Submitted:
29 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
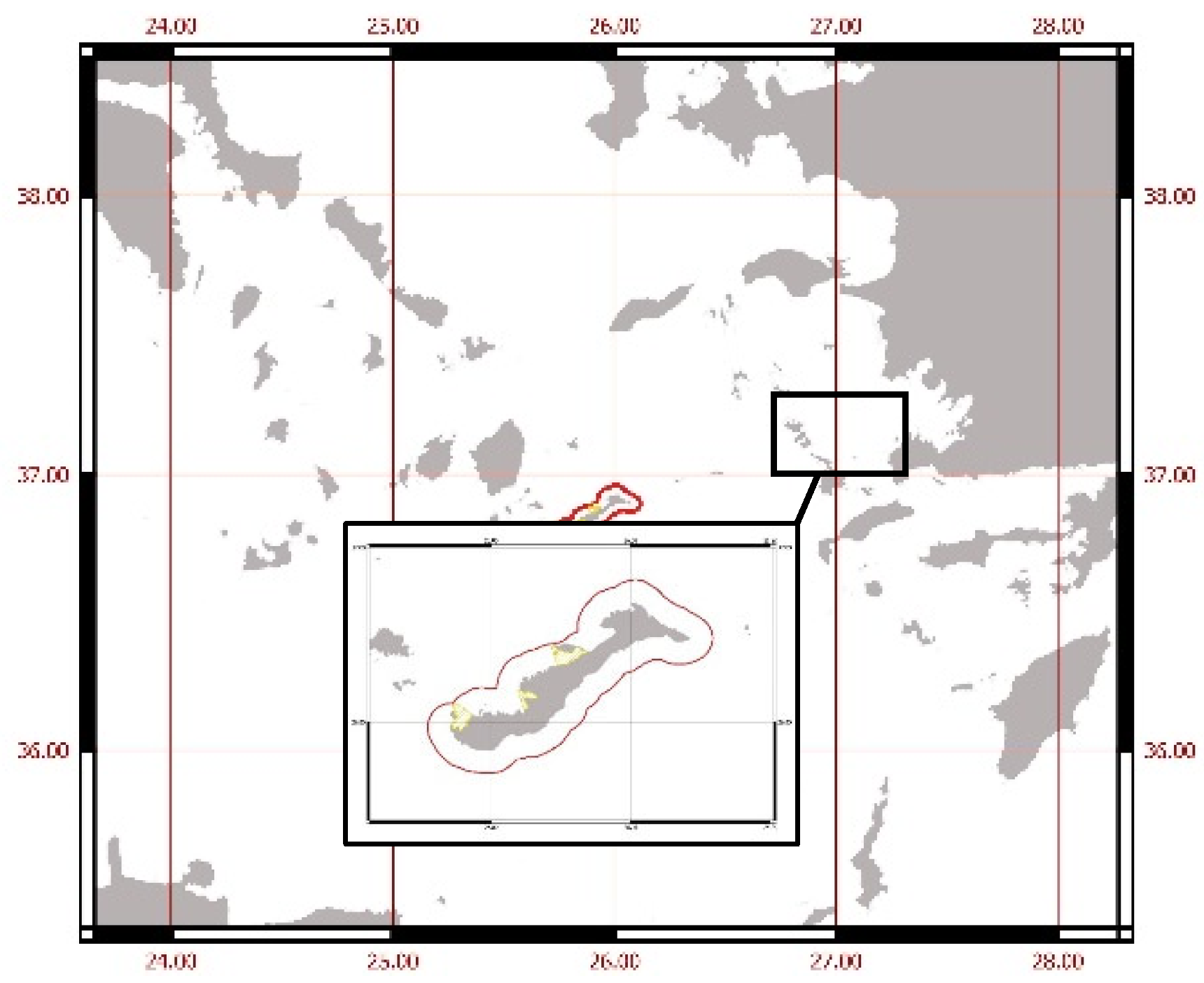
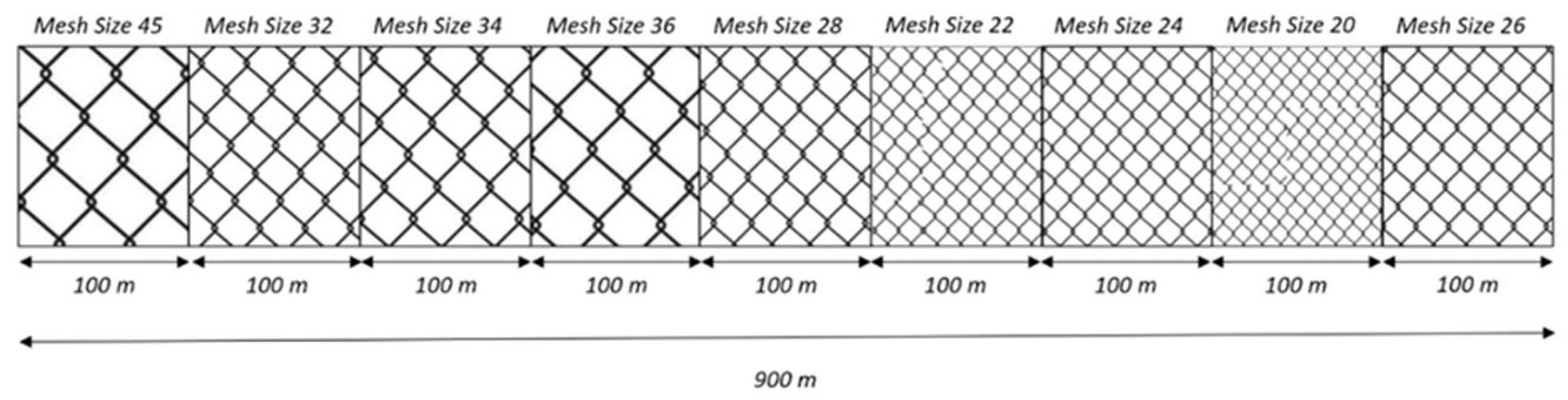
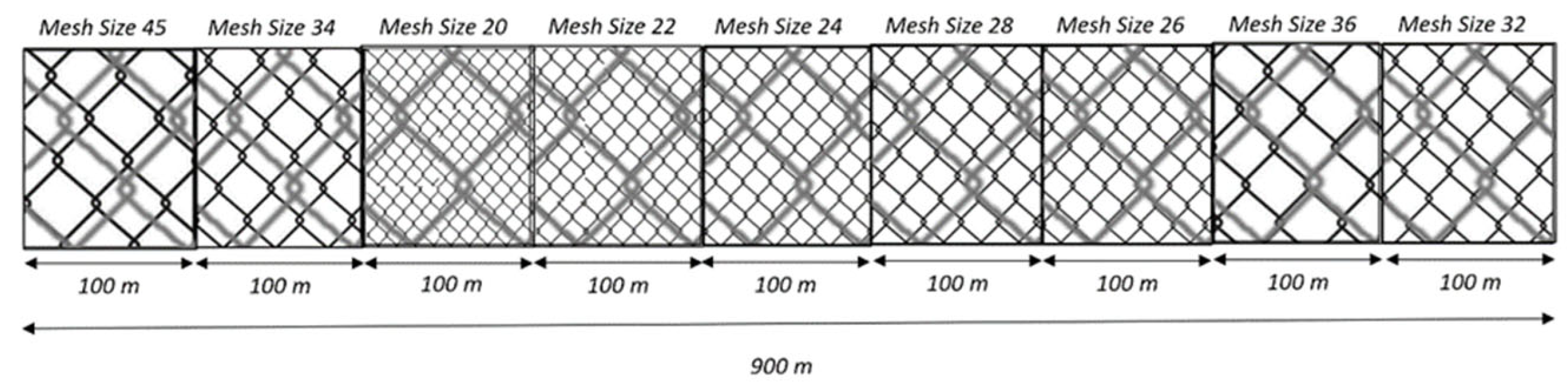
Sampling Methodology
Identification of the Species
Stomach Content and Sex Maturity
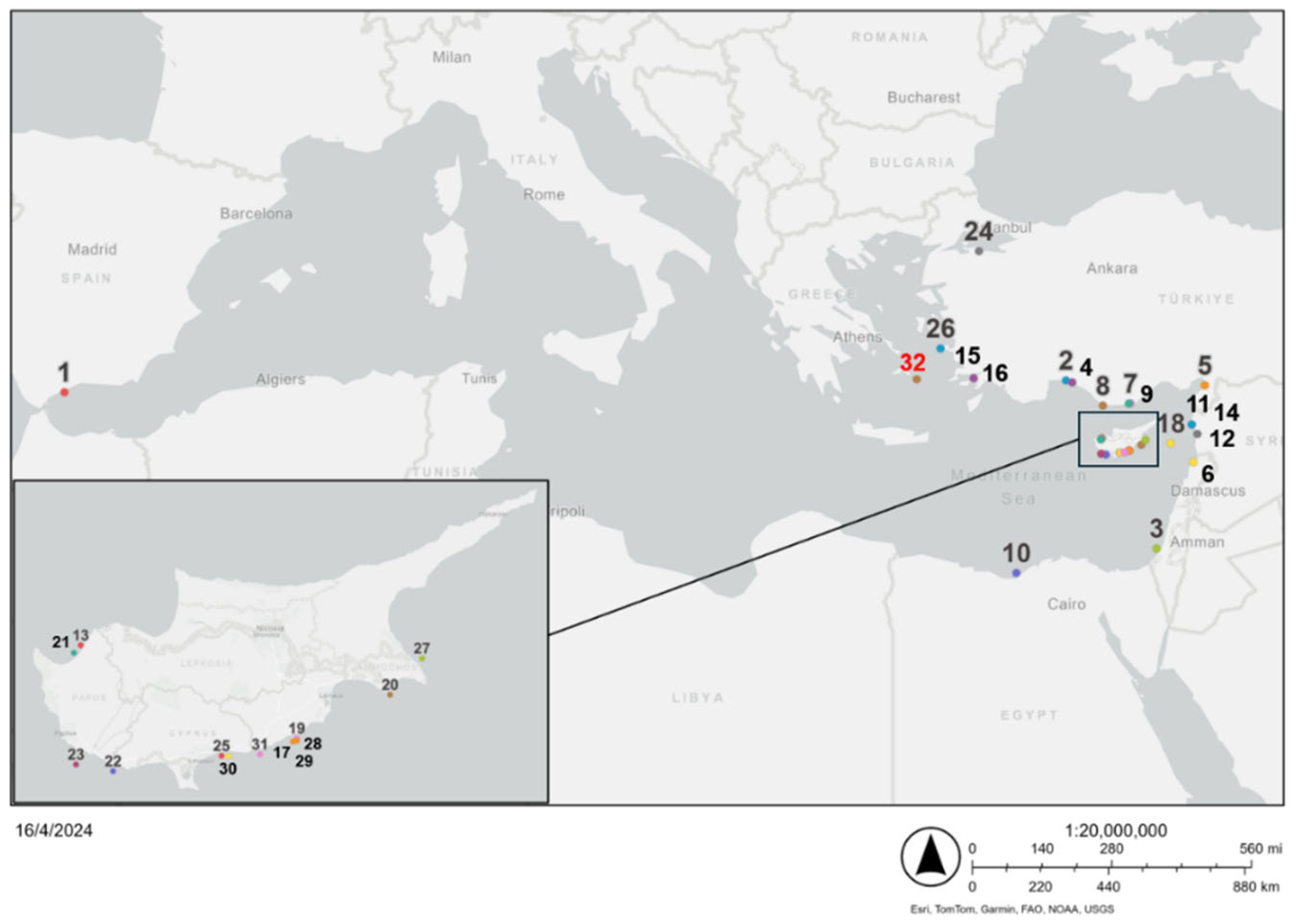
Distibution of F.petimba in the Mediterranean Sea
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Agency, E. e. Climate change, impacts and vulnerability in Europe EEA 2012. https://www.eea.europa.eu/publications/climate-impacts-and-vulnerability-2012.
- Lewis, S. L.; Maslin, M. A. Defining the Anthropocene. Nature 2015, 519, 171–180. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P. E.; Simberloff, D.; Bacher, S.; Blackburn, T. M.; Carlton, J. T.; Dawson, W.; Essl, F.; Foxcroft, L. C.; Genovesi, P.; et al. Scientists' warning on invasive alien species. Biol Rev Camb Philos Soc 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Genner, M. J.; Sims, D. W.; Wearmouth, V. J.; Southall, E. J.; Southward, A. J.; Henderson, P. A.; Hawkins, S. J. Regional climatic warming drives long-term community changes of British marine fish. Proc Biol Sci 2004, 271, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Munday, P. L.; Jones, G. P.; Pratchett, M. S.; Williams, A. J. Climate change and the future for coral reef fishes. Fish and Fisheries 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Simpson, Stephen D. ; Jennings, S.; Johnson, Mark P.; Blanchard, Julia L.; Schön, P.-J.; Sims, David W.; Genner, Martin J. Continental Shelf-Wide Response of a Fish Assemblage to Rapid Warming of the Sea. Current Biology 2011, 21, 1565–1570. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing Down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Jennings, S.; Alvsvåg, J.; Cotter, A. J. R.; Ehrich, S.; Greenstreet, S. P. R.; Jarre-Teichmann, A.; Mergardt, N.; Rijnsdorp, A. D.; Smedstad, O. Fishing effects in northeast Atlantic shelf seas: patterns in fishing effort, diversity and community structure. III. International trawling effort in the North Sea: an analysis of spatial and temporal trends. Fisheries Research 1999, 40, 125–134. [Google Scholar] [CrossRef]
- Daan, N.; Gislason, H.; G. Pope, J.; C. Rice, J. Changes in the North Sea fish community: evidence of indirect effects of fishing? ICES Journal of Marine Science 2005, 62, 177–188. [Google Scholar] [CrossRef]
- Jones, G. P.; McCormick, M. I.; Srinivasan, M.; Eagle, J. V. Coral decline threatens fish biodiversity in marine reserves. Proceedings of the National Academy of Sciences 2004, 101, 8251–8253. [Google Scholar] [CrossRef]
- Airoldi, L.; Balata, D.; Beck, M. W. The Gray Zone: Relationships between habitat loss and marine diversity and their applications in conservation. Journal of Experimental Marine Biology and Ecology 2008, 366, 8–15. [Google Scholar] [CrossRef]
- WILSON, S. K.; FISHER, R.; PRATCHETT, M. S.; GRAHAM, N. A. J.; DULVY, N. K.; TURNER, R. A.; CAKACAKA, A.; POLUNIN, N. V. C.; RUSHTON, S. P. Exploitation and habitat degradation as agents of change within coral reef fish communities. Global Change Biology 2008, 14, 2796–2809. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R. A.; Mittermeier, C. G.; da Fonseca, G. A. B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C. N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M. E.; Garcia Raso, J. E.; Bianchi, C. N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterranean Marine Science 2010, 11. [Google Scholar] [CrossRef]
- Costello, M. J.; Coll, M.; Danovaro, R.; Halpin, P.; Ojaveer, H.; Miloslavich, P. A Census of Marine Biodiversity Knowledge, Resources, and Future Challenges. PLOS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, A. O. Biotic Invasions in a Mediterranean Lagoon. Biological Invasions 2000, 2, 165–176. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Marchini, A.; Cantone, G.; Castelli, A.; Chimenz, C.; Cormaci, M.; Froglia, C.; Furnari, G.; Gambi, M. C.; Giaccone, G.; et al. Alien species along the Italian coasts: an overview. Biological Invasions 2011, 13, 215–237. [Google Scholar] [CrossRef]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M. C. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Mar Pollut Bull 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Goren, M.; Galil, B. S. A review of changes in the fish assemblages of Levantine inland and marine ecosystems following the introduction of non-native fishes. Journal of Applied Ichthyology 2005, 21, 364–370. [Google Scholar] [CrossRef]
- Harmelin, M.; Bitar, G.; Harmelin, J.-G.; Monestiez, P. The littoral fish community of the Lebanese rocky coast (eastern Mediterranean Sea) with emphasis on Red Sea immigrants. Biological Invasions 2005, 7, 625–637. [Google Scholar] [CrossRef]
- Parravicini, V.; Azzurro, E.; Kulbicki, M.; Belmaker, J. Niche shift can impair the ability to predict invasion risk in the marine realm: an illustration using Mediterranean fish invaders. Ecology Letters 2015, 18, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Raitsos, D.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci, A.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnology and oceanography 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Kalogirou, S.; Azzurro, E.; Bariche, M. The Ongoing Shift of Mediterranean Coastal Fish Assemblages and the Spread of Non-Indigenous Species. 2012.
- Cinar, M. E.; Bilecenoglu, M.; Ozturk, Β.; Katagan, Τ.; Yokes, Μ. Β.; Aysel, V.; Dagli, E.; Acik, S.; Ozcan, T.; Erdogan, H. An updated review of alien species on the coasts of Turkey. Mediterranean Marine Science 2011, 12, 257–315. [Google Scholar] [CrossRef]
- Golani, D. The Sandy Shore of the Red Sea-Launching Pad for Lessepsian (Suez Canal) Migrant Fish Colonizers of the Eastern Mediterranean. Journal of Biogeography 1993, 20, 579–585. [Google Scholar] [CrossRef]
- Galil, B. S.; Mienis, H. K.; Hoffman, R.; Goren, M. Non-indigenous species along the Israeli Mediterranean coast: tally, policy, outlook. Hydrobiologia 2021, 848, 2011–2029. [Google Scholar] [CrossRef]
- Por, F. D. Lessepsian Migration: The Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal; Springer, 1978. [CrossRef]
- Stern, N. I. R.; Paz, G. U. Y.; Yudkovsky, Y.; Lubinevsky, H.; Rinkevich, B. The arrival of a second ‘Lessepsian sprinter’? A first record of the red cornetfish Fistularia petimba in the Eastern Mediterranean. Mediterranean Marine Science 2018, 18, 524–528. [Google Scholar] [CrossRef]
- Ragheb, E. Morphometric and meristic characteristics of the first record Fistularia petimba (Lacepède, 1803) and Fistularia commersonii (Rüppell, 1838) (Piscès: Fistulariidae) from the Egyptian Mediterranean waters (West Alexandria). The Egyptian Journal of Aquatic Research 2022, 48, 143–150. [Google Scholar] [CrossRef]
- Fritzsche, R. A. A REVIEW OF THE CORNETFISHES, GENUS FISTULARIA (FISTULARIIDAE), WITH A DISCUSSION OF INTRAGENERIC RELATIONSHIPS AND ZOOGEOGRAPHY. BULLETIN OF MARINE SCIENCE 1976, 26, 106–204. [Google Scholar]
- Fischer, W. B.; G., *!!! REPLACE !!!* (Eds.) ;Fisheries and Aquaculture Management Division. FAO Species identification sheets for fishery purposes. Western Indian Ocean: fishing area 51. 1984.
- Sánchez-Tocino, L.; Hidalgo, F.; Pontes, M. Primera cita de Fistularia commersonii Ruppell, 1838 (Osteichtyes: Fistulariidae) en aguas mediterráneas de la Península Ibérica. Zoologica Baetica 2007, 18, 79–84. [Google Scholar]
- Kalogirou, S.; Corsini-Foka, M.; Kondylatos, G.; Wennhage, H. Diet of the invasive piscivorous fish Fistularia commersonii in a recently colonized area of eastern Mediterranean. Biological Invasions 2007, 9, 887–896. [Google Scholar] [CrossRef]
- Bañón, R.; Sande, C. First record of the red cornetfish Fistularia petimba (Syngnathiformes: Fistularidae) in Galician waters: a northernmost occurrence in the eastern Atlantic. Journal of Applied Ichthyology 2008, 24, 106–107. [Google Scholar] [CrossRef]
- Cárdenas, S.; Alvarez-Berastegui, D.; Ortiz, J. M. First record of Fistularia petimba Laccepéde, 1803 (Pisces FIstulariidae) off the coast of Cadiz (southern Iberian Peninsula). Boletín del Instituto Español de Oceanografía 1997, 13, 83–86. [Google Scholar]
- Ünlüoğlu, A.; Akalın, S.; Dal, İ.; Tıraşın, E.; Aydın, C. First record of red cornetfish Fistularia petimba (Syngnathiformes: Fistulariidae) from Antalya and İskenderun Bays along Turkish coasts of the Mediterranean Sea. Journal of Applied Ichthyology 2018, 34, 977–980. [Google Scholar] [CrossRef]
- Soydemir Çiftçi, N.; Ayas, D.; Doğangün, M. New locality record for Fistularia petimba Lacepède, 1803 from the Northeastern Mediterranean Sea (Mersin Bay). 2019.
- Karan, S.; Uyan, A.; Doğdu, S.; Gürlek, M.; Ergüden, D.; Turan, C. Genetic confirmation of Red cornetfish, Fistularia petimba (Syngnathiformes: Fistularidae) occurrence in Turkish marine waters. 2019, 4, 125-129.
- Frost, W. E. Observations on the Food of Eels (Anguilla anguilla) from the Windermere Catchment Area. Journal of Animal Ecology 1946, 15, 43–53. [Google Scholar] [CrossRef]
- Hyslop, E. J. Stomach contents analysis—a review of methods and their application. Journal of Fish Biology 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Hynes, H. B. N. THE FOOD OF THE FRESHWATER STICKLEBACKS (GASTEROSTEUS ACULIEATUS AND PYGOSTEUS PUNGITIUS) WITH A REVIEW OF METHODS USED N THE STUDIES OF THE FOOD OF FISHES. Journal of Animal Ecology 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Brown-Peterson, N. J.; Wyanski, D. M.; Saborido-Rey, F.; Macewicz, B. J.; Lowerre-Barbieri, S. K. A Standardized Terminology for Describing Reproductive Development in Fishes. Marine and Coastal Fisheries 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Ungaro, N. Field manual on macroscopic identification of maturity stages for the Mediterranean fishery resources.; Rome, 2008. https://www.faomedsudmed.org/pdf/publications/TD21.pdf.
- Follesa, C. , Carbonara, P. Atlas of the maturity stages of Mediterranean fishery resources, /: Italy, 2019. https, 2019. [Google Scholar]
- Esri. ArcGIS Online | Cloud-Based GIS Mapping Platform. 2018. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-online/overview (accessed on 26 March 2024).
- Floerl, O.; Inglis, G. J. Starting the invasion pathway: the interaction between source populations and human transport vectors. Biological Invasions 2005, 7, 589–606. [Google Scholar] [CrossRef]
- Ferrario, J.; Caronni, S.; Occhipinti-Ambrogi, A.; Marchini, A. Role of commercial harbours and recreational marinas in the spread of non-indigenous fouling species. Biofouling 2017, 33, 651–660. [Google Scholar] [CrossRef]
- Drake, J. M.; Lodge, D. M. Global hot spots of biological invasions: evaluating options for ballast–water management. Proceedings of the Royal Society of London. Series B: Biological Sciences 2004, 271, 575–580. [Google Scholar] [CrossRef]
- Galil, B. S.; Boero, F.; Campbell, M. L.; Carlton, J. T.; Cook, E.; Fraschetti, S.; Gollasch, S.; Hewitt, C. L.; Jelmert, A.; Macpherson, E.; et al. ‘Double trouble’: the expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biological Invasions 2015, 17, 973–976. [Google Scholar] [CrossRef]
- Ulman, A.; Ferrario, J.; Occhipinti, A.; Arvanitidis, C.; Bandi, A.; Bertolino, M.; Bogi, C.; Chatzigeorgiou, G.; Çiçek, B.; Içek, C.; et al. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S. , Wallentinus, I. , Zenetos,A., Leppäkoski,E., Çinar,M.E., Oztürk,B., Grabowski,M., Golani, D., Cardoso,A. C. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review Aquatic Invasions 2014, 9, 391–423. [Google Scholar]
- Crocetta, F.; Al Mabruk, S. A. A.; Azzurro, E.; Bakiu, R.; Bariche, M.; Batjakas, I. E.; Bejaoui, T.; Ben Souissi, J.; Cauchi, J.; Corsini-Foka, M.; et al. “New Alien Mediterranean Biodiversity Records” (November 2021). Mediterranean Marine Science 2021, 22, 724–746. [Google Scholar] [CrossRef]
- Chirine, H.; Ibrahim, A.; Alshawy, F. First record of Red cornetfish, Fistularia petimba Lacepède, 1803 (Actinopterygii: Fistulariidae) from the Syrian coast. International Journal of Aquatic Biology 2019, 7, 175–179. [Google Scholar] [CrossRef]
- Ibrahim, A.; Alshawy, F.; Hussein, C. Confirmation records and new distribution of the red cornet fish fistularia petimba lacepède, 1803 (actinopterygii: fistulariidae) in the Syrian Marine Waters (Eastern Mediterranean). Species 2020, 21, 95–100. [Google Scholar]
- Dragicevic, B.; Anadoli, O.; Angel, D.; Benabdi, M.; Bitar, G.; Castriota, L.; Crocetta, F.; Deidun, A.; Dulčić, J.; Edelist, D. New Mediterranean Biodiversity Records (19). 2019. 20 December.
- Cerim, H.; Yapıcı, S.; Gülşahin, A.; Soykan, O.; Bilge, G. The First Record of the Red Cornetfısh (Fistularia petimba Lacepède, 1803) in the Aegean Sea. Düzce Üniversitesi Bilim ve Teknoloji Dergisi 2021, 9, 607–615. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Resaikos, V.; Petrou, A. A preliminary assessment of Fistularia petimba (Lacepède, 1803) migration in the Mediterranean Sea: historical and new data from Cyprus (Levantine Sea) with biological notes. Mediterranean Marine Science 2023, 24, 446–453. [Google Scholar] [CrossRef]

| Morphometrics | Measurement (mm or g) |
|---|---|
| Total Length without filament (TL) | 395.00 |
| Total Length with filament (TLf) | 530.00 |
| Filament Length (fL) | 124.23 |
| Standard Length (SL) | 378.00 |
| Fork Length (FL) | 383.00 |
| Body Deth (BD) | 7.98 |
| Head Length (HL) | 142.00 |
| Eye Diameter (ED) | 10.97 |
| Snout Length (SN) | 114.00 |
| Dorsal Fin Length (DFL) | 15.41 |
| Dorsal Fin Height (DFH) | 28.89 |
| Pectoral Fin Length (PFL) | 6.66 |
| Pelvic Fin Height (PFH) | 16.81 |
| Dorsal Fin Length (PvFL) | 2.54 |
| Pelvic Fin Height (PvFH) | 6.83 |
| Caudal Fin Length (CFL) | 19.01 |
| Caudal Fin Height (CFH) | 5.10 |
| Anal Fin Length (AFL) | 15.16 |
| Anal Fin Height (AFH) | 27.37 |
| Pre-dorsal Fin Length (pDFL) | 67.00 |
| Pre-pectoral Fin Length (pPFL) | 319.00 |
| Pre-pelvic Fin Length (pPvFL) | 190.00 |
| Pre-anal Fin Length (pAFL) | 310.00 |
| Total Wet Weight (TWW) | 34.00 |
| No | Location | Latitude | Longitude | Date (capture) | Depth (m) | Gear Type | Sample size |
|---|---|---|---|---|---|---|---|
| 1 | Cadiz, Spain [36] | 36.455097 | -4.703372 | 23/06/1996 | 50 | Gillnet | 1 |
| 2 | Antalya Bay, Turkey [37] | 36.793556 | 31.209167 | 28/10/2016 | 35 – 43 | Bottom trawl | 1 |
| 3 | Ashdod, Israel [29] | 31.813950 | 34.459717 | 12/11/2016 | 80 | Bottom trawl | 1 |
| 4 | Antalya Bay, Turkey [37] | 36.737417 | 31.434361 | 26/11/2016 | 30 | Bottom trawl | 1 |
| 5 | Iskenderun, Turkey [37] | 36.654400 | 36.186183 | 21/05/2017 | 35 – 38 | Bottom trawl | 2 |
| 6 | Tripoli, Lebanon [53] | 34.410000 | 35.770000 | 15/11/2017 | N/A | Gillnet | 1 |
| 7 | Mersin Bay, Turkey [39] | 36.128833 | 33.520667 | 22/11/2017 | 95 | Bottom trawl | 1 |
| 8 | Antalya Bay, Turkey [39] | 36.061867 | 32.534233 | 09/01/2018 | 70 | Bottom trawl | 2 |
| 9 | Büyükeceli Coast (Mersin Bay)Turkey [38] | 36.123139 | 33.467944 | 05/10/2018 | 150 | Bottom trawl | 2 |
| 10 | Egypt [30] | El-Hamam - Sidi Kirayr. | 09/03/2019 | 40 – 60 | Bottom trawl | 1 | |
| 11 | Lattakia, Syria [54] | 35.518325 | 35.713492 | 29/07/2019 | 45 | Gillnet | 1 |
| 12 | Lattakia, Syria [55] | 35.243086 | 35.920000 | 24/09/2019 | 30 | Gillnet | 1 |
| 13 | Gialia, Cyprus [56] | 35.110000 | 32.490000 | 26/09/2019 | 55 | Gillnet | 1 |
| 14 | Banyas, Syria [55] | 35.518325 | 35.713492 | 29/09/2019 | 45 | Gillnet | 2 |
| 15 | Gökova Bay, Turkey [57] | 36.857889 | 27.896556 | 19/10/2019 | 15 – 20 | Longline | 1 |
| 16 | Güllük Bay, Turkey [57] | 36.857883 | 27.896561 | 17/11/2019 | 65 | Bottom trawl | 4 |
| 17 | Cyprus [58] | 34.747367 | 33.463400 | 14/07/2020 | 55 | Bottom trawl | 3 |
| 18 | Cyprus [58] | 34.964500 | 34.964500 | 15/07/2020 | 48 | Bottom trawl | 1 |
| 19 | Cyprus [58] | 34.759617 | 33.480650 | 16/07/2020 | 33 | Bottom trawl | 1 |
| 20 | Cyprus [58] | 34.924100 | 33.908050 | 24/07/2020 | 79 | Bottom trawl | 11 |
| 21 | Cyprus [58] | 35.081733 | 32.458700 | 24/07/2020 | 43 | Bottom trawl | 29 |
| 22 | Cyprus [58] | 34.635717 | 32.638517 | 27/03/2021 | 46 | Bottom trawl | 10 |
| 23 | Cyprus [58] | 34.661300 | 32.468650 | 27/03/2021 | 93 | Bottom trawl | 4 |
| 24 | Bandirma Bay, Turkey [53] | 40.416950 | 28.084000 | 11/06/2021 | 32 | Trammel net | 1 |
| 25 | Cyprus [58] | 34.693983 | 33.135567 | 04/08/2021 | 44 | Bottom trawl | 26 |
| 26 | Samos, Greece [53] | 37.706583 | 26.708783 | 07/11/2021 | 20 | Trammel net | 1 |
| 27 | Cyprus [58] | 35.060833 | 34.054383 | 08/08/2021 | 86 | Bottom trawl | 4 |
| 28 | Cyprus [58] | 34.750917 | 33.480933 | 08/08/2021 | 55 | Bottom trawl | 7 |
| 29 | Cyprus [58] | 34.750917 | 33.480933 | 08/08/2021 | 33 | Bottom trawl | 2 |
| 30 | Cyprus [58] | 34.692267 | 33.166750 | 08/08/2021 | 56 | Bottom trawl | 1 |
| 31 | Cyprus [58] | 34.699333 | 33.311817 | 13/09/2021 | 13 | Trammel net | 1 |
| 32 | Amorgos, Greece (current study) | 36.82760281 | 25.85879922 | 28/05/2023 | 24.5 - 30.6 | Trammel net | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





