1. Introduction
Duckweed, also known as Lemnaceae, is a rapidly proliferating aquatic monocot angiosperm. The common habitat of Lemnaceae constitutes still or slow-moving freshwater environments including ponds, lakes, and marshes. Lemnaceae species are very tiny with a size ranging from 1-10 millimeters in length consisting of floating leaves called fronds and delicate root forms referred to as rootlets. With the relatively small structure, Lemnaceae species can double the biomass with high nutritional value within a short period under favorable growth conditions. Recently, Duckweed has served as a model organism for understanding physiological aspects relative to growth and development due to its simple morphology, rapid growth, and ease of cultivation. Moreover, Duckweed possesses unique adaptive characteristics that enable it to survive in diverse environmental conditions including low light conditions, fluctuating nutrient levels, and different ranges of pH [
1]. Furthermore, Duckweed has an efficient potential to remove heavy metals, pesticides, and organic compounds from wastewater sources. Therefore, Duckweeds have gained attention as a promising candidate for investigating plant stress responses, and environmental interactions due to their distinct ability to accumulate a variety of pollutants and to survive in nutrient-rich and polluted waters, their high protein content, and their potential as a source of biofuel [
2]. Although the
Lemnaceae family is extensively diverse and consists of more than 30 species with five genera
Lemna,
Landoltia,
Spirodela, Wolffia, and
Wolfiella, most of the research has been focused on the
Lemna genus in bioaccumulation and tolerance to environmental stressors studies [
3]. Additionally, the genome size of the Lemnaceae species is relatively small compared to the other monocots which allows Duckweed to further contribute to genomics and transcriptomics studies. The understanding of genomics and transcriptomics in Duckweed enables the biotechnological assessment of genetic modifications and their impact on innovative sustainable solutions for environmental and agricultural challenges.
2. Advantages of Duckweed over Arabidopsis
Arabidopsis thaliana (Arabidopsis), a small cruciferous dicot plant, is a universally accepted model plant for plant biology research [
4]. Arabidopsis offers many advantages to be used as a functional and classical model for studying various aspects of plant research from physiology to muti-omics. It has a high reproductive potential and due to its small size, it can be grown on a large scale in a very small space. Additionally, it can be a self-pollinated plant, and thus it is easier to regulate the extraneous variables during experiments in comparison to cross-pollinated plants [
5]. Also, the completion of the entire genome sequencing of Arabidopsis by the Arabidopsis Genome Initiative in the year 2000 makes it a popular experimental model [
6]. Although Arabidopsis exhibits ample advantages, it has certain limitations in understanding the aspects of crop plants, particularly the monocots. Recently, Duckweed has re-emerged as a compelling alternative or complement to the Arabidopsis for the monocot model system. The remarkable growth rate and nutrient acquisition of Duckweed highlight the significant advantage as a model organism [
7]. Rapid growth enables quicker experiments and high-throughput screening, especially for genetics and large-scale studies. Subsequently, it acquires a small genome size, typically around 150 Mbp, which simplifies genomic analysis and genetic manipulation [
8]. In addition, Duckweed offers distinct advantages over Arabidopsis, including adaptability to various environmental conditions, and potential for bioenergy production [
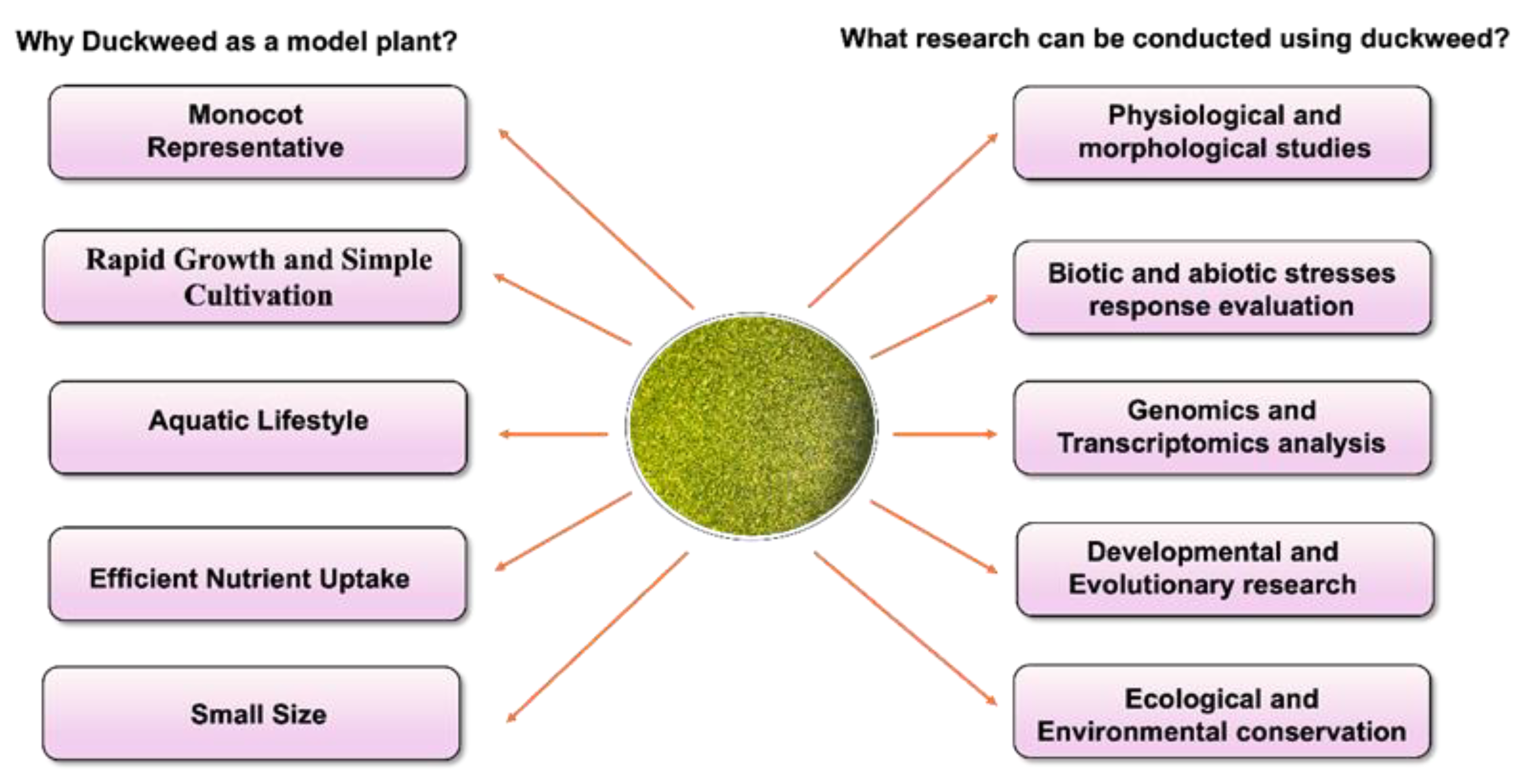
9]. Duckweed can be an excellent model organism for biological studies, including genetics, genomics, ecology, and renewable energy research. Considering the several advantages associated with Duckweed, it can be used for different research studies (
Figure 1).
3. Importance of Duckweed in Physiological Research
3.1. Nutrient Uptake and Stress Tolerance
With relatively minimal size and remarkable growth and adaptations, Duckweed has become the interest of plant physiological studies. The focus on Duckweed has been expanded due to its efficient and rapid nutrient absorption from the surrounding ecosystem with an extensive growth rate. Like terrestrial plants, Duckweed can uptake nutrients including nitrogen, and phosphorus that are essential for growth and metabolism through passive diffusion, active transport, and symbiotic interactions with microorganisms. However, the nutrient uptake in Duckweed is a non-continuous process and is solely affected by environmental abundance [
10]. The regulatory mechanisms governing nutrient uptake processes in Duckweed are not well understood. The research approach with particular attention on physiological and developmental processes, can provide a scope with enormous advantage in nutrient cycling and sustainable agriculture. Additionally, Duckweed has significant photosynthetic capabilities with high rates of carbon fixation and efficient light energy utilization [
9]. An essential aspect of understanding the photosynthetic system is the light adaptation potential. Several physiological studies have shown the effect of different light intensities on the growth and adaptation of diverse Lemnaceae species. With high-intensity light, Duckweed species reduce the physiological growth rate alternatively, diverting the growth to defense regulation with increased production of protective pigments such as carotenoids showing their strategic adjustments to light conditions [
11]. In addition, prior investigations have elucidated the response of Duckweed to environmental stressors encompassing heavy metal exposure, salinity variations, and temperature oscillations [
12]. In
Lemna minor species, the increased accumulation of macro and micronutrients exhibited the proficiencies of Duckweed to overcome salinity-induced stress [
13]. The ability to tolerate environmental stressors is another promising characteristic of Duckweed that provides insight into plant stress resistance responses.
3.2. Ion Transport in Duckweed
Duckweed has made substantial contributions to understanding the aspect of physiological ion transport. In a groundbreaking study, the dynamic response of calcium signaling in Duckweed under Cadmium (Cd) stress conditions was elucidated through the utilization of a Ca2+-sensing fluorescent reporter (GCaMP3) in transgenic Duckweed (
Lemna turionifera 5511). The notable accumulation of Ca2+ in vacuoles revealed the subcellular calcium localization during Cd stress. Furthermore, the Ca2+ inflow study has suggested that Ca2+ inflow was stable at low speed, however, the treatment of Cd treatment changed to high-speed efflux. Additionally, the introduction of exogenous γ-aminobutyric acid (GABA) to Duckweed resulted in the stabilization of the Ca2+ signal, highlighting its significant regulatory function in managing the Ca2+ signal under Cd-induced stress [
14]. In addition to Cd, cobalt (Co2+) is another metal ion that has a phyto-modulatory effect in Duckweed species. The hyperaccumulation of cobalt in fronds inhibits the vegetative growth of
L. minor with no distinctive alterations in the iron (Fe) content in fronds. The chlorophyll content and photosynthetic efficiency initially remained constant but gradually decreased over time with the acquisition of Co2+ suggesting the inhibition of biosynthesis rather than promoting the degradation of existing pigment molecules. This finding indicates the strength of
L. minor as a model system for understanding the mechanistic effect of heavy metal metabolism and bioaccumulation at the cellular level [
15].
Apart from heavy metals, the transport and impact of other ions such as phosphate (Pi), Nitrate (NO3-), and Ammonium (NH4+) have been investigated in different Duckweed species. In the pre-anthropogenic era, phosphate (Pi) was the limiting mineral factor for floating aquatic plants including Duckweeds under natural conditions. Therefore, Duckweed and other macrophytes, have evolved to be particularly proficient in assimilating and storing this ion. Moreover, phosphorus (Pi) deficiency in Duckweeds has been shown to exert significant impacts on growth and metabolic processes. The induction of a glycosylphosphatidylinositol-anchored purple acid phosphatase (PAP) in
L. punctata in response to Pi deficiency facilitates enhanced Pi uptake and storage in vacuoles to support growth. Furthermore, Duckweeds like
L. minor and
L. gibba employ diverse strategies for Pi storage, including the accumulation of various phosphate forms as short- and long-term Pi reserves [
1]. Therefore, Duckweed can survive under phosphorus deficiency highlighting its adaptability and making it an invaluable model for studying nutrient acquisition and storage mechanisms in aquatic plants.
3.4. Signaling Mechanisms in Duckweed
In addition to ion transport, Duckweed (Lemnaceae) has been a promising system for studying plant signaling responses to various environmental cues and stressors. Several studies have investigated the signaling pathways involved in the growth, development, and stress tolerance mechanisms of Duckweed. Previous findings suggested the roles of phytohormones, including auxins, cytokinins, abscisic acid (ABA), and gibberellins, in regulating key processes such as cell division, differentiation, and stress responses in Duckweed [
17,
18,
19,
20,
21] Additionally, the involvement of calcium ions (Ca2+), reactive oxygen species (ROS), and protein kinases in mediating signal transduction pathways have been identified in Duckweed, particularly in response to environmental stresses such as salinity, drought, and heavy metal toxicity [
22,
23,
24,
25] Furthermore, the signaling components, such as receptors, transcription factors, and downstream effectors, involved in developmental processes, responses to pathogens and environmental stimuli has been characterized through genomics and transcriptomics analysis [
26,
27,
28]. The understanding of Duckweed signaling networks can give scope to develop strategies to improve its resilience and productivity under adverse environmental conditions.
4. Phytoremediation and Wastewater Treatment through Duckweed
Duckweed has garnered attention for its potential applications in phytoremediation and wastewater treatment due to its ability to efficiently remove nutrients, heavy metals, and organic pollutants from aquatic environments [
29]. Research has demonstrated that Duckweed can effectively absorb and accumulate nutrients such as nitrogen and phosphorus, thereby mitigating eutrophication in water bodies [
30]. Additionally, Duckweed can uptake and sequester heavy metals such as cadmium, lead, and arsenic, contributing to the remediation of contaminated water and soil [
12]. Furthermore, Duckweed has the potential to degrade organic pollutants through mechanisms such as phytodegradation and rhizofiltration, where pollutants are metabolized by Duckweed or microbial communities associated with its roots. Moreover, Duckweed-based constructed wetlands and wastewater treatment systems have been developed and optimized for the treatment of various types of wastewater, including municipal, agricultural, and industrial effluents [
31,
32,
33]. Through bioremediation and wastewater treatment applications, Duckweed offers a cost-effective, sustainable, and environmentally friendly solution for addressing water pollution and resource management challenges.
5. Genome Complexity of Lemnaceae Species
Although Duckweed has promising characteristics, a few challenges hamper the application of Duckweed for agricultural and biotechnological applications. The limited genomics and transcriptomics information of Duckweed restricts this powerful model system from flourishing in plant stress research. Moreover, the genomic content of Duckweed is found to be enriched with repetitive elements, sequences that act as a confounding factor for sequencing and assembly efforts [
36,
37,
38,
39]. Therefore, it is required to utilize advanced sequencing technologies with sophisticated bioinformatics tools designed to analyze the genomic complexities of
Lemnaceae. Advanced sequencing techniques need to be applied to thoroughly understand its genome. One such advanced sequencing method is PacBio sequencing which can provide long, detailed reads of the genome, crucial for assembling complex genomes like Duckweed [
40]. These long reads allow for more complete and accurate assemblies, better gene characterization, and variant detection within the Duckweed population. However, PacBio sequencing is not allowed to reveal the 3D organization of the genome. To analyze the 3D architecture of the genome, Hi-C sequencing comes into play to capture the interaction pattern of different regions of chromosomes, providing a blueprint for the physical structure of the genome [
41]. This information helps to direct PacBio sequences into their proper chromosomal positions and relative spatial gene interaction potentially influencing stress tolerance [
42]. A powerful and inclusive understanding of the Duckweed genome can be achieved by combining the detailed sequencing of PacBio with the 3D architecture from Hi-C insights.
6. Transgenic Development of Duckweed
Studies on transgenic development in Lemnaceae have showcased its potential as a versatile platform for genetic engineering and biotechnological applications. The transgenic Duckweed lines with enhanced traits for biomass production, nutrient uptake, stress tolerance, and biofuel production have been developed through different transformation approaches [
43,
44,
45]. Genetic engineering approaches have been employed to modulate key metabolic pathways, regulatory genes, and signaling pathways in Duckweed, aiming to improve its productivity and sustainability. For instance, transgenic Duckweed lines with increased expression of genes involved in starch biosynthesis have been developed to enhance starch accumulation for bioethanol production [
46]. Similarly, genetic modifications targeting genes associated with dormant structure development have led to the development of Duckweed lines allowing to study of the extreme temperature response [
47]. Moreover, transgenic Duckweed expressing stress-responsive genes or transcription factors have shown enhanced tolerance to various abiotic stresses, such as salinity, drought, and heavy metal toxicity, expanding its potential for phytoremediation and ecological restoration. Furthermore, advances in genome editing technologies, such as CRISPR-Cas9, have enabled precise and targeted modifications of Duckweed genomes, accelerating the development of transgenic lines with desired traits [
48,
49,
50,
51,
52]. Through these transgenic development studies, Duckweed emerges as a promising platform for sustainable biotechnological applications, including bioenergy production, wastewater treatment, and environmental remediation.
7. Development of Novel Methods for Duckweed Germplasm Conservation
The development of new cryopreservation methods for Duckweed has aimed to establish efficient techniques for the long-term preservation of genetic diversity and conservation of valuable germplasm. Cryopreservation is crucial for maintaining the genetic resources of Duckweed species, especially those with unique traits of interest for biotechnological and agricultural applications. Traditional cryopreservation methods, such as slow freezing and vitrification, have been adapted and optimized for Duckweed, but they often suffer from low recovery rates and genetic stability issues. Consequently, researchers have explored novel cryopreservation approaches tailored to the unique physiology and morphology of Duckweed. One promising technique is encapsulation-dehydration, where Duckweed tissues are encapsulated in protective matrices and dehydrated before cryopreservation, enabling better cell survival and recovery post-thaw. Additionally, advancements in cryoprotectant solutions and protocols have improved the viability and regrowth of cryopreserved Duckweed samples. Furthermore, studies have investigated the use of cryogenic storage techniques, such as liquid nitrogen immersion and droplet freezing, to preserve Duckweed cultures in suspended animation at ultra-low temperatures [
53,
54]. These innovative cryopreservation methods offer practical solutions for the long-term storage and conservation of Duckweed germplasm, facilitating future research and biotechnological applications. Continued research efforts are needed to optimize protocols, enhance recovery rates, and ensure genetic stability for the widespread adoption of cryopreservation in Duckweed conservation programs and biobanking initiatives. Moreover, Duckweed has also acquired significant interest as a sustainable food source due to its high nutritional composition. Therefore, the preservation of the genetic diversity of Duckweed is crucial for plant research and potential food security. The traditional liquid and solid culture media have been used as methods for germplasm preservation and in vitro maintenance of Duckweed plants in the laboratory. The germplasm conservation can be efficiently extended through a novel synthetic seed technology approach. Developing Duckweed synthetic seeds (SynSeeds) can be a distinct germplasm conservation method for enabling a consistent supply of Duckweed plants for sustainable food production and research.
8. Discussion and Future Perspectives
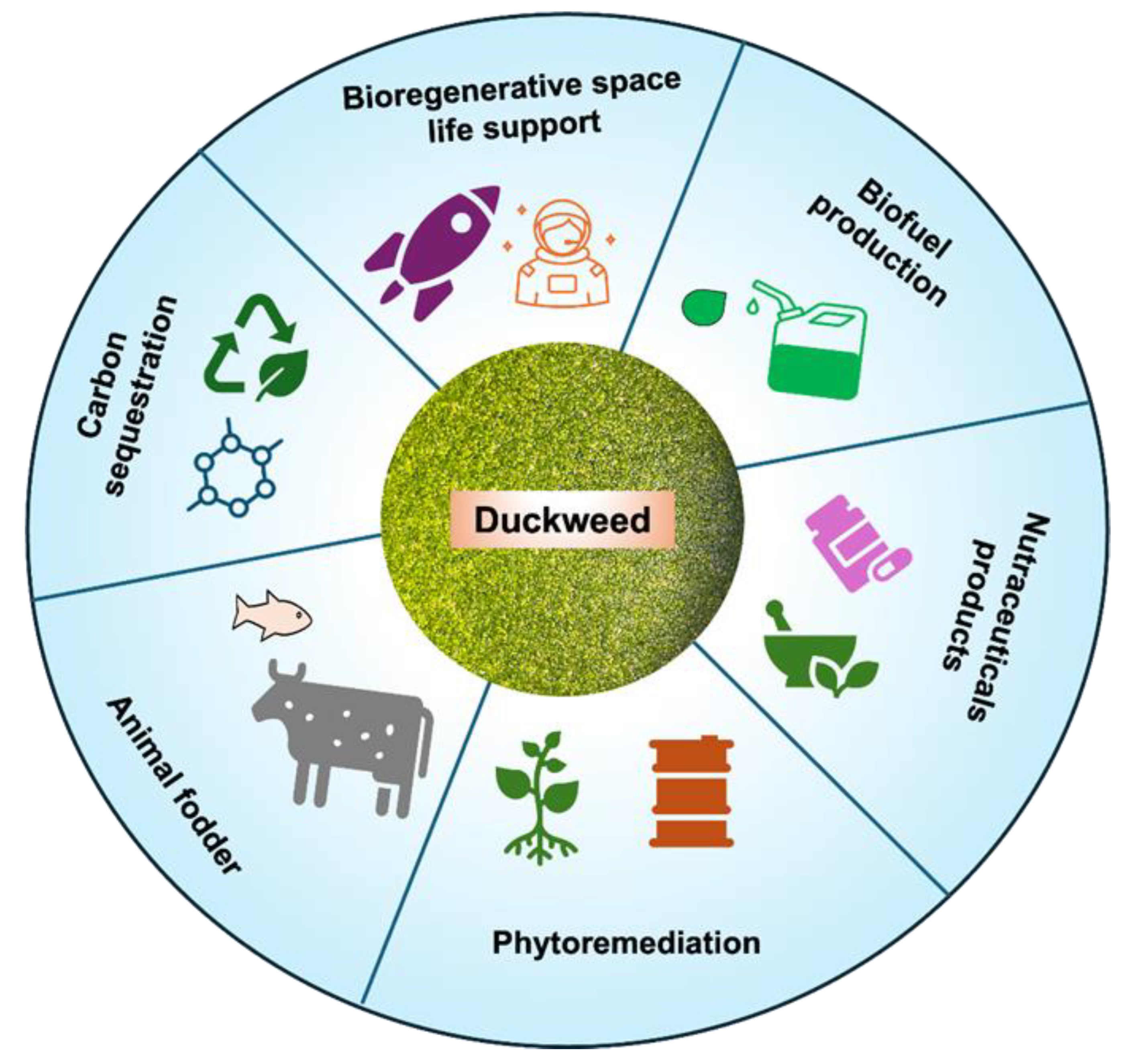
Recent developments in Duckweed research have advanced its potential applications across different fields showing enormous potential for this versatile plant in the near future. The traits of rapid growth, high nutrient uptake efficiency, and tolerance to environmental stresses of Duckweed present advantages to exploit it to its full potential. Profound insights into the genetic makeup of Duckweed's unique traits can be studied with the current advancements in genomic sequencing and molecular biology techniques. The elucidation of Duckweed's genetic makeup can facilitate the development of genetic engineering techniques aimed at enhancing desirable traits in Duckweed and other monocot plants. Genetic manipulation can be used to enhance traits such as increased biomass production, nutrient uptake, and stress resistance to maximize the utility of Duckweed in various applications. Moreover, innovations in cultivation technologies such as photobioreactors and specialized wastewater treatment techniques have optimized Duckweed cultivation for applications in bioenergy production, bioremediation of polluted water bodies, and sustainable agricultural practices. These technologies enable the efficient cultivation of Duckweed on a large scale and can be used for addressing pressing environmental challenges. Furthermore, beyond its traditional applications, Duckweeds are being investigated as a promising medium for biopharmaceutical production, phytoremediation of contaminated sites, and ecological restoration efforts [
55,
56]. These diverse applications highlight the versatility of Duckweed and its potential to contribute to multiple aspects of human well-being and environmental sustainability (
Figure 2). Interestingly, Duckweed shows tremendous promise for boosting space research by providing sustainable solutions for life support and resource management beyond Earth (
Appendix A). Overall, the future of Duckweed research holds promise for further developments in genetic manipulation, cultivation methods, and downstream processing technologies. Continued innovation in these areas is anticipated to establish Duckweed as an efficient model system in addition to its adoption as a sustainable solution to global challenges related to food security, environmental degradation, and the transition to renewable energy sources.
Author Contributions
Conceptualization, D.T., K.M.P-M. and M.S.M.; writing—original draft preparation, D.T.; writing—review and editing, K.M.P-M. and M.S.M.; visualization, D.T.; supervision, K.M.P-M. and M.S.M.; project administration, K.M.P-M. and M.S.M..; funding acquisition, K.M.P-M. and M.S.M. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by NSF award IOS-2038872 to K.M.P-M. and M.S.M.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ziegler, P.; Appenroth, K.J.; Sree, K.S. Survival Strategies of Duckweeds, the World’s Smallest Angiosperms. Plants 2023, 12, 2215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Saeed, M.; Choi, H.K. Duckweeds: their utilization, metabolites and cultivation. Applied Biological Chemistry 2021, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.R.; Marais, D.L.D.; McKAY, J.K.; Richards, J.H.; Juenger, T.E.; Keitt, T.H. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Mol. Ecol. 2012, 21, 5512–5529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.-H.; Su, F.; Chen, L.; Huang, T.; Cai, Y.-D. A Shortest-Path-Based Method for the Analysis and Prediction of Fruit-Related Genes in Arabidopsis thaliana. PLOS ONE 2016, 11, e0159519. [Google Scholar] [CrossRef] [PubMed]
- Olivas, N.H.D.; Frago, E.; Thoen, M.P.M.; Kloth, K.J.; Becker, F.F.M.; van Loon, J.J.A.; Gort, G.; Keurentjes, J.J.B.; van Heerwaarden, J.; Dicke, M. Natural variation in life history strategy of Arabidopsis thaliana determines stress responses to drought and insects of different feeding guilds. Mol. Ecol. 2017, 26, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Hejný, S. The Family of Lemnaceae-A Monographic Study: Biosystematic Investigations in the Family of Duckweeds (Lemnaceae) 1992, 4, 336. 4.
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: duckweed as a model plant system in the genomics and postgenomics era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Laird, R.A.; Barks, P.M. Skimming the surface: duckweed as a model system in ecology and evolution. Am. J. Bot. 2018, 105, 1962–1966. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and nutrient removal efficiency of duckweed (lemna minor) from synthetic and dumpsite leachate under artificial and natural conditions. PLOS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams III, W.W.; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Frontiers in Plant Science 2020, 11, 480. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.; Appenroth, K.-J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2015, 98, 1127–1154. [Google Scholar] [CrossRef]
- Ullah, H.; Gul, B.; Khan, H.; Zeb, U. Effect of salt stress on proximate composition of duckweed (Lemna minor L.). Heliyon 2021, 7, e07399. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, Z.; Xue, Y.; Yang, R.; Ma, X.; Sun, J.; Wang, J.; Lin, S.; Wang, W.; Yang, L.; et al. Mechanism of calcium signal response to cadmium stress in duckweed. Plant Signal. Behav. 2022, 17, 2119340. [Google Scholar] [CrossRef]
- Sree, K.S.; Keresztes, Á.; Mueller-Roeber, B.; Brandt, R.; Eberius, M.; Fischer, W.; Appenroth, K.J. Phytotoxicity of cobalt ions on the duckweed Lemna minor–Morphology, ion uptake, and starch accumulation. Chemosphere 2015, 131, 149–156. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Y.; Jin, Y.; Yi, Z.; Li, J.; Du, A.; He, K.; Huang, Y.; Zhao, H. Ammonium detoxification mechanism of ammonium-tolerant duckweed (Landoltia punctata) revealed by carbon and nitrogen metabolism under ammonium stress. Environ. Pollut. 2021, 277, 116834. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J.; Ziegler, P.; Sree, K.S. Accumulation of starch in duckweeds (Lemnaceae), potential energy plants. Physiol. Mol. Biol. Plants 2021, 27, 2621–2633. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Zhang, Y.; Huang, M.; Zhao, H. The influence of different plant hormones on biomass and starch accumulation of duckweed: A renewable feedstock for bioethanol production. Renew. Energy 2019, 138, 659–665. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Cytokinin-induced growth in the duckweeds Lemna gibba and Spirodela polyrhiza. Plant Growth Regul. 2018, 86, 477–486. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Yan, C.; Wu, J.; Wang, Y.; Ren, Q.; Wang, S.; Ma, X.; Zhao, L.; Sun, J. Regeneration of duckweed (Lemna turonifera) involves genetic molecular regulation and cyclohexane release. PLOS ONE 2022, 17, e0254265. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Fang, Y.; Liu, Y.; Jin, Y.; Sun, J.; Tao, X.; Ma, X.; He, K.; Zhao, H. Using proteomic analysis to investigate uniconazole-induced phytohormone variation and starch accumulation in duckweed (Landoltia punctata). BMC Biotechnol. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Tao, X.; Fang, Y.; Xiao, Y.; Jin, Y.-L.; Ma, X.-R.; Zhao, Y.; He, K.-Z.; Zhao, H.; Wang, H.-Y. Comparative transcriptome analysis to investigate the high starch accumulation of duckweed (Landoltia punctata) under nutrient starvation. Biotechnol. Biofuels 2013, 6, 72–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ding, Z.; Sun, X.; Zhang, J. Physiological and Transcriptomic Analysis Reveals Distorted Ion Homeostasis and Responses in the Freshwater Plant Spirodela polyrhiza L. under Salt Stress. Genes 2019, 10, 743. [Google Scholar] [CrossRef]
- Yang, L.; Yao, J.; Sun, J.; Shi, L.; Chen, Y.; Sun, J. The Ca2+ signaling, Glu, and GABA responds to Cd stress in duckweed. Aquat. Toxicol. 2020, 218, 105352. [Google Scholar] [CrossRef] [PubMed]
- Razinger, J.; Dermastia, M.; Koce, J.D.; Zrimec, A. Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environ. Pollut. 2008, 153, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Baggs, E.L.; Tiersma, M.B.; Abramson, B.W.; Michael, T.P.; Krasileva, K.V. Characterization of defense responses against bacterial pathogens in duckweeds lacking EDS1. New Phytol. 2022, 236, 1838–1855. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Taoka, K.-I.; Hosaka, A.; Tanaka, K.; Kobayashi, H.; Muranaka, T.; Toyooka, K.; Oyama, T.; Tsuji, H. Characterization of Frond and Flower Development and Identification of FT and FD Genes From Duckweed Lemna aequinoctialis Nd. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Xu, H.; Yu, C.; Xia, X.; Li, M.; Li, H.; Wang, Y.; Wang, S.; Wang, C.; Ma, Y.; Zhou, G. Comparative transcriptome analysis of duckweed (Landoltia punctata) in response to cadmium provides insights into molecular mechanisms underlying hyperaccumulation. Chemosphere 2018, 190, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fang, Y.; Huang, J.; Zhao, Y.; Li, Q.; Lai, F.; Xu, Y.; Tian, X.; He, K.; Jin, Y.; Tan, L. Duckweed systems for eutrophic water purification through converting wastewater nutrients to high-starch biomass: comparative evaluation of three different genera (Spirodela polyrhiza, Lemna minor and Landoltia punctata) in monoculture or polyculture. RSC advances 2023, 8, 17927–17937. [Google Scholar] [CrossRef]
- Körner, S.; Vermaat, J.E.; Veenstra, S. The capacity of duckweed to treat wastewater: ecological considerations for a sound design. Journal of environmental quality 2003, 32, 1583–1590. [Google Scholar] [CrossRef]
- A Ubuza, L.J.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; O Arazo, R. Assessment of the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation in stationary and recirculated set-ups. Environ. Eng. Res. 2019, 25, 977–982. [Google Scholar] [CrossRef]
- Ahmadi, A.W.; Dursun, S. Assessing the Efficiency and Role of Duckweed (Lemna Minor) in the Removal of Pollutants from Wastewater Treatment Plant Secondary Clarifier Tanks: A Comprehensive Review. Central Asian J. Water Res. 2024. [Google Scholar] [CrossRef]
- An, D.; Li, C.; Zhou, Y.; Wu, Y.; Wang, W. Genomes and Transcriptomes of Duckweeds. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Zhu, Q.; Tang, X.; Zhao, Q. Transcriptomic and physiological analysis of common duckweed Lemna minor responses to NH4 + toxicity. BMC Plant Biol. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Li, C.; Zhou, Y.; Wu, Y.; Wang, W. Genomes and Transcriptomes of Duckweeds. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Harkess, A.; Bewick, A.J.; Lu, Z.; Fourounjian, P.; Michael, T.P.; Schmitz, R.J.; Meyers, B.C. The unusual predominance of maintenance DNA methylation in Spirodela polyrhiza. G3 Genes|Genomes|Genetics 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeck, A.; Horemans, N.; Monsieurs, P.; Cao, H.X.; Vandenhove, H.; Blust, R. The first draft genome of the aquatic model plant Lemna minor opens the route for future stress physiology research and biotechnological applications. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Hoang, P.T.N.; Fiebig, A.; Novák, P.; Macas, J.; Cao, H.X.; Stepanenko, A.; Chen, G.; Borisjuk, N.; Scholz, U.; Schubert, I. Chromosome-scale genome assembly for the duckweed Spirodela intermedia, integrating cytogenetic maps, PacBio and Oxford Nanopore libraries. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio Sequencing and Its Applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef]
- Schöpflin, R.; Melo, U.S.; Moeinzadeh, H.; Heller, D.; Laupert, V.; Hertzberg, J.; Holtgrewe, M.; Alavi, N.; Klever, M.-K.; Jungnitsch, J.; et al. Integration of Hi-C with short and long-read genome sequencing reveals the structure of germline rearranged genomes. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Belton, J.-M.; McCord, R.P.; Gibcus, J.H.; Naumova, N.; Zhan, Y.; Dekker, J. Hi–C: A comprehensive technique to capture the conformation of genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Y.; Xu, S.; Tang, X.; Zhao, J.; Yu, C.; He, G.; Xu, H.; Wang, S.; Tang, Y.; et al. Efficient genetic transformation and CRISPR /Cas9-mediated genome editing in Lemna aequinoctialis. Plant Biotechnol. J. 2019, 17, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Sun, H.-J.; Oh, M.J.; Song, I.-J.; Kim, M.; Sin, H.-S.; Goh, C.-H.; Kim, Y.-W.; Lim, P.-O.; Lee, H.-Y.; et al. Expression of the protective antigen for PEDV in transgenic duckweed, Lemna minor. Hortic. Environ. Biotechnol. 2011, 52, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, S.; Fang, Y.; Liu, P.; Hu, Z.; Jin, Y.; Yi, Z.; He, K.; Li, X.; Zhao, L.; et al. Rapid and Highly Efficient Genetic Transformation and Application of Interleukin-17B Expressed in Duckweed as Mucosal Vaccine Adjuvant. Biomolecules 2022, 12, 1881. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, X.; Anaokar, S.; Shi, H.; Dahl, W.B.; Cai, Y.; Luo, G.; Chai, J.; Cai, Y.; Mollá-Morales, A.; et al. Engineering triacylglycerol accumulation in duckweed (Lemna japonica). Plant Biotechnol. J. 2022, 21, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Chanroj, S.; Jaiprasert, A.; Issaro, N. A novel technique for recombinant protein expression in duckweed (Spirodela polyrhiza) turions. J. Plant Biotechnol. 2021, 48, 156–164. [Google Scholar] [CrossRef]
- Yamamoto, Y.T.; Rajbhandari, N.; Lin, X.; Bergmann, B.A.; Nishimura, Y.; Stomp, A.-M. Genetic transformation of duckweed Lemna gibba and Lemna minor. In Vitro Cell Dev. Biol. Plant 2001, 37, 349–353. [Google Scholar] [CrossRef]
- Firsov, A.; Tarasenko, I.; Mitiouchkina, T.; Shaloiko, L.; Kozlov, O.; Vinokurov, L.; Rasskazova, E.; Murashev, A.; Vainstein, A.; Dolgov, S. Expression and Immunogenicity of M2e Peptide of Avian Influenza Virus H5N1 Fused to Ricin Toxin B Chain Produced in Duckweed Plants. Front. Chem. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J.J.; Himmel, M.E.; Skory, C.D.; Adney, W.S.; Thomas, S.R.; Tisserat, B.; Nishimura, Y.; Yamamoto, Y.T. Expression and characterization of Acidothermus cellulolyticus E1 endoglucanase in transgenic duckweed Lemna minor 8627. Bioresour. Technol. 2007, 98, 2866–2872. [Google Scholar] [CrossRef]
- Hemalatha, M.; Mohan, S.V. Duckweed biorefinery – Potential to remediate dairy wastewater in integration with microbial protein production. Bioresour. Technol. 2021, 346, 126499. [Google Scholar] [CrossRef]
- Sońta, M.; Łozicki, A.; Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; van Pruissen, G.W.P.; Cornelissen, R.L. Duckweed from a Biorefinery System: Nutrient Recovery Efficiency and Forage Value. Energies 2020, 13, 5261. [Google Scholar] [CrossRef]
- Peterson, A.; Kishchenko, O.; Kuhlmann, M.; Tschiersch, H.; Fuchs, J.; Tikhenko, N.; Schubert, I.; Nagel, M. Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants. Plants 2023, 12, 3302. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Wingate, V. , Biolex Therapeutics Inc, 2012. Methods and compositions for the cryopreservation of duckweed. U.S. Patent Application 13/379, 959.
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, H.; Stomp, A.-M.; Cheng, J.J. The production of duckweed as a source of biofuels. Biofuels 2012, 3, 589–601. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).