Submitted:
29 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
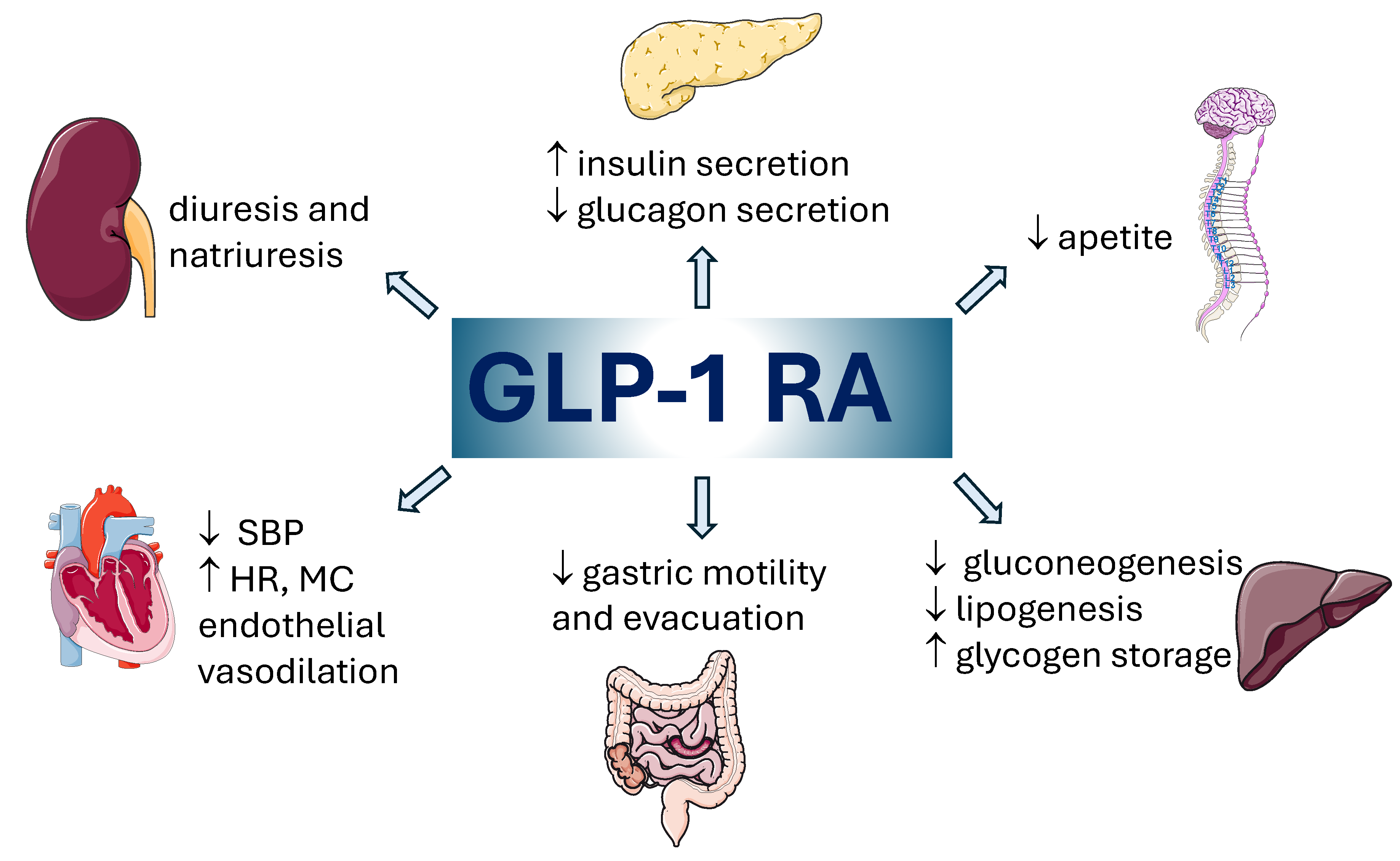
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Materials
2.3. Data Analysis
3. Results
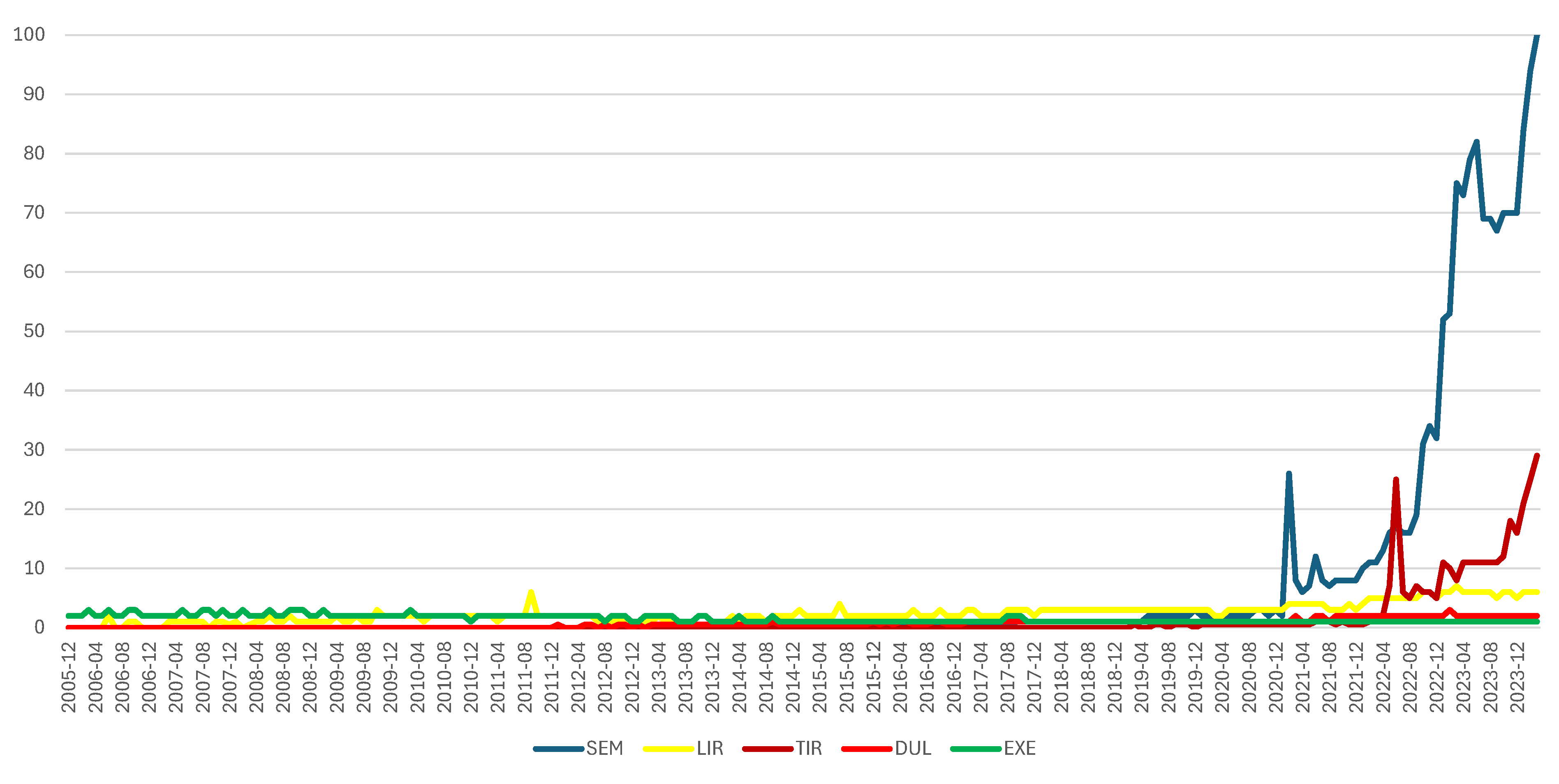
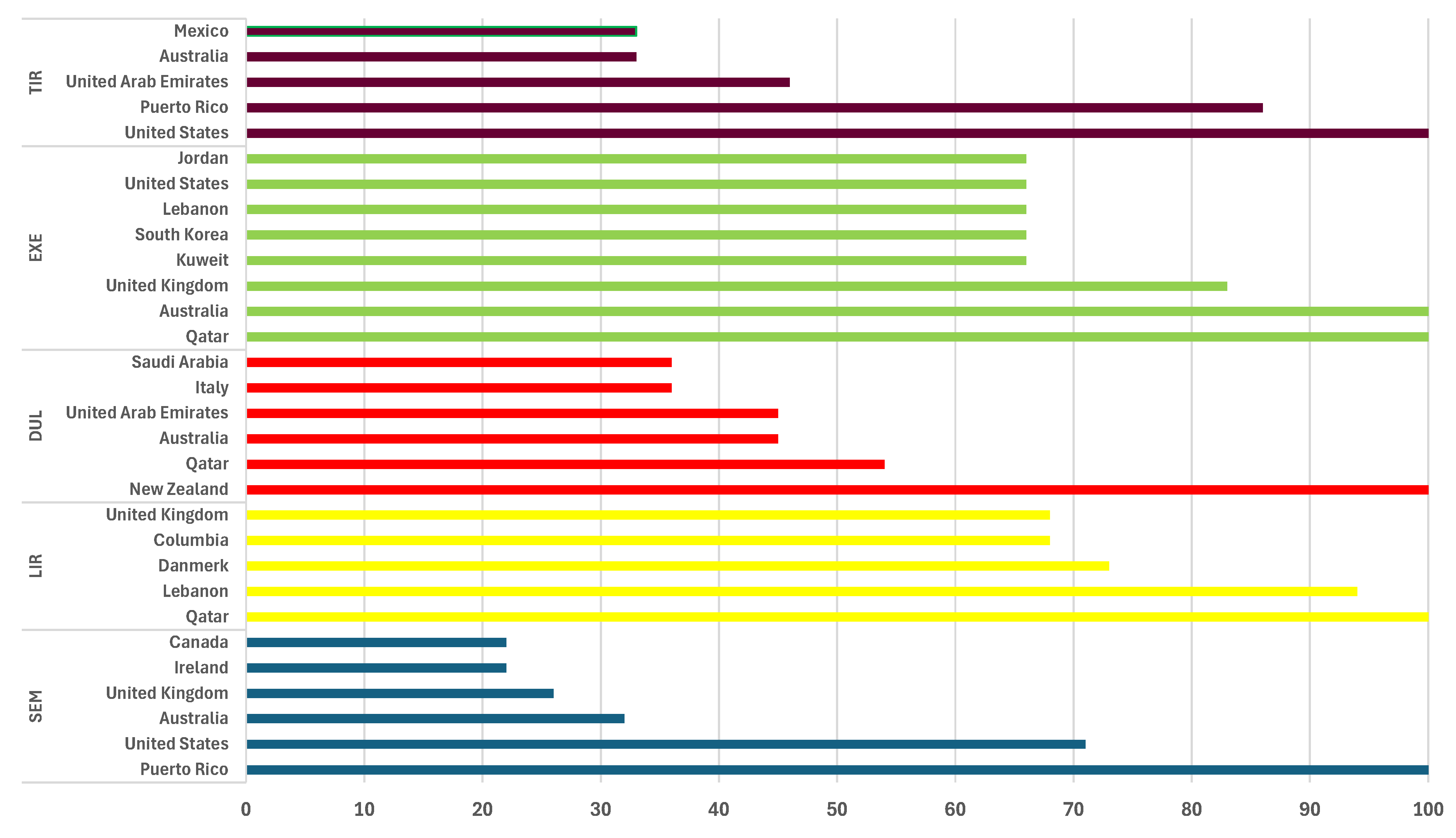
3.1. Analysis of Searching Google Popularity
| Terms associated with “weight loss” | Terms associated with “side effects” | |
|---|---|---|
| SEM | weight loss semaglutide | side effects semaglutide |
| semaglutide for weight loss | ||
| ozempic weight loss | ||
| LIR | liraglutide weight loss | liraglutide side effects |
| liraglutide for weight loss | ||
| TIR | tirzepatide weight loss | tirzepatide side effects |
| tirzepatide for weight loss | ||
| semaglutide weight loss | ||
| DUL | dulaglutide weight loss | dulaglutide side effects |
| EXE | exenatide weight loss | exenatide side effects |
3.2. Descriptive Analysis
3.2.1. Analysis of ICSRs
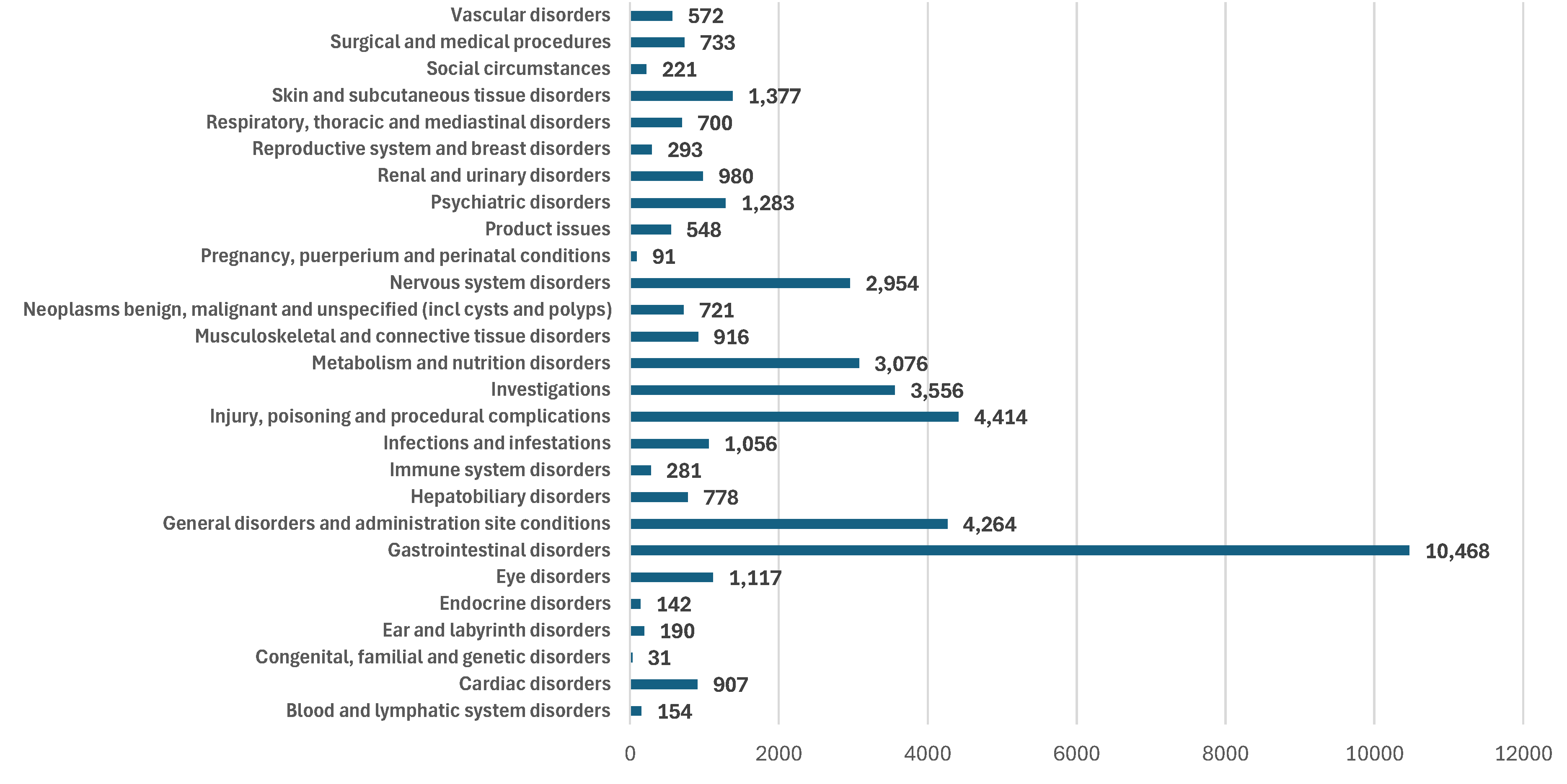
3.2.2. Comparative Evaluation of ADRs Grouped in SOC
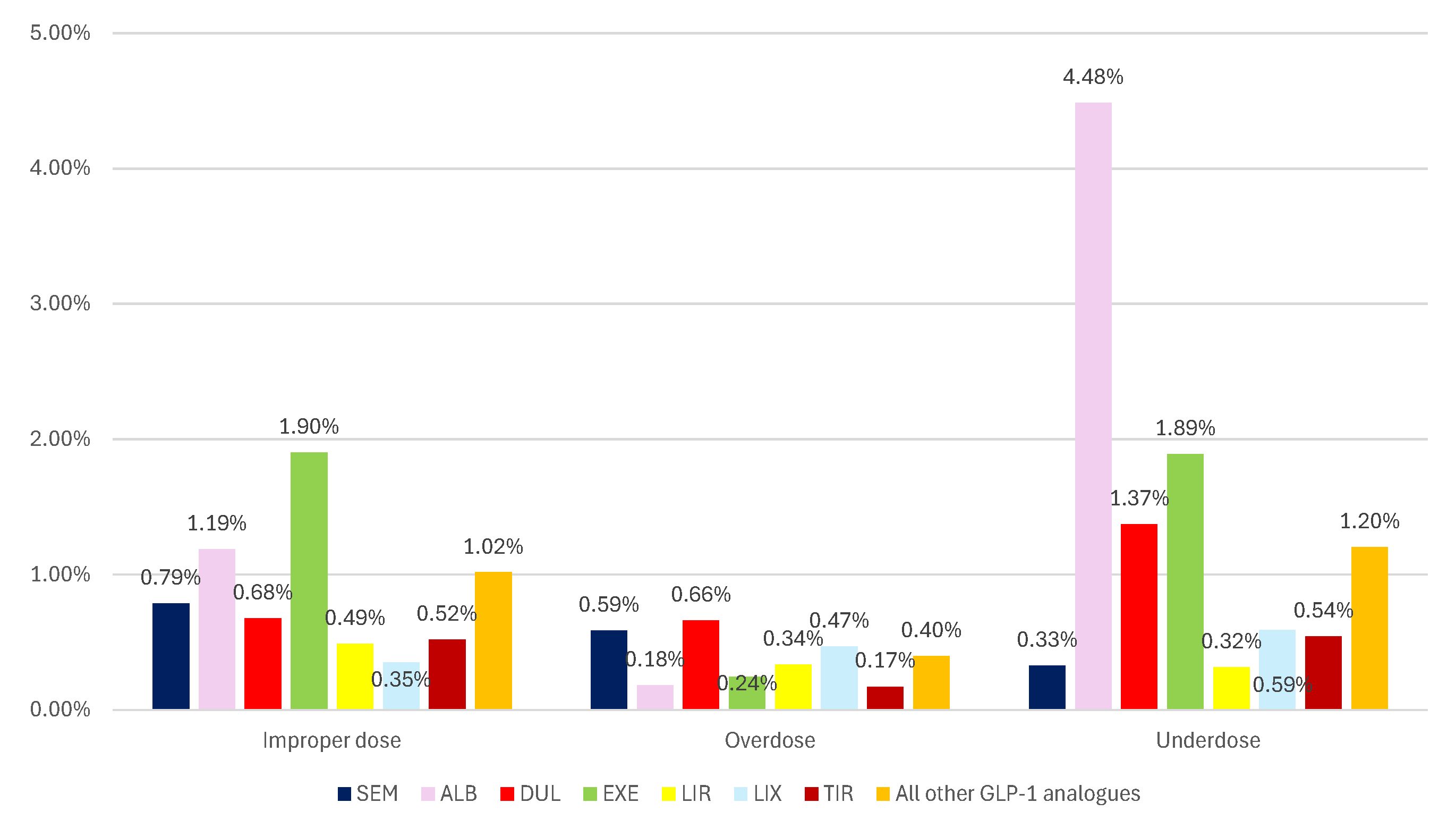
3.2.3. ADRs Reported for Incorrect Dosage
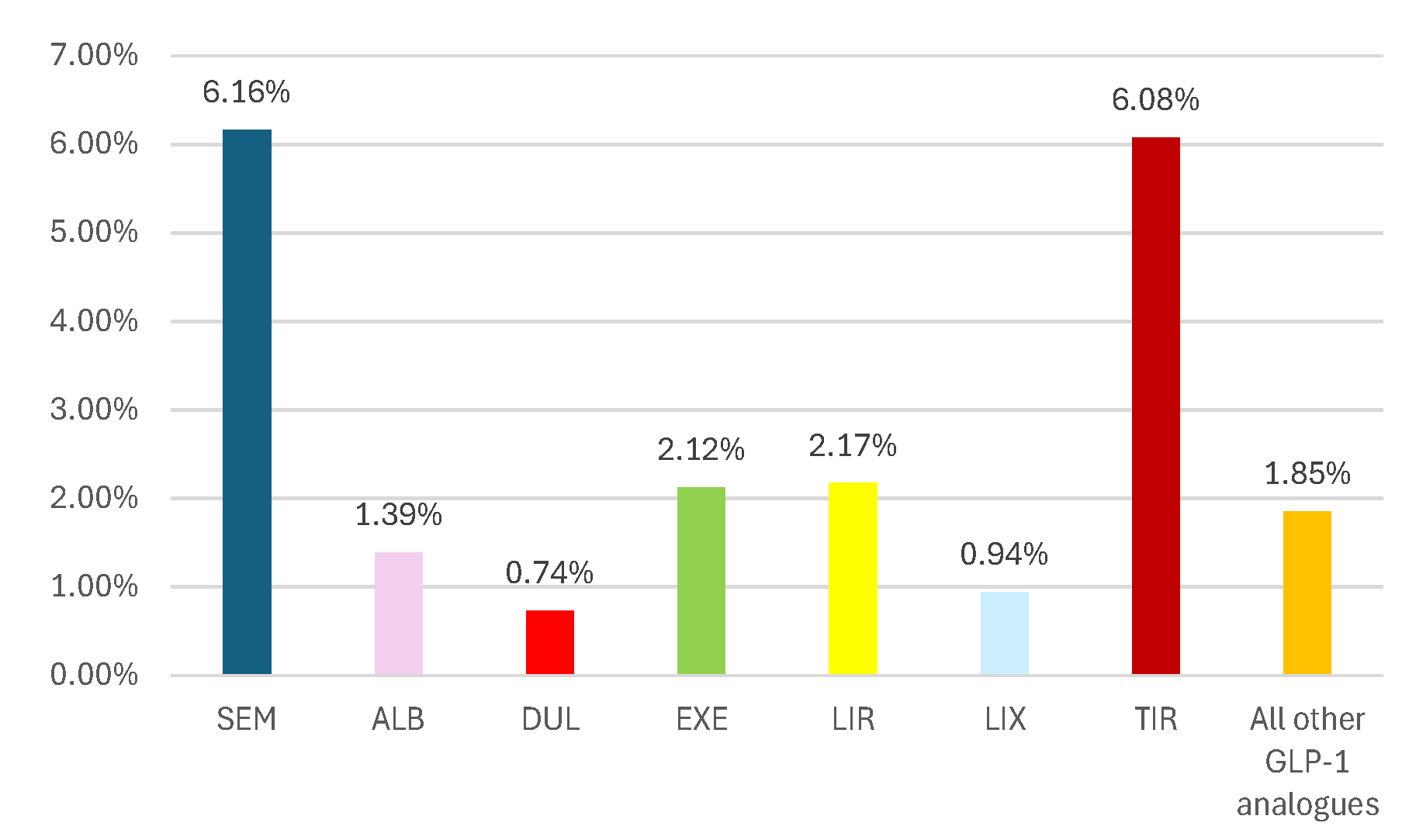
3.2.4. ADRs Reported as “Off-Label Use”
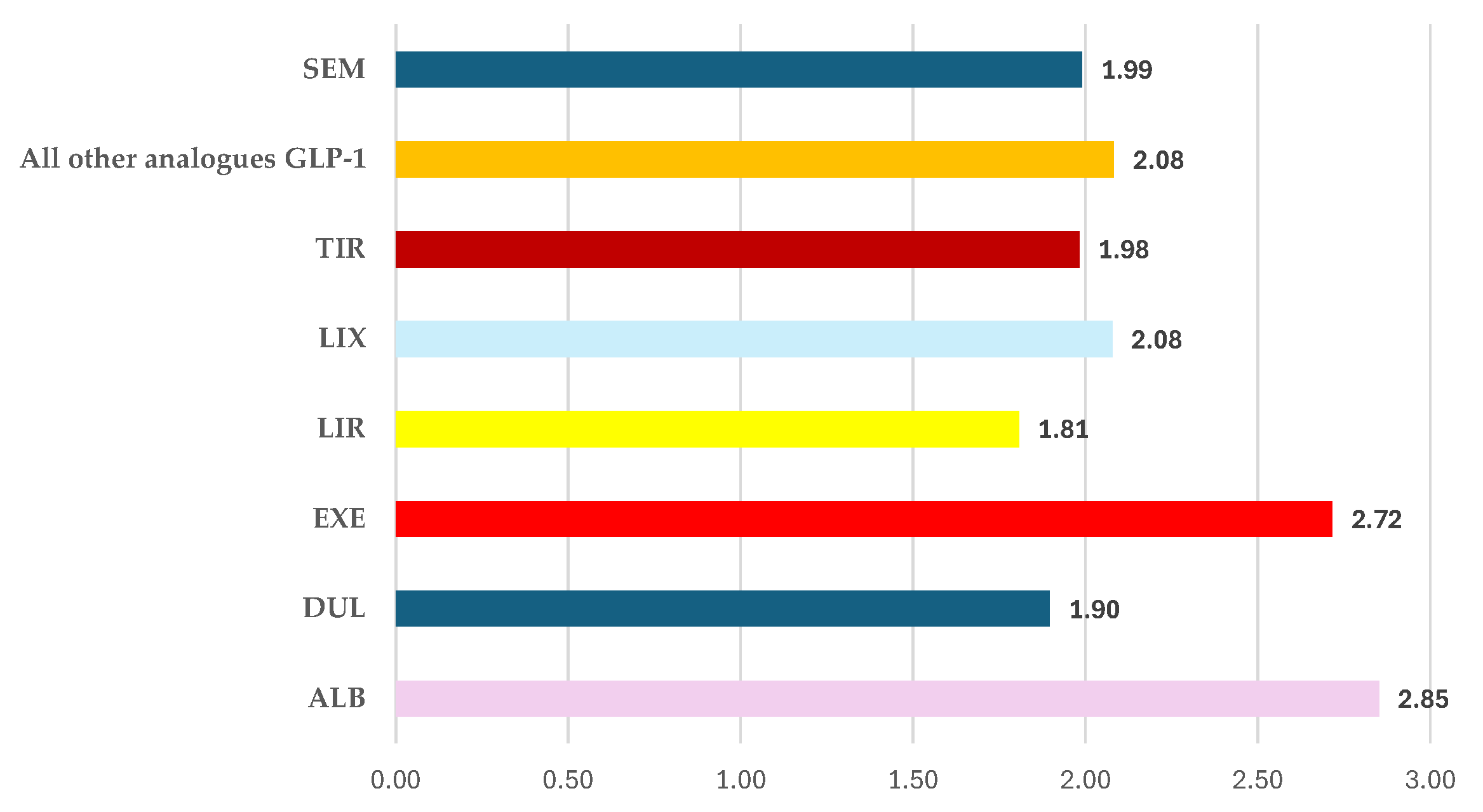
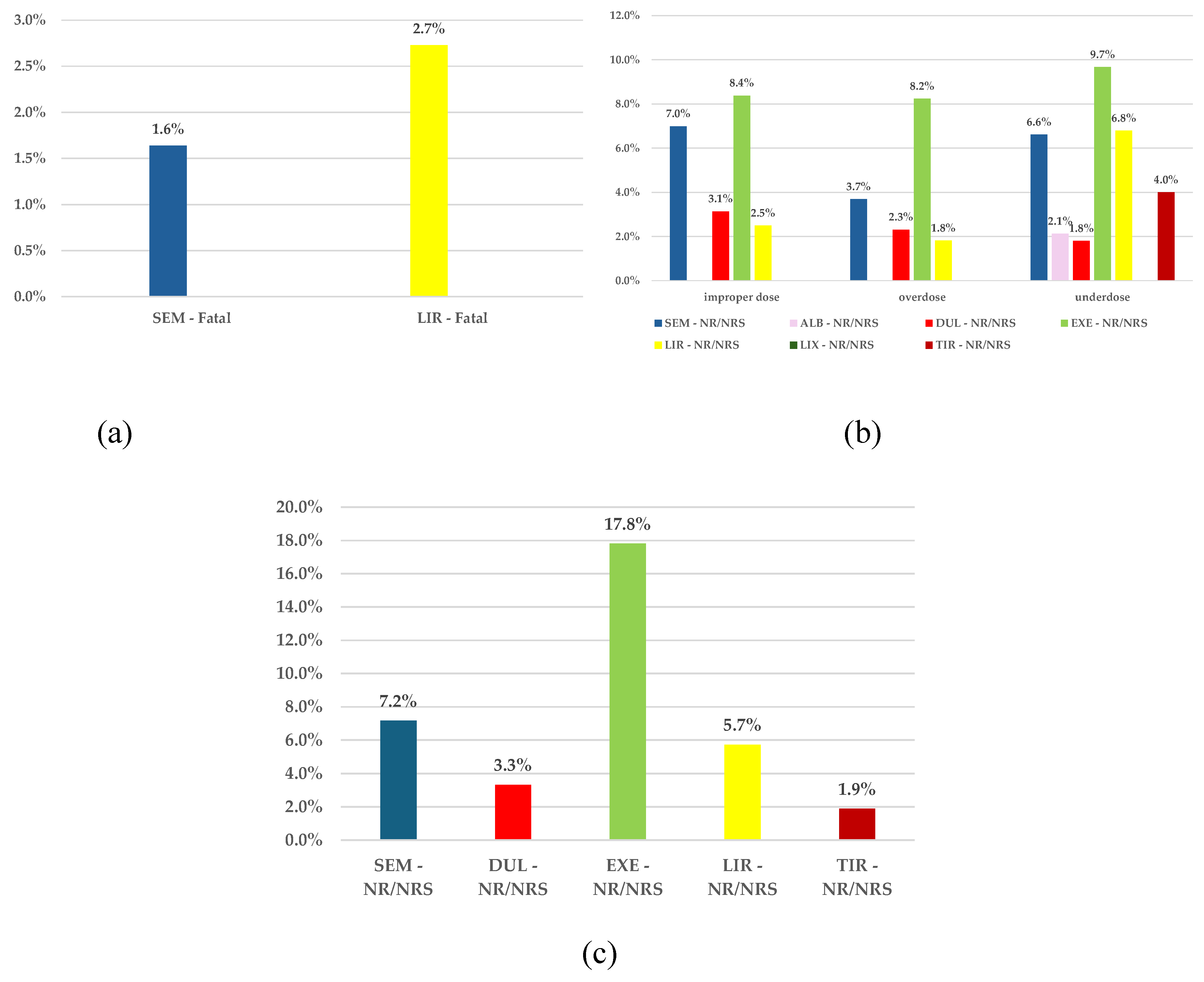
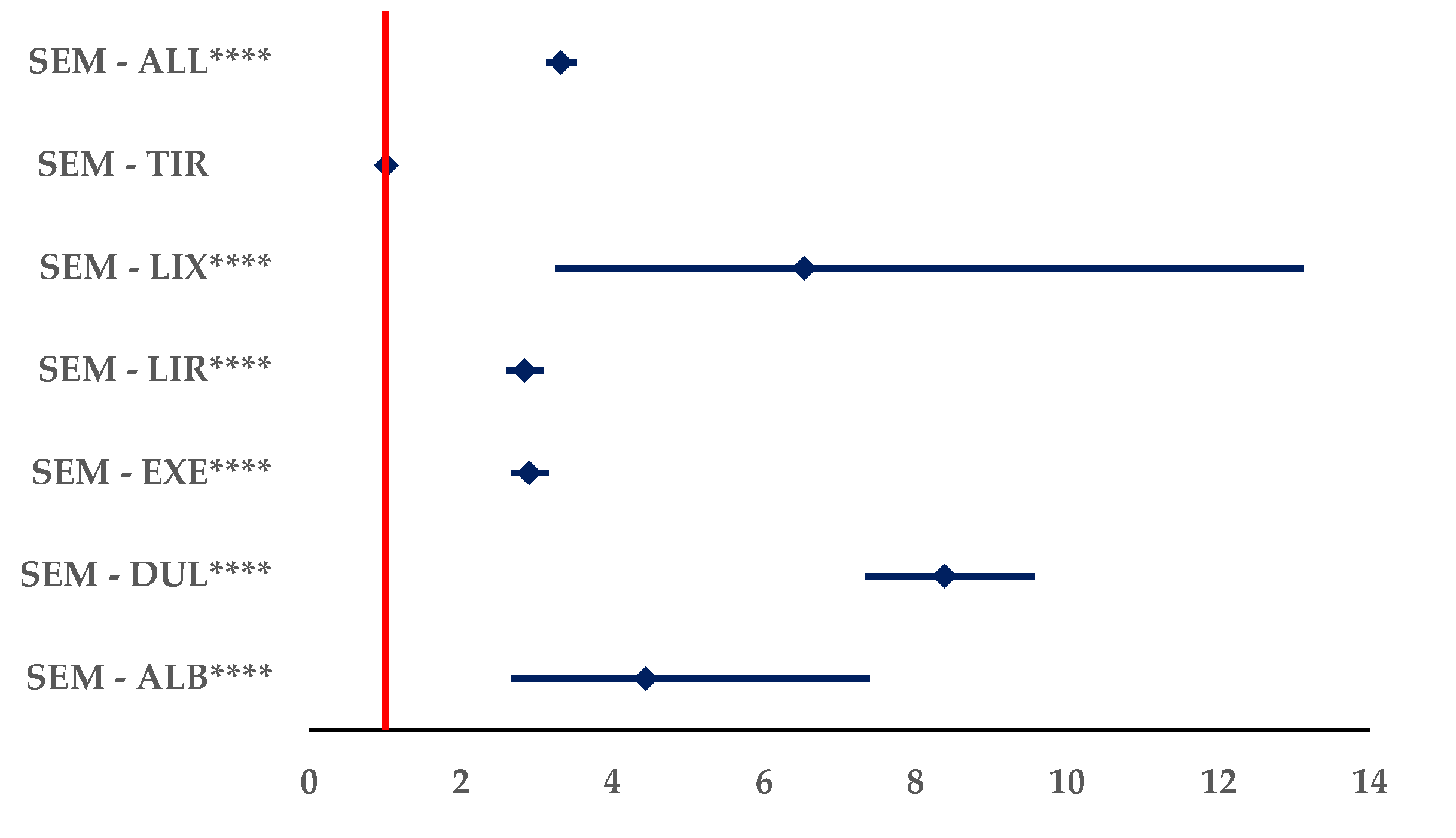
3.2.5. Distribution of ADRs by Outcome
ADRs Reported for SEM
The Frequency of ADRs with Unfavourable Outcomes Reported for SEM Compared to All Other GLP-1 RAs
- improper doses: SEM – 7.0% and EXE – 8.4%
- overdose: SEM - 3.7% and EXE – 8.2%
- underdose: SEM – 6.6%, EXE – 9.7%, and LIR – 6.8%
3.3. Disproportionality Analysis
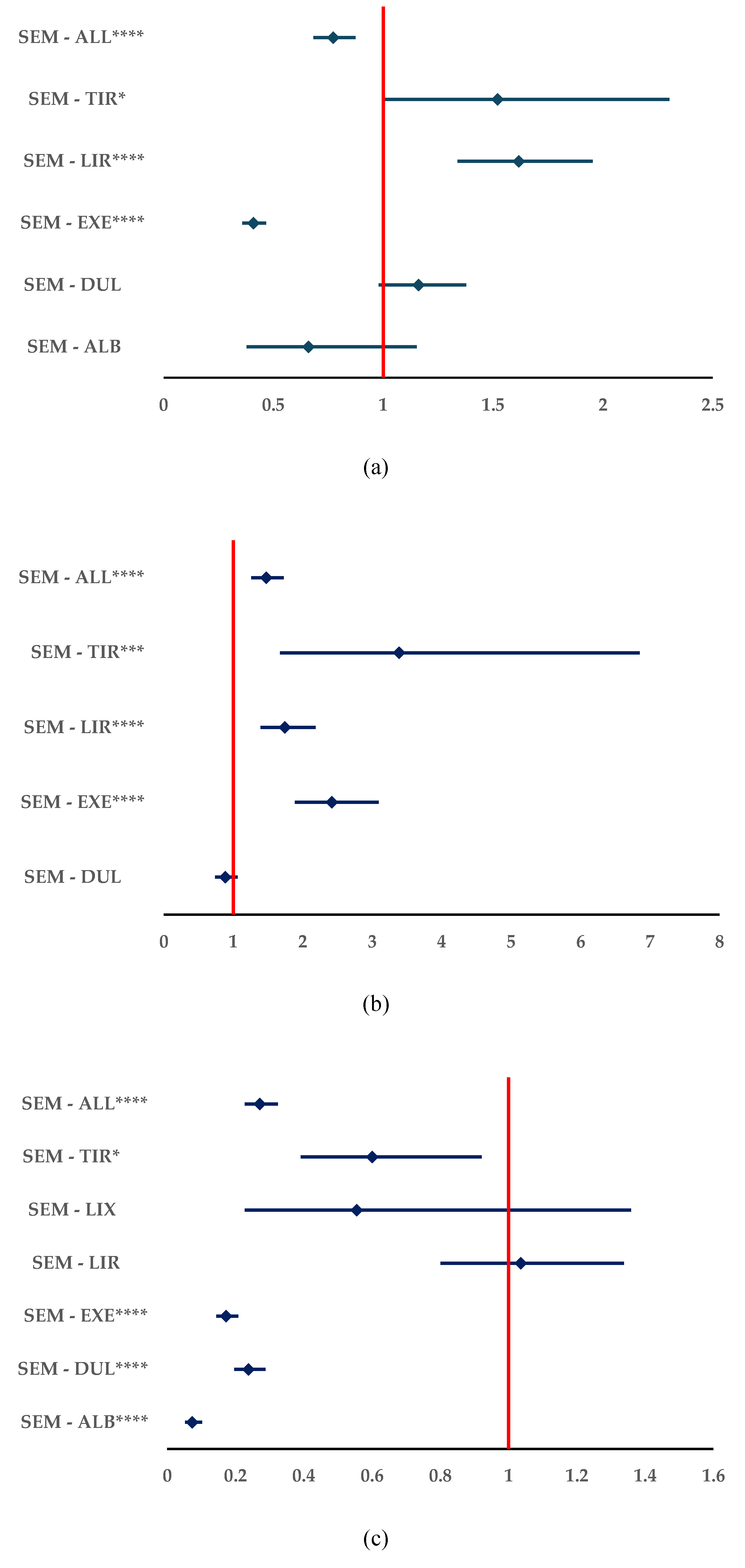
3.3.1. Incorrect Doses
3.3.2. Off-Label Use
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prasad-Reddy, L.; Isaacs, D. A Clinical Review of GLP-1 Receptor Agonists: Efficacy and Safety in Diabetes and Beyond. Drugs Context 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, L.; Crişan, D.; Morgovan, C.; Avram, L.; Ghibu, S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. Int. J. Mol. Sci. 2024, Vol. 25, Page 794 2024, 25, 794. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Ștefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, Vol. 9, Page 1073 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yang, J.; Deng, C.; Ruan, Q.; Duan, K. Efficacy and Safety of Semaglutide 2.4 Mg for Weight Loss in Overweight or Obese Adults without Diabetes: An Updated Systematic Review and Meta-Analysis Including the 2-Year STEP 5 Trial. Diabetes. Obes. Metab. 2024, 26, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 April 2024).
- Han, S.H.; Safeek, R.; Ockerman, K.; Trieu, N.; Mars, P.; Klenke, A.; Furnas, H.; Sorice-Virk, S. Public Interest in the Off-Label Use of Glucagon-like Peptide 1 Agonists (Ozempic) for Cosmetic Weight Loss: A Google Trends Analysis. Aesthetic Surg. J. 2023, 44, 60–67. [Google Scholar] [CrossRef]
- Morgovan, C.; Cosma, S.A.; Valeanu, M.; Juncan, A.M.; Rus, L.L.; Gligor, F.G.; Butuca, A.; Tit, D.M.; Bungau, S.; Ghibu, S. An Exploratory Research of 18 Years on the Economic Burden of Diabetes for the Romanian National Health Insurance System. Int. J. Environ. Res. Public Heal. 2020, Vol. 17, Page 4456 2020, 17, 4456. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Vickers-Smith, R.; Harris, D.; Papanti Pelletier, G.D.; Corkery, J.M.; Guirguis, A.; Martinotti, G.; Sensi, S.L.; Schifano, F. Is There a Risk for Semaglutide Misuse? Focus on the Food and Drug Administration’s FDA Adverse Events Reporting System (FAERS) Pharmacovigilance Dataset. Pharm. 2023, Vol. 16, Page 994 2023, 16, 994. [Google Scholar] [CrossRef]
- Larsen, P.J. Mechanisms behind GLP-1 Induced Weight Loss. 2008, 8, S34–S41. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-Marketing Withdrawal of Anti-Obesity Medicinal Products Because of Adverse Drug Reactions: A Systematic Review. BMC Med. 2016, 14. [Google Scholar] [CrossRef]
- Czernichow, S.; Batty, D. Withdrawal of Sibutramine for Weight Loss: Where Does This Leave Clinicians? Obes. Facts 2010, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- EMA Confirms Recommendation to Withdraw Marketing Authorisations for Amfepramone Medicines | European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/ema-confirms-recommendation-withdraw-marketing-authorisations-amfepramone-medicines-0 (accessed on 11 April 2024).
- Waldrop, S.W.; Johnson, V.R.; Stanford, F.C. Inequalities in the Provision of GLP-1 Receptor Agonists for the Treatment of Obesity. Nat. Med. 2024 301 2024, 30, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Rohani, P.; Malekpour Alamdari, N.; Bagheri, S.E.; Hekmatdoost, A.; Sohouli, M.H. The Effects of Subcutaneous Tirzepatide on Obesity and Overweight: A Systematic Review and Meta-regression Analysis of Randomized Controlled Trials. Front. Endocrinol. (Lausanne). 2023, 14, 1230206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent Updates on GLP-1 Agonists: Current Advancements & Challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, K.; Salman Roghani, R.; Camilleri, M. Effects of GLP-1 Agonists on Proportion of Weight Loss in Obesity with or without Diabetes: Systematic Review and Meta-Analysis. Obes. Med. 2022, 35, 100456. [Google Scholar] [CrossRef]
- Evolution of GLP-1 Receptor Agonists for Diabetes Treatment | Biopharma PEG. Available online: https://www.biochempeg.com/article/299.html (accessed on 11 April 2024).
- Prasad-Reddy, L.; Isaacs, D. A Clinical Review of GLP-1 Receptor Agonists: Efficacy and Safety in Diabetes and Beyond. Drugs Context 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Raj, R.; Elshimy, G.; Zapata, I.; Kannan, L.; Majety, P.; Edem, D.; Correa, R. Adverse Events Related to Tirzepatide. J. Endocr. Soc. 2023, 7. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetol. 2022 6512 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Tiuca, R.A.; Burlacu, A.; Varga, A. A 2021 Update on the Use of Liraglutide in the Modern Treatment of ‘Diabesity’: A Narrative Review. Med. 2021, 57. [Google Scholar] [CrossRef]
- Taylor, S.I. GLP-1 Receptor Agonists: Differentiation within the Class. Lancet Diabetes Endocrinol. 2018, 6, 83–85. [Google Scholar] [CrossRef]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef]
- Committee, A.D.A.P.P. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef] [PubMed]
- Blind, E.; Janssen, H.; Dunder, K.; de Graeff, P.A. The European Medicines Agency’s Approval of New Medicines for Type 2 Diabetes. Diabetes. Obes. Metab. 2018, 20, 2059. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, E.W.; Torekov, S.S.; Holst, J.J. Liraglutide for Type 2 Diabetes and Obesity: A 2015 Update. Expert Rev. Cardiovasc. Ther. 2015, 13, 753–767. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M.; Burton, D.; O’Neill, J.; Eckert, D.; Carlson, P.; Zinsmeister, A.R. Exenatide in Obesity with Accelerated Gastric Emptying: A Randomized, Pharmacodynamics Study. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Chudleigh, R.A.; Platts, J.; Bain, S.C. Comparative Effectiveness of Long-Acting GLP-1 Receptor Agonists in Type 2 Diabetes: A Short Review on the Emerging Data. Diabetes, Metab. Syndr. Obes. Targets Ther. 2020, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- UK Government Letters Sent to Healthcare Professionals in September 2017 - GOV.UK. Available online: https://www.gov.uk/drug-safety-update/letters-sent-to-healthcare-professionals-in-september-2017 (accessed on 11 April 2024).
- Hughes, S.; Neumiller, J.J. Oral Semaglutide. Clin. Diabetes 2020, 38, 109. [Google Scholar] [CrossRef]
- Singh, G.; Krauthamer, M.; Bjalme-Evans, M. Wegovy (Semaglutide): A New Weight Loss Drug for Chronic Weight Management. J. Investig. Med. 2022, 70, 5–13. [Google Scholar] [CrossRef]
- Ahmann, A.J.; Capehorn, M.; Charpentier, G.; Dotta, F.; Henkel, E.; Lingvay, I.; Holst, A.G.; Annett, M.P.; Aroda, V.R. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018, 41, 258–266. [Google Scholar] [CrossRef]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus Dulaglutide Once Weekly in Patients with Type 2 Diabetes (SUSTAIN 7): A Randomised, Open-Label, Phase 3b Trial. lancet. Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Tan, Y.; Chen, Y.; Zhou, X.; Liu, S.; Yu, J. GLP-1RAs Caused Gastrointestinal Adverse Reactions of Drug Withdrawal: A System Review and Network Meta-Analysis. Front. Endocrinol. (Lausanne). 2023, 14, 1149328. [Google Scholar] [CrossRef]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse Drug Reactions of GLP-1 Agonists: A Systematic Review of Case Reports. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102427. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Panagiotopoulou, T. V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202. [Google Scholar] [CrossRef]
- European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/ (accessed on 11 April 2024).
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef]
- Medicines Agency, E. Guideline on Good Pharmacovigilance Practices (GVP) - Product- or Population-Specific Considerations IV: Paediatric Population. 2018. [Google Scholar]
- Guo, Y.; Shen, Z.; Zhao, W.; Lu, J.; Song, Y.; Shen, L.; Lu, Y.; Wu, M.; Shi, Q.; Zhuang, W.; et al. Rational Identification of Novel Antibody-Drug Conjugate with High Bystander Killing Effect against Heterogeneous Tumors. Adv. Sci. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharm. 2023, Vol. 16, Page 455 2023, 16, 455. [Google Scholar] [CrossRef] [PubMed]
- Medicines Agency, E. Note for Guidance-EudraVigilance Human-Processing of Safety Messages and Individual Case Safety Reports (ICSRs). 2010. [Google Scholar]
- Medicines Agency, E. GUIDELINE ON THE USE OF STATISTICAL SIGNAL DETECTION METHODS IN THE EUDRAVIGILANCE DATA ANALYSIS SYSTEM. 2006. [Google Scholar]
- Google Trends: Semaglutide, Liraglutide, Tirzepatide, Dulaglutide, Exenatide. Available online: https://trends.google.com/trends/explore?date=2005-12-01 2024-03-31&q=semaglutide,liraglutide,tirzepatide,dulaglutide,exenatide&hl=ro (accessed on 20 April 2024).
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Isaacs, D.M.; Kruger, D.F.; Spollett, G.R. Optimizing Therapeutic Outcomes With Oral Semaglutide: A Patient-Centered Approach. Diabetes Spectr. 2021, 34, 7. [Google Scholar] [CrossRef]
- Kalra, S.; Sahay, R. A Review on Semaglutide: An Oral Glucagon-Like Peptide 1 Receptor Agonist in Management of Type 2 Diabetes Mellitus. Diabetes Ther. 2020, 11, 1965–1982. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Blonde, L.; Pratley, R.E. A New Era for Oral Peptides: SNAC and the Development of Oral Semaglutide for the Treatment of Type 2 Diabetes. Rev. Endocr. Metab. Disord. 2022, 23, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.; Miętkiewska-Dolecka, M.; Kręgielska-Narożna, M.; Bogdański, P. Popularity of Surgical and Pharmacological Obesity Treatment Methods Searched by Google Users: The Retrospective Analysis of Google Trends Statistics in 2004–2022. Obes. Surg. 2024, 34, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Basch, C.H.; Narayanan, S.; Tang, H.; Fera, J.; Basch, C.E. Descriptive Analysis of TikTok Videos Posted under the Hashtag #Ozempic. J. Med. Surgery, Public Heal. 2023, 1, 100013. [Google Scholar] [CrossRef]
- Liraglutide and Semaglutide Market Share Report, 2023-2032. Available online: https://www.gminsights.com/industry-analysis/liraglutide-and-semaglutide-market (accessed on 14 April 2024).
- Miriam, E. Tucker Semaglutide Prescribing Surged in the Past Year. Available online: https://www.medscape.com/viewarticle/997956?form=fpf (accessed on 14 April 2024).
- Patoulias, D.; Popovic, D.S.; Stoian, A.P.; Janez, A.; Sahebkar, A.; Rizzo, M. Effect of Semaglutide versus Other Glucagon-like Peptide-1 Receptor Agonists on Cardio-Metabolic Risk Factors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Head-to-Head, Phase 3, Randomized Controlled Trials. J. Diabetes Complications 2023, 37, 108529. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lin, Y.H.; Dai, L.Z.; Lin, C.S.; Huang, Y.; Liu, S.Y. Efficacy and Safety of GLP-1 Receptor Agonists versus SGLT-2 Inhibitors in Overweight/Obese Patients with or without Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. BMJ Open 2023, 13, e061807. [Google Scholar] [CrossRef] [PubMed]
- Chubb, B.; Gupta, P.; Gupta, J.; Nuhoho, S.; Kallenbach, K.; Orme, M. Once-Daily Oral Semaglutide Versus Injectable GLP-1 RAs in People with Type 2 Diabetes Inadequately Controlled on Basal Insulin: Systematic Review and Network Meta-Analysis. Diabetes Ther. 2021, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.Z.; Chen, J.Q.; Ren, T.S.; Zhang, Y.S.; Wang, Y.N.; Zhao, Q.C. Comparative Efficacy and Safety of Glucagon-like Peptide 1 Receptor Agonists for the Treatment of Type 2 Diabetes: A Network Meta-Analysis. Medicine (Baltimore). 2023, 102, E34122. [Google Scholar] [CrossRef] [PubMed]
- Azuri, J.; Hammerman, A.; Aboalhasan, E.; Sluckis, B.; Arbel, R. Liraglutide versus Semaglutide for Weight Reduction—a Cost Needed to Treat Analysis. Obesity 2023, 31, 1510–1513. [Google Scholar] [CrossRef]
- Chiappini, S.; Vickers-Smith, R.; Harris, D.; Papanti Pelletier, G.D.; Corkery, J.M.; Guirguis, A.; Martinotti, G.; Sensi, S.L.; Schifano, F. Is There a Risk for Semaglutide Misuse? Focus on the Food and Drug Administration’s FDA Adverse Events Reporting System (FAERS) Pharmacovigilance Dataset. Pharmaceuticals 2023, 16, 1–13. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. (Lausanne). 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Sugimoto, D.; Wharton, S. Efficacy and Safety of Semaglutide for Weight Management: Evidence from the STEP Program. Postgrad. Med. 2022, 134, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Poison Centers See Nearly 1,500% Increase in Calls Related to Injected Weight-Loss Drugs as People Accidentally Overdose | CNN. Available online: https://edition.cnn.com/2023/12/13/health/semaglutide-overdoses-wellness/index.html (accessed on 14 April 2024).
- Lambson, J.E.; Flegal, S.C.; Johnson, A.R. Administration Errors of Compounded Semaglutide Reported to a Poison Control Center—Case Series. J. Am. Pharm. Assoc. 2023, 63, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Wiener, B.G.; Gnirke, M.; Vassallo, S.; Smith, S.W.; Su, M.K. Challenges with Glucagon-like Peptide-1 (GLP-1) Agonist Initiation: A Case Series of Semaglutide Overdose Administration Errors. Clin. Toxicol. 2024, 1–3. [Google Scholar] [CrossRef]
- Surge in Fake Ozempic Reveals Dark Side of Weight-Loss Frenzy. Available online: https://medwatch.com/News/other/article16935969.ece (accessed on 16 April 2024).
- News Details. Available online: https://www.novonordisk-us.com/media/news-archive/news-details.html?id=166119 (accessed on 16 April 2024).
- Counterfeit Ozempic Found in US Retail Pharmacy - National Association of Boards of Pharmacy. Available online: https://nabp.pharmacy/news/blog/regulatory_news/counterfeit-ozempic-found-in-us-retail-pharmacy/ (accessed on 16 April 2024).
- EMA Alerts EU Patients and Healthcare Professionals to Reports of Falsified Ozempic Pens | European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/ema-alerts-eu-patients-healthcare-professionals-reports-falsified-ozempic-pens (accessed on 16 April 2024).


| HLT | PT |
|---|---|
| modified dose | Dose calculation error |
| Dose calculation error associated with device* | |
| Drug dose titration not performed | |
| Drug titration error | |
| Incorrect dosage administered | |
| Incorrect dose administered | |
| Incorrect dose administered by device | |
| Incorrect dose administered by product | |
| Incorrect product dosage form administered* | |
| Product dosage form confusion* | |
| Wrong dose | |
| overdose | Accidental overdose |
| Intentional overdose | |
| Extra dose administered | |
| Overdose | |
| Prescribed overdose | |
| underdose | Accidental underdose |
| Drug dose omission by device | |
| Incomplete dose administered* | |
| Intentional dose omission | |
| Intentional underdose | |
| Prescribed underdose | |
| Product dose omission* | |
| Product dose omission in error | |
| Product dose omission issue | |
| Underdose | |
| off label use | Contraindicated product administered |
| Contraindicated product prescribed | |
| Drug effective for unapproved indication* | |
| Off label use | |
| Off label use of device | |
| Product use in unapproved therapeutic environment* | |
| Product use in unapproved indication | |
| Product used for unknown indication* | |
| Unintentional use for unapproved indication |
| SEM | ALB | DUL | EXE | LIR | LIX | TIR | All GLP-1 RA | |
|---|---|---|---|---|---|---|---|---|
| n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
| Total | 21,012 | 384 | 17,332 | 12,926 | 18,149 | 412 | 2,333 | 51,536 |
| Age category | ||||||||
| NS | 7,942 | 148 | 5,476 | 4,160 | 6,668 | 105 | 1,138 | 17,695 |
| (37.8) | (38.5) | (31.6) | (32.2) | (36.7) | (25.5) | (48.8) | (34.3) | |
| 0-1 Month | 1 | 0 | 1 | 6 | 4 | 0 | 0 | 11 |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| 2 Months - 2 | 2 | 0 | 3 | 1 | 4 | 0 | 0 | 8 |
| Years | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) |
| 3-11 Years | 9 | 0 | 2 | 1 | 9 | 0 | 0 | 12 |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| 12-17 Years | 23 | 0 | 6 | 6 | 64 | 0 | 2 | 78 |
| (0.1) | (0.0) | (0.0) | (0.0) | (0.4) | (0.0) | (0.0) | (0.2) | |
| 18-64 Years | 8,345 | 162 | 6,576 | 5,519 | 8,236 | 186 | 863 | 21,542 |
| (39.7) | (42.2) | (37.9) | (42.7) | (45.4) | (45.1) | (37.0) | (41.8) | |
| 65-85 Years | 4,546 | 72 | 4,951 | 3,153 | 3,104 | 118 | 307 | 11,705 |
| (21.6) | (18.8) | (28.6) | (24.4) | (17.1) | (28.6) | (13.2) | (22.7) | |
| More than 85 | 144 | 2 | 317 | 80 | 60 | 3 | 23 | 485 |
| Years | (0.7) | (0.5) | (1.8) | (0.6) | (0.3) | (0.7) | (1.0) | (0.9) |
| Sex | ||||||||
| Female | 12,122 | 211 | 8,443 | 6,741 | 10,851 | 210 | 1,180 | 27,636 |
| (57.7) | (54.9) | (48.7) | (52.2) | (59.8) | (51.0) | (50.6) | (53.6) | |
| Male | 8,206 | 158 | 7,676 | 5,825 | 6,189 | 169 | 685 | 20,702 |
| (39.1) | (41.1) | (44.3) | (45.1) | (34.1) | (41.0) | (29.4) | (40.2) | |
| NS | 684 | 15 | 1,213 | 360 | 1,109 | 33 | 468 | 3,198 |
| (3.3) | (3.9) | (7.0) | (2.8) | (6.1) | (8.0) | (20.1) | (6.2) | |
| Geographic origin | ||||||||
| EEA | 11,060 | 12 | 8,864 | 3,226 | 7,309 | 265 | 115 | 19,791 |
| (52.6) | (3.1) | (51.1) | (25.0) | (40.3) | (64.3) | (4.9) | (38.4) | |
| NON-EEA | 9,952 | 372 | 8,468 | 9,700 | 10,839 | 147 | 2,218 | 31,744 |
| (47.4) | (96.9) | (48.9) | (75.0) | (59.7) | (35.7) | (95.1) | (61.6) | |
| NS | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Reporter | ||||||||
| HP | 12,877 | 175 | 11,001 | 8,751 | 12,316 | 333 | 1,470 | 34,046 |
| (61.3) | (45.6) | (63.5) | (67.7) | (67.9) | (80.8) | (63.0) | (66.1) | |
| NHP | 8,135 | 209 | 6,331 | 4,168 | 5,832 | 79 | 863 | 17,482 |
| (38.7) | (54.4) | (36.5) | (32.2) | (32.1) | (19.2) | (37.0) | (33.9) | |
| NS | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 8 |
| (0.0) | (0.0) | (0.0) | (0.1) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Seriousness | ||||||||
| Non serious | 8,983 | 5 | 7,051 | 1,383 | 4,637 | 119 | 120 | 13,315 |
| (42.8) | (1.3) | (40.7) | (10.7) | (25.5) | (28.9) | (5.1) | (25.8) | |
| NS | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 6 |
| (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Serious | 12,029 | 379 | 10,281 | 11,540 | 13,509 | 293 | 2,213 | 38,215 |
| (57.2) | (98.7) | (59.3) | (89.3) | (74.4) | (71.1) | (94.9) | (74.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





