Submitted:
28 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
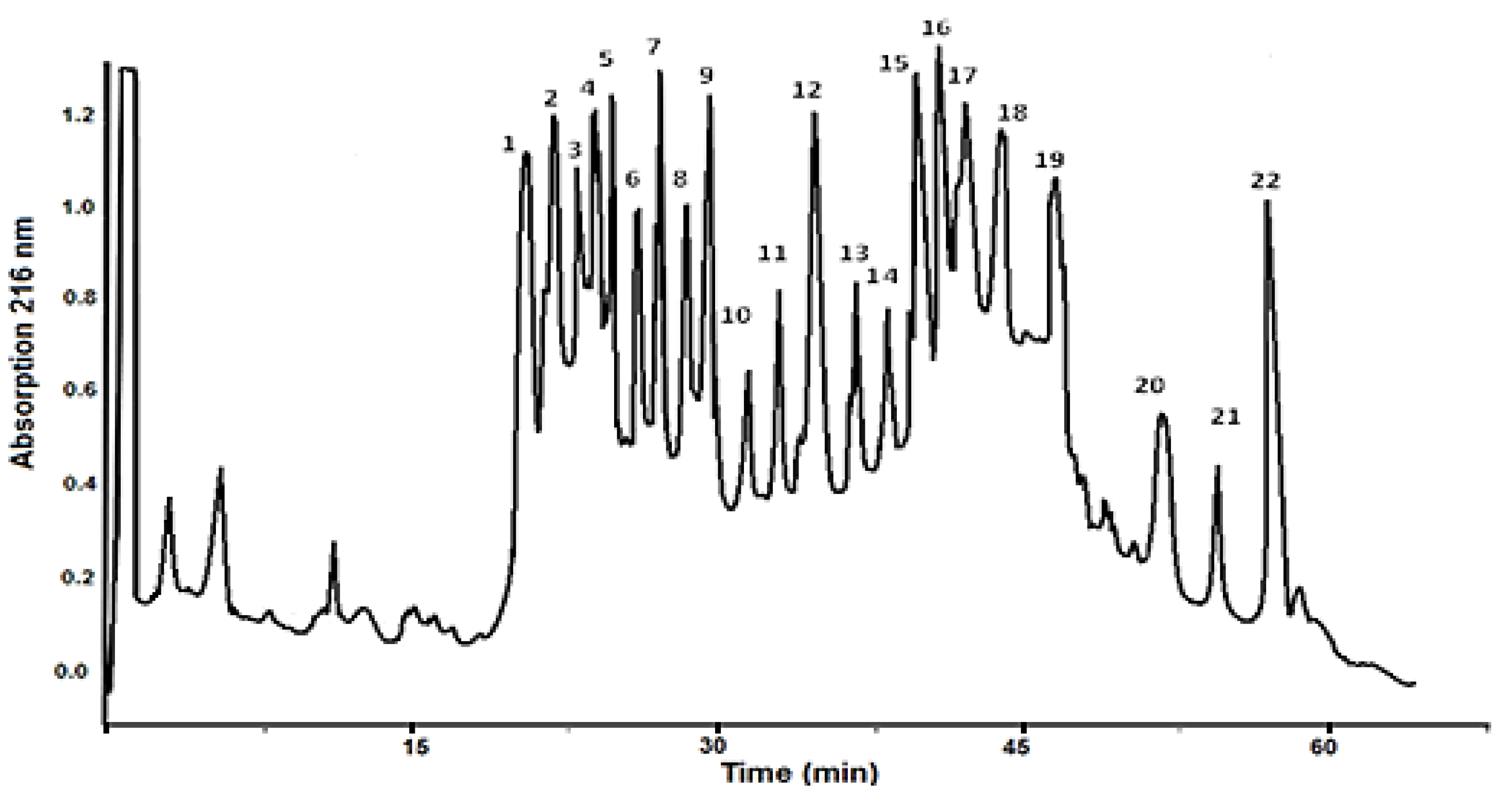
2.1. Preparation of Snail Mucus Extract
2.2. Analysis and Characteristics of the Isolated Mucus Fraction with MW < 20 kDa
2.2.1. Molecular Mass Analysis and de Novo Sequencing of Peptides by Mass Spectrometry
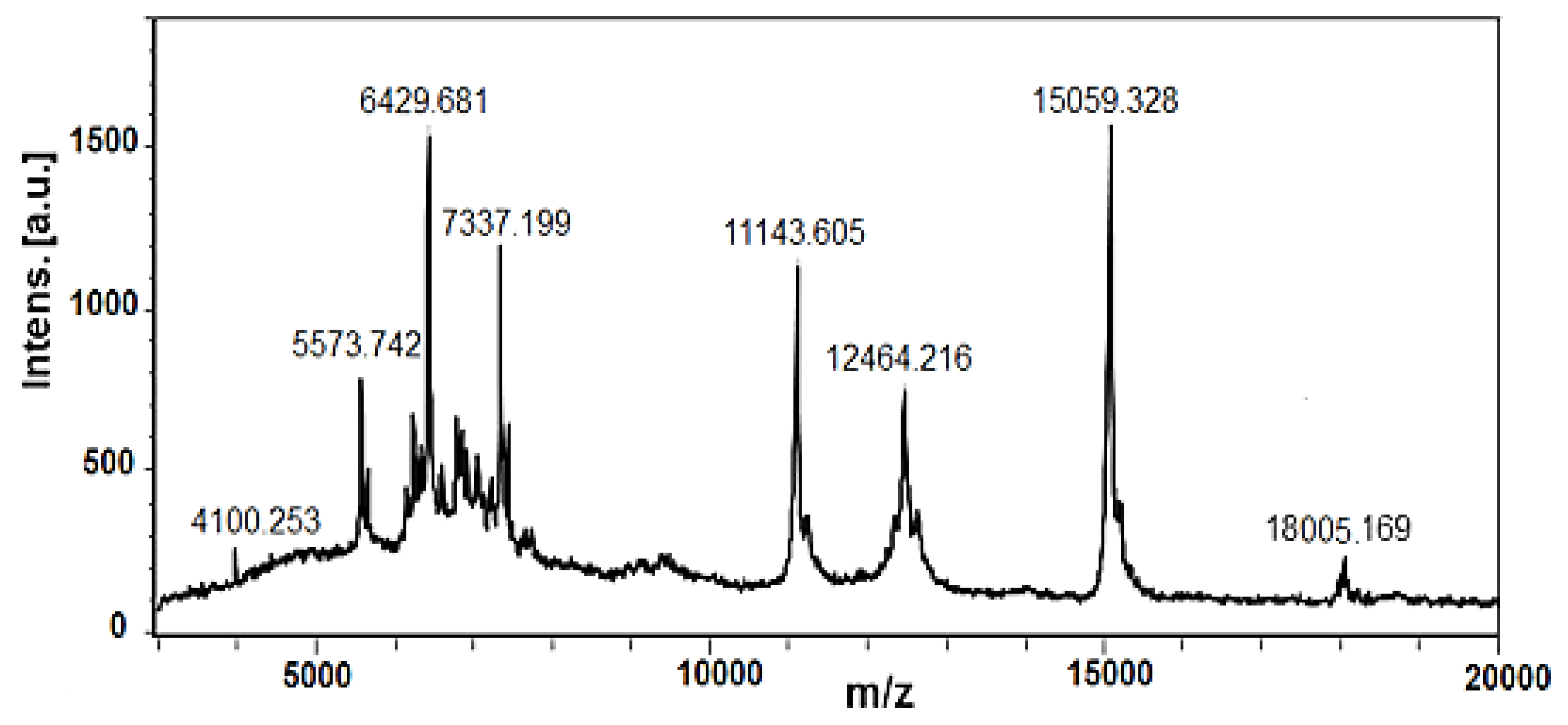
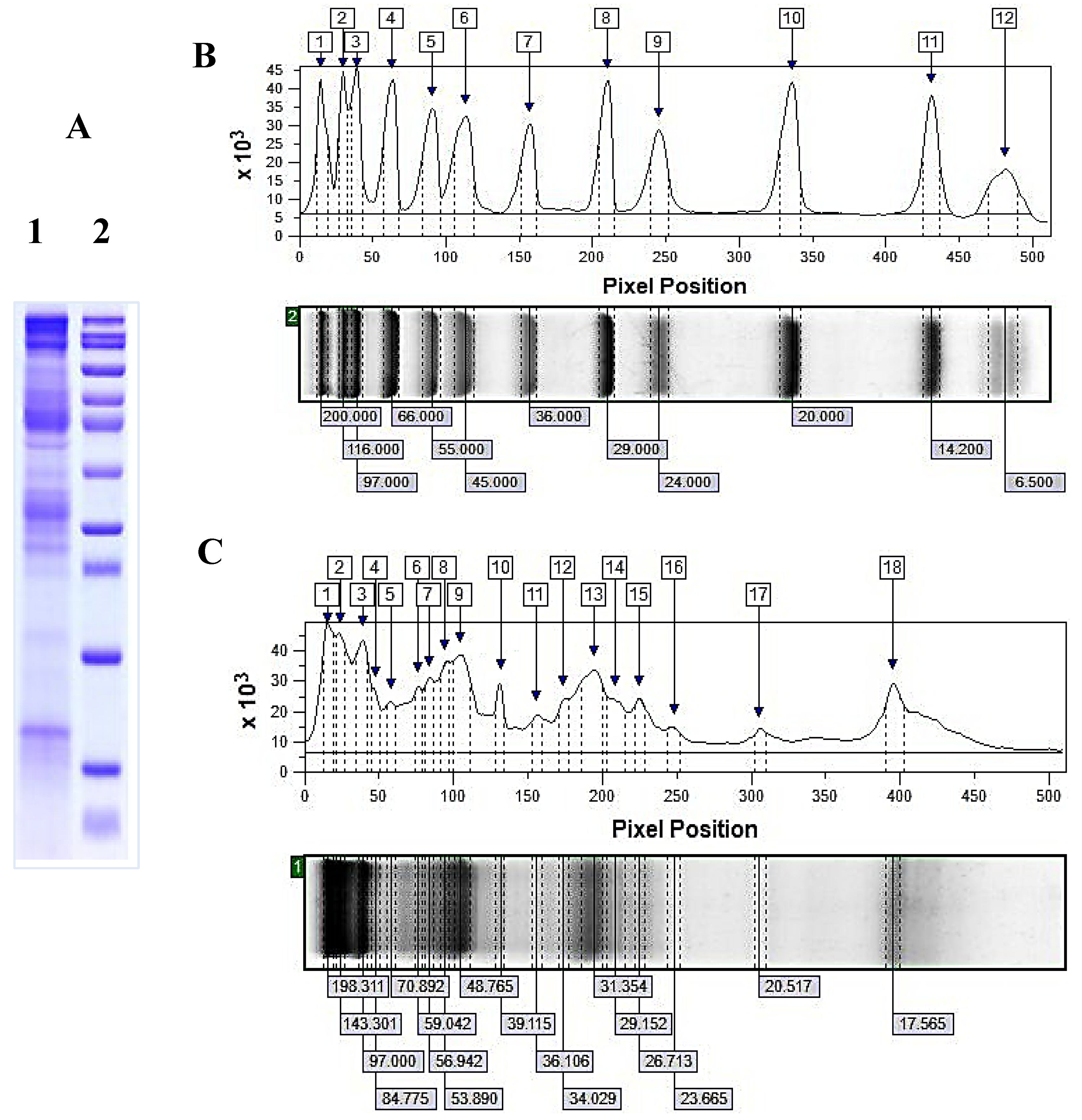
2.2.2. Characterisation of Mucus Fraction with MW > 20 kDa by Electrophoretic and MALDI-MS Analyses
2.2.3. Identification of Proteins in Mucus Fraction with MW > 20 kDa from C. aspersum Snail
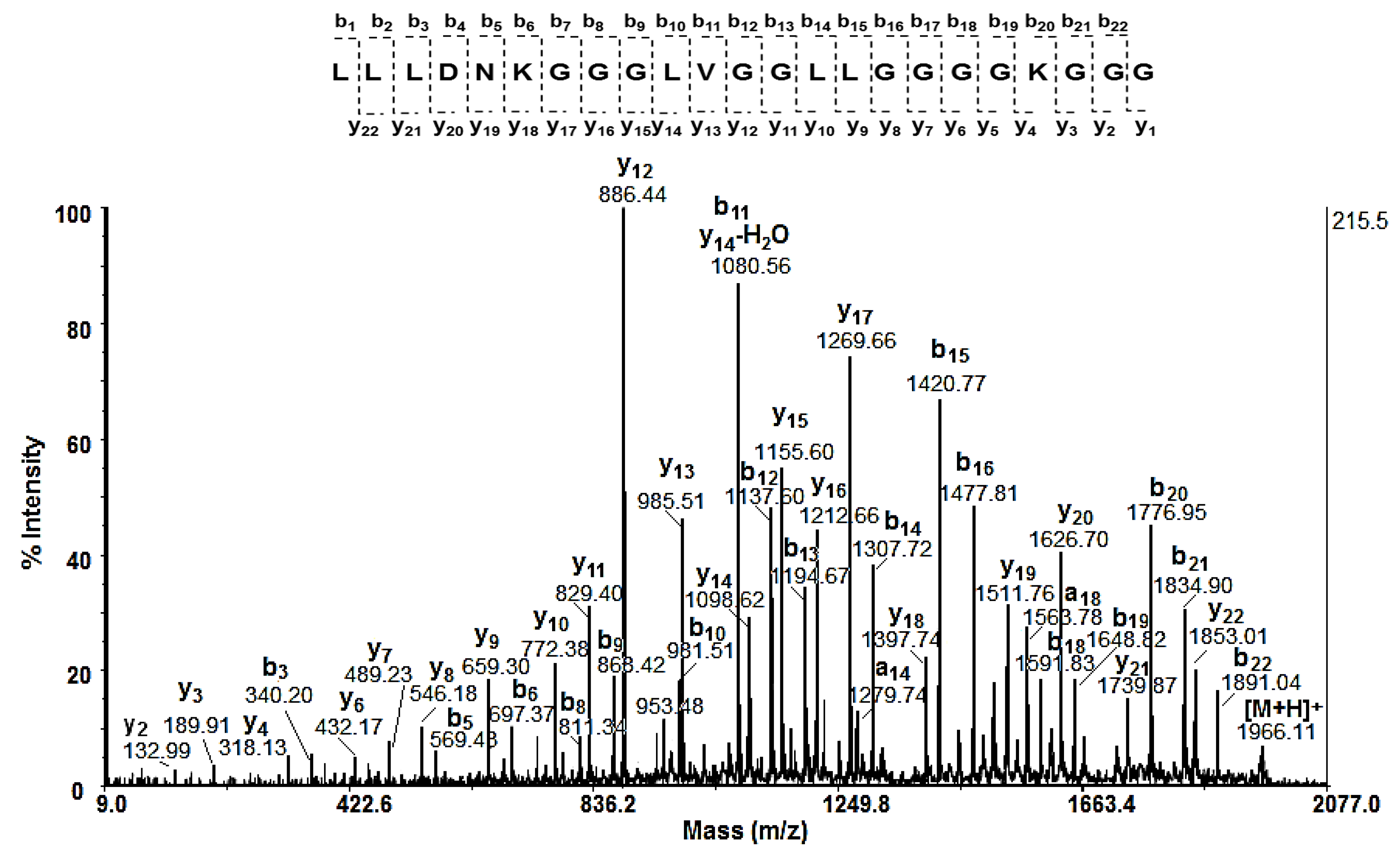
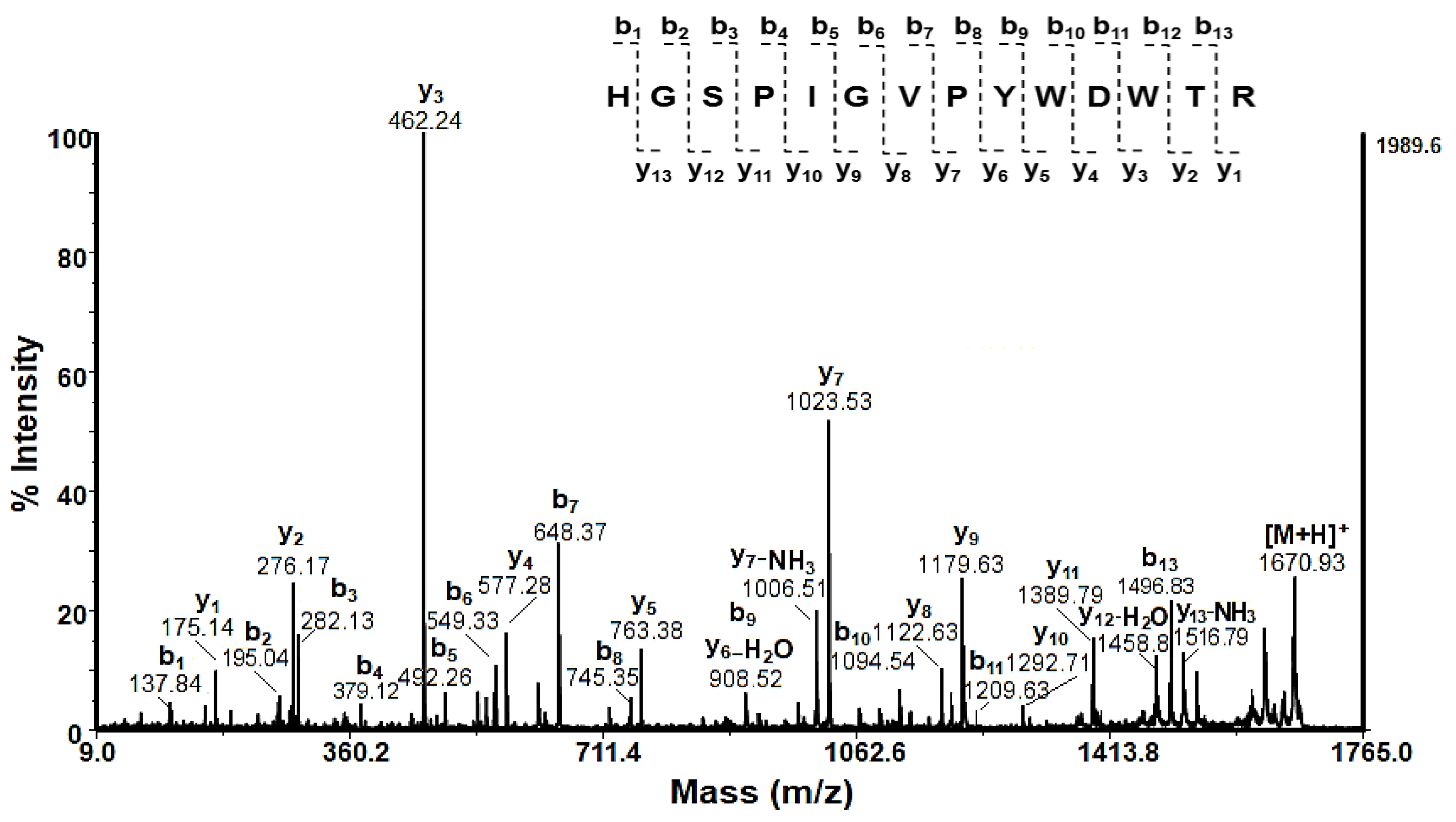
2.2.4. Characterisation of Mucus Fraction with MW > 20 kDa by Tandem Mass Spectrometrical Analyses of 12% SDS-PAGE
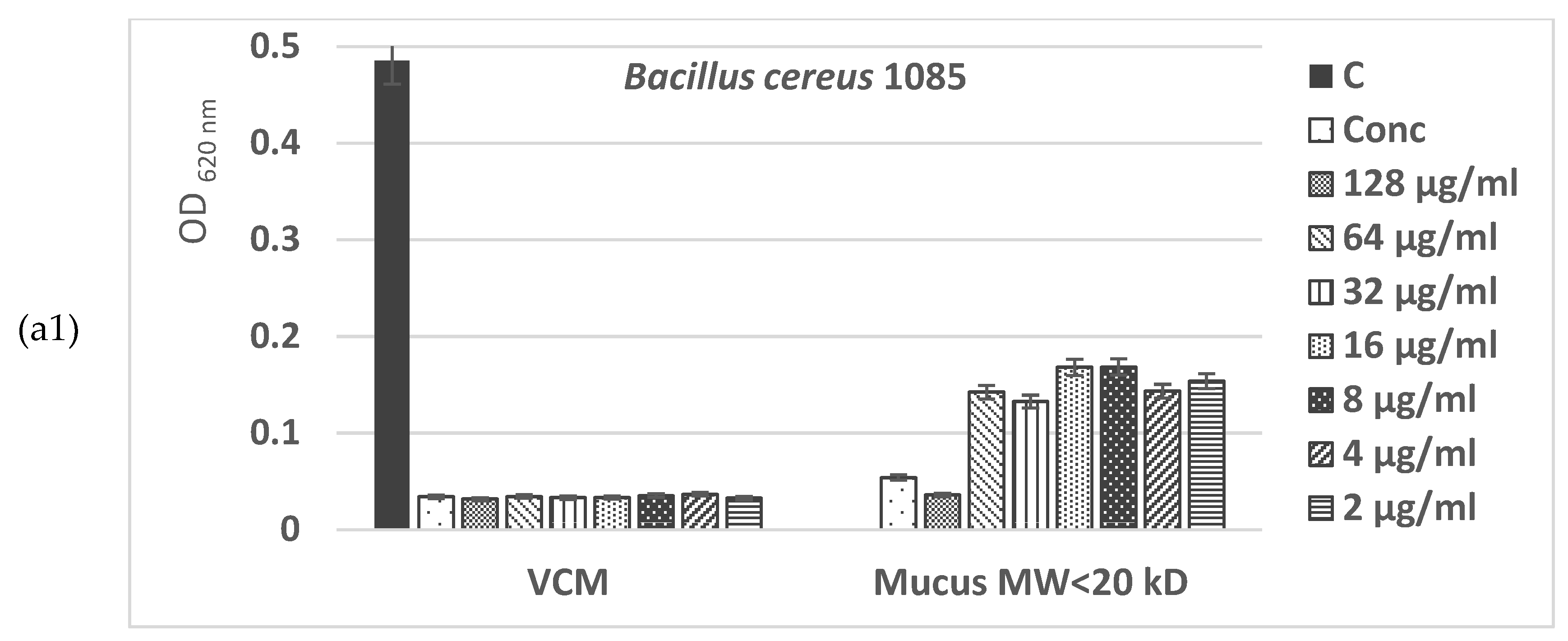
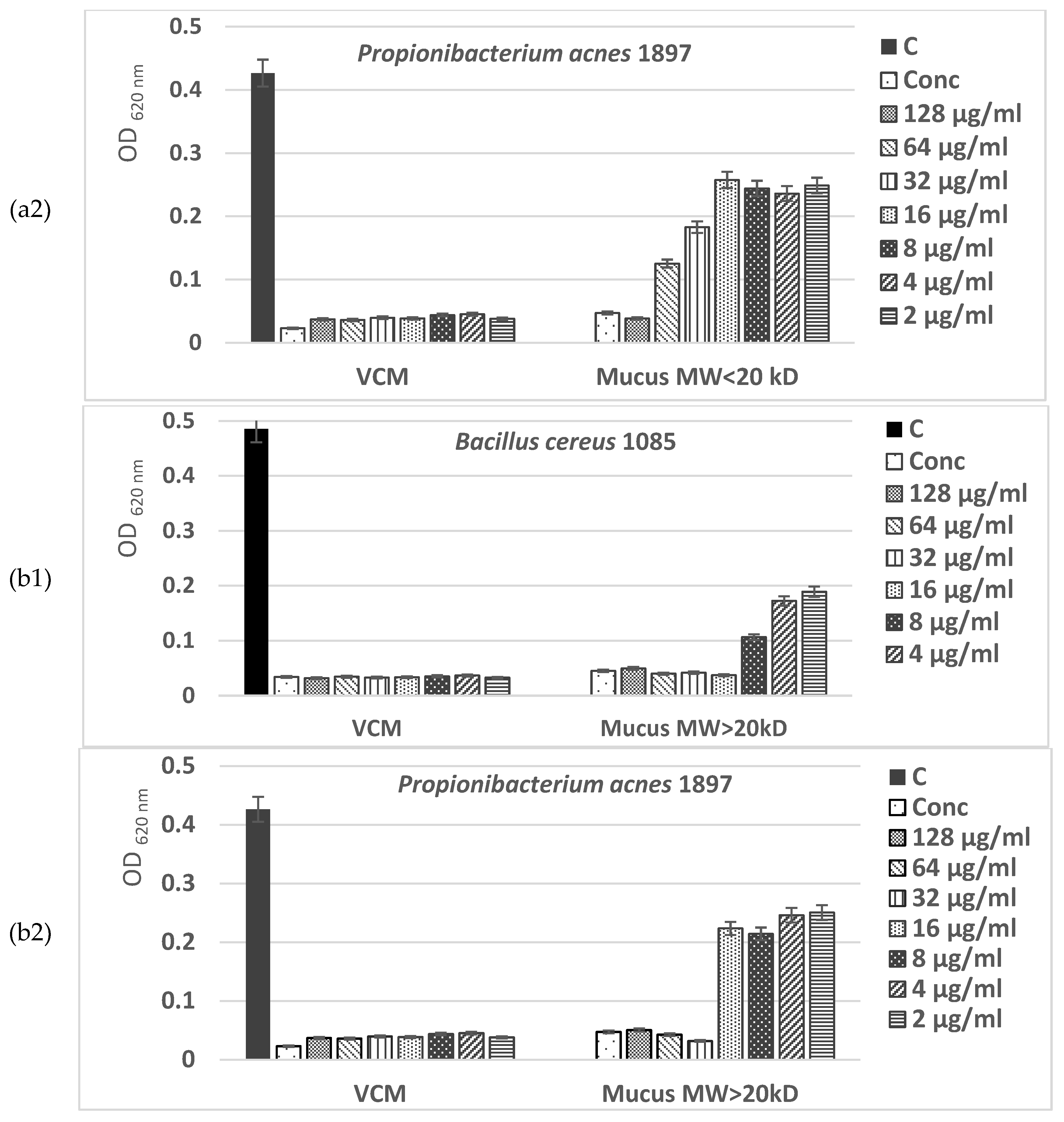
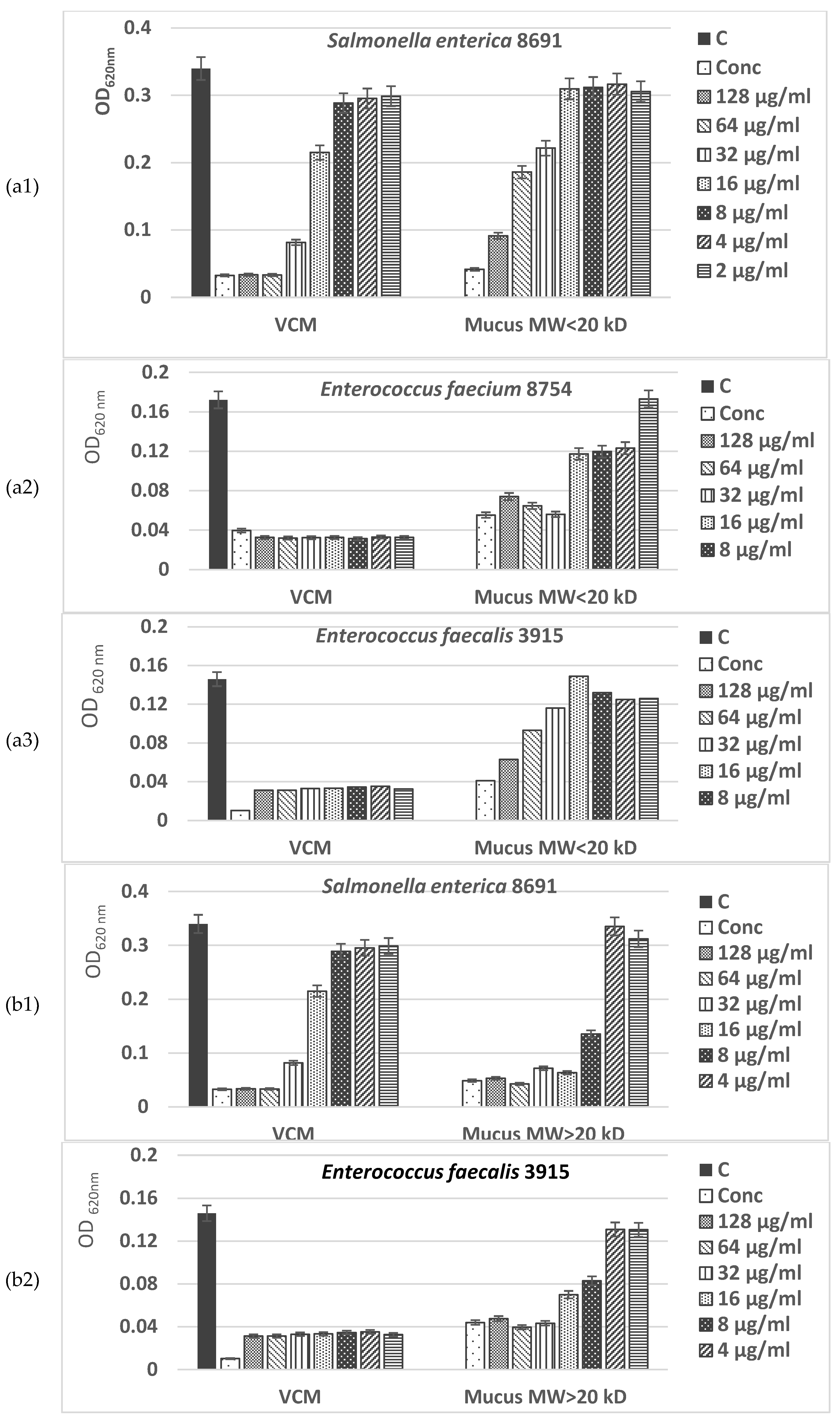
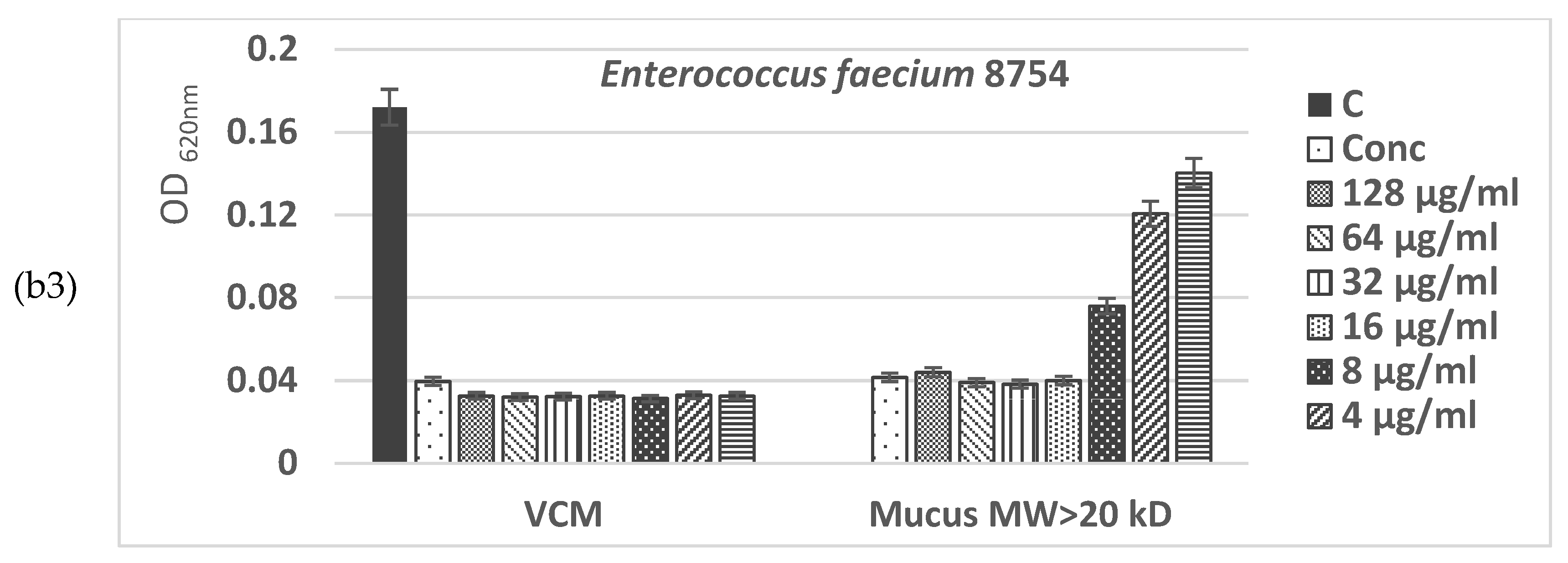
2.3. Antimicrobial Effect of Two Fractions Isolated from C. aspersum Mucus
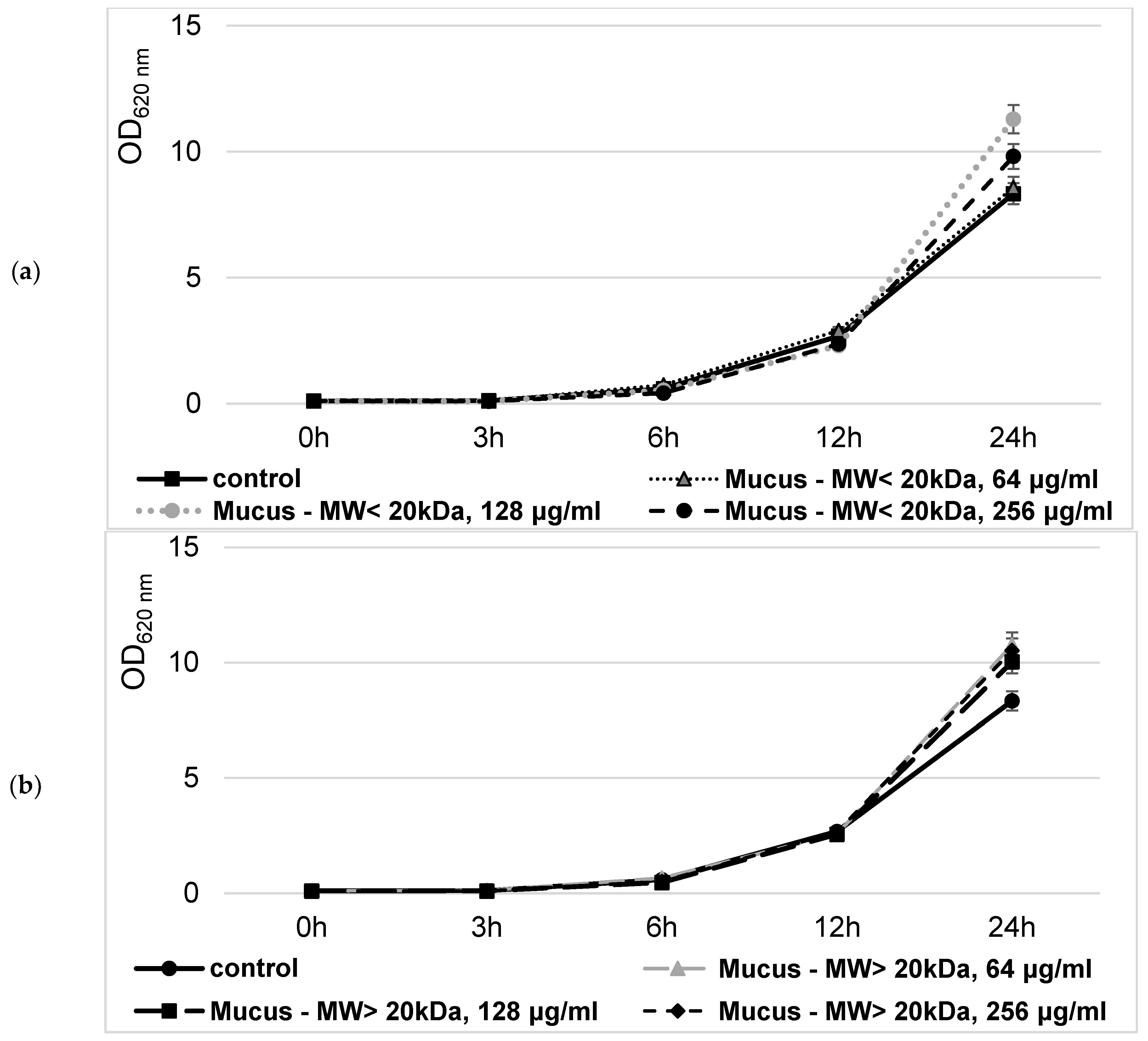
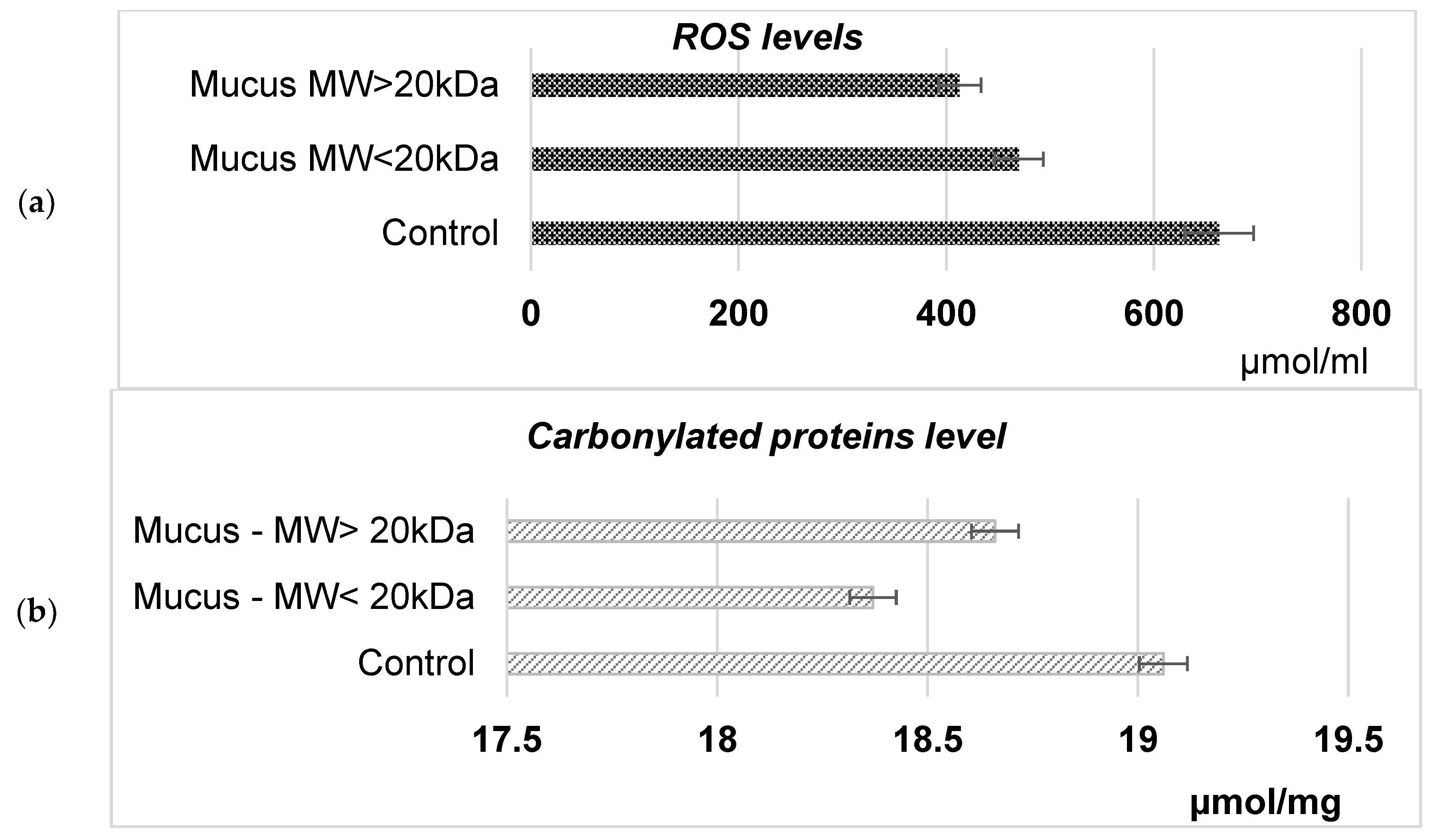
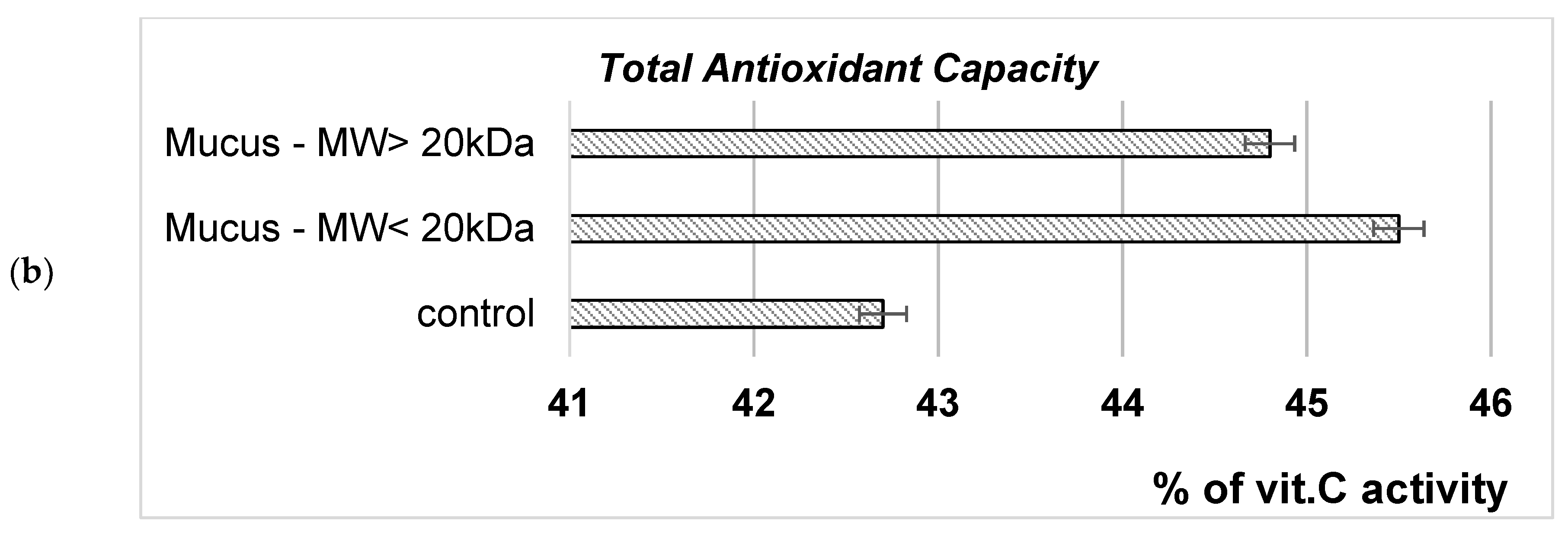
2.4. Studying the in Vitro and in Vivo Effects of Snail Mucus on Eukaryotic Cell
3. Discussion
4. Materials and Methods
4.1. Preparation of Mucus Extract
4.2. HPLC Purification of Peptides from the Fraction Below 20 kDa
4.3. Molecular Mass Analysis and De Novo Sequencing of Peptides by Mass Spectrometry
4.4. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Image Analysis of 10% SDS-PAGE by ImageQuant™ TL v8.2.0 Software
4.6. Tryptic In-Gel Digestion and Peptide Extraction
4.7. Antibacterial Activity Assay
| Bacteria strain | Causative agents |
| Bacillus cereus NBIMCC 1085 | Foodborne disease |
| Propionibacterium acnes DSM 1897 | Skin condition of acne, chronic endophthalmitis, corneal ulcers, sarcoidosis, etc. |
| Salmonella enterica NBIMCC 8691 | Salmonellosis |
| Enterococcus faecalis NBIMCC 3915 | Endocarditis, sepsis, urinary tract infections (UTIs), meningitis, etc. |
| Enterococcus faecium NBIMMCC 8754 | Bloodstream infections, urinary tract infections (UTIs), and wound infections associated with catheters or surgery are the most common infections. |
4.8. In vivo and In Vitro Effect on Eukaryotic Cell
4.8.1. Cell Treatment and Viability Assay
4.8.2. Measurement of the Mucus Cytotoxic Effect
4.8.3. Determination of Cytoprotective Effect
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garvey, M. Antimicrobial Peptides Demonstrate Activity against Resistant Bacterial Pathogens. Infect. Dis. Rep. 2023, 15, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Antibiotic Resistance: Moving From Individual Health Norms to Social Norms in One Health and Global Health. Front Microbiol. 2020, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Final Report DRUG-RESISTANT INFECTIONS A Threat to Our Economic Future, March 2017, Jonas and World Bank Group Team, 2017.
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med 2023, 20, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Orlek, A.; Anjum, M.F.; Mather, A.E.; Stoesser, N.; Walker, A.S. Factors associated with plasmid antibiotic resistance gene carriage revealed using large-scale multivariable analysis. Sci Rep. 2023, 13, 2500. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Imtiaz, M.; ul Haq, I. Emergent crisis of antibiotic resistance: A silent pandemic threat to 21st century. Microb. Pathog. 2023, 174, 105923. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, C.; Bechinger, B. Molecular packing of amphipathic peptides on the surface of lipid membranes. Langmuir 2014, 30, 10374–83. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Antimicrobial Peptides Demonstrate Activity against Resistant Bacterial Pathogens. Infect. Dis. Rep. 2023, 15, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Muñoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Muñoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. J. Fungi 2022, 7, 1173. [Google Scholar] [CrossRef]
- Tan, L.T.H.; Chan, K.G.; Pusparajah, P.; Lee, W.L.; Chuah, L.H.; Khan, T.M.; Goh, B.H. Targeting Membrane Lipid a Potential Cancer Cure? Front. Pharmacol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, L.; Zhou, Z.; Song, L. Research progress on the mollusc immunity in China. Dev. Comp. Immunol. 2013, 39, 2–10. [Google Scholar] [CrossRef]
- Li, H.; Parisi, M.G.; Parrinello, N.; Cammarata, M.; Roch, P. Molluscan antimicrobial peptides, a review from activity-based evidences to computer-assisted sequences. Invertebr. Surviv. J. 2011, 8, 85–97. [Google Scholar]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Velkova, L.; Stevanovic, S.; Molin, L.; Traldi, P.; Velikova, R.; Voelter, W. Bioactive compounds isolated from garden Snails. J. BioSci. Biotechnol. 2015, 147–155. [Google Scholar]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial Activity of Molluscan Hemocyanins from Helix and Rapana Snails. Curr Pharm Biotechnol. 2016, 17, 263–70. [Google Scholar] [CrossRef]
- Kirilova, M.; Topalova, Y.; Velkova, L.; Dolashki, A.; Kaynarov, D.; Daskalova, E.; Zheleva, N. Antibacterial Action of Protein Fraction Isolated from Rapana venosa Hemolymph against Escherichia coli NBIMCC 8785. Pharmaceuticals 2024, 17, 68. [Google Scholar] [CrossRef]
- Dolashki, A. , Velkova L., Daskalova E., Zheleva N., Topalova Y., Atanasov V., Voelter W., Dolashka P. Antimicrobial Activities of Different Fractions from Mucus of the Garden Snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef]
- Pitt, S.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.; Pereira, A.; Hidalgo, W.; Uribe, N. Antibacterial, Antibiofilm and Anti-Virulence Activity of Biactive Fractions from Mucus Secretion of Giant African Snail Achatina fulica against Staphylococcus aureus Strains. Antibiotics 2021, 10, 1548. [Google Scholar] [CrossRef]
- Etim, L.B.; Aleruchi, C.; Obande, G.A. Antibacterial Properties of Snail Mucus on Bacteria Isolated from Patients with Wound Infection. Br. Microbiol. Res. J. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Santana, W.; Melo, C.; Cardoso, J.; Pereira-Filho, R.; Rabelo, A.; Reis, F.; Albuquerque, R.L.C. Assessment of antimicrobial activity and healing potential of mucous secretion of Achatina fulica. Int. J. Morphol. 2012, 30, 365–373. [Google Scholar] [CrossRef]
- Pitt, S.J.; Hawthorne, J.A.; Garcia-Maya, M.; Alexandrovich, A.; Symonds, R.C.; Gunn, A. Identification and characterisation of anti-Pseudomonas aeruginosa proteins in mucus of the brown garden snail, Cornu aspersum. Br. J. Biomed. Sci. 2019, 76, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Brieva, A.; Philips, N.; Tejedor, R.; Guerrero, A.; Pivel, J.P.; Alonso-Lebrero, J.L.; Gonzalez, S. Molecular basis for the regenerative properties of a secretion of the mollusk Cryptomphalus aspersa. Ski. Pharmacol Physiol 2008, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, N.; Voynikov, Y.; Dolashki, A.; Krumova, E.; Abrashev, R.; Kowalewski, D.; Stevanovic, S.; Velkova, L.; Velikova, R.; Dolashka, P. Antioxidative screening of fractions from the mucus of garden snail Cornu aspersum. Bul. Chem. Com. 2018, 50C, 176–183. [Google Scholar]
- Petrov, L.; Kachaunov, M.; Alexandrova, A.; Tsvetanova, E.; Georgieva, A.; Dolashki, A.; Velkova, L.; Dolashka, P. Snail Mucus Protective Effect on Ethanol-Induced Gastric Ulcers in Mice. Life 2022, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhang, Z.; Li, G.; Sun, J.; Gu, T.; Ain, N.U.; Zhang, X.; Li, D. Extraction, structure, pharmacological activities and applications of polysaccharides and proteins isolated from snail mucus. Int J Biol Macromol. 2024, 258 Pt 1, 128878. [Google Scholar] [CrossRef]
- Deng, T.; Gao, D.; Song, X.; Zhou, Z.; Zhou, L.; Tao, M.; Jiang, Z.; Yang, L.; Luo, L.; Zhou, A.; Hu, L.; Qin, H.; Wu, M. A natural biological adhesive from snail mucus for wound repair. Nat Commun 2023, 14, 396. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F. Antimicrobial properties of terrestrial snail and slug mucus. J Complement Integr Med. 2018, 15. [Google Scholar] [CrossRef]
- Ulagesan, S.; Kim, H.J. Antibacterial and Antifungal Activities of Proteins Extracted from Seven Different Snails. Appl. Sci. 2018, 8, 1362. [Google Scholar] [CrossRef]
- Cerullo, A.R.; McDermott, M.B.; Pepi, L.E.; et al. Comparative mucomic analysis of three functionally distinct Cornu aspersum Secretions. Nat Commun 2023, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, A.; Bujacz, A.; Mak, P.; Potempa, B.; Niedziela, T. Structural studies of Helix aspersa agglutinin complexed with GalNAc: a lectin that serves as a diagnostic tool. Int J Biol Macromol. 2015, 81, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Greistorfer, S.; Klepal, W.; Cyran, N.; Gugumuck, A.; Rudoll, L.; Suppan, J.; von Byern, J. Snail mucus − glandular origin and composition in Helix pomatia. Zoology 2017, 122, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Herluinus Mafranenda, D.N.H.; Kriswandini, I.L.; Arijani, R.E. Antimicrobial proteins of Snail mucus (Achatina fulica) against Streptococcus mutans and Aggregatibacter actinomycetemcomitans. Dent. J. 2014, 47, 31–36. [Google Scholar] [CrossRef]
- Dolashki, A.; Nissimova, A.; Daskalova, E.; Velkova, L.; Topalova, Y.; Hristova, P.; Traldi, P.; Voelter, W.; Dolashka, P. Structure and antibacterial activity of isolated peptides from the mucus of garden snail Cornu aspersum. Bulg. Chem. Commun. 2018, 50C, 195–200. [Google Scholar]
- Topalova, Y.; Belouhova, M.; Velkova, L.; Dolashki, A.; Zheleva, N.; Daskalova, E.; Kaynarov, D.; Voelter, W.; Dolashka, P. Effect and Mechanisms of Antibacterial Peptide Fraction from Mucus of C. aspersum against Escherichia coli NBIMCC 8785. Biomedicines 2022, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Nissimova, A.; Dolashki, A.; Daskalova, E.; Dolashka, P.; Topalova, Y. Glycine-rich peptides from C. aspersum snail with antibacterial activity. Bulg. Chem. Commun. 2018, 50, 169–175. [Google Scholar]
- Meher, P.; Sahu, T.; Saini, V.; Rao, A.R. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou’s general PseAAC. Sci. Rep. 2017, 7, 42362. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, J.M.; Pease, L.B.; Pierce, C.M.; Startz, T.P.; Zhang, Y.; Smith, A.M. The effect of molluscan glue proteins on gel mechanics. J. Exp. Biol. 2004, 207, 1127–1135. [Google Scholar] [CrossRef]
- Tachapuripunya, V.; Roytrakul, S.; Chumnanpuen, P.; E-kobon, T. Unveiling Putative Functions of Mucus Proteins and Their Tryptic Peptides in Seven Gastropod Species Using Comparative Proteomics and Machine Learning-Based Bioinformatics Predictions. Molecules 2021, 26, 3475. [Google Scholar] [CrossRef]
- Li, D.; Graham, L.D. Epiphragmin, the major protein of epiphragm mucus from the vineyard snail, Cernuella virgata. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2007, 148, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.R.; Klein, A.H.; Hayes, R.A.; Wang, T.; Cummins, S.F. The protein and volatile components of trail mucus in the Common Garden Snail, Cornu aspersum. PLoS ONE 2021, 16, e0251565. [Google Scholar] [CrossRef]
- González García, M.; Rodríguez, A.; Alba, A.; Vázquez, A.A.; Morales Vicente, F.E.; Pérez-Erviti, J.; Spellerberg, B.; Stenger, S.; Grieshober, M.; Conzelmann, C.; et al. New Antibacterial Peptides from the Freshwater Mollusk Pomacea poeyana (Pilsbry, 1927). Biomolecules 2020, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ebou, A.; Koua, D.; Addablah, A.; Kakou-Ngazoa, S.; Dutertre, S. Combined Proteotranscriptomic-Based Strategy to Discover Novel Antimicrobial Peptides from Cone Snails. Biomedicines 2021, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.G.; Simova, S.D.; Dangalov, M.; Velkova, L.; Atanasov, V.; Dolashki, A.; Dolashka, P. An 1H NMR- and MS-Based Study of Metabolites Profiling of Garden Snail Helix aspersa Mucus. Metabolites 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.; Berto, R.F.; Gois, E.A.; Fontenele-Cardi, N.C.; Honório-Júnior, J.E.; Konno, K.; Richardson, M.; Rocha, M.F.; Camargo, A.A.; Pimenta, D.C.; Cardi, B.A.; Carvalho, K.M. Leptoglycin: A new Glycine/Leucine-rich antimicrobial peptide isolated from the skin secretion of the South American frog Leptodactylus pentadactylus (Leptodactylidae). Toxicon 2009, 54, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta. 2009, 1788, 1687–92. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef]
- Breitenbach Barroso Coelho, L.C.; Marcelino Dos Santos Silva, P.; Felix de Oliveira, W.; de Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; Dos Santos Correia, M.T. Lectins as antimicrobial agents. J Appl Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Pietrzyk-Brzezinska, A.J.; Bujacz, A. H-type lectins - Structural characteristics and their applications in diagnostics, analytics and drug delivery. Int J Biol Macromol. 2020, 152, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Prokop, O.; Uhlenbruck, G.; Köhler, W. A new source of antibody-like substances having anti-blood group specificity: a discussion on the specificity of Helix agglutinins. Vox Sang. 1968, 14, 321–33. [Google Scholar] [CrossRef]
- Ito, S.; Shimizu, M.; Nagatsuka, M.; Kitajima, S.; Honda, M.; Tsuchiya, T.; Kanzawa, N. High molecular weight lectin isolated from the mucus of the giant African snail Achatina fulica. Biosci Biotechnol Biochem. 2011, 75, 20–5. [Google Scholar] [CrossRef]
- Maji, S.; Datta, U.; Lal Hembram, M. A new sperm agglutinin factor from marine snail Telescopium telescopium: An evaluation with goat (Capra hircus) cauda epididymal spermatozoa. Iran. J. Reprod. Med. 2010, 8, 10–17. [Google Scholar]
- 58 Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Costa-Paiva, E.M. Multifunctional Roles of Hemocyanins. Subcell Biochem. 2020, 94, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.S. Exploring the Antimicrobial Potential and Biofilm Inhibitory Properties of Hemocyanin from Hemifusus pugilinus (Born, 1778). Int J Mol Sci. 2023, 24, 11494. [Google Scholar] [CrossRef]
- Yang, S.; Huang, H.; Wang, F.; Aweya, J.J.; Zheng, Z.; Zhang, Y. Prediction and characterization of a novel hemocyanin-derived antimicrobial peptide from shrimp Litopenaeus vannamei. Amino Acids. 2018, 50, 995–1005. [Google Scholar] [CrossRef]
- Zhuang, J.; Coates, C.J.; Zhu, H.; Zhu, P.; Wu, Z.; Xie, L. Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol. 2015, 49, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ehara, T.; Kitajima, S.; Kanzawa, N.; Tamiya, T.; Tsuchiya, T. Antimicrobial action of achacin is mediated by L-amino acid oxidase activity. FEBS Lett. 2002, 531, 509–512. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Cerullo, A.R.; Parziale, J.; Achrak, E.; Sultana, S.; Ferd, J.; Samad, S.; Deng, W.; Braunschweig, A.B.; Holford, M. Advancing discovery of snail mucins function and application. Front Bioeng Biotech. 2021, 9, 734023. [Google Scholar] [CrossRef]
- Rashad, M.; Sampò, S.; Cataldi, A.; Zara, S. Biological activities of gastropods secretions: snail and slug slime. Nat Prod Bioprospect 2023, 13, 42. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A Biologically Relevant Glycan Barrier in Mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection. NPJ Biofilms Microbiome 2018, 4, 14. [Google Scholar] [CrossRef]
- Okeniyi, F.A.; Oghenochuko, O.M.; Olawoye, S.O.; Animashahun, R.A.; Adeyonu, A.G.; Akpor, O.B. Antimicrobial potentials of mucus mucin from different species of giant African land snails on some typed culture pathogenic bacteria. Asian J Agric & Biol. 2022, 4, 202107294. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Eliseikina, M.G.; Balakirev, E.S.; Ayala, F.J. Multiple bacterial partners in symbiosis with the nudibranch mollusk Rostanga alisae. Sci Rep. 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Madian, A.G.; Regnier, F.E. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010, 9, 3766–80. [Google Scholar] [CrossRef]
- Phrompanya, P.; Suriyaruean, N.; Nantarat, N.; Saenphet, S.; Tragoolpua, Y.; Saenphet, K. Biological properties of mucus from land snails (Lissachatina fulica) and freshwater snails (Pomacea canaliculata) and histochemical study of mucous cells in their foot. Peer J. 2023, 11, e15827. [Google Scholar] [CrossRef]
- Wang, W.; Yi, J.; Ke, S.; Halmela, M. Gastropod biological fluid, method of making and refining and use. 2010. United States Patent Application 2010023 3111.
- Wang, L.; Yang, J.; Wang, Y.; Zhang, J.; Gao, Y.; Yuan, J.; Su, A.; Ju, X. Study on antioxidant activity and amino acid analysis of rapeseed protein hydrolysates. Int. J. Food Prop. 2016, 19, 1899–1911. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. 1970, 227, 680–5. [Google Scholar] [CrossRef] [PubMed]
- Lincz, L.F.; Scorgie, F.E.; Garg, M.B.; Gilbert, J.; Sakoff, J.A. A simplified method to calculate telomere length from Southern blot images of terminal restriction fragment lengths. Biotechniques. 2020, 68, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.; Capdevielle, J.; Guillemot, J.C.; Ferrara, P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992, 203, 173–179. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing; Approved Standard-34th edition; March 2024.
- Daskalova, A.; Petrova, V.; Velkova, L.; Kujumdzieva, A.; Tomova, A.; Voelter, W.; Dolashka, P. Investigation of protein expression of Saccharomyces cerevisiae cells in quiescent and proliferating state before and after toxic stress. Biotechnol. Biotechnol. Equip. 2021, 35, 366–376. [Google Scholar] [CrossRef]
- Kostova, I.; Traykova, M.; Rastogi, V.K. New lanthanide complexes with antioxidant activity. Medicinal Chemistry 2008, 4, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 485, 69–71. [Google Scholar] [CrossRef]
- Zhang, Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis 2000, 21, 1175–1182. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Anti-oxidant activity of polyphenols from Phyllanthus debilis Klein ex Willd. J. Nat. Remedies 2006, 6, 141–146. [Google Scholar]
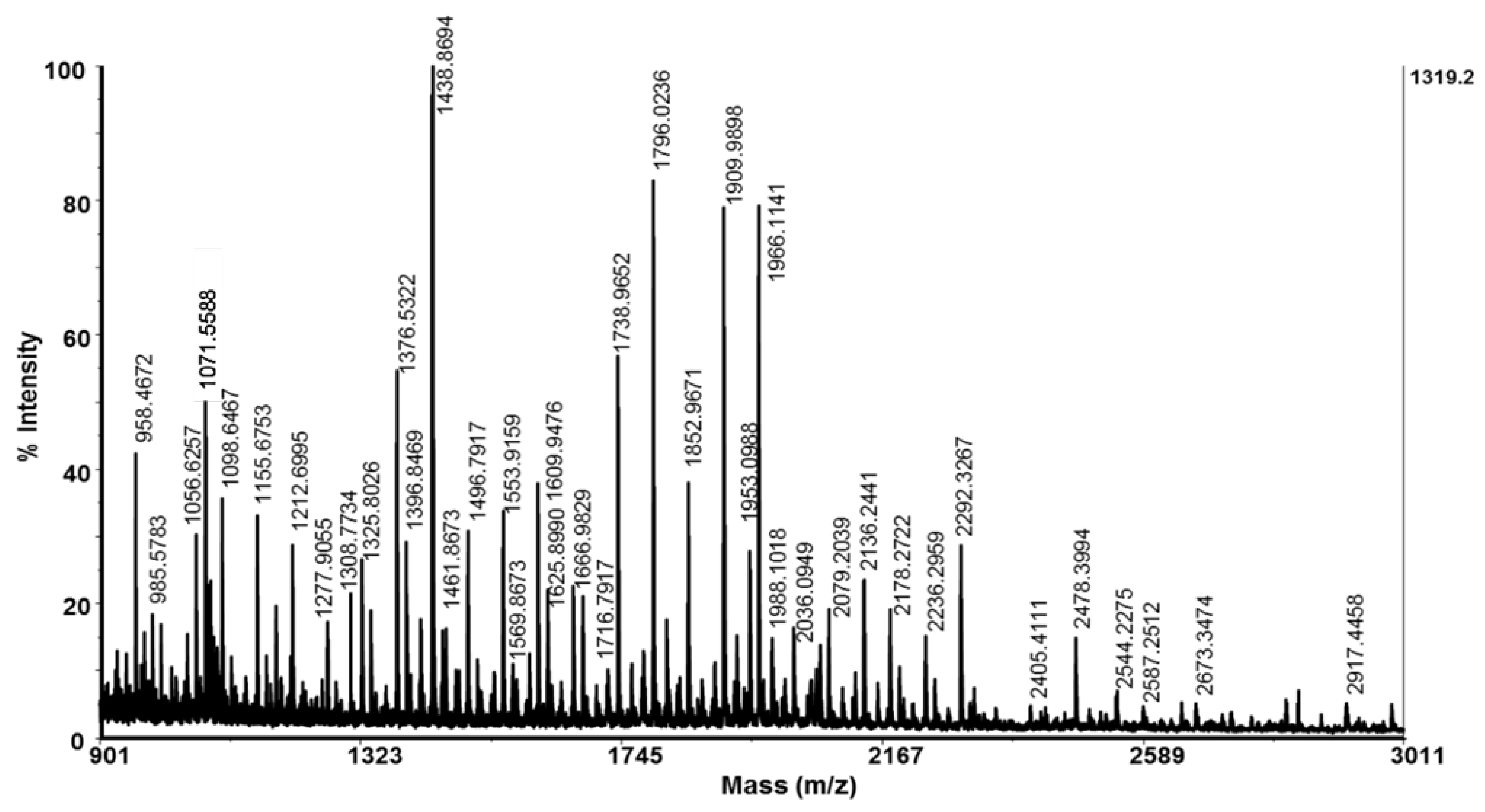

| No | Amino Acid Sequence of Peptides | [M+H]+ Da |
Calcul. monois. mass Da | pI | GRAVY | Net Charge | Predicted by iAMPpred software | ||
|---|---|---|---|---|---|---|---|---|---|
| Аntibacterial (%) | Аntiviral (%) | Аntifungal (%) | |||||||
| 1 | LLPFKEPDL | 1071.60 | 1070.60 | 4.37 | −0.600 | −2/+1 | 28 | 51 | 19 |
| 2 | ACGATLQLENCG | 1179.77 | 1178.51 | 4.00 | +0.350 | -1/0 | 29.3 | 35.7 | 43.7 |
| 3 | LNLGGNGANGLVGG | 1212.76 | 1211.63 | 5.52 | +0.321 | 0/0 | 74.0 | 43.1 | 74.3 |
| 4 | AGVGGAAGNPSTYVG | 1277.70 | 1276.60 | 5.57 | +0.260 | 0/0 | 25.1 | 7.4 | 11.3 |
| 5 | GGGMVKEDGSCLGV | 1308.77 | 1307.58 | 4.37 | +0.207 | −2/+1 | 40.4 | 31.4 | 33.7 |
| 6a | MLGGGVNSLRPPK | 1325.80 | 1324.73 | 11.0 | −0.262 | 0/+2 | 22.8 | 14.0 | 8.9 |
| 7 | CVGGAGGHGDSCAKGT | 1376.53 | 1375.56 | 6.73 | −0.106 | −1/+1 | 85.2 | 48.8 | 74.4 |
| 8 | GGGGYHTWGEGGKF | 1409.48 | 1408.62 | 6.75 | −0.964 | −1/+1 | 69.0 | 62.8 | 72.6 |
| 9 | MLNVAVNKGEVKH | 1438.86 | 1437.78 | 8.37 | −0.138 | −1/+2 | 56.4 | 38.0 | 19.7 |
| 10b | NLVGGSGGGGRGGANPLG | 1496.79 | 1495.75 | 9.75 | −0.217 | 0/+1 | 66.0 | 33.7 | 48.2 |
| 11 | GTMSPAGGEMGPVTAGVG | 1576.04 | 1574.71 | 4.00 | +0.250 | −1/0 | 13.1 | 24.8 | 8.3 |
| 12 | GTKGCGPGSCPPGDTVAGVG | 1716.79 | 1715.76 | 5.82 | −0.100 | −1/+1 | 23.8 | 20.2 | 25.8 |
| 13c | ACSLLLGGGGVGGGKGGGGHAG | 1738.96 | 1737.86 | 8.27 | +0.409 | 0/+1 | 82.6 | 49.5 | 67.0 |
| 14a | LLLDGFGGGLLVEHDPGS | 1796.00 | 1794.92 | 4.02 | +0.439 | -3/0 | 37 | 45 | 10 |
| 15d | MGGWGGLGGGHNGGWMPPK | 1852.96 | 1851.83 | 8.52 | −0.611 | 0/+1 | 69 | 56.0 | 57.0 |
| 16c | ACLTPVDHFFAGMPCGGGP | 1876.88 | 1875.81 | 5.08 | +0.542 | −1/0 | 32.4 | 42.6 | 20.1 |
| 17c | NGLFGGLGGGGHGGGGKGPGEGGG | 1909.98 | 1908.88 | 6.75 | −0.487 | −1/+1 | 89.5 | 67.2 | 79.5 |
| 18 | LLLDNKGGGLVGGLLGGGGKGGG | 1966.11 | 1965.10 | 8.59 | +0.322 | −1/+2 | 93 | 56 | 81 |
| 19 | GMVLLHCSPALDFHKTPAV | 2036.09 | 2035.04 | 6.91 | +0.616 | −1/+1 | 18 | 53 | 14 |
| 20 | LPFLLGVGGLLGGSVGGGGGGGGAPL | 2136.24 | 2135.17 | 5.52 | +1.023 | 0/0 | 66 | 33 | 36 |
| 21 | MVLDGKGGGGLLGGVLGGGKDAHLGG | 2292.32 | 2291.21 | 6.50 | +0.319 | −2/+2 | 84.3 | 59.0 | 71 |
| 22 | LLKDNGVGGLLGGGGAGGGGLVGGNLGGGAG | 2478.39 | 2477.30 | 5.84 | +0.439 | −1/+1 | 86.4 | 54 | 66 |
| 23 | KTSKLMVYLAGGGGGLLGGVGGGGGGAGGGGPGGL | 2843.76 | 2842.48 | 9.70 | +0.374 | 0/+2 | 76 | 47 | 67 |
| Band кDa |
AAS of peptide | Mass [M+H]+ | Protein name | UniProt ID | Identities |
|---|---|---|---|---|---|
| 17.5 | TAAFTEDTSVVTGR | 1454.63 | mucus protein [Cornu aspersum] | QEG59314.1 | 86%, E=8e-05 |
| FTAAFTEDTSVAEGR | 1601.74 | mucus protein [C. aspersum] | QEG59314.1 | 100%, E=2e-09 | |
| GNVAFTAAFTEDTSVAEGR | 1942.91 | mucus protein [C. aspersum] | QEG59314.1 | 100%, E=4e-13 | |
| GALNGNVAFTAAFTEDTSVAEGR | 2298.10 | mucus protein [C. aspersum] | QEG59314.1 | 100%,E=2e-11 | |
| 23.6 | GSCNWPMTILMLESLR | 1850.90 | NADH subunit 6 [Albinaria caerulea] | P48922 | 78%, E=0.005 |
| QYLQITWPSPR | 1388.7 | H-type lectin domain-cont. Protein B. glabrata | KAI8776186.1 | 100%,E=0.041 | |
| 26.7 | VPSDDPGR | 842.40 | Chain A, H. aspersa agglutinin[C. aspersum] | 4Q56_A | 100%,E=0.003 |
| DITFASPYCR | 1172.54 | Chain A, H. aspersa agglutinin C. aspersum | 4Q56_A | 90%,E=1e-04 | |
| NGGLVHPGPR | 1003.54 | uncharacterized protein [Pomacea canaliculata] | XP_025078819.1 | 89%, E=4.5 | |
| 31.35 | DWTLYVNTPLAPAR | 1616.84 | unnamed protein, partial [C. unifasciata] mucin 18b [Plakobranchus ocellatus] |
GFO09821.1 | 78%, E=0.33 75%, E=27 |
| FCPNAQRTR | 1092.54 | glutathione S-transferase omega-1-like [P. Canaliculata] [Elysia marginata] |
XP_025114871.1 GFR70608.1 |
89%, E=0.31 89%, E=0.31 |
|
| NPVGSVPVLELDGK | 1423.78 | glutathione S-transferase omega-1[P. Canaliculata] [B. Glabrata] containing protein [B.glabrata] |
XP_025099831.1 KAI8762368.1 |
86%, E=0.013 79%, E=0.02 |
|
| 33.0 | GPCFTPHTYTNWSWLR | 1965.91 | unnamed protein, Candidula unifasciata] “Bacterial protein of unknown function (HtrL_YibB) | CAG5121511.1 | 63%, E=4e-04 |
| DFLPPASLPDFAPSPPRVAER | 2279.18 | unnamed protein product, [Candidula unifasciata] | CAG5136685.1 | 53%, E=0.34 | |
| 39.1 | AGYLQITWPSPR | 1388.72 | mucus protein [C. aspersum] H-type lectin domain- |
QEG59312.1 KAI8776186.1 |
100%,E=1e-08 100% E=0.055 |
| ASVTGDLSNK | 991.51 | mucus protein [C. aspersum] | QEG59312.1 | 100%,E=8e-04 | |
| ELLGNVYRAAFTEDTSVAEGR | 2298.14 | mucus protein [C. aspersum] | QEG59314.1 | 77%, E=3e-08 | |
| VGSNGAR | 660.34 | mucus protein [C. aspersum] | QEG59312.1 | 100%E=0.004 | |
| ANVTFDLSEK | 1123.56 | von Willebrand factor A domain-containing protein 3B-like isoform X1 [B. glabrata] protein 3B isoform X2, [B. glabrata] |
XP_013069929.2 XP_013069930.2 |
100%, E=0.59 100%, E=0.59 |
|
| LFSTASGGR | 895.4632 | Collagen alpha-6(VI) chain, partial [Biomphalaria pfeifferi] | KAK0040452.1 | 89% E=1.9 | |
| LPLYEDPKLDVSSLR | 1744.83 | uncharacterized protein LOC101853718 [A. californica] | XP_012941625.1 | 83%, E=0.27 | |
| 48.7 | FDANPFFSGR | 1157.59 | Hemocyanin, βC chain unit D [Helix pomatia] | P12031.2 | 89%, E=9e-06 |
| HGSPIGVPYWDWTR | 1670.93 | Hemocyanin α N [C. aspersum] | AYO86684.1 | 100%, E=2e-09 | |
| LISEATYFNSR | 1300.71 | hemocyanin β [C. aspersum] βC chain unit D [H. pomatia] |
AYO86685.1, P12031.2 |
100%, E=2e-04 82%, E=6e-05 |
|
| FDPNPFFSGR | 1183.5 | Hemocyanin, βC chain unit D [H. pomatia] | P12031.2 | 100%,E=3e-07 | |
| EVFEQVEHALLAR | 1540.81 | Hemocyanin β [C. aspersum] βC chain unit D [H. pomatia] |
AYO86685.1 P12031.2 |
100%, E=0.006 88%, E=0.050 |
|
| QYLQITWSPPR | 1388.73 | fibrinogen-like protein A isoform [B. glabrata] | XP_055864456.1 | 78%, E=1.4 | |
| 52.8 | TDVTSALLGARCNEGGTNTHSPLR | 2470.21 | unnamed protein, partial [C. unifasciata] unnamed protein C. unifasciata |
CAG5131631.1 CAG5131621.1 |
70%, E=0.006 63%, E=0.26 |
| LTQHFNVGSNGAR | 1400.70 | mucus protein [C. aspersum] unnamed protein C. unifasciata unnamed protein partial [C. unifasciata] |
QEG59312.1 CAG5131621.1 CAG5131631.1 |
92%,E=0.001 77%, E=0.094 75%, E= 0.38 |
|
| MPDYDCGCCNGSNGSYGSGGGGGGGR | 2384.84 | unnamed protein, partial [C.unifasciata] unnamed protein product [C. unifasciata] |
CAG5126455.1 CAG5131834.1 |
80% , Е=0.23 63% Е=0.23 |
|
| 56.9 | DGMSAAPQAENAFALK | 1620.77 | Achacin; Flags: Precursor [L. Fulica] L-amino-acid oxidase (Escapin) A. Californica |
P35903.1 Q6IWZ0.1 |
71%, E=3e-04 63%, Е=0.95 |
| SGFPTTSKDR | 1095.54 | L-amino-acid oxidase (Escapin) [A. Californica | Q6IWZ0.1 | 100%, E=0.080 | |
| VGGGPSGVELDEFEARVELK | 2088.0 | Achacin ; Flags : Precursor [Lissachatina fulica] | P35903.1 | 41% E=0.40 | |
| AELKGDMQYTYADSEKVR | 2104.00 | fibrillin-3, partial [Biomphalaria pfeifferi] fibrillin-3, partial [B. Glabrata] |
KAK0048148.1 KAK6970092.1 |
48%, Е=0.22 48%, Е=0.22 |
|
| 70.8 | AKVQVIGVPDDRLLLR | 1792.08 | acyl-CoA synthetase family member 2, mito- chondrial-like, partial [Pomacea canaliculata] unnamed protein product, [C. unifasciata] |
XP_025086706.1 CAG5136482 |
91%, E=0.005 91%, E=0.005 |
| HGGGGGGFGGGGFGSR | 1320.65 | uncharacterized protein LOC124113905 [H. rufescens fibroin heavy chain isoform X1 [A. Californica] elastin-like [Ostrea edulis] Glycine, alanine asparagine -rich protein [Haliotis asinina] |
XP_046330350.2 XP_012943828.1 XP_056017499.1 P86732.1 |
100%,E=9e-04 81%, E=0.018 87%, E=0.038 65%, E=0.004 |
|
| 84.7 | YVLEDLSAADLELSR | 1693.86 | FMRFamide-activatedamiloride-sensitive sodium channel [C. aspersum] | Q25011.1 | 100%, E=0.14 |
| GSSVSLCVWDWAVLPR | 1774.8 | FMRFamide-activatedamiloride-sensitive sodium channel [C. aspersum] | Q25011.1 | 71%, E= 1.9 | |
| 97.0 | MVTSLYPGEDWAMLPR | 1865.89 | mucin-5AC-like, partial [Haliotis rufescens] | XP_048239711.1 | 56%, E= 0.15 |
| ETVSPGYSEGECTCASITQK | 2089.91 | mucin-5B-like isoform X4 [H. rubra] | XP_046552239.1 | 62%, E= 6.7 | |
| VFADFELHNIGASADVR | 1860.92 | hemocyanin β [C. aspersum] | AYO86685.1 | 100%, E= 2e-10 | |
| CQVSLPYWDWAVPLR | 1832.92 | hemocyanin, βC chain unit G [H. pomatia] | P56823.1 | 100%, E= 5e-04 | |
| LSGYDVSKVNEILK | 1564.86 | hemocyanin β [C. aspersum] | AYO86685.1 | 85%, E=8e-05 | |
| HLWLLHCFEHDLNGYEYDNLR | 2687.25 | hemocyanin β [C. aspersum] | AYO86685.1 | 87% E=8e-06 | |
| ANNARLTDASHDNPFSSYTLR | 2350.12 | hemocyanin β [C. aspersum] | AYO86685.1 | 94%, E=1e-09 | |
| 143.3 | VGRIESESYVVKKSK | 1708.96 | zinc finger protein 502-like [B. glabrata] 184 [B. glabrata] |
XP_055863608 KAI8795828.1 |
77%, E=0.12 77%, E=0.12 |
| VLTFYQSCDCVVDHCHR | 2024.88 | zinc finger protein 664-like [Patella vulgata] | XP_050401404.1 | 75%, E=1.5 | |
| GEPGSTGPPGLK | 1096.56 | collagen alpha-1(IV) chain-like, partial [P. canaliculata] | XP_025114158.1 | 100%, E=2e-04 | |
| NGPSGVELDEFEARVELK | 1988.99 | collagen alpha-1(XIII) chain – like isoform X7 [H. rubra] | XP_046573418.1 | 59%, E=2.5 | |
| FVDMDGMFSSAAGGRPEAR | 2000.89 | collagen α-4(VI) chain-like X2 [P. acuta] α-1(XII) chain-like [P. acuta] α-1(XII) chain-like isoform X6 [B. glabrata] |
XP_059154404.1 XP_059163536.1 XP_055860588.1 |
73%, E= 0.012 75%, E=0.26 68%, E=0.26 |
|
| RAYTDGMFSSAAGGRPEAR | 1999.94 | collagen α-4(VI) chain-like isoform [P. acuta] collagen α-4(VI) chain-like isoform [P. acuta] |
XP_059154404.1 XP_059154396.1 |
79%,E=0.031 79%, E=0.031 |
|
| CNEGGTNTHSPLR | 1385.62 | collagen α-6(VI) chain-like [Physella acuta] | XP_059164377.1 | 89%, E=6.1 | |
| 198.31 | NPGYLVSKVNEILK | 1573.89 | mucin-2-like [P. acuta] serine/arginine repetitive matrix protein 2 [B. glabrata] | XP_059140993.1 KAI8735946.1 |
71%, E=0.84; 71%, E=0.84 |
| DCRVRVGGPLFAPSPYLFER | 2279.1 | mucin-17-like [Haliotis rubra] | XP_046554219.1 | 52%,E=0.78 | |
| FNFLPLSADALESLR | 1692.90 | E3 ubiquitin-protein ligase HERC2-like isoform X2 [B. glabrata] | XP_055894969.1 | 83%, E= 0.50 | |
| ASSGSCDFSSSTAANR | 1547.64 | mucin-5AC-like isoform X2 [H. rufescens] mucin-5AC-like isoform X1 [H. rufescens] |
XP_048252836.1 XP_048252834.1 |
85%,E= 0.45 85%,E= 0.45 |
|
| GCAGCPQAGSK | 978.41 | fibrillin-1-like [B. glabrata] | XP_055888733.1 | 89%, Е=2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





