1. Introduction
Despite the many lives saved by the discovery of antibiotics (ABs), their use also favours the advancement of bacterial resistance. The excessive use of ABs in veterinary and agricultural settings, as well as their environmental presence lead to the development and spread of resistance genes. But, in addition to this panorama, another well-known and highly relevant driver of antimicrobial resistance (AMR) is indiscriminate exposure to these medicines in clinical settings (inappropriate prescribing, self-medication) [
1,
2,
3,
4,
5,
6,
7,
8].
The challenges in overcoming the global progression of AMR through the promotion of an accurate selection and adherence to the guidelines include the following: i) problems with the standardization of data and monitoring tools; ii) difficulty accessing adequate care for diagnosis and treatment; and iii) an increase in infections caused by resistant pathogens and the need for more effective treatments [
9]. Additionally, dispensing without prescription is a widespread practice difficult to be controlled [
10]. Low- and middle-income countries (LMICs) are most affected, especially with regard to investments in structure, specialized laboratories, and trained personnel for the diagnosis, monitoring, and evaluation (M&E) of AMR [
11].
The World Health Organization Global Antimicrobial Resistance and Use Surveillance System (GLASS) was released in 2015 to try to fill gaps in coping with AMR [
12]. In 2021, of the 216 countries, territories and areas in the world, only 55 (25%) provided data on antimicrobial consumption, and 87 (40%) provided bacterial identification results [
13] Since the main cause of AMR is the inappropriate use of ABs, it is equally important to know (i) the prescription profile of these drugs; (ii) the degree of adherence to the treatment guidelines; and (iii) the pattern of nonprescription dispensing, as well as other situations that contribute to both excessive use and irrational consumption. To this end, M&E of the use at the national level and studies on the use of medicines at different levels of the supply chain that provide data at the local level are recommended [
12,
14].
The World Health Organization (WHO) developed the Access, Watch, and Reserve (AWaRE) classification to define appropriate prescription and use strategies and to facilitate the collection and comparison of data for the surveillance of AB consumption. This classification is based on the risk of resistance to different categories of antibiotics. The adoption of the AWaRE classification in national antibiotic management policies assists in the M&E of incorporated strategies to reduce AMR. The goal established by the WHO is for 60% of ABs consumed in a country to be by those considered to have a lower risk of AMR (the “Access” category) [
15].
The consumption of ABs varies—between and within countries and regions—and is influenced by cultural, economic, and political factors [
16,
17]. In high-income countries, the consumption of AB decreased in the Access category between 2013 and 2018 [
18]. The same was not observed in LMICs [
19,
20]. A recent review indicated that the general increase in the consumption of ABs in these countries, especially those in the Watch category, worsened during the COVID-19 pandemic [
20]. In Brazilian intensive care units, for example, the consumption of Reserve AB reached 40% [
17]. Consistently and uniformly evaluating antimicrobial consumption is a challenge in many countries, such as those in Latin America [
21] and Africa [
22]. The combined efforts of the most diverse areas of science are recommended, as resistance mechanisms are known for all ABs used in the clinic, and the discovery of promising new classes to treat bacterial infections is increasingly rare [
23].
The guide for local policies of integrated antimicrobial stewardship (AMS), put together by the WHO, is based on five pillars: developing national coordination; ensuring access to and regulation of antimicrobials; improving awareness, education, and training; strengthening infection control through efforts focussed on the water supply, sanitation, and hygiene; and surveillance, M&E [
24]. However, close and continuous surveillance for quick detection of inappropriate use and the appearance of AMR can be difficult, especially because well-equipped laboratories with trained personnel can be scarce in certain settings [
9,
25]. Hence, another helpful and inexpensive strategy has been suggested for challenging scenarios: the use of pharmacovigilance (PV) data to report suspected cases of AMR, therapeutic failure, and inappropriate or off-label use of AB [
26,
27,
28,
29,
30,
31].
This narrative review aims to synthesise the contributions of PV as an auxiliary tool for identifying and monitoring suspected ineffectiveness, resistance, and inappropriate use of antibiotics. In conducting this review, we aimed to identify studies that illustrated how PV strategies can be useful in: i) the timely identification of possible AMR clusters that guide authorities for specific testing, and ii) contributing to monitor ABs use.
The searches were performed in the PubMed, Embase, and Lilacs databases between December 2023 and March 2024 as follows: i) defining the search: (“adverse drug reaction reporting system” OR “pharmacovigilance”) AND (“anti-bacterial agents” OR “antibiotics”) AND (“drug misuse” OR “off-label” OR “therapeutic failure” OR “lack of effectiveness” OR “antibiotic resistance” OR “drug ineffective”). This search returned approximately 40 titles, but there were only a few articles of interest; ii) in the second step, a broader search strategy was used, focussing on studies published in the last five years (from 2019), with the terms (“adverse drug reaction reporting system” OR “pharmacovigilance”) AND (“anti-bacterial agents” OR “antibiotics”). Approximately 600 titles were screened from the 3 databases searched and; iii) additionally, related articles and references cited by the studies of interest were checked to find more eligible studies. Articles of interest in the English, Portuguese, and Spanish languages were identified. After reading them, the information they contained was organized and summarized under the following topics about the possible strategies within PV to identify ineffectiveness and inappropriate use of ABs.
2. The Role of PV
Beginning in the 1960s, after the thalidomide tragedy, the concepts of adverse drug reactions (ADRs) and PV were established and refined [
32]. For more than 50 years, the WHO has contributed to the creation of PV systems based on the notification of suspected ADRs, with the objective of quickly identifying signals of unknown reactions and preventing new disasters such as those caused by thalidomide. Today, the WHO Program for International Drug Monitoring (WHO-PIDM) has been established in more than 170 countries [
33]. Due to this network structure of countries covering 99% of the World population, the WHO-PIDM is the most extensive global network linking healthcare professionals and patients who can report any suspicion related to medicines use and safety through a simple, quick system that reaches the WHO-PIDM database [
34].
According to the WHO, PV is “the science and the activities related to the detection, assessment, understanding, and prevention of adverse effects or any other medicine or vaccine-related problem” [
35]. These activities consist of monitoring “events”, their collection, and research to detect any causal relationship between the medicine and the observed harm, i.e., an adverse “reaction”, defined as “a harmful effect suspected to be caused by a medicine” [
36]. PV is based on the monitoring of large populations by the reports of multiple observers (health professionals, patients, and manufacturers). This same information allows the evaluation of the use of the involved medicines in different situations, an insight into the behavioural/epidemiological changes that populations are experiencing, and in the indirect evaluation of the observed risks and benefits [
28,
37].
Data from spontaneous reports are key sources for the identification of safety signals related to the use of medicines after they have been approved and commercialized, i.e., their use in real life [
38,
39,
40]. One of the features of spontaneous reporting is the use of internationally standardized terminologies, allowing for better evaluation and comparison between different countries/regions [
38,
41]. In addition to the adverse effect itself, the information collected in the standard and simple report form lets us recognise the reason for using the medicine, medicines taken concomitantly, and the underlying diseases of patients affected by an ADR.
Like all types of studies, those based on PV reports have limitations, namely, potential biases due to incomplete data, errors in the classification/coding of adverse events (AEs), and, especially, underreporting [
42,
43,
44] Still, PV is a useful method for quickly collecting reports of suspected events that, when they reach a critical number, become a signal that can be further investigated in detail, such as with additional methodologically sound studies when needed.
As the field of PV has evolved, large databases have been built at the national (for example, the Food and Drug Administration Adverse Event Reporting System (FAERS) in the United States), regional (for example, EudraVigilance), and global levels (VigiBase, in WHO-PIDM). In the specific field of ABs, the literature is rich in PV studies that evaluated the profile of AEs of these medicines. These studies aimed to identify the main substances related to a given event, or the reverse, the main events related to an AB, the frequency of observation of an AE/AB pair, and the evaluation of rare events or drug-drug interactions involving an AB, in addition to the identification of populations at risk. Supplementary Table S1 lists recent studies that exemplify the recognized goals of PV [
38,
39,
40,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89].
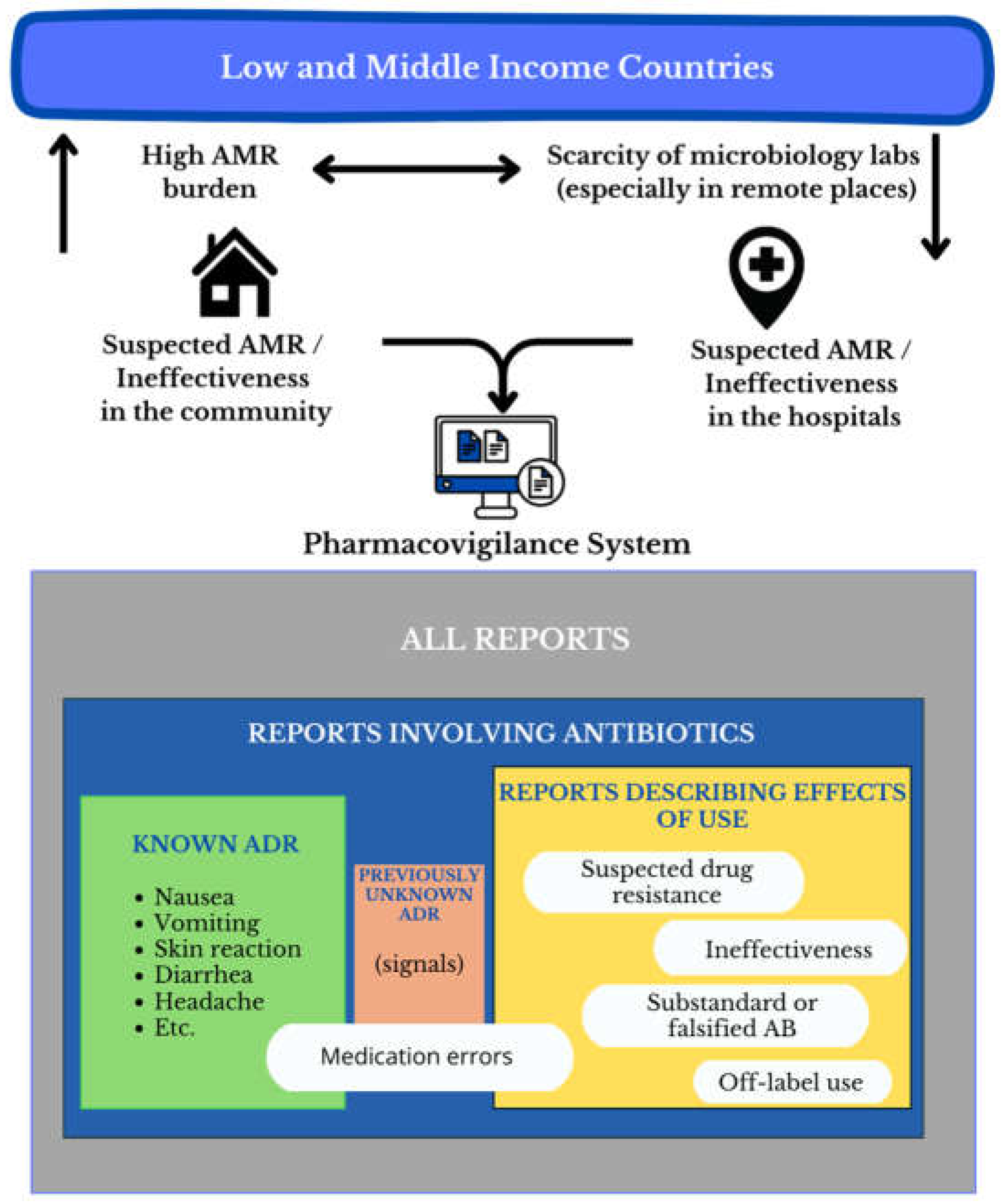
3. PV Strategies Useful for Monitoring the Use of ABs and Combating AMR
ABs cause many of the AE reported in PV programs [
90,
91,
92,
93,
94], especially those in the Watch category [
20,
31,
90]. Interestingly, in addition to the adverse reactions themselves (for example, nausea, vomiting, diarrhoea, hypersensitivity, headache, etc.), the dictionaries that describe AEs include terms useful for identifying aspects related to their use. Thus, events such as “lack of the expected therapeutic effect”, “lack of efficacy”, “inappropriate use”, “use out of indication”, and even “suspicion of resistance to ABs” are already coded as Medical Dictionary for Regulatory Activities (MedDRA) terms [
26,
27,
28,
29,
30,
37,
95,
96]
. This terminology is internationally standardized and widely used in health device information systems and VC databases worldwide [
97]. Some researchers have noted that the identification of reports involving these terms in PV databases could be used as a proxy for inappropriate use or suspicion of resistance in the absence of specific methods for the detection of these problems [
26,
27,
95,
96,
98,
99].
A pilot study showed the feasibility of identifying treatment failures due to AMR in reports of ADRs in VigiBase [
99]. Another study showed how PV data may be relevant in the monitoring of AMR by relating data from suspected AE reports to ABs used to treat carbapenemase-producing
Klebsiella pneumoniae (KPC) infections in three different databases in two countries, with high (Italy) and low prevalence of KPC (United Kingdom), based on data on culture isolates provided by the European Center for Disease Prevention and Control. The positive correlations showed an overall increase in AE, as well as serious AEs related to KPC outbreaks in both countries, showing that it is possible to identify AMR outbreaks earlier by monitoring the evolution of AE notifications related to ABs [
28].
Figure 1 summarizes the potential of PV databases as another tool in scenarios with few resources.
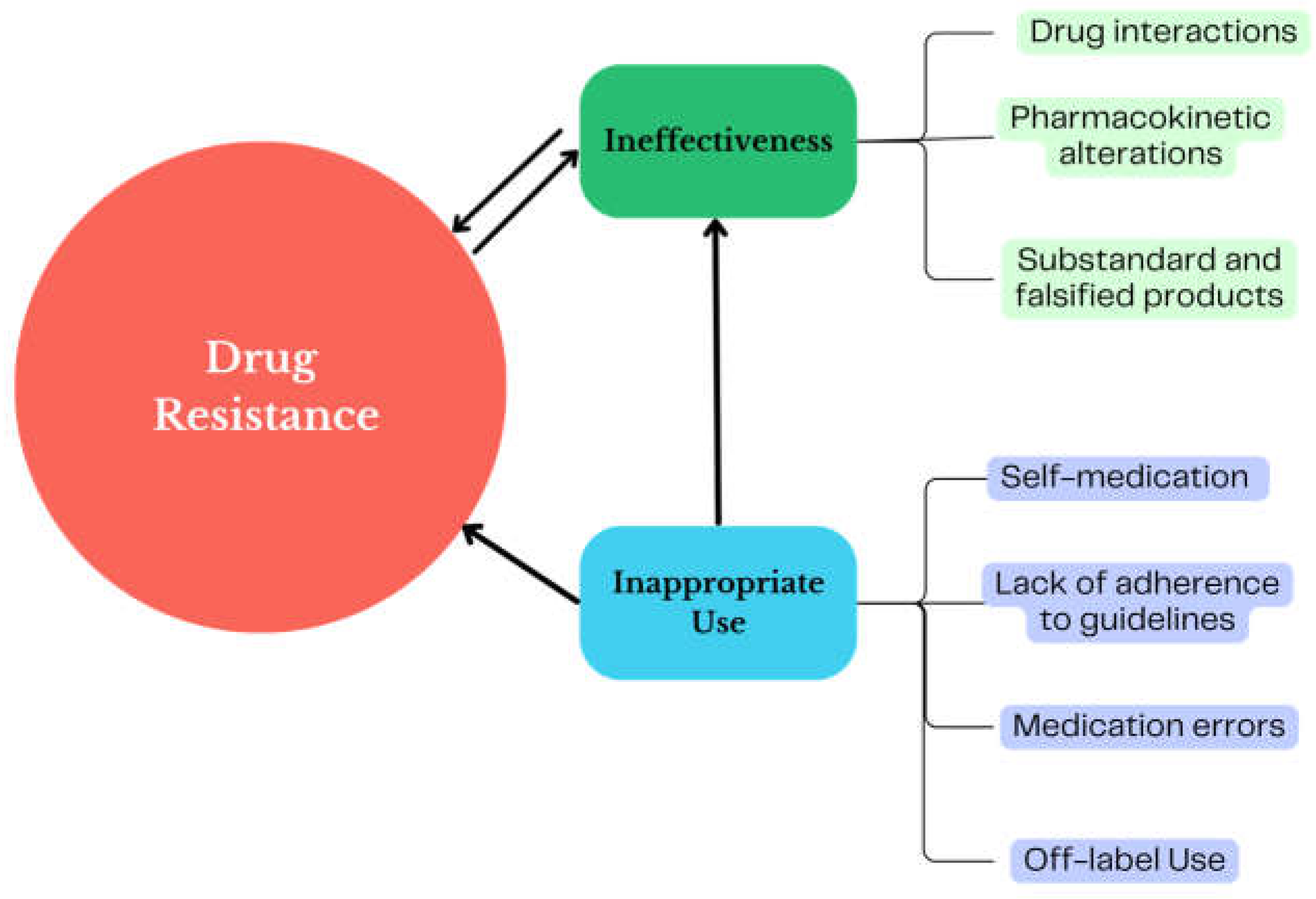
Figure 2 presents a scheme of the connections between the factors that influence the use of antibiotics in real life and their relationship with AMR that can be identified in PV studies.
4. PV as a Tool to Identify the Ineffectiveness of Antibiotics
There are many reasons why a medicine may not achieve its goal. Some of these issues are related to incidents such as the prescription of an inadequate dose, a choice of AB that disregards the clinical guidelines, or nonadherence to treatment. Other causes include drug-drug interactions, comorbidities (e.g., pharmacokinetic changes in critically ill patients), the nutritional status of the user, and the use of counterfeit drugs or drugs with low doses of the active ingredient, in addition to biopharmaceutical problems [
100,
101,
102,
103,
104,
105,
106,
107].
Previous research highlighted specific MedDRA terms in PV databases denoting ineffectiveness, such as “drug ineffective,” “treatment failure,” “decreased drug efficacy,” and “therapy nonresponder” [
95]. Monitoring signs related to these events can be crucial in the investigation of their causes. In addition, AEs related to ineffectiveness may also be a trigger to suspect resistance to the involved AB, which is precisely the cause of therapeutic failure [
30]. The following is a summary of the findings related to these events and ABs described in PV systems in several countries.
A comprehensive VigiBase analysis (1968-2018) of AB-related AEs (n = 1,170,751) found 15,250 (1.3%) reports that reported 17 MedDRA preferred terms (PT) of interest for AMR. The six most reported medicines, which corresponded to 38% of these notifications (n = 5806), were amoxicillin (n = 873; 5.7%; Access), cephalothin (n = 151; 1%; Access), ciprofloxacin (n = 1748; 11.5%; Watch), clarithromycin (n = 991; 6.5%; Watch), levofloxacin (n = 1342; 8.8%; Watch), and daptomycin (n = 701; 4.6%; Reserve). The terms most frequently reported in these notifications were “drug ineffective” (n = 6959; 45.6%), “off-label use” (n = 1455; 9.5%), and “pathogen resistance” (n = 1327; 9.0%) [
95].
In the Netherlands PV centre, 252 notifications were identified between 1998 and 2019 with MedDRA PTs that are considered relevant for AMR. The frequencies of “off-label” (n = 91; 36.1%), “drug ineffective” (n = 71; 28.2%), “product use in inappropriate indications” (n = 28; 11, 1%), “pathogen resistance” (n = 14; 5.6%), and “drug resistance” (n = 13; 5.2%) were detected. That study also described the involved ABs: tobramycin (n = 89; 35%; Watch), colistin (n = 30; 11.9%; Reserve), ciprofloxacin (n = 16; 6.3%; Watch), doxycycline (n = 14; 5.5%; Access), and aztreonam (n = 12; 4.8%; Reserve) [
30].
A study conducted in the European PV database (Eudravigilance – through 2022) described the relationship between MedDRA terms that indicated “drug resistance” and “drug ineffectiveness” and three ABs commonly used in critically ill patients: meropenem (n = 8,864; Watch), colistin (n = 983; Reserve), and linezolid (n = 13,381; Reserve). The terms associated with “drug resistance” were more frequent for colistin (n = 83; 8.4%) than for meropenem (n = 316; 3.6%) or linezolid (n = 319; 2.4%). Colistin was also the one most related to “drug ineffectiveness” (n = 100; 10.1%), followed by meropenem (n = 838; 9.5%) and linezolid (n = 556; 4.2%). Suspected bacterial resistance was related to the outcome of death in 20% of the records associated with meropenem, 24% with colistin, and 6% with linezolid. In the “drug ineffective” group, death was reported in 28%, 35%, and 19% of the records, respectively. The authors of that study also suggested that colistin was more likely to be associated with reports of events related to resistance and inefficacy than any ABs other than ceftazidime/avibactam [
37].
One study in the Portuguese database described 59,022 reports (2017-2019) related to the most widely used antibiotics in the country for the treatment of upper respiratory tract infections, both in outpatient and hospital settings. The term “drug ineffective” was among the 10 most frequently found MedDRA PTs related to these ABs, with greater emphasis on amoxicillin (n = 105; 0.18%; Access), amoxicillin/clavulanic acid (n = 295; 0.5%; Access), azithromycin (n = 206; 0.35%; Watch), cefazolin (n = 30; 0.05%; Access), ciprofloxacin (n = 299; 0.51%; Watch), clarithromycin (n = 140; 0.24%; Watch), and levofloxacin (n = 220; 0.37%; Watch) [
108].
In an analysis of 1,722 notifications of AEs involving amoxicillin from 1988-2014 in the Korea Adverse Event Reporting System database, the third most reported AE was “drug ineffective” (n = 174; 10%). The authors attributed the ineffectiveness of amoxicillin to the failure of patients to take the drug for the prescribed time [
109].
In Brazil, 12,665 cases of AEs reported between 2018 and 2021 in VigiMed, the Brazilian PV database, were analysed, and the main drugs reported were vancomycin (n = 1,733; 14%; Watch), ceftriaxone (n = 1,274; 10%; Watch), piperacillin/tazobactam (n = 1,024; 8%; Watch), ciprofloxacin (n = 936; 7%; Watch), and azithromycin (n = 870; 7%; Watch). The event “drug ineffective” was identified as a safety signal associated with azithromycin (n = 8; 1%), cefuroxime (n = 7; 3.3%), amoxicillin (n = 4; 1.3%), and amoxicillin/clavulanic acid (n = 8; 1.8%) [
110].
Mhaidat et al. evaluated 539 notifications from Jordan (2003-2022). The most frequently reported ABs were tetracyclines (n = 101; 19%), fluoroquinolones (n = 54; 10%), third-generation cephalosporins (n = 48; 9%), and carbapenems (n = 42; 8%). In 279 reports, it was possible to characterize the AEs, and the “drug ineffective” MedDRA PT (n = 28; 5.19%) was most often related to carbapenems (n = 9; 32%), followed by tetracyclines (n = 8; 28%). The study also found “off-label” reports (n = 13; 2.41%), “incorrect route of administration” (n = 10; 1.89%), and “unapproved indication” (n = 6; 1.11%) [
111].
In India, 1,980 reports of suspected AEs were identified and evaluated for causality at a PV monitoring centre (2013-2019). Antimicrobials drew attention as the class most involved in suspicions (29%). Among the relevant AEs found, “multidrug resistance” was related to ceftriaxone/tazobactam (n = 15; 0.76%; the Not recommended category) and amikacin (n = 9; 0.45%; Access) [
112].
An investigation of tigecycline-related AEs reported to FAERS between 2004 and 2009 found 1,182 occurrences. “drug ineffective” emerged as one of the most reported events (n = 63; 5.33%), along with “pathogen resistance” (n = 22; 1.86%) [
113]. Another study done on the same database (2015-2018) found 5,899 notifications related to carbapenems, and again, “drug ineffective” was the most prevalent event (n = 620; 10.51%) [
64].
5. PV as a Tool to Identify Inappropriate Uses of Antibiotics
In addition to the consumed amount of AB, the quality of consumption in terms of appropriateness of the selection and adherence to clinical recommendations can provide valuable information because, as already mentioned, the inappropriate use of ABs is one of the main causes of AMR. In Africa, for example, a continent highly affected by infectious diseases, high consumption and inappropriate use of ABs were associated with low rates of adherence to prescribing guidelines, high rates of prolonged AB prophylaxis and/or prophylaxis with more than one AB, and high consumption of AB in the Watch group [
114,
115,
116].
In the National PV Database of Vietnam, 6,385 notifications related to ABs were found, totalling 11,652 AE/AB pairs in a 1-year period (2018-2019). Among the 10 most reported suspected ABs, eight are classified in the Watch group, namely, cefotaxime (n = 1,142; 17.9%), ceftriaxone (n = 395; 9.9%), ceftazidime (n = 394; 6.2%), cefoperazone (n = 293; 4.6%), cefuroxime (n = 242; 3.8%), ciprofloxacin (n = 651; 10.2%), levofloxacin (n = 287; 4.5%), and vancomycin (n = 306; 4.8%), while two belong to the Access group, amoxicillin (n = 491; 7.7%) and ampicillin (n = 261; 4.1%). Serious events accounted for 49% of the AEs. A total of 889 AEs were considered preventable, 13.4% of which were related to amoxicillin, 10.2% to cefotaxime, and 7.4% to ciprofloxacin. Interestingly, the main causes of these preventable AEs were related to prescribing, which included “inappropriate prescribing” (n = 352; 40%), “indicating antibiotics for patients with no sign of infection” (n = 272; 31%), “inappropriate indication for antimicrobial prophylaxis” (n = 49; 6%), “inappropriate indication” (n = 31; 4%), “readministration of antibiotics causing prior allergy/allergies” (n = 99; 11%), and “inappropriate dosing” (n = 26; 3%). These results reveal evidence of AB overuse that the authors say is related to problems such as difficulty in making an adequate diagnosis, high infection pressure, and problems related to prescribers (personal experience and financial interests) [
94].
Ballon et al. conducted a study in the French PV Database (2010-2019) comparing the profile of AEs in pregnant (n = 911) and nonpregnant women (n = 3,358) and reported that anti-infective medicines (n = 141; 18.3%) were among the drugs most often associated with these events. The AEs grouped into “lesions, poisoning, and surgical complications”, represented mainly by the MedDRA PTs associated with medication errors, were more frequent among pregnant women (n = 71; 8.1%) than among nonpregnant women (n = 55; 1.6%) [
117].
The Russian PV database (2012-2014) included 3,608 reports of beta-lactams: penicillins (n = 1,123; 31%), cephalosporins (n = 2,324; 64%), and carbapenems (n = 161; 4%). The experts analysed the narrative descriptions of each patient and identified prescription problems and medication errors in 1,043 reports (28.9%). Notably, only 29 of these notifications described these problems in the AE field. The age group most involved in this study was children (n = 457; 43.8%). The most frequent prescription problems were “administration of an antibiotic in the absence of indications/wrong indication” (n = 395; 32.5%), and “contraindicated administration” (n = 210; 17.3%); medication errors were “inappropriate dose or dose regimen” (n = 360; 29.7%), and “incorrect preparation of an antibiotic solution” (n = 27; 2.2%). A relationship with too low dose frequency was found among the eight (0.22%) patients who experienced treatment failure. Other types of reported prescription problems that deserve attention were “late discontinuation of a drug in a patient developing an ADR” (n = 80; 6.6%), “irrational switch of an antibiotic” (n = 59 4.9%), “late or irrational change of antibiotic in case of treatment failure” (n = 31; 2.6%), and “administration of an inadequate treatment regimen for the disease/treatment strategy” (n = 11; 0.9%) [
118].
The study conducted in India, described in the previous section, which related “multidrug resistance” to ceftriaxone/tazobactam and amikacin, also called attention to evidence of inappropriate use, since most indications were for empirical prophylaxis, and susceptibility tests for ABs were requested only after treatment failure of the ABs initially prescribed. The observed resistance to ceftriaxone/tazobactam led to substitution with meropenem (Watch) or linezolid (Reserve) [
112].
Ren et al. analysed the safety of cephalosporins in two PV databases in China (2009-2010) and reported 1,337 AEs. Misuse, especially an “inappropriate dosing regimen”, was observed in 93% of the patients (n = 1,243), and overuse was observed in 18% (n = 249), the latter being more impactful among children [
119].
Looking further back in the literature, some studies cited issues related to the misuse of ABs. According to the Iranian PV database (1998-2009), 15.2% (n = 183) of the reports for ceftriaxone (n = 1205; Watch) were considered preventable [
120]. In another study, reports received by the Italian Interregional Group of PV (1988-2004) suggested a higher reporting rate for amoxicillin/clavulanic acid (measured by the number of reports/1 000 000 defined daily doses/year) compared to amoxicillin (2.11 versus 1.52, in the period 200-2004) and an indiscriminate increase in amoxicillin/clavulanic acid, although there are guidelines to make this the second priority [
121].
5.1. Off-Label Use of Antibiotics
Off-label use is not necessarily inappropriate; in certain cases of complicated patients with resistant or uncommon bacteria, empiric use of AB is possible, but always under the close supervision of microbiologists. However, AB treatment without a registered indication and disconnected from evidence of effectiveness against a particular microbial agent, population, and site of action is frequent. Children, pregnant women, and the elderly are populations rarely included in preapproval clinical trials of drugs [
122,
123,
124,
125], despite this, medicines are often used in these circumstances just extrapolating the indications and dosage from adult patients without comorbidities, changes in metabolism or development that would influence pharmacokinetics and pharmacodynamics.
From data in the Swedish database, a study concluded that medicines with off-label use are more subject to AEs than medicines used as indicated. Furthermore, the paediatric population is more affected than adults [
126]. Off-label prescriptions are frequent in children and are more strongly related to severe AEs [
127,
128].
In Germany, 20,854 reports (2000-2019) were evaluated in the 0-17-year age group, and 3.5% of the respondents had records of off-label use [
129]. In Brazil, a study that evaluated 3,330 notifications for children up to 12 years of age found that 30% of the reports of fatal cases described an off-label use (n = 22). In that study, anti-infective drugs were the most reported class (n = 1,602; 41%) [
130].
An analysis of AEs associated with oxazolidinones reported to FAERS (2018-2020) revealed a more critical safety profile for tedizolid (the newest antibiotic on the market) than expected for serious events such as myelotoxicity, lactic acidosis, and peripheral neuropathy. The authors associated the findings with frequent off-label use, especially regarding the use of this AB for up to 3 weeks, which was longer than what was initially approved (only 6 days) [
39].
Table 1 summarizes the most relevant MedDRA terms and AWaRe categories of associated ABs cited in the studies included in this review.
6. Discussion
The revised studies reinforce the potential added utility of the already existing PV systems to collaborate in AMS initiatives by pointing to inappropriate use of ABs and suggesting the existence of possible AMR in remote places with difficult access to laboratory testing and cultures. As already suggested in the literature [
26,
27,
28,
95,
99] this can be achieved through the promotion of reporting by using the MedDRA PTs of interest for AMR. These studies show how an in-depth analysis of the available data already included in PV databases can provide useful elements for AMS teams at the local or national level and help define antibiotic policies in the country.
In a regional database (Eudravigilance), a higher probability of notification of an antimicrobial (in the cited example, colistin) with terms related to resistance and inefficacy was observed than for other antibiotics [
37]. Studies such as this one may help doctors choose an AB with a lower probability of resistance/ineffectiveness in a given local reality, which is a key tool for developing AMS. According to a Korean study [
109], the suspicion that the ineffectiveness of amoxicillin was due to inadequate treatment duration shows that encouraging the identification and notification of specific events of interest for in-depth investigations of the causes of ineffective drugs may help us improve the use of ABs. These are some practical examples of the use of the information provided by PV.
Terms such as “drug ineffective,” “therapeutic failure,” “drug resistance,” “pathogen resistance”, and “multidrug resistance” in PV databases encompass a range of problems that should be better investigated and proven useful in warning about possible causes of AMR before they are used in studies aiming to clarify the contributing factors to be considered in measures to contain AMR.
Table 1 reinforces the greater involvement of the Watch category in problems of use and suspicion of AMR and the relevance of the terms listed in identifying these problems.
Although the off-label use of ABs to treat infections caused by resistant pathogens has shown positive results in some situations, the cost of such use must be weighed carefully [
131], and it should always be guided by a microbiologist or an infectologist. The studies summarised here emphasise that the term “off-label,” in addition to issues of safety and vulnerability of at-risk populations, frequently appears in PV databases and permeates events of interest to AMR.
Weaknesses in antibiotic therapy and treatment failures related to inadequate dose regimens can be identified, as exemplified in studies in Russia and China [
118,
119]. For example, some of the PV studies included in this review suggest the need to review local protocols to ensure that they follow the latest available evidence, the local patterns of AMR, as well as the pharmacokinetics and pharmacodynamics of each AB. Different studies included in this revision describe the use of inappropriate doses in treatment failure and bacterial resistance [
101,
105,
132,
133]. In other words, the analysis of data included in PV reports may provide clues about inappropriate use in a given place, which allows the design of interventions to improve or reverse this use.
Preventing AEs is an important part of the safe and appropriate use of medicines; furthermore, in the case of ABs, more appropriate use can contain AMR. On the one hand, there are inappropriate or irrational prescriptions (for example, one of the commonest practices in primary health care: using an AB for mild viral upper respiratory conditions). On the other hand, there are medication errors caused by mistakes or confusions in names, dosages or duration of treatments. Both cases lead to preventable situations that directly increase the risk of AMR. Concerning medication errors, there is a fear on the part of professionals to record errors in notifications, as demonstrated in the Russian study in which only 2.8% of problems identified were notified [
118]. Institutions with higher patient safety culture scores, which report and treat errors, have lower rates of healthcare events [
134]. Concerning irrational prescription, it is wider than the prescription of ABs, and it involves many factors beyond inappropriate or outdated knowledge, as psychological, economic and political aspects also take part of it, together with the characteristics of the healthcare system structure. Still, the PV systems have proven useful for indicating points of improvement in the use of health care despite the incompleteness of the records and the underreporting.
Studies to monitor the use of ABs, within the scope of PV, prepared according to quality criteria [
44,
135], can identify a range of associated problems, such as self-medication practices, common practices of inadequate prescriptions and what may influence them, the most prevalent errors, and the ABs most associated with problems of use. The identification of these issues makes it possible to reflect on two aspects of PV: (i) the regulatory aspect, for which measures to control the use and change the leaflet, policies and protocols for the use of AB can be established; and (ii) the aspect of the care process, which guides process-improvement actions to minimize medication errors and the development of education programs for health professionals and the population.
Given its structure at the local, national, and global levels, the use of PV systems can be a useful part of strategies to contain AMR, obviously not as a substitute for microbiological laboratory testing but as a system for identifying potential problems of antibiotic efficacy in certain places. Once reported, health authorities and national antimicrobial surveillance teams will have a basis to design the appropriate actions to either obtain microbiological confirmation of the suspicion in the case of AMR or design specific studies to reverse the inappropriate use of antibiotics by using local real-world data. Local/national analyses provide more detailed data reflecting the local epidemiological reality, along with higher-powered application of timely and effective intervention strategies
Another advantage of PV analysis is that it can be performed over time series derived from local systems and may reflect important changes in use while signalling attention to certain classes of AB. Therapeutic failure analyses may point to the possibility of counterfeiting and substandard products, which are common practices in LMICs and cause great harm to public health [
102,
136,
137]. In addition to the low cost of PV studies, their structure is already widespread and based on international guidelines, and there are PV practices based on active searches, so studies have focussed on specific MedDRA PTs and utilization [
138].
The limitation of this review is that, due to the method chosen, some studies relevant to the topic may have been missed, which may have caused some selection bias. On the other hand, it enabled a broader search so we could explore the recent themes of PV. It was also limited to citing the most frequent terms found, which do not exhaust the topic, as a range of terms that indicate ineffectiveness and inappropriate use can be found in the various PV databases according to the culture and training of the reporters.
Although the strategies were divided into two major topics (ineffectiveness, and inappropriate use) to facilitate data organization, in reality, they are found together, all signalling problems related to ABs. Despite PV studies not being definitive in their conclusions, as they are, by nature, about suspected AEs, they may indicate guidelines and paths to be followed and investigated to confirm possible AMR.
7. Conclusion
This review strongly showed support for the idea that PV studies may be useful in the use of ABs, in addition to the already known description of the safety profile of these medicines: (1) PV can help identify AB ineffectiveness, and (2) it can identify inappropriate use. This use of well-known tools already consolidated in many countries can help guide AMS and drug-related policies so that healthcare providers can be alerted about a possible lack of response due to AB resistance.
In a scenario where access to adequate microbiological diagnosis and monitoring is difficult, it is necessary to take advantage of every possible source of available information, such as PV programs and their databases. The studies presented in this review showed not only the presence but the frequent appearance of terms of interest about AMR on a local, national, and international basis. These strategies can be incorporated as indicators of inappropriate use and AB resistance in AMS. Knowing the roots of the causes of these types of events and encouraging specific notifications can make transformative contributions to stewardship programs.
The main aim of this review is to show the feasibility of using a well-known and consolidated public health tool to contribute to antimicrobial stewardship activities and to address any resistance problems.
From this perspective, strengthening PV systems and encouraging reportings of specific AEs related to AMR could be important indicators for monitoring the use of ABs and advancing AMR-related national policies and stewardship programs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Studies that analysed signs and associations between adverse events and antibiotics in PV databases (2019-2023).
Author Contributions
Conceptualization, V.S., A.F. and E.L.; methodology, V.S., A.F. and E.L.; formal analysis, V.S.; writing—original draft preparation, V.S.; writing—review and editing, A.F. and A.L.; supervision, A.F. and E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, grant number E-26/201.373/2022.
Acknowledgments
We thank Rebeca Lacerda and Alice Ramos for the support given and assistance with the figures and the supplementary table.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kua, K. Antibiotics and Antibiotic Resistance- Flipsides of the Same Coin. Current Pharmaceutical Design 2022, 28, 2312–2329. [CrossRef]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr Pharm Des 2023, 29, 356–364. [CrossRef]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic Perturbations to the Gut Microbiome. Nat Rev Microbiol 2023, 21, 772–788. [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food Chem Toxicol 2019, 125, 462–466. [CrossRef]
- Muloi, D.M.; Kurui, P.; Sharma, G.; Ochieng, L.; Nganga, F.; Gudda, F.; Muthini, J.M.; Grace, D.; Dione, M.; Moodley, A.; et al. Antibiotic Quality and Use Practices amongst Dairy Farmers and Drug Retailers in Central Kenyan Highlands. Sci Rep 2023, 13, 23101. [CrossRef]
- Matin, M.A.; Khan, W.A.; Karim, M.M.; Ahmed, S.; John-Langba, J.; Sankoh, O.A.; Gyapong, M.; Kinsman, J.; Wertheim, H. What Influences Antibiotic Sales in Rural Bangladesh? A Drug Dispensers’ Perspective. J Pharm Policy Pract 2020, 13, 20. [CrossRef]
- Malik, F.; Figueras, A. Analysis of the Antimicrobial Market in Pakistan: Is It Really Necessary Such a Vast Offering of “Watch” Antimicrobials? Antibiotics (Basel) 2019, 8, 189. [CrossRef]
- Ateshim, Y.; Bereket, B.; Major, F.; Emun, Y.; Woldai, B.; Pasha, I.; Habte, E.; Russom, M. Prevalence of Self-Medication with Antibiotics and Associated Factors in the Community of Asmara, Eritrea: A Descriptive Cross Sectional Survey. BMC Public Health 2019, 19, 726. [CrossRef]
- Kirchhelle, C.; Atkinson, P.; Broom, A.; Chuengsatiansup, K.; Ferreira, J.P.; Fortané, N.; Frost, I.; Gradmann, C.; Hinchliffe, S.; Hoffman, S.J.; et al. Setting the Standard: Multidisciplinary Hallmarks for Structural, Equitable and Tracked Antibiotic Policy. BMJ Glob Health 2020, 5, e003091. [CrossRef]
- Guinovart, M.C.; Figueras, A.; Llop, J.C.; Llor, C. Obtaining Antibiotics without Prescription in Spain in 2014: Even Easier Now than 6 Years Ago. J Antimicrob Chemother 2015, 70, 1270–1271. [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of Antimicrobial Resistance in Low- and Middle-Income Countries: A Scattered Picture. Antimicrob Resist Infect Control 2021, 10, 63. [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 10 February 2024).
- World Health Organization. GLASS Dashboard. Available online: https://worldhealthorg.shinyapps.io/glass-dashboard/_w_270f2329/#!/home (accessed on 12 April 2024).
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Available online: https://reliefweb.int/report/world/global-antimicrobial-resistance-and-use-surveillance-system-glass-report-2022 (accessed on 8 March 2024).
- World Health Organization. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; Geneva, 2022.
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Hospital Antibiotic Prescribing Patterns in Adult Patients According to the WHO Access, Watch and Reserve Classification (AWaRe): Results from a Worldwide Point Prevalence Survey in 69 Countries. J Antimicrob Chemother 2021, 76, 1614–1624. [CrossRef]
- Ramos, O. S. A.; Xavier, B. B. C.; Soares, R. R.; Fernandez-Llimos, F.; Costa Lima, E. Geographical Variation in Antimicrobial Use and Multiresistant Pathogens in Brazilian Intensive Care Units: A Nationwide Study. J Infect Dev Ctries 2023, 17, 485–493. [CrossRef]
- Simmons, B.; Ariyoshi, K.; Ohmagari, N.; Pulcini, C.; Huttner, B.; Gandra, S.; Satta, G.; Moja, L.; Sharland, M.; Magrini, N.; et al. Progress towards Antibiotic Use Targets in Eight High-Income Countries. Bull World Health Organ 2021, 99, 550–561. [CrossRef]
- Talaat, M.; Tolba, S.; Abdou, E.; Sarhan, M.; Gomaa, M.; Hutin, Y.J.-F. Over-Prescription and Overuse of Antimicrobials in the Eastern Mediterranean Region: The Urgent Need for Antimicrobial Stewardship Programs with Access, Watch, and Reserve Adoption. Antibiotics (Basel) 2022, 11, 1773. [CrossRef]
- Kanan, M.; Ramadan, M.; Haif, H.; Abdullah, B.; Mubarak, J.; Ahmad, W.; Mari, S.; Hassan, S.; Eid, R.; Hasan, M.; et al. Empowering Low- and Middle-Income Countries to Combat AMR by Minimal Use of Antibiotics: A Way Forward. Antibiotics (Basel) 2023, 12, 1504. [CrossRef]
- Marin, G.H.; Giangreco, L.; Dorati, C.; Mordujovich, P.; Boni, S.; Mantilla-Ponte, H.; Alfonso Arvez, M.J.; López Peña, M.; Aldunate González, M.F.; Ching Fung, S.M.; et al. Antimicrobial Consumption in Latin American Countries: First Steps of a Long Road Ahead. J Prim Care Community Health 2022, 13, 21501319221082346. [CrossRef]
- Sohaili, A.; Asin, J.; Thomas, P.P.M. The Fragmented Picture of Antimicrobial Resistance in Kenya: A Situational Analysis of Antimicrobial Consumption and the Imperative for Antimicrobial Stewardship. Antibiotics (Basel) 2024, 13, 197. [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat Rev Microbiol 2023, 21, 280–295. [CrossRef]
- World Health Organization. WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities 2021. Available online: https://www.who.int/publications/i/item/9789240025530 (accessed on 10 February 2024).
- Lim, C.; Ashley, E.A.; Hamers, R.L.; Turner, P.; Kesteman, T.; Akech, S.; Corso, A.; Mayxay, M.; Okeke, I.N.; Limmathurotsakul, D.; et al. Surveillance Strategies Using Routine Microbiology for Antimicrobial Resistance in Low- and Middle-Income Countries. Clin Microbiol Infect 2021, 27, 1391–1399. [CrossRef]
- Agrawal, V.; Shrivastava, T.P.; Adusumilli, P.K.; Vivekanandan, K.; Thota, P.; Bhushan, S. Pivotal Role of Pharmacovigilance Programme of India in Containment of Antimicrobial Resistance in India. Perspect Clin Res 2019, 10, 140–144. [CrossRef]
- Bairy, L.K.; Nayak, V.; A, A.; Kunder, S.K. Advances in Pharmacovigilance Initiatives Surrounding Antimicrobial Resistance-Indian Perspective. Expert Opin Drug Saf 2016, 15, 1055–1062. [CrossRef]
- Gatti, M.; Raschi, E.; De Ponti, F. Relationship between Adverse Drug Reactions to Antibacterial Agents and the Klebsiella Pneumoniae Carbapenemase-Producing (KPC) Klebsiella Pneumoniae Outbreak: Insight from a Pharmacovigilance Study. BMC Pharmacol Toxicol 2019, 20, 65. [CrossRef]
- Habarugira, J.M.V.; Figueras, A. Antimicrobial Stewardship: Can We Add Pharmacovigilance Networks to the Toolbox? Eur J Clin Pharmacol 2021, 77, 787–790. [CrossRef]
- Habarugira, J.M.V.; Härmark, L.; Figueras, A. Pharmacovigilance Data as a Trigger to Identify Antimicrobial Resistance and Inappropriate Use of Antibiotics: A Study Using Reports from The Netherlands Pharmacovigilance Centre. Antibiotics (Basel) 2021, 10. [CrossRef]
- Habarugira, J.M.V.; Harmark, L.; Figueras, A. Adverse Drug Reaction Reports Containing AMR-Relevant MedDRA Terms in the Dutch Pharmacovigilance Database. 2021. [CrossRef]
- World Health Organization. The Importance of Pharmacovigilance. Available online: https://www.who.int/publications-detail-redirect/10665-42493 (accessed on 11 April 2024).
- World Health Organization. Programme for International Drug Monitoring. Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/networks/pidm (accessed on 8 March 2024).
- Wang, H.; Marquez, P.V.; Figueras, A.; Bieliaieva, K. Why Is the Safety of Medicines Important for Resilient Health Systems? A Synthesis Report. World Bank 2023. [CrossRef]
- World Health Organization. Pharmacovigilance. Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance (accessed on 30 January 2024).
- Uppsala Monitoring Centre Glossary. Available online: https://who-umc.org/pharmacovigilance-communications/glossary/ (accessed on 12 February 2024).
- Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. [CrossRef]
- M Shaju, A.; Panicker, N.; Chandni, V.; Lakshmi Prasanna, V.M.; Nair, G.; Subeesh, V. Drugs-Associated with Red Man Syndrome: An Integrative Approach Using Disproportionality Analysis and Pharmip. J Clin Pharm Ther 2022, 47, 1650–1658. [CrossRef]
- Gatti, M.; Fusaroli, M.; Raschi, E.; Moretti, U.; Poluzzi, E.; De Ponti, F. Serious Adverse Events with Tedizolid and Linezolid: Pharmacovigilance Insights through the FDA Adverse Event Reporting System. Expert Opin Drug Saf 2021, 20, 1421–1431. [CrossRef]
- Gatti M; Raschi E; De Ponti F. Serious Adverse Events with Novel Beta-Lactam/Beta-Lactamase Inhibitor Combinations: A Large-Scale Pharmacovigilance Analysis. Eur J Clin Microbiol Infect Dis 2021, 40, 1169–1176. [CrossRef]
- Sabaté, M.; Montané, E. Pharmacoepidemiology: An Overview. Journal of Clinical Medicine 2023, 12, 7033. [CrossRef]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf 2023, 46, 625–636. [CrossRef]
- Alomar, M.; Palaian, S.; Al-Tabakha, M.M. Pharmacovigilance in Perspective: Drug Withdrawals, Data Mining and Policy Implications. F1000Res 2019, 8, 2109. [CrossRef]
- Christ, P.; Dubrall, D.; Schmid, M.; Sachs, B. Comparative Analysis of Information Provided in German Adverse Drug Reaction Reports Sent by Physicians, Pharmacists and Consumers. Drug Saf 2023, 46, 1363–1379. [CrossRef]
- Zou, D.; Zhang, R.; Yu, L.; Hu, T.; Wu, B. Seizures Associated with Antibiotics: A Real-World Disproportionality Analysis of FAERS Database. Expert Opin Drug Saf 2023, 22, 1143–1148. [CrossRef]
- Zhou, L.; Yang, J.; Xiao, M.; Shan, H.; Liu, M.; Lu, Y.; Zou, Y.; Wu, B. Severe Cutaneous Adverse Reactions Due to Antibiotics Therapy: A Pharmacovigilance Analysis of FDA Adverse Event Reporting System Events. Expert Opin Drug Saf 2023, 1–8. [CrossRef]
- Li, D.; Song, Y.; Bai, Z.; Xi, X.; Liu, F.; Zhang, Y.; Qin, C.; Du, D.; Du, Q.; Liu, S. Real-World Data in Pharmacovigilance Database Provides a New Perspective for Understanding the Risk of Clostridium Difficile Infection Associated with Antibacterial Drug Exposure. Antibiotics (Basel) 2023, 12. [CrossRef]
- Shao, H.; Shi, D.; Dai, Y. Linezolid and the Risk of QT Interval Prolongation: A Pharmacovigilance Study of the Food and Drug Administration Adverse Event Reporting System. Br J Clin Pharmacol 2023, 89, 1386–1392. [CrossRef]
- Liu, X.; Xu, Z.; Ma, J.; Zhang, A.; Li, Z.; Qi, G.; Li, Z.; Wei, F.; Zhong, L. Hepatobiliary Calculi Associated with Ceftriaxone Treatment: An Analysis of FAERS Data from 2004 to 2021. J Infect Chemother 2023, 29, 136–142. [CrossRef]
- Seo, H.; Kim, E. Electrolyte Disorders Associated with Piperacillin/Tazobactam: A Pharmacovigilance Study Using the FAERS Database. Antibiotics (Basel) 2023, 12. [CrossRef]
- Chen, J.J.; Huo, X.C.; Wang, S.X.; Wang, F.; Zhao, Q. Data Mining for Adverse Drug Reaction Signals of Daptomycin Based on Real-World Data: A Disproportionality Analysis of the US Food and Drug Administration Adverse Event Reporting System. Int J Clin Pharm 2022, 44, 1351–1360. [CrossRef]
- Tang, R.; Lopes, V.L.; Caffrey, A.R. Colistin-Associated Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Reactions: A Retrospective Case-Non-Case Pharmacovigilance Study. Expert Opin Drug Saf 2022, 21, 1121–1126. [CrossRef]
- Heo, J.Y.; Cho, M.K.; Kim, S. Data Mining for Detecting Signals of Adverse Drug Reaction of Doxycycline Using the Korea Adverse Event Reporting System Database. J Dermatolog Treat 2022, 33, 2192–2197. [CrossRef]
- Recht, J.; Chansamouth, V.; White, N.J.; Ashley, E.A. Nitrofurantoin and Glucose-6-Phosphate Dehydrogenase Deficiency: A Safety Review. JAC Antimicrob Resist 2022, 4, dlac045. [CrossRef]
- Yamada, T.; Mitsuboshi, S.; Suzuki, K.; Nishihara, M.; Neo, M. Analysis of the Frequency of Ceftriaxone-Induced Encephalopathy Using the Japanese Adverse Drug Event Report Database. Int J Clin Pharm 2022, 44, 1067–1071. [CrossRef]
- Kuula, L.S.M.; Backman, J.T.; Blom, M.L. Healthcare Costs and Mortality Associated with Serious Fluoroquinolone-Related Adverse Reactions. Pharmacol Res Perspect 2022, 10, e00931. [CrossRef]
- Gatti, M.; Fusaroli, M.; Raschi, E.; Capelli, I.; Poluzzi, E.; De Ponti, F. Crystal Nephropathy and Amoxicillin: Insights from International Spontaneous Reporting Systems. J Nephrol 2022, 35, 1017–1027. [CrossRef]
- Taher, M.K.; Alami, A.; Gravel, C.A.; Tsui, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic Quinolones and Risk of Retinal Detachment I: Analysis of Data from the US FDA Adverse Event Reporting System. Expert Opin Drug Saf 2022, 21, 269–276. [CrossRef]
- Mitsuboshi, S.; Katagiri, H. Risk of Kidney Injury in Patients on Concomitant Oral Vancomycin and Piperacillin-Tazobactam: Analysis of the Pharmacovigilance Database in Japan. Basic Clin Pharmacol Toxicol 2022, 130, 208–212. [CrossRef]
- Rey, A.; Gras, V.; Moragny, J.; Choukroun, G.; Masmoudi, K.; Liabeuf, S. Use of the Capture-Recapture Method to Estimate the Frequency of Community- and Hospital-Acquired Drug-Induced Acute Kidney Injuries in French Databases. Front Pharmacol 2022, 13, 899164. [CrossRef]
- Jo, H.-G.; Jeong, K.; Ryu, J.-Y.; Park, S.; Choi, Y.-S.; Kwack, W.-G.; Choi, Y.-J.; Chung, E.-K. Fatal Events Associated with Adverse Drug Reactions in the Korean National Pharmacovigilance Database. J Pers Med 2021, 12. [CrossRef]
- Asai, Y.; Yamamoto, T.; Abe, Y. Evaluation of the Expression Profile of Antibiotic-Induced Thrombocytopenia Using the Japanese Adverse Drug Event Report Database. Int J Toxicol 2021, 40, 542–550. [CrossRef]
- Largeau, B.; Agier, M.-S.; Beau-Salinas, F.; Pariente, A.; Maruani, A.; Vial, T.; Jonville-Béra, A.-P. Specific Features of Amoxicillin-Associated Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Nationwide Study. J Eur Acad Dermatol Venereol 2021, 35, 2415–2420. [CrossRef]
- Ge, W.; Hu, H.; Li, C.; Wang, L.; Xia, J. Safety Profile of Carbapenems: Data Mining of the FDA Adverse Events Reporting System. Int J Clin Pharmacol Ther 2021, 59, 594–602. [CrossRef]
- Yamada, T.; Mitsuboshi, S.; Suzuki, K.; Nishihara, M.; Uchiyama, K. Risk of Muscle Toxicity Events for Daptomycin with and without Statins: Analysis of the Japanese Adverse Event Report Database. Basic Clin Pharmacol Toxicol 2021, 129, 268–272. [CrossRef]
- Nakao, S.; Hasegawa, S.; Umetsu, R.; Shimada, K.; Mukai, R.; Tanaka, M.; Matsumoto, K.; Yoshida, Y.; Inoue, M.; Satake, R.; et al. Pharmacovigilance Study of Anti-Infective-Related Acute Kidney Injury Using the Japanese Adverse Drug Event Report Database. BMC Pharmacol Toxicol 2021, 22, 47. [CrossRef]
- Nguyen, K.-D.; Vu, D.-H.; Nguyen, H.-A.; Dao, V.-T.; Montastruc, J.-L.; Bagheri, H. Risk Comparison of Beta-Lactam-Induced Anaphylaxis: Therapeutic Stratification Analysis in a Vietnamese Pharmacovigilance Database. J Clin Pharm Ther 2021, 46, 950–956. [CrossRef]
- Rudolph, A.; Dahmke, H.; Kupferschmidt, H.; Burden, A.; Weiler, S. Coadministration of Tizanidine and Ciprofloxacin: A Retrospective Analysis of the WHO Pharmacovigilance Database. Eur J Clin Pharmacol 2021, 77, 895–902. [CrossRef]
- Lacroix, C.; Bera-Jonville, A.-P.; Montastruc, F.; Velly, L.; Micallef, J.; Guilhaumou, R. Serious Neurological Adverse Events of Ceftriaxone. Antibiotics (Basel) 2021, 10. [CrossRef]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of Antibiotic-Induced Taste and Smell Disorders Using the FDA Adverse Event Reporting System Database. Sci Rep 2021, 11, 9625. [CrossRef]
- Contejean, A.; Tisseyre, M.; Canouï, E.; Treluyer, J.-M.; Kerneis, S.; Chouchana, L. Combination of Vancomycin plus Piperacillin and Risk of Acute Kidney Injury: A Worldwide Pharmacovigilance Database Analysis. J Antimicrob Chemother 2021, 76, 1311–1314. [CrossRef]
- Gatti, M.; Raschi, E.; De Ponti, F. Serotonin Syndrome by Drug Interactions with Linezolid: Clues from Pharmacovigilance-Pharmacokinetic/Pharmacodynamic Analysis. Eur J Clin Pharmacol 2021, 77, 233–239. [CrossRef]
- Akimoto, H.; Nagashima, T.; Minagawa, K.; Hayakawa, T.; Takahashi, Y.; Asai, S. Signal Detection of Potential Hepatotoxic Drugs: Case-Control Study Using Both a Spontaneous Reporting System and Electronic Medical Records. Biol Pharm Bull 2021, 44, 1514–1523. [CrossRef]
- Dai, Y.; Wang, Y.; Zeng, Y.; Zhang, C.; Zhou, Z.; Shi, D. Linezolid and the Risk of Lactic Acidosis: Data Mining and Analysis of the FDA Adverse Event Reporting System. J Clin Pharm Ther 2020, 45, 1422–1426. [CrossRef]
- Scavone, C.; Mascolo, A.; Ruggiero, R.; Sportiello, L.; Rafaniello, C.; Berrino, L.; Capuano, A. Quinolones-Induced Musculoskeletal, Neurological, and Psychiatric ADRs: A Pharmacovigilance Study Based on Data From the Italian Spontaneous Reporting System. Front Pharmacol 2020, 11, 428. [CrossRef]
- Villa Zapata, L.; Hansten, P.D.; Horn, J.R.; Boyce, R.D.; Gephart, S.; Subbian, V.; Romero, A.; Malone, D.C. Evidence of Clinically Meaningful Drug-Drug Interaction With Concomitant Use of Colchicine and Clarithromycin. Drug Saf 2020, 43, 661–668. [CrossRef]
- Zelmat, Y.; Rousseau, V.; Chebane, L.; Montastruc, J.-L.; Bagheri, H.; Sommet, A. Fluoroquinolone-Induced Photosensitivity: A Chemical Fragment-Based Approach by a Case/Non-Case Study in VigiBase(®). Drug Saf 2020, 43, 561–566. [CrossRef]
- Kennedy, K.E.; Teng, C.; Patek, T.M.; Frei, C.R. Hypoglycemia Associated with Antibiotics Alone and in Combination with Sulfonylureas and Meglitinides: An Epidemiologic Surveillance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf 2020, 43, 363–369. [CrossRef]
- Timbrook, T.T.; McKay, L.; Sutton, J.D.; Spivak, E.S. Disproportionality Analysis of Safety with Nafcillin and Oxacillin with the FDA Adverse Event Reporting System (FAERS). Antimicrob Agents Chemother 2020, 64. [CrossRef]
- Patek, T.M.; Teng, C.; Kennedy, K.E.; Alvarez, C.A.; Frei, C.R. Comparing Acute Kidney Injury Reports Among Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf 2020, 43, 17–22. [CrossRef]
- Bonaldo, G.; Andriani, L.A.; D’Annibali, O.; Motola, D.; Vaccheri, A. Cardiovascular Safety of Macrolide and Fluoroquinolone Antibiotics: An Analysis of the WHO Database of Adverse Drug Reactions. Pharmacoepidemiol Drug Saf 2019, 28, 1457–1463. [CrossRef]
- Thornhill, M.H.; Dayer, M.J.; Durkin, M.J.; Lockhart, P.B.; Baddour, L.M. Risk of Adverse Reactions to Oral Antibiotics Prescribed by Dentists. J Dent Res 2019, 98, 1081–1087. [CrossRef]
- Orion, K.; Mack, J.; Kullak-Ublick, G.A.; Weiler, S. Kounis Syndrome: A Retrospective Analysis of Individual Case Safety Reports from the International WHO Database in Pharmacovigilance. Int J Clin Pharmacol Ther 2019, 57, 240–248. [CrossRef]
- Sommet, A.; Bénévent, J.; Rousseau, V.; Chebane, L.; Douros, A.; Montastruc, J.-L.; Montastruc, F. What Fluoroquinolones Have the Highest Risk of Aortic Aneurysm? A Case/Non-Case Study in VigiBase®. J Gen Intern Med 2019, 34, 502–503. [CrossRef]
- Lacroix, C.; Kheloufi, F.; Montastruc, F.; Bennis, Y.; Pizzoglio, V.; Micallef, J. Serious Central Nervous System Side Effects of Cephalosporins: A National Analysis of Serious Reports Registered in the French Pharmacovigilance Database. J Neurol Sci 2019, 398, 196–201. [CrossRef]
- Chandler, R.E. Increased Risk for Aseptic Meningitis after Amoxicillin or Amoxicillin-Clavulanic Acid in Males: A Signal Revealed by Subset Disproportionality Analysis within a Global Database of Suspected Adverse Drug Reactions. Pharmacoepidemiol Drug Saf 2019, 28, 389–395. [CrossRef]
- Teng, C.; Reveles, K.R.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Clostridium Difficile Infection Risk with Important Antibiotic Classes: An Analysis of the FDA Adverse Event Reporting System. Int J Med Sci 2019, 16, 630–635. [CrossRef]
- Morales, D.R.; Slattery, J.; Pacurariu, A.; Pinheiro, L.; McGettigan, P.; Kurz, X. Relative and Absolute Risk of Tendon Rupture with Fluoroquinolone and Concomitant Fluoroquinolone/Corticosteroid Therapy: Population-Based Nested Case-Control Study. Clin Drug Investig 2019, 39, 205–213. [CrossRef]
- Teng, C.; Walter, E.A.; Gaspar, D.K.S.; Obodozie-Ofoegbu, O.O.; Frei, C.R. Torsades de Pointes and QT Prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System. Int J Med Sci 2019, 16, 1018–1022. [CrossRef]
- Abu Esba, L.C.; Al Mardawi, G.; AlJasser, M.I.; Aljohani, B.; Abu Alburak, A. Adverse Drug Reactions Spontaneously Reported at a Tertiary Care Hospital and Preventable Measures Implemented. J Clin Pharm Ther 2021, 46, 460–469. [CrossRef]
- Kaur, K.; Kanwal, P.; Goyal, P.; Singh, P.; Yakhmi, S.; Jain, S.; Kaushal, S. Spontaneous Adverse Drug Reaction Monitoring in a Tertiary Care Centre. Curr Drug Saf 2020, 15, 215–221. [CrossRef]
- Aagaard, L.; Hansen, E.H. Adverse Drug Reactions Reported for Systemic Antibacterials in Danish Children over a Decade. Br J Clin Pharmacol 2010, 70, 765–768. [CrossRef]
- Rosli, R.; Ming, L.C.; Abd Aziz, N.; Manan, M.M. A Retrospective Analysis of Spontaneous Adverse Drug Reactions Reports Relating to Paediatric Patients. PLoS ONE 2016, 11, e0155385. [CrossRef]
- Tran, H.N.; Nguyen, T.N.T.; Tran, N.T.K.; Nguyen, L.T.; Vu, H.D.; Nguyen, A.H.; Trinh, N.T.H. Preventability of Adverse Drug Reactions Related to Antibiotics: An Assessment Based on Spontaneous Reporting System. Ther Innov Regul Sci 2023, 57, 1104–1112. [CrossRef]
- Habarugira, J.M.V.; Figueras, A. Pharmacovigilance Network as an Additional Tool for the Surveillance of Antimicrobial Resistance. Pharmacoepidemiol Drug Saf 2021, 30, 1123–1131. [CrossRef]
- World Health Organization. The Evolving Threat of Antimicrobial Resistance : Options for Action. In The evolving threat of antimicrobial resistance : Options for action; 2012.
- English | MedDRA. Available online: https://www.meddra.org/how-to-use/support-documentation/english/welcome (accessed on 14 July 2023).
- Uppsala monitoring Centre. The WHO Programme for International Drug Monitoring. Available online: https://who-umc.org/about-the-who-programme-for-international-drug-monitoring/ (accessed on 14 July 2023).
- Jasovský, D.; Aagaard, H.; Zorzet, A. Uppsala Reports / Uppsala Monitoring Centre. January 2017, 74, pp. 8–10.
- Ruíz-Garzón, J.A.; Calderón-Ospina, C.A. Consideraciones acerca del reporte y la evaluación del fallo terapéutico en farmacovigilancia. Rev. Fac. Med. 2019, 67, 475–480. [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic Issues for Antibiotics in the Critically Ill Patient. Crit Care Med 2009, 37, 840–851; quiz 859. [CrossRef]
- Gallelli, L.; Palleria, C.; De Vuono, A.; Mumoli, L.; Vasapollo, P.; Piro, B.; Russo, E. Safety and Efficacy of Generic Drugs with Respect to Brand Formulation. J Pharmacol Pharmacother 2013, 4, S110-114. [CrossRef]
- Johnston, A.; Holt, D.W. Substandard Drugs: A Potential Crisis for Public Health. Br J Clin Pharmacol 2014, 78, 218–243. [CrossRef]
- World Health Organization. 1 in 10 Medical Products in Developing Countries Is Substandard or Falsified. Available online: https://www.who.int/news/item/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified (accessed on 28 February 2024).
- Roberts, J.A.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic Resistance--What’s Dosing Got to Do with It? Crit Care Med 2008, 36, 2433–2440. [CrossRef]
- Martins, T.S. de S.; Figueras, A.; Souza, L. dos R. de; Santos, K.C.O. dos; Oliveira, E.M. de; Secoli, S.R. Nonadherence to Treatment Recommendations Is a Factor Contributing to the Clinical Failure of Daptomycin: Cohort Study in Brazil. Braz. J. Pharm. Sci. (Online) 2020, 56, e17184–e17184. [CrossRef]
- Mvalo, T.; Smith, A.G.; Eckerle, M.; Hosseinipour, M.C.; Kondowe, D.; Vaidya, D.; Liu, Y.; Corbett, K.; Nansongole, D.; Mtimaukanena, T.A.; et al. Antibiotic Treatment Failure in Children Aged 1 to 59 Months with World Health Organization-Defined Severe Pneumonia in Malawi: A CPAP IMPACT Trial Secondary Analysis. PLoS ONE 2022, 17. [CrossRef]
- Ferreira, J.; Placido, A.I.; Afreixo, V.; Ribeiro-Vaz, I.; Roque, F.; Herdeiro, M.T. Descriptive Analysis of Adverse Drug Reactions Reports of the Most Consumed Antibiotics in Portugal, Prescribed for Upper Airway Infections. Antibiotics (Basel) 2022, 11, 477. [CrossRef]
- Soukavong, M.; Kim, J.; Park, K.; Yang, B.R.; Lee, J.; Jin, X.M.; Park, B.J. Signal Detection of Adverse Drug Reaction of Amoxicillin Using the Korea Adverse Event Reporting System Database. J Korean Med Sci 2016, 31, 1355–1361. [CrossRef]
- Barbosa, L.H.L.A.; Silva, A.R.O.; Carvalho-Assef, A.P.D.; Lima, E.C.; da Silva, F.A.B. Potential Safety Signals for Antibacterial Agents from the Brazilian National Pharmacovigilance Database (Vigimed/VigiFlow). Front Pharmacol 2022, 13, 948339. [CrossRef]
- Mhaidat, N.M.; Al-Azzam, S.; Banat, H.A.; Jaber, J.M.; Araydah, M.; Alshogran, O.Y.; Aldeyab, M.A. Reporting Antimicrobial-Related Adverse Drug Events in Jordan: An Analysis from the VigiBase Database. Antibiotics (Basel) 2023, 12. [CrossRef]
- Sharma, M.; Baghel, R.; Thakur, S.; Adwal, S. Surveillance of Adverse Drug Reactions at an Adverse Drug Reaction Monitoring Centre in Central India: A 7-Year Surveillance Study. BMJ Open 2021, 11, e052737. [CrossRef]
- Kadoyama, K.; Sakaeda, T.; Tamon, A.; Okuno, Y. Adverse Event Profile of Tigecycline: Data Mining of the Public Version of the U.S. Food and Drug Administration Adverse Event Reporting System. Biol Pharm Bull 2012, 35, 967–970.
- Saleem, Z.; Ahsan, U.; Haseeb, A.; Altaf, U.; Batool, N.; Rani, H.; Jaffer, J.; Shahid, F.; Hussain, M.; Amir, A.; et al. Antibiotic Utilization Patterns for Different Wound Types among Surgical Patients: Findings and Implications. Antibiotics (Basel) 2023, 12. [CrossRef]
- Saleem, Z.; Godman, B.; Cook, A.; Khan, M.A.; Campbell, S.M.; Seaton, R.A.; Siachalinga, L.; Haseeb, A.; Amir, A.; Kurdi, A.; et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics (Basel) 2022, 11, 1824. [CrossRef]
- Kamara, I.F.; Kanu, J.; Maruta, A.; Fofanah, B.D.; Kamara, K.N.; Sheriff, B.; Katawera, V.; D’Almeida, S.A.; Musoke, R.; Nuwagira, I.; et al. Antibiotic Use among Hospitalised Patients in Sierra Leone: A National Point Prevalence Survey Using the WHO Survey Methodology. BMJ Open 2023, 13, e078367. [CrossRef]
- Balon, M.; Tessier, S.; Damase-Michel, C.; Cottin, J.; Lambert, A.; Thompson, M.-A.; Benevent, J.; Lacroix, I. Adverse Drug Reactions in Pregnant Women: Do They Differ from Those in Non-Pregnant Women of Childbearing Age? Therapie 2023, 78, 165–173. [CrossRef]
- Kuzmina, A.V.; Asetskaya, I.L.; Zyryanov, S.K.; Polivanov, V.A. Detecting Medication Errors Associated with the Use of Beta-Lactams in the Russian Pharmacovigilance Database. BMC Pharmacol Toxicol 2021, 22, 5. [CrossRef]
- Ren, X.; Liu, D.; Ding, N.; Huang, K.; Xiong, Y.; Du, G.; Zeng, F. Safety Evaluation of Cephalosporins Based on Utilization and Adverse Drug Events: Analysis of Two Databases in China. Expert Opin Drug Saf 2012, 11, 689–697. [CrossRef]
- Shalviri, G.; Yousefian, S.; Gholami, K. Adverse Events Induced by Ceftriaxone: A Ten Years Review of Reported Cases to Iranian Pharmacovigilance Centre. Pharmacoepidemiol. Drug Saf. 2010, 19, S136–S137. [CrossRef]
- Salvo, F.; Polimeni, G.; Moretti, U.; Conforti, A.; Leone, R.; Leoni, O.; Motola, D.; Dusi, G.; Caputi, A.P. Adverse Drug Reactions Related to Amoxicillin Alone and in Association with Clavulanic Acid: Data from Spontaneous Reporting in Italy. J Antimicrob Chemother 2007, 60, 121–126.
- Thompson, G.; Barker, C.I.; Folgori, L.; Bielicki, J.A.; Bradley, J.S.; Lutsar, I.; Sharland, M. Global Shortage of Neonatal and Paediatric Antibiotic Trials: Rapid Review. BMJ Open 2017, 7, e016293. [CrossRef]
- Garazzino, S.; Lutsar, I.; Bertaina, C.; Tovo, P.-A.; Sharland, M. New Antibiotics for Paediatric Use: A Review of a Decade of Regulatory Trials Submitted to the European Medicines Agency from 2000--Why Aren’t We Doing Better? Int J Antimicrob Agents 2013, 42, 99–118. [CrossRef]
- Thomas, S.H.L.; Yates, L.M. Prescribing without Evidence - Pregnancy. Br J Clin Pharmacol 2012, 74, 691–697. [CrossRef]
- Li, Y.; Wu, Y.; Jiang, T.; Xing, H.; Xu, J.; Li, C.; Ni, R.; Zhang, N.; Xiang, G.; Li, L.; et al. Opportunities and Challenges of Pharmacovigilance in Special Populations: A Narrative Review of the Literature. Ther Adv Drug Saf 2023, 14, 20420986231200746. [CrossRef]
- Wallerstedt, S.M.; Brunlöf, G.; Sundström, A. Rates of Spontaneous Reports of Adverse Drug Reactions for Drugs Reported in Children. Drug-Safety 2011, 34, 669–682. [CrossRef]
- Jaberi, E.; Boussaha, I.; Dode, X.; Grenet, G.; Kassai, B.; Nguyen, K.A. Unlicensed/Off-Label Drug Prescriptions at Hospital Discharge in Children: An Observational Study Using Routinely Collected Health Data. Healthcare (Basel) 2024, 12, 208. [CrossRef]
- Ufer, M.; Kimland, E.; Bergman, U. Adverse Drug Reactions and Off-Label Prescribing for Paediatric Outpatients: A One-Year Survey of Spontaneous Reports in Sweden. Pharmacoepidemiol Drug Saf 2004, 13, 147–152. [CrossRef]
- Dubrall, D.; Leitzen, S.; Toni, I.; Stingl, J.; Schulz, M.; Schmid, M.; Neubert, A.; Sachs, B. Descriptive Analysis of Adverse Drug Reaction Reports in Children and Adolescents from Germany: Frequently Reported Reactions and Suspected Drugs. BMC Pharmacology and Toxicology 2021, 22, 56. [CrossRef]
- Lima, E. da C.; Matos, G.C. de; Vieira, J.M. de L.; Gonçalves, I.C. da C.R.; Cabral, L.M.; Turner, M.A. Suspected Adverse Drug Reactions Reported for Brazilian Children: Cross-Sectional Study. J Pediatr (Rio J) 2019, 95, 682–688. [CrossRef]
- Jean, S.-S.; Liu, I.-M.; Hsieh, P.-C.; Kuo, D.-H.; Liu, Y.-L.; Hsueh, P.-R. Off-Label Use versus Formal Recommendations of Conventional and Novel Antibiotics for the Treatment of Infections Caused by Multidrug-Resistant Bacteria. International Journal of Antimicrobial Agents 2023, 61, 106763. [CrossRef]
- Shenkutie, A.M.; Zhang, J.; Yao, M.; Asrat, D.; Chow, F.W.N.; Leung, P.H.M. Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter Baumannii Sequence Type 1894. Int J Mol Sci 2022, 23, 12705. [CrossRef]
- Ismail, B.; Shafei, M.N.; Harun, A.; Ali, S.; Omar, M.; Deris, Z.Z. Predictors of Polymyxin B Treatment Failure in Gram-Negative Healthcare-Associated Infections among Critically Ill Patients. J. Microbiol. Immunol. Infect. 2018, 51, 763–769. [CrossRef]
- Vikan, M.; Haugen, A.S.; Bjørnnes, A.K.; Valeberg, B.T.; Deilkås, E.C.T.; Danielsen, S.O. The Association between Patient Safety Culture and Adverse Events - a Scoping Review. BMC Health Serv Res 2023, 23, 300. [CrossRef]
- Conteh, T.A.; Thomas, F.; Abiri, O.T.; Komeh, J.P.; Kanu, A.; Kanu, J.S.; Fofanah, B.D.; Thekkur, P.; Zachariah, R. Quality of Reporting of Adverse Drug Reactions to Antimicrobials Improved Following Operational Research: A before-and-after Study in Sierra Leone (2017-2023). Trop Med Infect Dis 2023, 8. [CrossRef]
- Zabala, G.A.; Bellingham, K.; Vidhamaly, V.; Boupha, P.; Boutsamay, K.; Newton, P.N.; Caillet, C. Substandard and Falsified Antibiotics: Neglected Drivers of Antimicrobial Resistance? BMJ Glob Health 2022, 7, e008587. [CrossRef]
- Kyriacos, S.; Mroueh, M.; Chahine, R.P.; Khouzam, O. Quality of Amoxicillin Formulations in Some Arab Countries. J Clin Pharm Ther 2008, 33, 375–379. [CrossRef]
- Dittrich, A.T.M.; Smeets, N.J.L.; de Jong, E.F.M.; Kämink, J.L.; Kroeze, Y.; Draaisma, J.M.T.; van Puijenbroek, E.P.; te Loo, D.M.W.M. Quality of Active versus Spontaneous Reporting of Adverse Drug Reactions in Pediatric Patients: Relevance for Pharmacovigilance and Knowledge in Pediatric Medical Care. Pharmaceuticals 2022, 15, 1148. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).