasty using the orbicularis muscle flap. Aesthetic Plast Surg. 2012 Feb and 21674294., 36(1):18-22. [CrossRef]
- Cigna E, Pierazzi DM, Sereni S, Marcasciano M, Losco L, Bolletta A. Lymphatico-venous anastomosis in chronic ulcer with venous insufficiency: A case report. Microsurgery. 2021 Sep and 10.1002/micr.30753., 41(6):574-578.
- Baker HP, Straszewski AJ, Dahm JS, Dickherber JL, Krishnan P, Dillman DB, Strelzow JA. Gunshot-related lower extremity nerve injuries. Eur J Orthop Surg Traumatol. 2023 May and 7., 33(4):851-856. [CrossRef]
- Maruccia M, Onesti MG, Sorvillo V, Albano A, Dessy LA, Carlesimo B, Tarallo M, Marcasciano M, Giudice G, Cigna E, Ribuffo D. An Alternative Treatment Strategy for Complicated Chronic Wounds: Negative Pressure Therapy over Mesh Skin Graft. Biomed Res Int.
- Inoue, T., Maeda, K., Nagano, A., Shimizu, A., Ueshima, J., Murotani, K., Sato, K., & Tsu-baki, A. (2020). Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients.
- Trujillo JM, Logue ME, Kunkel R, Demas CP. Off-label Usage of Absorbable Beads Containing Antibiotics for Prevention of Surgical Site Infections. Wounds. 2017 Oct and 29(10):E84-E87.
- Kenna DM, Irojah BB, Mudge K, Eveler K. Absorbable Antibiotic Beads Prophylaxis in Immediate Breast Reconstruction. Plast Reconstr Surg. 2018 Apr and 141(4):486e-492e.
- Sherif, RD, Ingargiola M, Sanati-Mehrizy P, Torina PJ, Harmaty MA. Use of antibiotic beads to salvage infected breast implants. J Plast Reconstr Aesthet Surg. 2017 Oct;70(10):1386-1390.
- Khansa I, Barker JC, Ghatak PD, Sen CK, Gordillo GM. Use of antibiotic impregnated resorbable beads reduces pressure ulcer recurrence: A retrospective analysis. Wound Repair Regen. 2018 Mar;26(2):221-227.
- S. Nealon, A. Baumgarten, R. Carrion, J. Parker, 297 The Use of Antibiotic Impregnated Beads in Setting of Penile Implant Infection: A Single Institution Experience, The Journal of Sexual Medicine, Volume 16.
- Robert Morley, MSc, Matt Rothwell, MSc, John Stephenson, PhD, Liza McIlvenny, MSc, Frank Webb, MSc, Aaron Barber, MSc, Complex Foot Infection Treated With Surgical Debridement and Antibiotic Loaded Calcium Sulfate—A Retrospective Cohort Study of 137.
- Chadwick P, Ahmad N, Dunn G et al. (2022) Local antibiotic delivery: early intervention in infection management strategy. The Diabetic Foot Journal 25(2): 44–52.
- Ahmed, Shahnur MD; Lee, Jason T. C. MD, MSc; Roth, Dylan BS; Sinha, Mithuan PhD; Fisher, Carla MD; Fan, Betty DO; Imeokparia, Folasade MD; Ludwig, Kandice MD; Lester, Mary E. MD; Hassanein, Aladdin H. MD, MMSc. Prophylactic Absorbable Antibiotic Beads for HIgh Risk, Implants-based Prepectoral Reconstruction. Plastic & Reconstructive Surgery- Global Open.
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995 and 49:711–745.
- Patel P, Singh R, Agarwal A et al. (2021) Diabetic foot ulcers and osteomyelitis: use of biodegradable calcium sulphate beads with antibiotics for treatment of multidrug-resisteant organists. Wounds 33(3): 70–6.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveil- lance definition of health care associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008 and 36:309–332.
- Marco Marcasciano, Juste Kaciulyte, Riccardo Di Giuli, Fabio Marcasciano, Federico Lo Torto, Maristella Guerra, Giovanni Dal Prà, Leonardo Barellini, Marco Mazzocchi, Donato Casella, Diego Ribuffo, “Just Pulse it!” Introduction of a conservative implant.
- Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013 and 132:1319–1328.
- Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013 and 100:768–774.
- Giordano S, Peltoniemi H, Lilius P, Salmi A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: A comparative study. Aesthet Surg J. 2013 and 33:675–680.
- Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant associated anaplastic large- cell lymphoma. Plast Reconstr Surg. 2016 and 137:1659–1669.
- Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015 and 59:111.
- Bamba R, Tran PC, Mailey BA, et al. Comparison of breast recon- struction outcomes using oxychlorosene versus triple antibiotic solution for pocket irrigation. Plast Reconstr Surg Glob Open. 2022 and 10:e3975.
- Dawson SE, Bamba R, Tran PC, et al. Implant-based breast recon- struction outcomes using oxychlorosene for pocket irrigation. Plast Reconstr Surg. 2021 and 148:518e–520e.
- Luchette FA, Bone LB, Born CT, et al. EAST Practice Man- agement Guidelines Workgroup: practice management guidelines for prophylactic antibiotic use in open fractures. Eastern As-sociation for the Surgery of Trauma. 2000.
| Criteria/author | Trujillo 2017 [22] | Kenna 2017 [23] | Rami 2017 [24] | Khansa 2018 [25] | Nealon 2019 [26] | Morley 2021 [27] | Chadwick 2022 [28] | Ahmed 2023 [29] |
| 1. Was the study question or objective clearly stated? | YES | YES | YES | YES | YES | YES | YES | YES |
| 2. Was the study population clearly and fully described, including a case definition? | NO | YES | YES | YES | NO | YES | YES | NO |
| 3. Were the cases consecutive? | NO | NO | YES | YES | NO | YES | YES | YES |
| 4. Were the subjects comparable? | YES | YES | YES | YES | YES | YES | YES | NO |
| 5. Was the intervention clearly described? | NO | YES | YES | YES | YES | YES | YES | YES |
| 6. Were the outcome measures clearly defined,valid, reliable, andimplemented consistentlyacross all study participants? | YES | YES | YES | YES | YES | YES | YES | NO |
| 7. Was the length of follow-up adequate? | YES | YES | YES | YES | NO | YES | YES | YES |
| 8. Were the statistical methods well-described? | NO | NO | YES | YES | NO | YES | YES | YES |
| 9. Were the results well-described? | YES | YES | YES | YES | YES | YES | YES | NO |
| Quality Rating, ≥ 7 = Good, 5-6 = fair, ≤ 4 = poor | FAIR | GOOD | GOOD | GOOD | FAIR | GOOD | GOOD | FAIR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





