Introduction
Viruses pose a serious challenge to human health, causing a wide range of diseases from the common cold to more severe illnesses such as HIV/AIDS and covid-19 (Sankaran & Weiss, 2021). The treatment and prevention of viral infections are crucial areas of research and have led to the development of various strategies to combat these pathogens (Cheng et al, 2017). Drug delivery methods for antiviral agents are an essential aspect of these strategies, enabling the effective targeting and elimination of viruses within the human body (Kausar et al, 2021).
One of the primary methods of treating viral infections is the use of antiviral drugs that inhibit the replication of the virus (Vardanayan & Hruby, 2016). These drugs work by targeting specific stages of the viral life cycle, such as entry into the host cell, replication, or release of new viral particles (De Clercq, 2007). The effectiveness of these drugs often depends on their ability to reach the site of infection in sufficient concentrations, making drug delivery systems an essential component of antiviral therapy (Sharma et al, 2012).
An innovative and increasingly research method involves the use of nanoparticles for enhanced delivery of antiviral agents (Milovanovic et al, 2017). Nanoparticles, due to their small size and the ability to be engineered with specific properties, offer a promising approach to overcoming the challenges associated with traditional drug delivery systems (Patel et al., 2019). These nanostructured materials can be designed to improve the solubility, stability, and bioavailability of antiviral drugs, as well as to facilitate targeted delivery to the site of viral infection, thereby maximizing therapeutic efficacy while minimizing side effects (Singh et al., 2017).
Nanoparticles can be made from a variety of materials, including lipids, polymers, and metals, and can be tailored to carry both small-molecule drugs and biologics, such as peptides, proteins, and nucleic acids (Siddiqi et al., 2018). Lipid-based nanoparticles, for example, have been extensively studied for their ability to encapsulate and deliver RNA-based therapeutics, a strategy that has proven pivotal in the rapid development and deployment of mRNA vaccines against COVID-19 (Zhang et al., 2020). These lipid nanoparticles protect the RNA molecules from degradation, facilitate their entry into host cells, and ensure the efficient expression of the viral antigen, eliciting a robust immune response (Hou et al., 2021).
Polymeric nanoparticles are another class of carriers that have shown promise in antiviral drug delivery. They can be engineered to release their payload in a controlled manner, ensuring sustained drug levels at the target site and reducing the frequency of dosing (Gupta et al., 2019). Additionally, the surface of these nanoparticles can be modified with ligands that recognize and bind to specific receptors on the surface of infected cells, thereby achieving targeted delivery of antiviral agents (Kumar et al., 2020).
Metallic nanoparticles, such as silver and gold nanoparticles, have also been explored for their antiviral properties. These nanoparticles can inactivate viruses directly or can be used as carriers for antiviral drugs, enhancing their therapeutic effectiveness (Elechiguerra et al., 2005). Furthermore, the unique optical properties of gold nanoparticles have been utilized in the development of diagnostic tools for viral infections, demonstrating the versatile applications of nanoparticles in the field of virology (Yeh et al., 2020).
In conclusion, the application of nanoparticles in the field of antiviral drug delivery represents a significant advancement in our ongoing battle against viral infections. By harnessing the unique properties of nanoparticles, including their small size, customizable surface characteristics, and ability to encapsulate a wide range of therapeutic agents, researchers are developing more effective, targeted, and less toxic antiviral therapies. As this systematic review will further explore, the innovative use of lipid, polymeric, and metallic nanoparticles offers promising strategies for enhancing the delivery and efficacy of antiviral agents. These nanotechnology-based approaches not only hold the potential to revolutionize the treatment of existing viral infections but also to rapidly respond to emerging viral threats, thereby contributing to global public health security and resilience.
Methods
Objective:
The objective of this systematic review is to evaluate and synthesize the advancements in nanoparticle-based vaccine technology for broad-spectrum protection against influenza viruses. Specifically, it aims to assess the efficacy, immunogenicity, and potential for universal vaccine development presented by the latest research on lipid nanoparticles and nanoparticle-enhanced vaccines targeting multiple influenza virus strains.
Search Strategy:
To identify relevant empirical studies on the use of nanoparticles for drug delivery in humans, particularly for combating influenza viruses, a comprehensive search was conducted in PubMed. The search covered a period from 2018 to 2024, focusing on the most recent and relevant studies in the field. The following keywords and Boolean operators were used to refine the search: ("Drug delivery methods in humans" AND "Virus" AND "nanoparticles" AND "Influenza").
Inclusion and Exclusion Criteria:Inclusion Criteria:
The review includes empirical studies focused on human subjects, specifically investigating the efficacy and mechanisms of nanoparticles in drug delivery systems for treating or preventing influenza. These studies must be published between 2018 and 2024 and accessible through PubMed, providing direct insights into the advancement of nanoparticle technology in virology.
Exclusion Criteria:
Excluded from the review are non-empirical articles such as reviews, editorials, and commentaries. Studies published outside the specified date range, research not involving human subjects or unrelated to drug delivery methods, and papers focusing on viruses other than influenza are also omitted. This delineation ensures the collected data is relevant and contributes meaningfully to understanding nanoparticle-based interventions against influenza.
Data Extraction: A matrix table has been used to display each selected paper. A descriptive analysis was done to review each article.
Data Analysis: The data was synthesised manually, to create a narrative discussion
Results
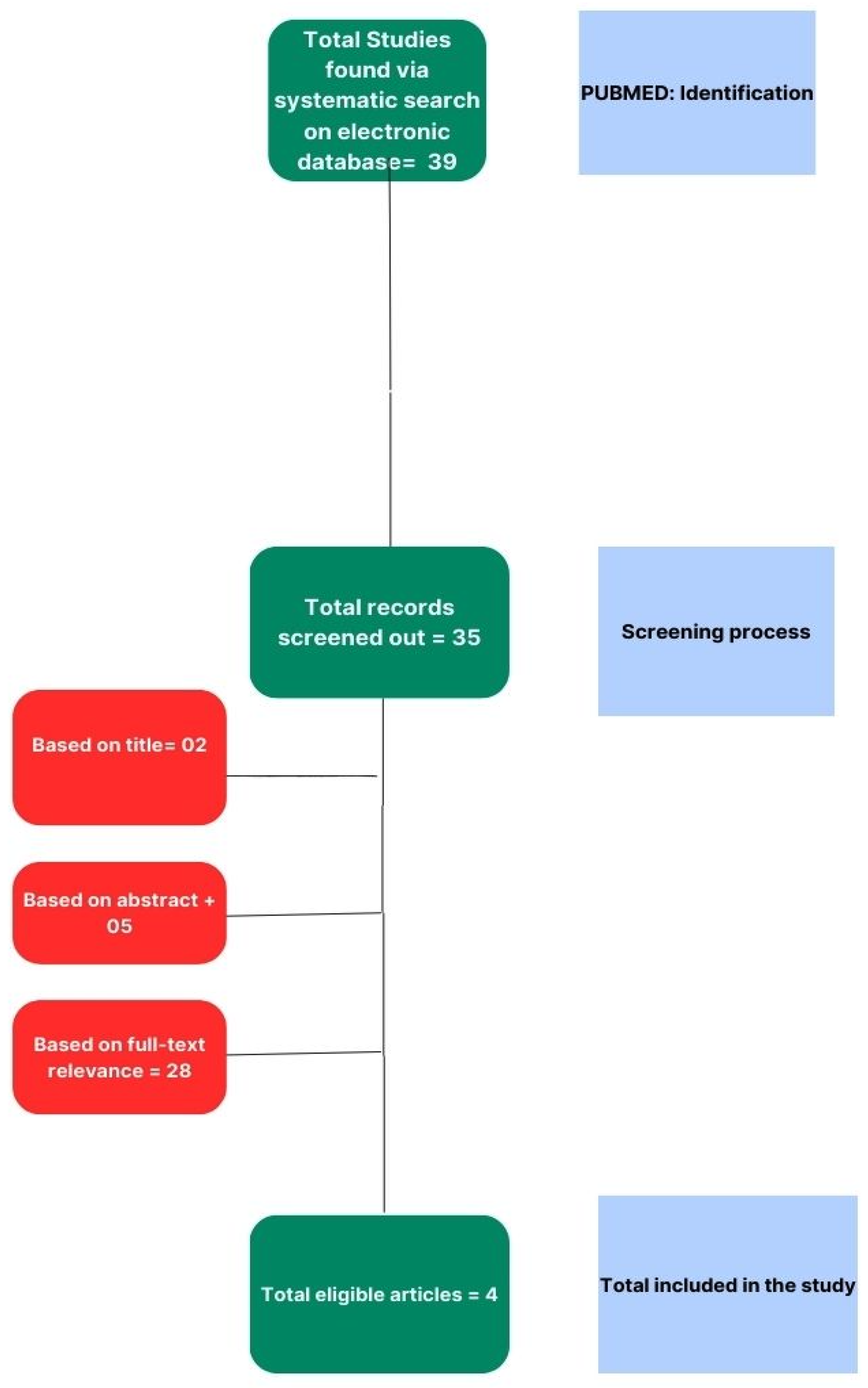
Our systematic review process was meticulously structured to ensure a comprehensive analysis of the literature pertinent to our research question. The initial stage of the process involved an exhaustive search of electronic databases to identify relevant studies. This primary search was conducted through PUBMED, which was chosen for its extensive repository of medical and life science journals. We employed a combination of keywords and MeSH terms tailored to our study objectives, yielding a total of 39 potential studies for inclusion.
Subsequent to the identification process, we implemented a rigorous screening procedure to assess the eligibility of the retrieved records. This involved a preliminary review of titles and abstracts, leading to the exclusion of studies that did not meet the predefined inclusion criteria. Records that were deemed irrelevant based on their titles (n=2) were first removed, followed by a more detailed examination of abstracts, resulting in a further exclusion of 5 studies.
The remaining articles underwent a thorough full-text review to determine their relevance to the research topic. A total of 28 studies were excluded at this stage based on criteria such as insufficient data, inappropriate study design, or lack of alignment with the research focus. The full-text review was conducted independently by two reviewers to ensure objectivity, with any disagreements resolved through discussion or consultation with a third reviewer.
Following the multi-level screening process, 4 studies were found to be eligible and were included in the final systematic review. The selection process is graphically summarized in the PRISMA flow diagram (
Figure 1).
The included studies were subjected to a qualitative synthesis, and where possible, a quantitative meta-analysis. Each study was evaluated for its methodological quality using appropriate appraisal tools, the results of which are presented in subsequent
Table 1.
Discussion
Development of Broad Spectrum Pentavalent Nucleoside-Modified mRNA Vaccine against Influenza B Viruses-
A new approach to developing an influenza B virus vaccine involves using lipid nanoparticle-encapsulated nucleoside-modified mRNA (mRNA-LNP). A single dose of a pentavalent vaccine containing five different antigens, including hemagglutinin and neuraminidase proteins, nucleoprotein and matrix protein, protected mice from a wide range of influenza B viruses. The mRNA-LNP is delivered to cells, where it is translated into proteins that stimulate the immune system to produce antibodies against the virus. Most monovalent mRNA-LNP vaccine formulations protect mice from challenges with most influenza B viruses.
B/Yamagata/16/1988-like lineage HA antigen:
Evaluation of the immunogenicity and protective efficacy of a B/Yamagata/16/1988-like lineage HA antigen was tested by vaccinating mice with 5 µg of the antigen and challenged with influenza B viruses. The antigen demonstrated broad protective immune responses and outperformed other antigen constructs, suggesting potential for further investigation as a vaccination regimen.
B/Victoria/2/1987-like lineage HA antigen:
The B/Victoria/2/1987-like lineage HA antigen was tested for its protective immunity. Mice vaccinated with 5 µg showed protection against most influenza B viruses after a single dose, and modest improvements in functional antibody responses indicated its potential effectiveness.
NA antigen:
A new influenza B virus vaccine was tested on mice which included the NA antigen. Vaccination with a B/Victoria/2/87-like NA from B/Colorado/06/2017 (V) provided protection against both B/Yamagata/16/88-like and B/Victoria/2/87-like viruses. Although the B/Yamagata/16/1988-like NA reduced viral titers, it did not produce measurable NAI activity.
NP antigen:
The NP antigen has been found to induce robust antibody responses and protect mice from influenza virus challenge, but it did not lead to the production of neutralizing antibodies. It was found that NP-mediated protection is not antibody-dependent, and targeting T cell epitopes has shown high efficacy in protecting mice. The B/Col NP mRNA-LNP vaccine induced strong antigen-specific CD4+ and CD8+ T cell responses after a single dose, with particularly strong IFN-γ+CD8+ T cell responses. The observed protection in NP mRNA-LNP-vaccinated animals likely stemmed from T cell-mediated immunity.
M2 antigen:
The M2 antigen was tested for its ability to induce broad protective immune responses in vaccines. Although not fully protective, it significantly reduced viral titers, suggesting M2 could be a valuable antigen for multivalent vaccines, though further research is needed to determine its efficacy.
Quadrivalent Influenza Nanoparticle Vaccines Induce Broad Protection-
Two-component nanoparticle immunogens were used, designed to display 20 hemagglutinin trimmers, to induce broad protection against various influenza viruses. These nanoparticles induced antibody responses comparable to or better than commercially available vaccines, and also broad protective responses against viruses different from the vaccine strains by targeting the conserved HA stem region. The findings suggest that nanoparticle immunogens could be a universal influenza vaccine.
Lipid Nanoparticles Enhance the Efficacy of mRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses-
Lipid nanoparticles (LNPs) as adjuvants were tested to enhance the immune response to vaccines. It reveals that LNPs can significantly improve the efficacy of mRNA and protein subunit vaccines, offering a promising new approach to vaccine development. LNPs can induce robust T follicular helper (Tfh) cells and humoral responses, which are crucial for activating B cells and generating long-lived memory B cells and antibody-producing plasma cells. The study shows that LNPs outperform other adjuvants like MF59 in stimulating both Tfh cell and humoral responses, suggesting that LNPs can significantly improve the effectiveness of mRNA and protein subunit vaccines. The research suggests that LNPs can be broadly applicable to various vaccine types, potentially improving the efficacy of a wide range of vaccines.
An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses-
Intranasal Multivalent Epitope-Based Nanoparticle vaccine is a novel approach to developing a broad-spectrum vaccine against influenza B viruses. The research uses lipid nanoparticles to deliver five different antigens from influenza B viruses, including hemagglutinin and neuraminidase proteins from the B/Yamagata and B/Victoria lineages, as well as nucleoprotein and matrix protein 2 from the B/Victoria lineage. This multivalent approach aims to target diverse influenza B strains and potentially overcome the limitations of current seasonal vaccines.
The development of universal anti-influenza vaccines requires the use of suitable epitopes, such as three conserved peptides from HA, NA, and M2e proteins. These peptides cover most mutations in current influenza strains. Combining these peptides on a ferritin nanoparticle simulates natural viruses, resulting in superior immune responses and cross reactive protection. The inclusion of multiple conserved epitopes from different virus proteins is a good strategy for the universal influenza vaccine goal. The study also found that tandem epitopes in HMNF perform better than their mixture array.
Limitations
Despite the comprehensive approach of this systematic review, it is important to acknowledge several limitations that might affect the generalizability and interpretation of the findings. The limitations are inherent in the research design, the selection of studies, and the nature of the subject matter.
Firstly, the review was restricted to studies available in the PubMed database and published between 2018 and 2024. Although PubMed is a widely recognized database for health-related research, the exclusion of studies from other databases may have resulted in publication bias, potentially overlooking significant contributions from studies published elsewhere or in languages other than English.
Secondly, the selection criteria necessitated that the studies involve human subjects and focus on the efficacy and mechanisms of nanoparticles in drug delivery systems for treating or preventing influenza. This requirement means preclinical studies, often essential in understanding the mechanistic underpinnings and in the early development of therapeutic strategies, were omitted. Consequently, the review does not encompass the full spectrum of research activities, such as animal model studies that could provide additional insights into the potential of nanoparticle-based vaccines.
Another limitation is the reliance on empirical studies that specifically address nanoparticles' role in drug delivery, which narrows the scope of the review and excludes a broader range of nanotechnologies that may be applicable to antiviral therapies. The rapid pace of development in nanomedicine means that emerging technologies may not be adequately represented in the current literature.
The data extraction was based on a narrative synthesis rather than a quantitative meta-analysis. This approach may introduce a subjective bias in interpreting the results, as the process relies heavily on the reviewers’ expertise and judgement. Quantitative data that could offer a more objective comparison between studies is thus underrepresented.
Lastly, the review acknowledges the innovative potential of intranasal multivalent epitope-based nanoparticle vaccines, but it also recognizes the need for further studies to assess safety and efficacy in humans. This highlights the nascent stage of some of the technologies discussed and implies that more comprehensive clinical trials are necessary to establish these approaches' clinical relevance.
Conclusion
In summary, recent developments in influenza B virus vaccination strategies have shown promise in providing broad-spectrum protection. The utilization of lipid nanoparticle-encapsulated nucleoside-modified mRNA (mRNA-LNP) technology to deliver a pentavalent vaccine has demonstrated potential in stimulating a comprehensive immune response, with encouraging outcomes in preclinical models.
Research findings indicate that antigens derived from both the B/Yamagata and B/Victoria lineages, at doses as low as 5 µg, are capable of eliciting robust protective immunity in mice, suggesting their potential incorporation into future vaccine formulations. The inclusion of the NA antigen, despite the absence of neuraminidase inhibitor activity, has reduced viral titers, hinting at alternative protective mechanisms.
The role of the NP antigen in eliciting a strong T cell-mediated immune response further diversifies the immune targets in vaccine design, emphasizing the importance of cellular immunity. Meanwhile, the partial protection offered by the M2 antigen supports its inclusion in a multivalent approach, warranting further study.
The success of nanoparticle immunogens in inducing broad protection by focusing on conserved regions of the virus suggests a step towards a universal influenza vaccine. Additionally, the enhancement of immune responses by lipid nanoparticles (LNPs) positions them as a highly effective adjuvant technology for both mRNA and protein subunit vaccines.
The innovative approach of an intranasal multivalent epitope-based nanoparticle vaccine also stands out as a promising strategy to counteract various influenza B strains. This underscores the potential for such vaccines to move beyond the limitations of current seasonal vaccines and offer more extensive protection.
Overall, the research on influenza B virus vaccines is moving towards multivalent and broadly protective strategies, integrating novel antigens, delivery systems, and adjuvants. These advances are laying the groundwork for the next generation of influenza vaccines, aiming for a wider range of efficacy and the prospect of universal protection against influenza viruses.
Note: This paper did not receive any external funding, and the authors declare no conflict of interest
References
-
1. Alameh, M. G., Tombácz, I., Bettini, E., Lederer, K., Sittplangkoon, C., Wilmore, J. R., Gaudette, B. T., Soliman, O. Y., Pine, M., Hicks, P., Manzoni, T. B., Knox, J. J., Johnson, J. L., Laczkó, D., Muramatsu, H., Davis, B., Meng, W., Rosenfeld, A. M., Strohmeier, S., Lin, P. J. C., … Pardi, N. (2021). Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity, 54(12), 2877–2892.e7. [CrossRef]
-
2. Boyoglu-Barnum, S., Ellis, D., Gillespie, R. A., Hutchinson, G. B., Park, Y. J., Moin, S. M., Acton, O. J., Ravichandran, R., Murphy, M., Pettie, D., Matheson, N., Carter, L., Creanga, A., Watson, M. J., Kephart, S., Ataca, S., Vaile, J. R., Ueda, G., Crank, M. C., Stewart, L., … Kanekiyo, M. (2021). Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature, 592(7855), 623–628. [CrossRef]
-
3. Cheng, V. C. C., Chan, J. F. W., Hung, I. F. N., & Yuen, K. Y. (2017). Viral Infections, an Overview with a Focus on Prevention of Transmission. International Encyclopedia of Public Health, 368–377. [CrossRef]
-
4. De Clercq E. (2007). The design of drugs for HIV and HCV. Nature reviews. Drug discovery, 6(12), 1001–1018. [CrossRef]
-
5. Elechiguerra, J. L., Burt, J. L., Morones, J. R., Camacho-Bragado, A., Gao, X., Lara, H. H., & Yacaman, M. J. (2005). Interaction of silver nanoparticles with HIV-1. Journal of nanobiotechnology, 3, 6. [CrossRef]
- Gupta, A., Mumtaz, S., Li, C. H., Hussain, I., & Rotello, V. M. (2019). Combatting antibiotic-resistant bacteria using nanomaterials. Chemical Society Reviews, 48(2), 415-427.
- Hou, X., Zaks, T., Langer, R., & Dong, Y. (2021). Lipid nanoparticles for mRNA delivery. Nature Reviews Materials, 6(12), 1078-1094.
-
8. Pan, J., Wang, Q., Qi, M., Chen, J., Wu, X., Zhang, X., Li, W., Zhang, X. E., & Cui, Z. (2023). An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses. ACS nano, 17(14), 13474–13487. [CrossRef]
-
9. Kausar, S., Said Khan, F., Ishaq Mujeeb Ur Rehman, M., Akram, M., Riaz, M., Rasool, G., Hamid Khan, A., Saleem, I., Shamim, S., & Malik, A. (2021). A review: Mechanism of action of antiviral drugs. International journal of immunopathology and pharmacology, 35, 20587384211002621. [CrossRef]
- Kumar, N., Sharma, S., Kumar, R., Tripathi, B. N., Barua, S., Ly, H., & Rouse, B. T. (2020). Host-directed antiviral therapy. Clinical microbiology reviews, 33(3), 10-1128.
-
11. Milovanovic, M., Arsenijevic, A., Milovanovic, J., Kanjevac, T., & Arsenijevic, N. (2017). Nanoparticles in Antiviral Therapy. Antimicrobial Nanoarchitectonics, 383–410. [CrossRef]
-
12. Pardi, N., Carreño, J.M., O’Dell, G. et al. (2022) Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat Commun 13, 4677. [CrossRef]
-
13. Patel, S., Ryals, R. C., Weller, K. K., Pennesi, M. E., & Sahay, G. (2019). Lipid nanoparticles for delivery of messenger RNA to the back of the eye. Journal of controlled release : official journal of the Controlled Release Society, 303, 91–100. [CrossRef]
-
14. Sankaran, N., & Weiss, R. A. (2021). Viruses: Impact on Science and Society. Encyclopedia of Virology, 671–680. [CrossRef]
-
15. Sharma, P., Chawla, A., Arora, S., & Pawar, P. (2012). Novel drug delivery approaches on antiviral and antiretroviral agents. Journal of advanced pharmaceutical technology & research, 3(3), 147–159. [CrossRef]
- Siddiqi, K. S., Husen, A., & Rao, R. A. (2018). A review on biosynthesis of silver nanoparticles and their biocidal properties. Journal of nanobiotechnology, 16, 1-28.
- Singh, L., Kruger, H. G., Maguire, G. E., Govender, T., & Parboosing, R. (2017). The role of nanotechnology in the treatment of viral infections. Therapeutic advances in infectious disease, 4(4), 105-131.
-
18. Vardanyan, R., & Hruby, V. (2016). Antiviral Drugs. Synthesis of Best-Seller Drugs, 687–736. [CrossRef]
-
19. Yeh, M. T., Bujaki, E., Dolan, P. T., Smith, M., Wahid, R., Konz, J., Weiner, A. J., Bandyopadhyay, A. S., Van Damme, P., De Coster, I., Revets, H., Macadam, A., & Andino, R. (2020). Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell host & microbe, 27(5), 736–751.e8. [CrossRef]
-
20. Zhang, N. N., Li, X. F., Deng, Y. Q., Zhao, H., Huang, Y. J., Yang, G., Huang, W. J., Gao, P., Zhou, C., Zhang, R. R., Guo, Y., Sun, S. H., Fan, H., Zu, S. L., Chen, Q., He, Q., Cao, T. S., Huang, X. Y., Qiu, H. Y., Nie, J. H., … Qin, C. F. (2020). A Thermostable mRNA Vaccine against COVID-19. Cell, 182(5), 1271–1283.e16. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).