1. Introduction
The eggs of teleosts are surrounded by a non-cellular egg envelope, the zona radata (ZR), which is usually divided into two or three layers (Anderson, 1974; Flegler 1977; Kobayashi and Yamamoto, 1981; Cotelli et al., 1986; Li et al., 2000). The inner layer is composed of a thick fibrous material, and the outer layer of a thin, highly electron-dense material (Wourms, 1976; Choi 2021). These ZR serve to protect against physical impacts and chemical penetration from the external environment, and their structure and thickness are species-specific and influenced by habitat and spawning grounds (Ivankov and Kurdyayeva, 1973; Kim et al., 1996; Riehl and Patzner, 1998). The subfamily Cultrinae of the family Cyprinidae has approximately 18 genera and 80 species known worldwide, mostly inhabiting water systems in East Asia, including China, Mongolia, Russia, Taiwan, and Korea (Dai and Yang, 2003; Dai et al., 2005; Nelson et al., 2016). Four species from three genera, Erythroculter, Culter, and Hemiculter, have been reported to inhabit Korea (Kim and Park, 2002; Chae et al., 2019).
Since the recent introduction of H. leucisculus for aquaculture purposes in Central Asia along the Caspian Sea coast, its population has rapidly increased, causing significant effects on freshwater ecosystems (Coad and Hussain, 2007; Jouladeh-Roudbar et al., 2015; Mousavji-Sabet et al., 2019). In Korea, it has been reported that E. erythropterus and Opsariichthys uncirostris amurensis have invaded the Nakdonggang River from their original habitat, adversely affecting freshwater ecosystems such as that of largemouth bass and bluegill, which are alien species (ME, 2016). E. erythropterus is recognized in China and Taiwan under four different genera and scientific names—Chanodichthys erythropterus, C. erythropterus, E. erythropterus, Culterichthys erythropterus, and C. brevicauda are recognized as C. alburnus, C. brevicauda, and E. adokii (Dai and Yang, 2003; Dai et al, 2005; Chen et al., 2017; Wang et al., 2021; Gu et al., 2022). In both these arguments, Cultrinae is creating a social problem in Korea. First, the anthropogenic introduction of E. erythropterus into the habitat of endangered C. brevicauda has reduced the population of C. brevicauda and established it as the top predator in each stream, much like largemouth bass. Second, there is still global confusion regarding the scientific names. This study aimed to preserve C. brevicauda populations in the face of the spread of E. erythropterus and to distinguish the three morphologically indistinguishable species using egg envelopes.
2. Materials and Methods
A gravid female of three species was collected using a triangle net from the Geumgang and Yeongsangang Rivers from May to July 2022 (spawning season)—E. erythropterus (36°18″14′ N, 126°55″18′ E, Hoam-ri, Gyuam-myeon, Buyeo-gun, Chungcheongnam-do, South Korea) in Geumgang River, and Culter brevicauda and Hemiculter eigenmanni (35°0″2′N, 126°41″6′E, Godong-ri, Geumcheon-myeon, Naju-si, Jeollanam-do, South Korea) in Yeongsangang River. The permission to catch C. brevicauda, an endangered species, was granted by the Ministry of Environment, South Korea (April 2022, license number: No. 2022-22). The study followed the Guide for the Care and Use of Laboratory Animals (2011), provided by the National Institutes of Health, USA. All experimental procedures were performed under the supervision of the Institutional Animal Care and Use Committee of the Chonbuk National University, South Korea. All the experiments were performed under MS-222 anesthesia, and all efforts were made to minimize pain in the animals.
After anesthetization with MS-222, fully grown oocytes were collected by pressing the abdomen of the fish. The extracted eggs were fixed in 10% neutral buffered formalin, dehydrated using a graded ethanol series (60–100%), and cleared in xylene. The samples were embedded in wax (Paraplast, Leica, Germany), and five μmsections were deparaffinized and stained with Harris’s hematoxylin and eosin (Gurr, 1956). For photographs and evaluation of the ZR, a light microscope (AX 10, Carl Zeiss, Germany) was used with AxioVision (LE REL 4.5, Carl Zeiss, Germany). For scanning electron microscopy (SEM), the fragments were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2. Post-fixation was performed using 1.0% osmium tetroxide in the same buffer. After dehydration in a graded ethanol series (60–100%) and drying to a critical point using tert-butyl alcohol, the dried samples were coated with osmium tetroxide using a plasma coater (HPC-1SW, Vacuum Device Inc., Tokyo, Japan) and then filmed with an SEM (S-300N, Hitachi, Japan) operating at 15 kV. For transmission electron microscopy (TEM), tissues that were fixed and dehydrated as described for the SEM were embedded in an Epon mixture (Epon 812, EMS, USA). The fragments were observed using a TEM (H-7650, Hitachi, Japan) operating at 100 kV. Epon blocks were sectioned at 0.8 μmusing an ultramicrotome (Leica, Reichert Ultracut S, Germany), and stained with 1% toluidine blue. The samples were examined using a light microscope (Carl Zeiss, AX10, Germany) and analyzed with AxioVision 4.5 (Carl Zeiss, AxioVision, Germany). All the data collected was subjected to statistical analysis using one-way analysis of variance (ANOVA) using SPSS ver. 29.0.
3. Results
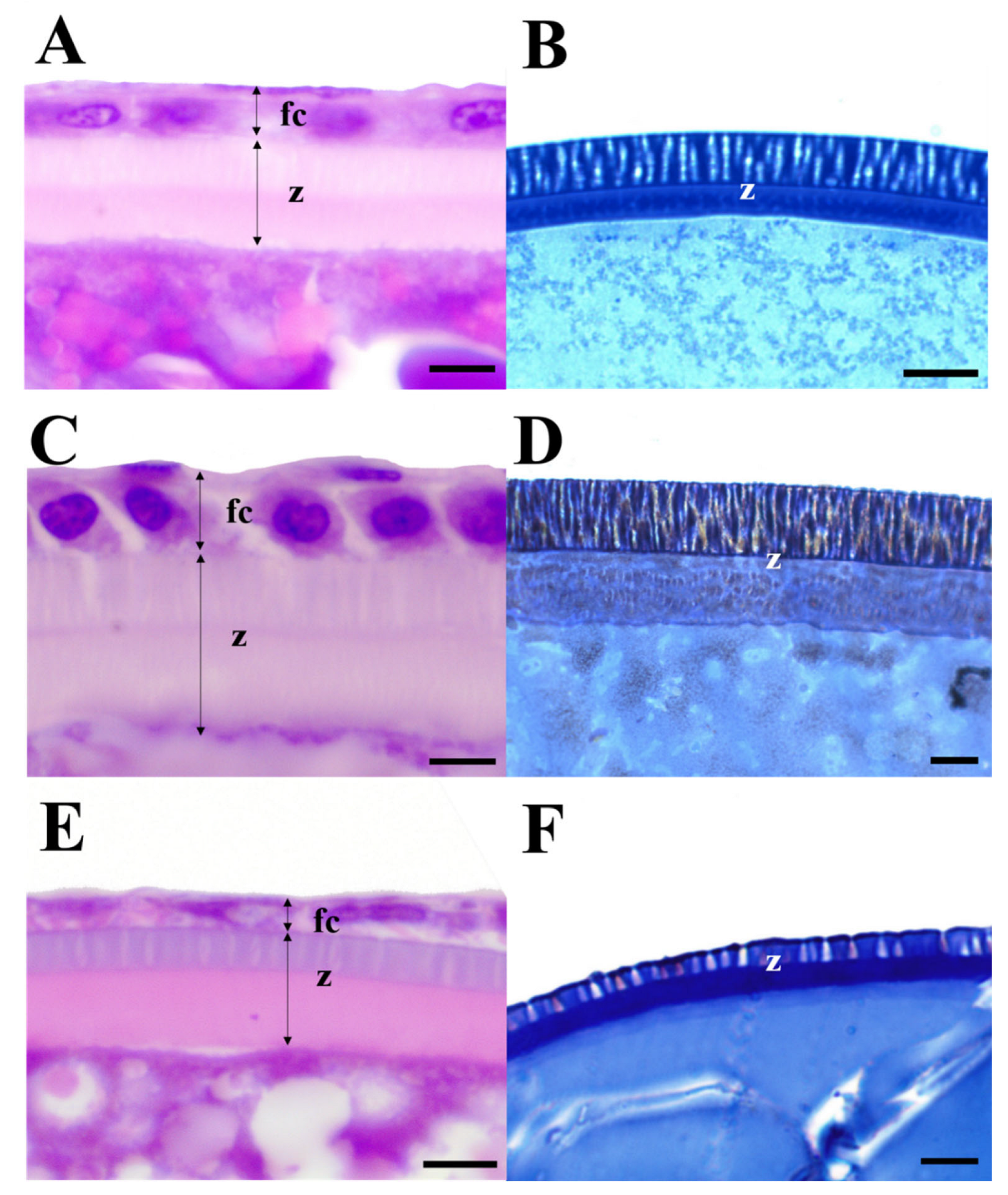
All three species,
E. erythropterus,
C. brevicauda, and
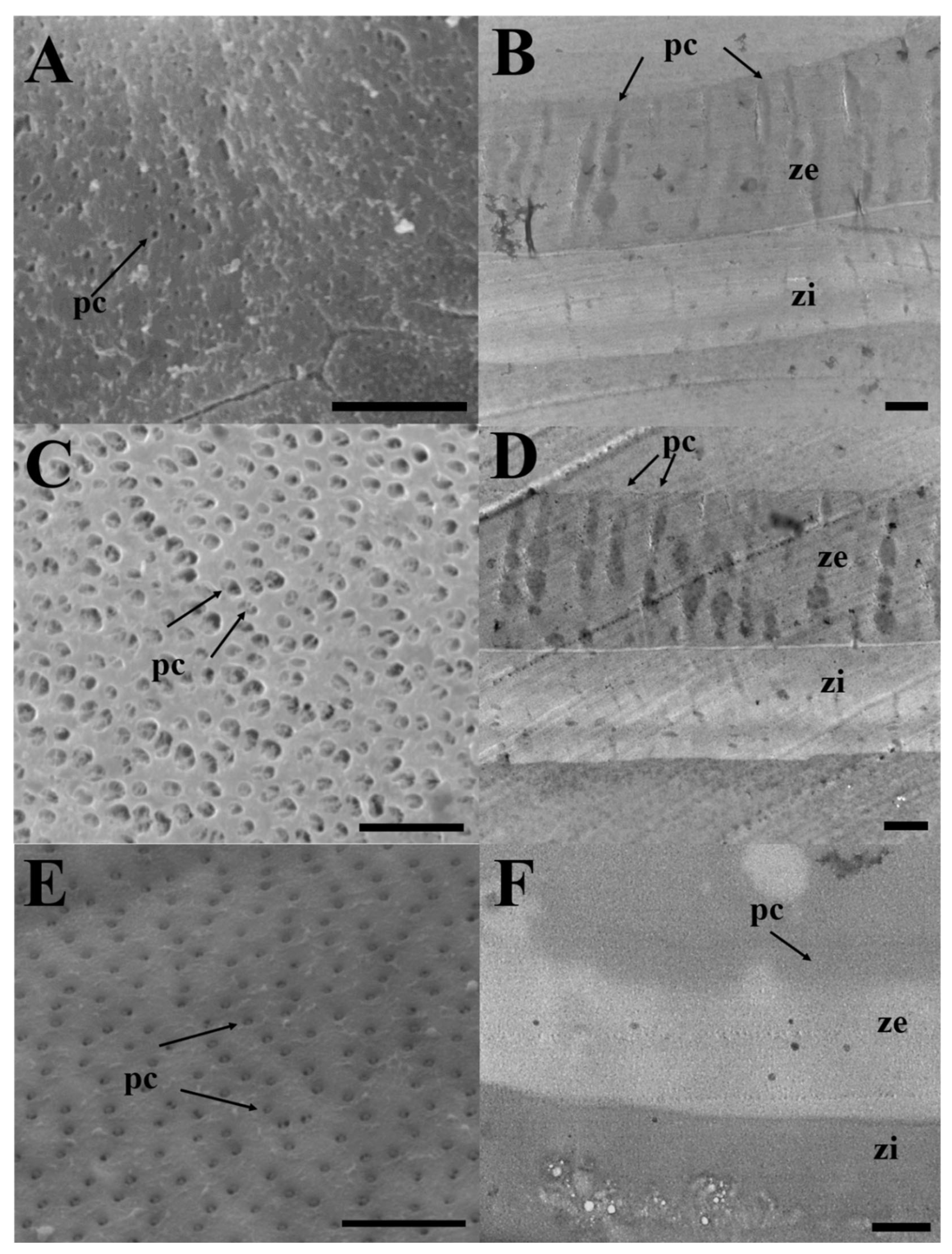
H. eigenmanni, have non-structural forms of egg envelopes on the outer surface, and no special structure other than the pore canal was identified on the outer side. Light microscopic analysis of cross-sections of mature eggs revealed striped ZR stained with eosin and 1% toluidine blue. Further, no structure was identified between the outer surface of egg envelopes and the follicular cell layer (Figure. 1). SEM analysis of the surface of mature eggs revealed a large number of pore canals in the egg envelopes—83 for
E. erythropterus, 75 for
C. brevicauda, and 58 for
H. eigenmanni per 10 μm2 (
Table 1,
Figure 2A,C,E). TEM analysis of the ZR of the three species was similar to the results of light and SEM; multiple pore canals were identified in the ZR of
E. erythropterus,
C. brevicauda, and
H. eigenmanni, which comprised an outer and inner layer. No other structures were identified on the outer surface of the egg envelopes (
Figure 2B,D,F). The thickness of the egg envelopes was measured to be 7.89±0.34 (7.43~8.28) μm in E
. erythropterus, 12.27±0.46 (11.57~13.26) μm in
C. brevicauda, and 7.42±0.24 (6.69~7.66) μm in
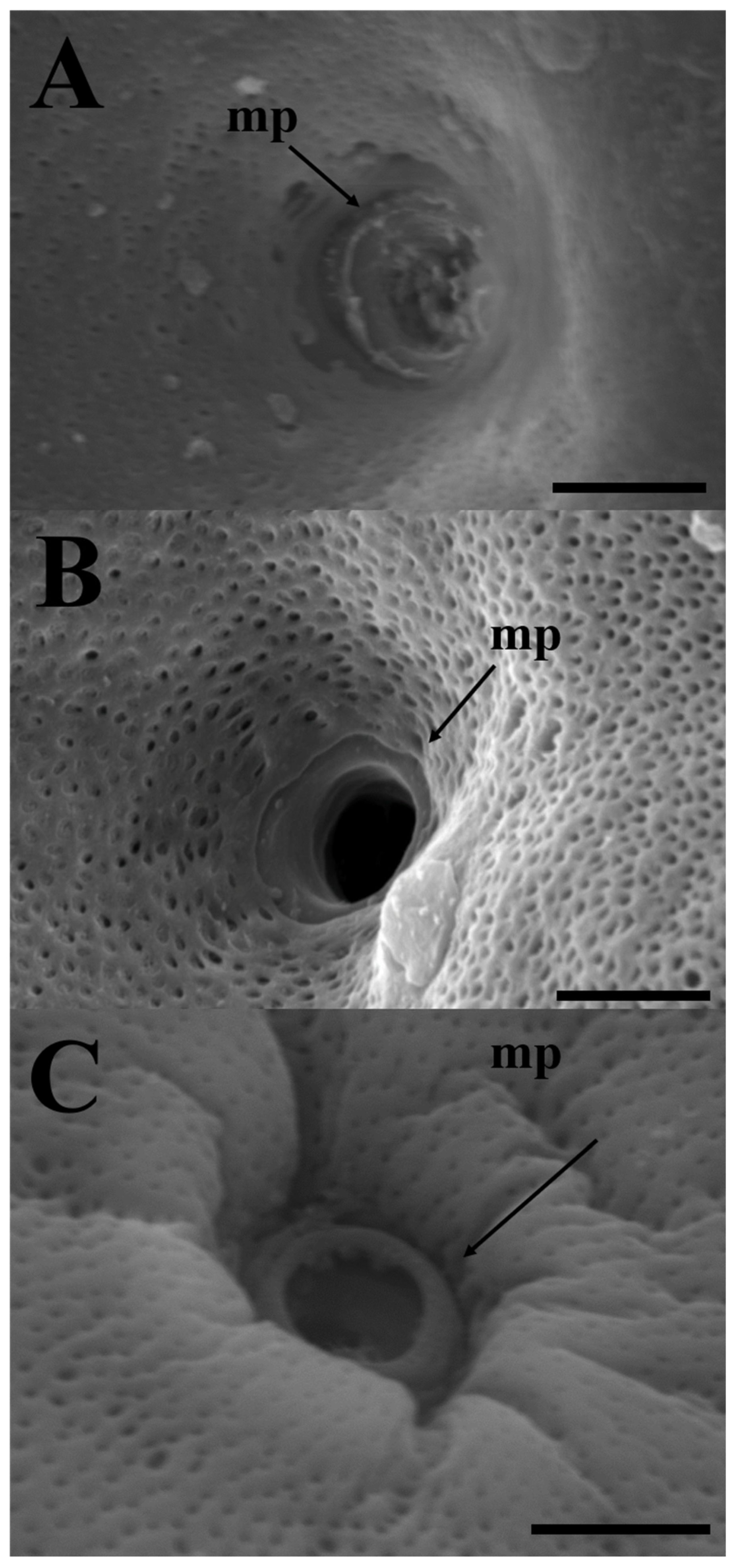
H. eigenmanni, and one funnel-shaped micropyle was identified at the tip of each animal pole (
Figure 3). The inner diameter of the micropyle was the largest among the three species with
E. erythropterus 6.62±0.29 (6.09~7.10) μm, followed by
C. brevicauda 4.19±0.39 (3.39~4.58) μm, and
H. eigenmanni 3.98±0.46 (3.47~4.93) μm(
Table 1).
Figure 1.
Light micrographs showing the zona radiata in the oocytes of three Cultrinae species based on (a, c, e) Harris’s hematoxylin and (b, d, f) eosin staining and 1% toluidine blue staining. (a) and (b) Erythroculter erythropterus; (c) and (d) Culter brevicauda; (e) and (f) Hemiculter eigenmanni fc, follicular layer; z, zona radiata. Scales indicate 5 μm(a), (c), (e), 10 μm(b), 1 μm(d), and (f), respectively.
Figure 1.
Light micrographs showing the zona radiata in the oocytes of three Cultrinae species based on (a, c, e) Harris’s hematoxylin and (b, d, f) eosin staining and 1% toluidine blue staining. (a) and (b) Erythroculter erythropterus; (c) and (d) Culter brevicauda; (e) and (f) Hemiculter eigenmanni fc, follicular layer; z, zona radiata. Scales indicate 5 μm(a), (c), (e), 10 μm(b), 1 μm(d), and (f), respectively.
4. Discussion
The outer surface of the egg envelope in fish is characterized by a variety of structures—non-structural, granular, villous, filamentous, saw-shaped, hillock-shaped, and fence-shaped (Park, 1996; Choi, 2021). These structures perform functions such as attachment, hydrostatic regulation, and embryo protection (Laale, 1980; Hiromi, 1984; Rizzo et al., 2002). The egg envelope structures additionally act as a species specificity, demonstrating a close ecological link with the habitat (Stehr and Hawkes, 1979; Groot and Alderdice, 1985; Berrada-Rkhami and Gabrion, 1990; Hirai, 1993; Rizzo et al., 2002). Park (1996) and Choi (2015) reported that the ultrastructure of an egg envelope is associated with spawning areas in fish. The non-structural form has been observed in Misgurnus anguilicaudatus, M. mizolepis, Lefua costata, Gobiobotia nakdongensis, and Microphysogobio yaluensis, which are known to have lentic habitats or spawning areas where the bottom structure comprises mud (Kim et al., 2017). Among the Acheilognathinae that spawn in freshwater bivalves, Acheilognathus lanceolatus, A. signifer, A. koreensis, A. somjinensis, A. yamatsutae, A. majusculus and Sarcocheilichthys nigripinnis morii of the genus Sarcocheilichthys exhibit a non-structural egg envelope, which was found to be favorable for deposition in the gills of bivalves by laying fusiform, pear-shaped, and mono-oval eggs (Choi, 2021). All three Cultrinae species have bottom structures composed of mud or sand and inhabit lentic waters, which is consistent with previous findings (Kim and Park, 2002). Additionally, differences in the thickness of the zona radiata of three species, E. erythropterus, C. brevicauda, and H. eigenmanni, demonstrated species specificity. C. alburnus, which lives in general streams in China, has a non-attached floating egg. However, the C. alburnus which lives in lakes is known to attach sinking eggs that show intraspecific specificity depending on the habitat environment (Cheng et al., 2023).
In this study, all three species had ZR comprising two layers—an outer and inner layer. The inner layer contains several microtubules composed of microfibers, which are involved in oocyte respiration and nutrient transport (Nagahama, 1983). A funnel-shaped micropyle with a larger outer diameter and a smaller inner diameter was observed in all three species of Cultrinae. Although the micropyle morphology of the three species was similar, there were differences observed between the species; E. erythropterus demonstrated the longest inner diameter, followed by C. brevicauda and H. eigenmanni. These micropyle are species-specific and function to prevent multi-fertilization during the spawning season and defend against invasion by sperm from other species (Grierson and Neville, 1981; Cameron and Hunter, 1984). Thus, C. brevicauda and H. eigenmanni, which have similar micropyle sizes, have ecological differences due to different spawning sites and times and E. erythropterus has adapted to have completely different micropyle sizes. Therefore, no cases of hybridization have been reported yet between different species. Some fish have two or more micropyles, that are used as taxonomic characteristics (Chen et al., 1999; Morisawa, 1999; Debus et al., 2002). The size of the micropyle is known to be closely related to sperm head size (Hart, 1990; Linhart and Kudo, 1997). The three species in this study live in very large rivers, making it difficult to collect them with conventional nets, and it would have been difficult to do without equipment such as SEM and TEM. If there is one regret, it is the analysis of Hemiculter leucisculus, which lives in Korea but was not included in this study because it was very difficult to collect. This species can only be found in the estuaries of large rivers in Korea, such as the Imjingang and Hangang River, which are relatively far north, so we were unable to collect it. Finally, further studies are needed to compare the sperm morphology, egg envelope, and micropyles of all four species of Cultrinae in Korea to define their habitats, interspecific hybridization defense mechanisms, and to assign accurate scientific names based on these data.
5. Conclusions
E. erythropterus is currently an invasive species in the Nakdonggang River in South Korea, where it is a top predator that disturbs aquatic ecosystems. Significant capital and manpower are, therefore, being invested in its extermination. On the other hand, C. brevicauda has been ecologically declining in the Nakdonggang River and is listed as Vulnerable on the IUCN Red List. H. eigenmanni is found in all water systems in Korea, so the study of their egg envelopes is, therefore, expected to provide basic data for ecology, extermination, and conservation, and it is a relatively large river species that is very difficult to study. This study is, therefore, significant in that it revealed that their egg envelopes are all non-structural, that the bottom structure of the stream is primarily composed of mud and sand, and that they live in slow-flowing water, the micropyle is funnel-shaped but differently sized in all three species, and that there are differences in the thickness of the zona radiata and number of pore canals. These differences may be used as morphological characters to distinguish between the species of Cultrinae globally, which is currently facing problems due to the lack of accurate scientific names. This will additionally serve as a basis for ecosystem recovery in Korea, such as that for the Nakdonggang River.
Author Contributions
Conceptualization, J.G.K. and C.W.P.; methodology, J.G.K. and C.W.P.; software, J.G.K.; validation, J.G.K.; formal analysis, J.G.K. and C.W.P.; investigation, J.G.K. and C.W.P.; resources, J.G.K. and C.W.P.; data curation, J.G.K. and C.W.P.; writing—original draft preparation, J.G.K.; writing—review and editing, J.G.K.; visualization, J.G.K.; supervision, J.G.K.; project administration, J.G.K. and C.W.P.; funding acquisition, J.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study followed the Guide for the Care and Use of Laboratory Animals (2011), provided by the National Institutes of Health, USA. All experimental procedures were performed under the supervision of the Institutional Animal Care and Use Committee of the Chonbuk National University, South Korea. (IACUC-22-1523) date of approval: 21 October 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anderson , E. Comparative aspects of the ultrastructure of the female gamete. Int Rev Cytol Suppl 1974, 4, 1–70. [Google Scholar]
- Berrada-Rkhami, O.; Gabrion, C. The fine structure of the embryonic envelopes before and after hatching in bothriocephalids: Physiological and ecological significance. Parasitol res. 1990, 76, 251–262. [Google Scholar] [CrossRef]
- Cameron, I.L.; Hunter, K.E. Regulation of the permeability of the medaka fish embryo chorion by exogeneous sodium and calcium ions. J Exp Zool 1984, 231, 447–454. [Google Scholar] [CrossRef]
- Chae, B.S.; Song, H.B.; Park, J.Y. A field guide to the freshwater fishes of Korea; LG Evergreen Foundation: Seoul, Korea, 2019; pp. 135–139. [Google Scholar]
- Chen, K.C.; Shao, K.T.; Yang, J.S. Using micropylar ultrastructure for species identification and phylogenetic inference among four species of Sparidae. J Fish Biol 1999, 55, 288–300. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, Z.; Dai, W.; Fan, Q.; He, S. Phylogeographic structure, cryptic speciation and demographic history of the sharpbelly (Hemiculter leucisculus), a freshwater habitat generalist from southern China. BMC Evol Biol 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, M.; Liu, S.; Zheng, J.; Chi, M.; Jiang, W.; Liu, Y.; Hang, X.; Peng, M.; Li, F.; Wang, D. Preliminary Analysis of Quality and Cryopreservation of Sex-Reversed Female Culter alburnus Sperm. Aquac Res 2023, 2023, 1672265. [Google Scholar] [CrossRef]
- Choi, S. J. Oogenesis and ultrastructure of the egg envelope in 15 Korean cyprinid fish (Pisces, Cypriniformes) spawning in bivalves. Doctoral Dissertation, Chonbuk National University, Korea, 2021; 122pp.
- Choi, W.S. The oogenesis and ultrastructure of egg envelope in 3 species of genus Gobiobotia (Pisces: Cyprinidae) from Korea. Master′s thesis, Chonbuk National University, 2015; 41pp.
- Coad, B.W.; Hussain, N.A. First record of the exotic species Hemiculter leucisculus (Actinopterygii: Cyprinidae) in Iraq. Zool Middle East 2007, 40, 107–109. [Google Scholar] [CrossRef]
- Cotelli, F.; Andronico, F.; Bassi., R.; Brivio, M.; Ceccagno, C.; Denis-Donini, S.; La Rosa, M.L.; Lamia Donin, C.L. Studies on the composition, structure and differentiation of fish egg chorion. Cell Biol Int Rep 1986, 10, 471. [Google Scholar] [CrossRef]
- Dai, Y.G.; Yang, J.X. Phylogeny and Zoogeography of the Cyprinid Hemicultrine Group (Cyprinidae: Cultrinae). Zool Stud 2003, 42, 73–92. [Google Scholar]
- Dai, Y.G.; Yang, J.X.; Chen, Y.R. Phylogeny and zoogeography of the subfamily Cultrinae (Cyprinidae). Acta Zootaxonomica Sinica 2005, 30, 213–233. [Google Scholar]
- Debus, L.; Winkler, M.; Billard, R. Structure of micropyle surface on oocytes and caviar grains in Sturgeons. Internat Rev Hydrobiol 2002, 87, 585–603. [Google Scholar] [CrossRef]
- Flegler, C. Electron microscopic studies on the development of the chorion of the viviparous teleost Dermogenys pusillus (Hemirhamphidae). Cell and Tissue Res 1977, 179, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Grierson, J.P.; Neville, A.C. Helicoidal architecture of fish eggshell. Cell and Tissue Res 1981, 13, 818–830. [Google Scholar] [CrossRef]
- Groot, E.P.; Alderdice, D.F. Fine structure of the external egg membranes of five species of Pacific salmon and steelhead trout. Can J Zool 1985, 63, 552–566. [Google Scholar] [CrossRef]
- Gu., Q.; Zhong, H.; Sun, Y.; Yuan, H.; Li, S.; Shen, Z.; Wen, M. Reanalysis on Phylogeographic Pattern of Sharpbelly Hemiculter leucisculus (Cyprinidae: Cultrinae) in China: A Review and the Implications for Conservation. Front Ecol Evol 2022, 10, 865089. [Google Scholar] [CrossRef]
- Gurr, E. A practical manual of medical and biological staining techniques, Interscience, New York, 1956; pp. 1–99.
- Hart, N.H. Fertilization in teleost fishes: Mechanisms of sperm-egg interactions. Int Rev Cytol 1990, 121, 1–66. [Google Scholar] [CrossRef]
- Hirai., A. Fine structure of the egg membrane in four species of Pleuronectinae. Jpn J Ichthyol 1993, 40, 227–235. [Google Scholar]
- Hiromi, O. Electron microscopic study on adhesive material of pacific herring (Clupea pallasi) eggs. Jpn J Ichthyol 1984, 30, 404–411. [Google Scholar] [CrossRef]
- Ivankov, V.N.; Kurdyayeva, V.P. Systematic differences and the ecological importance of the membranes in fish eggs. J Ichthyol 1973, 13, 864–873. [Google Scholar]
- Jouladeh-Roudbar, A.; Vatandoust, S.; Eagderi, S.; Jafari-Kenari, S.; Mousavi-Sabet, H. Freshwater fishes of Iran; an updated checklist. Aquac Aqar Conserv Legis 2015, 8, 855–909. [Google Scholar]
- Kim, D.H.; Reu, D.S.; Deung, Y.G. A comparative study on the ultrastructures of the egg envelope in fertilized eggs of fishes, Characidae, three species. Korean J Electron Microscopy 1996, 26, 277–291. [Google Scholar]
- Kim, I.S; Park, J.Y. Freshwater fishes of Korea. Kyohak Publishing Co. Ltd, Seoul. 2002; 467pp.
- Kim, J.G.; Reu, D.S.; Park, J.Y. Oogenesis of Microphysogobio yaluensis (Pisces, Cyprinidae) in the Korean endemic species. Kor J Icthyol 2017, 29, 252–257. [Google Scholar]
- Kobayashi, W.; Yamamoto, T.S. Fine structure of micropylar apparatus of the chum salmon egg, with a discussion of the mechanism for blocking polyspermy. J Exp Zool 1981, 217, 265–275. [Google Scholar] [CrossRef]
- Laale, H.W. The perivitelline space and egg envelopes of bony fishes, a review. Copeia 1980, 2, 210–226. [Google Scholar] [CrossRef]
- Li, Y.H.; Wu, C.C.; Yang, J.S. Comparative ultrastructural studies of the zona radiata of marine fish eggs in three genera in Perciformes. J Fish biol 2000, 56, 615–621. [Google Scholar] [CrossRef]
- Linhart, O.; Kudo, S. Surface structure of paddlefish eggs before and after fertilization. J Fish Biol 1997, 51, 573–582. [Google Scholar] [CrossRef]
- ME (Ministry of Environment). Study on the management method of invasive freshwater fishes. Ministry of Environment, Sejong, 2016; 157pp.
- Morisawa, S. Fine structure of micropylar region during late oogenesis in eggs of the hagfish Eptatretus burgeri (Agnatha). Develop Growth Differ 1999, 41, 611–618. [Google Scholar] [CrossRef]
- Mousavi-Sabet, H.; Heidari, A.; Salehi, M. Reproductive biology of the invasive sharpbelly, Hemiculter leucisculus (Basilewsky, 1855), from the southern Caspian Sea basin. Iran J Ichthyol 2019, 6, 31–40. [Google Scholar] [CrossRef]
- Nagahama, Y. The functional morphology of teleost gonads. In Fish physiology, Academic Press, 1983; 9: 223-275.
- Nelson, J.S; Grande, T.C; Wilson, M.V.H. Fishes of the world. John Wiley & sons, Inc, 2016; pp.185-186.
- Park, J.Y. A morphological study on the gonad of the species in the Family Cobitidae (Pisces: Cypriniformes) from Korea. Doctoral Dissertation, Chonbuk National University, Korea, 1996; 158pp.
- Riehl, R.; Patzner, R.A. Minireview: The modes of egg attachments in teleost fishes. Ital J Zool 1998, 65, 415–420. [Google Scholar] [CrossRef]
- Rizzo, E.; Sato, Y.; Barreto, B.P.; Godinho, H.P. Adhesiveness and surface patterns of eggs in neotropical freshwater teleosts. J Fish Biol 2002, 61, 615–632. [Google Scholar] [CrossRef]
- Stehr, C.M.; Hawkes, J.W. The comparative ultrastructure of the egg membrane and associated pore structure in the starry flounder, Platichthys stellatus (Pallas), and pink salmon, Oncorhynchus gorbuscha (Walbaum). Cell Tiss Res 1979, 202, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, L.; Tang, K.; Liu, M.; Xue, X.; Wang, G.; Wang, Z. Population genetic structure of sharpbelly Hemiculter leucisculus and morphological diversification along climate gradients in China. Ecol Evol 1855, 11, 6798–6813. [Google Scholar] [CrossRef] [PubMed]
- Wourms, J.P. Annual fish oogenesis: Differentiation of the mature oocyte and formation of the primary envelope. Devl Bio 1976, 50, 338–354. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).