Submitted:
29 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
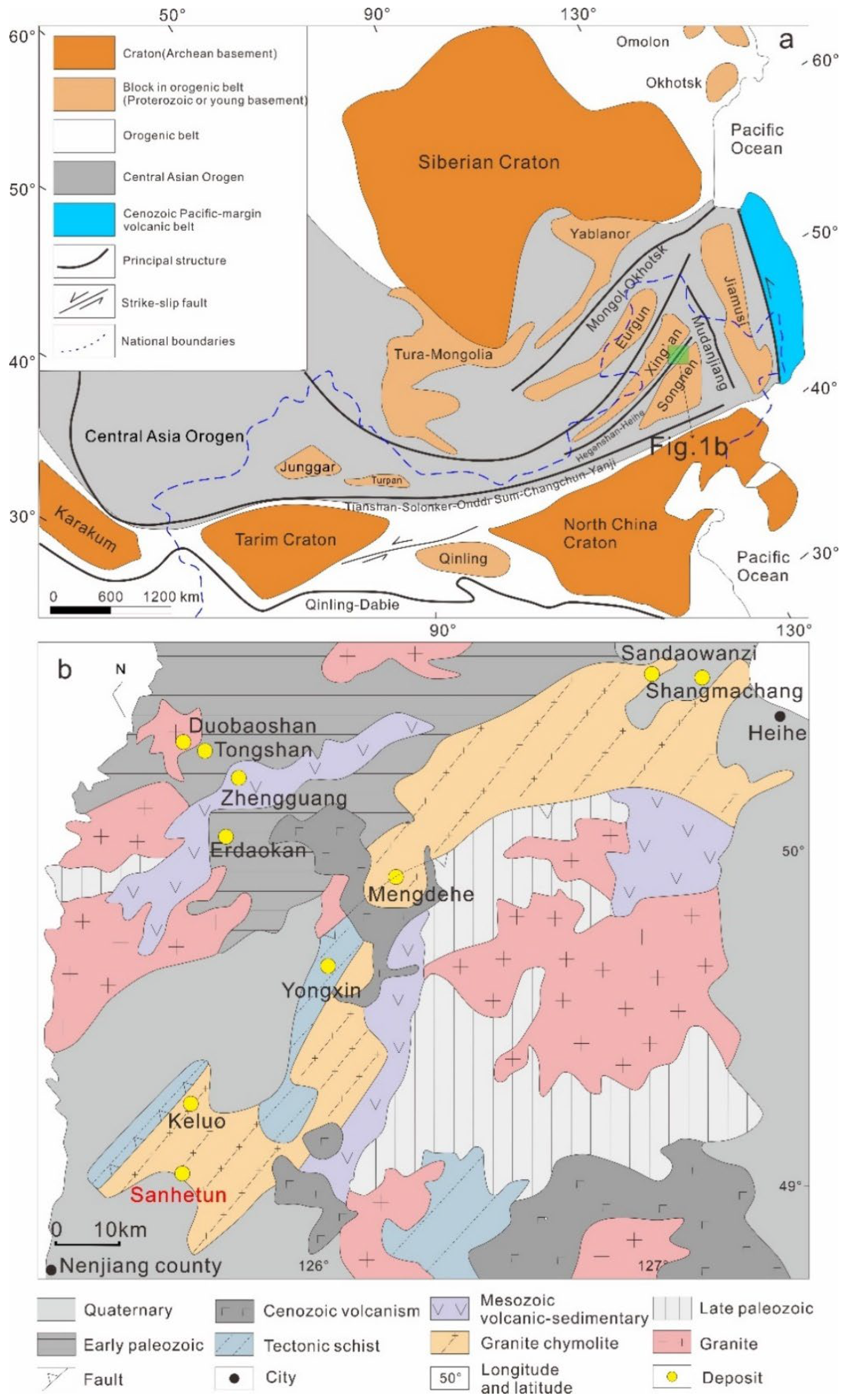
2. Geological Settting
2.1. Reginal Geology
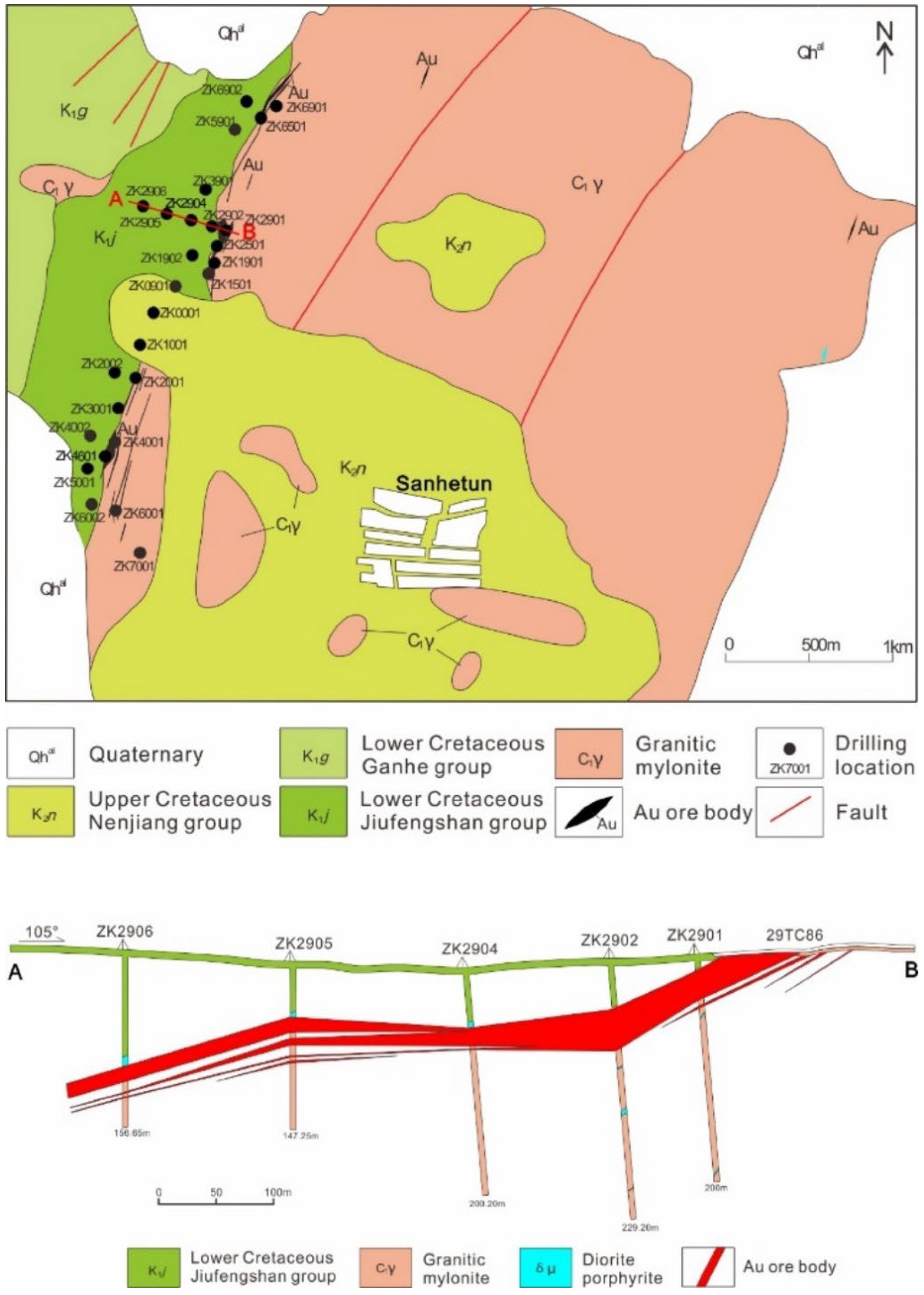
2.2. Deposit Geology
3. Materials and Methods
3.1. TIMA Analyses
3.2. SEM Analyses
3.3. EPMA Analyses
3.4. LA-ICP-MS Analyses
3.5. In Situ Sulfur Isotope Analyses
3.6. Calcite U-Pb Isotope Analyses
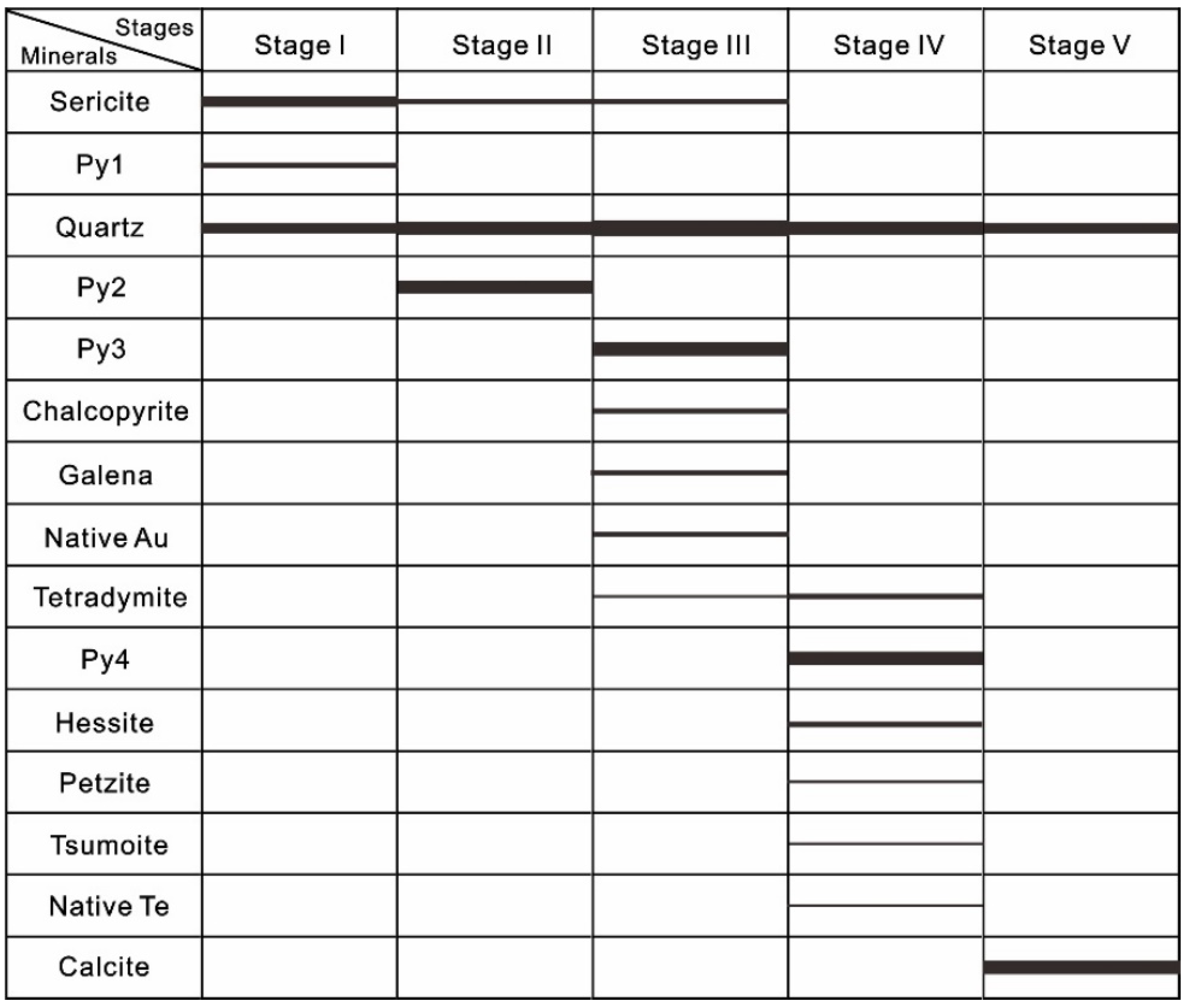
4. Results
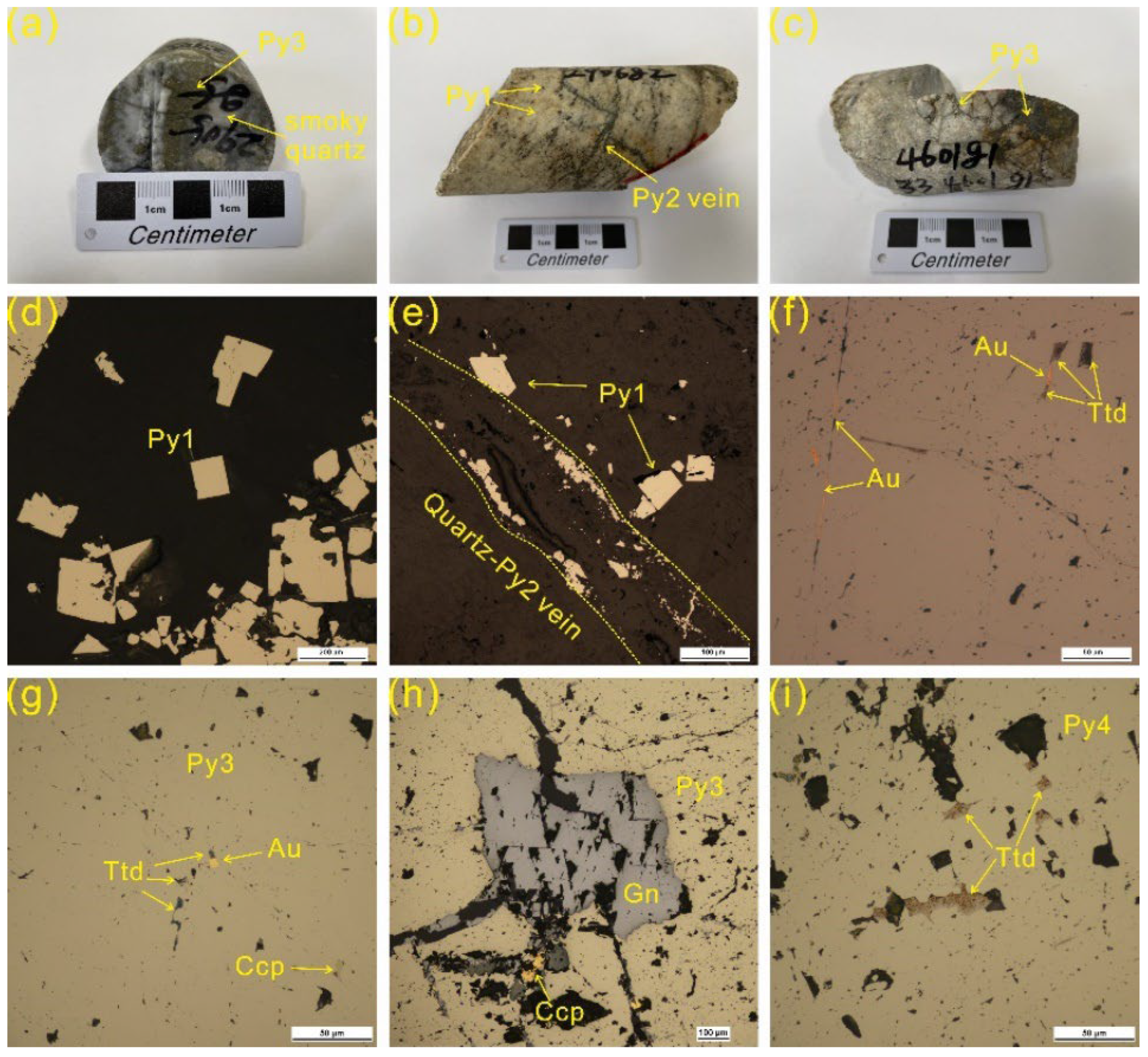
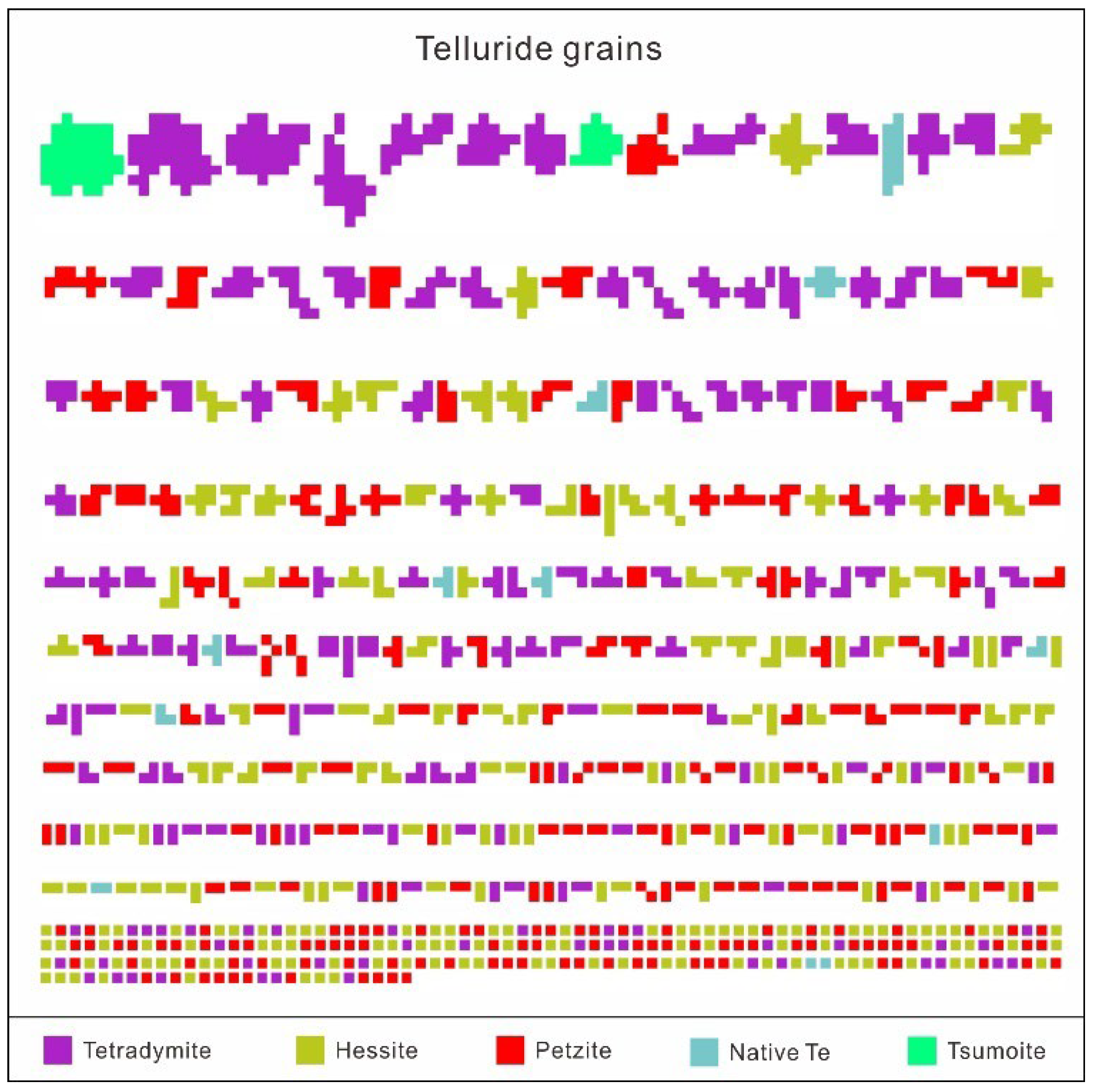
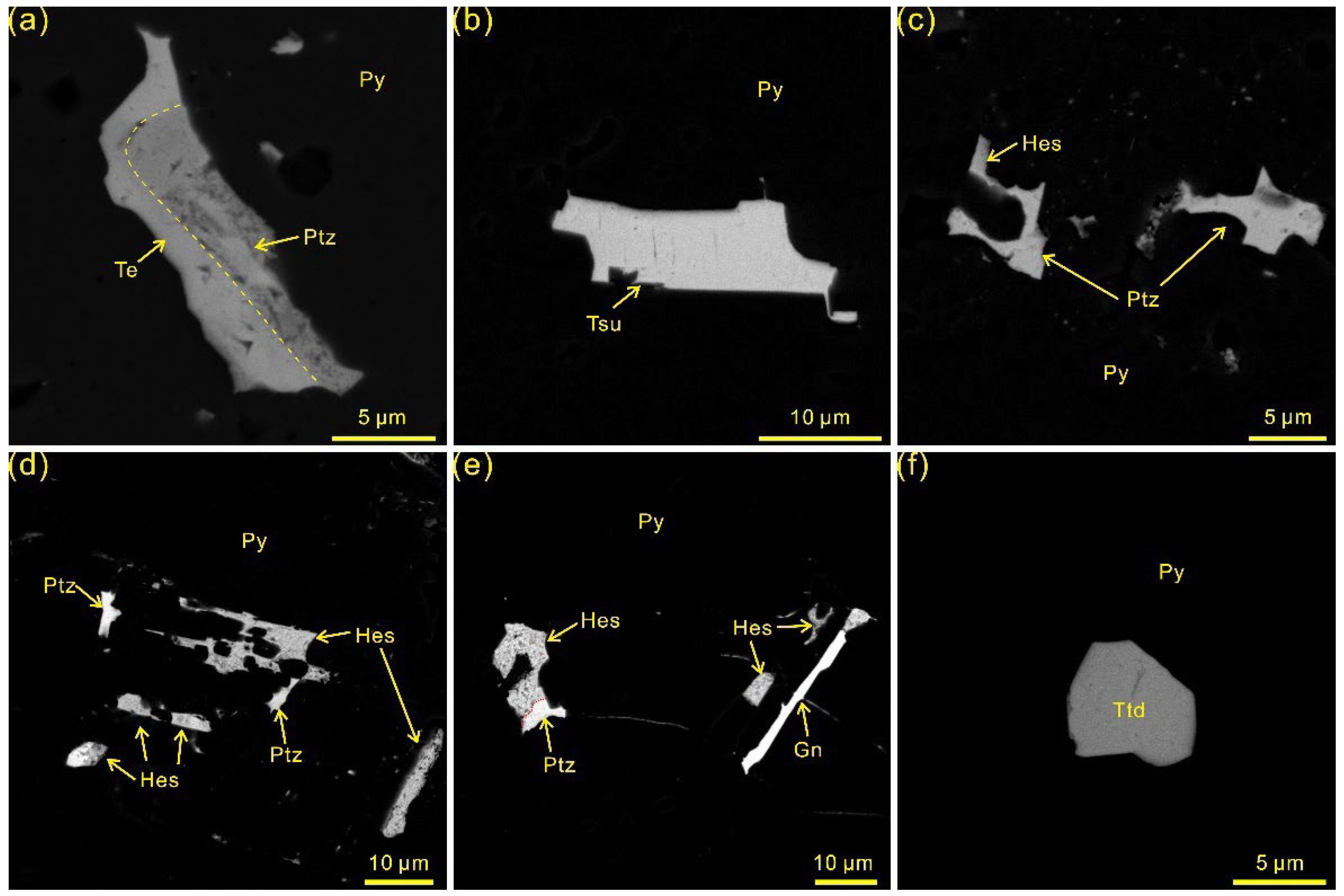
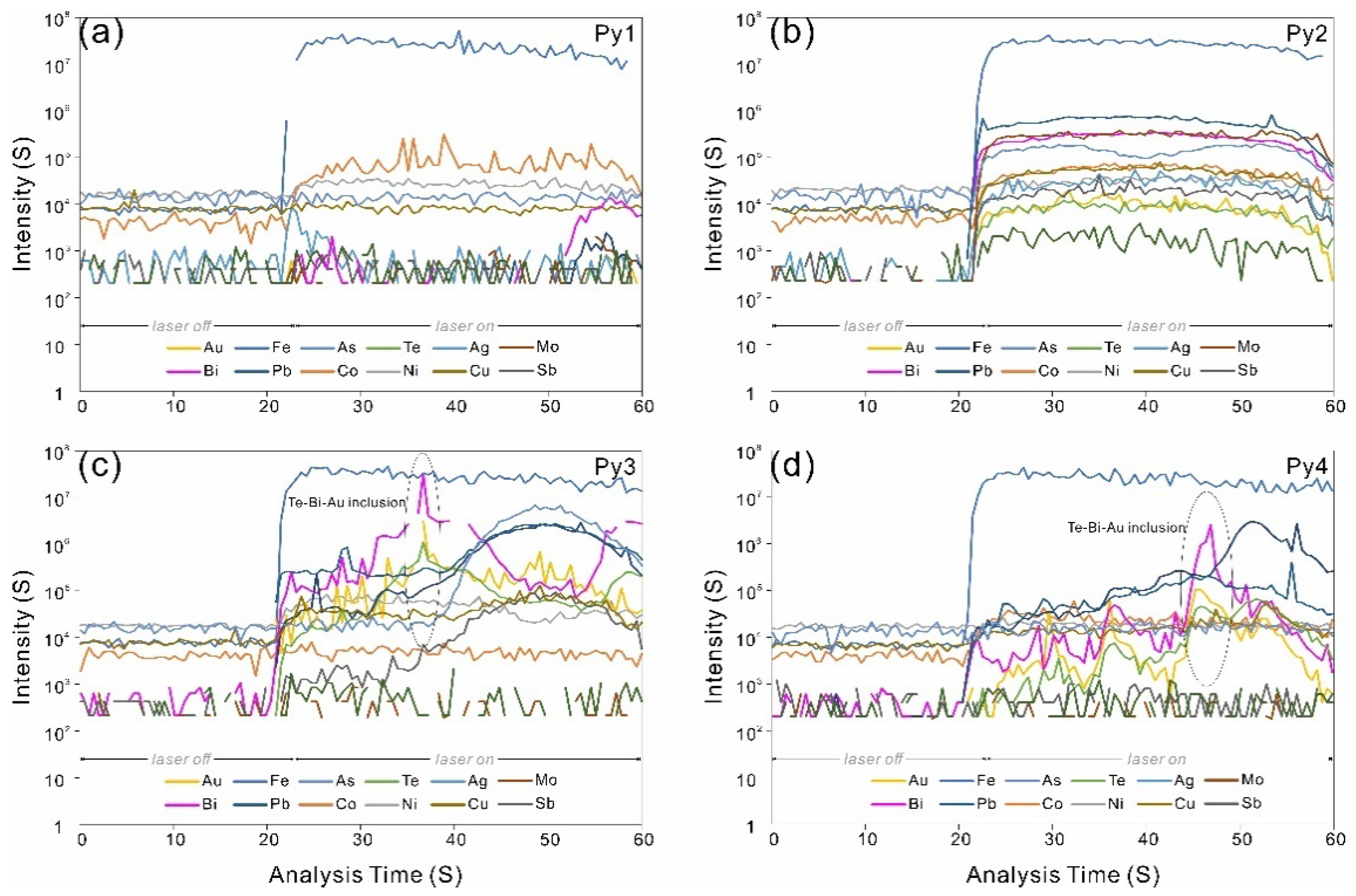
4.1. Telluride Mineralogy
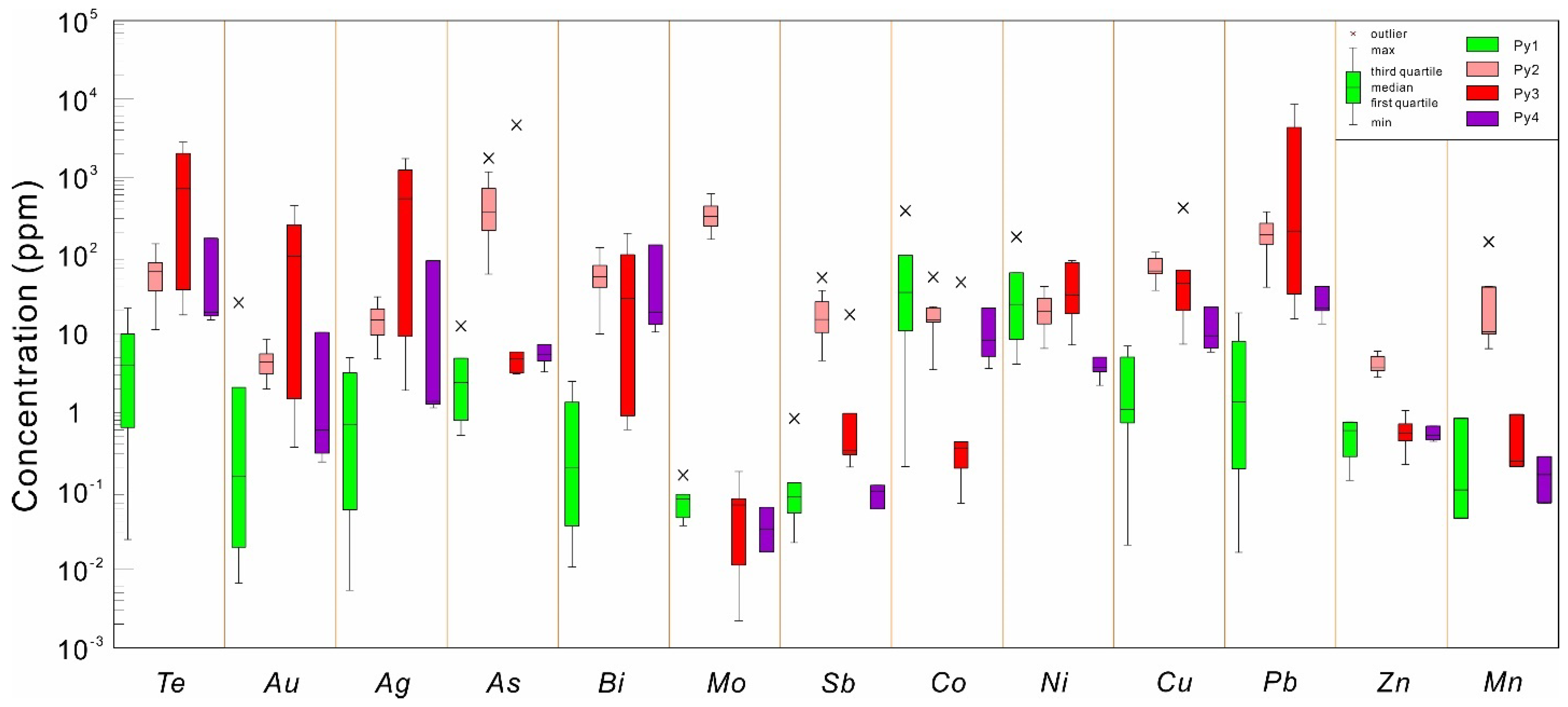
4.2. Pyrite Major and Trace Element Chemistry
4.3. In Situ Sulfur Isotopic Composition
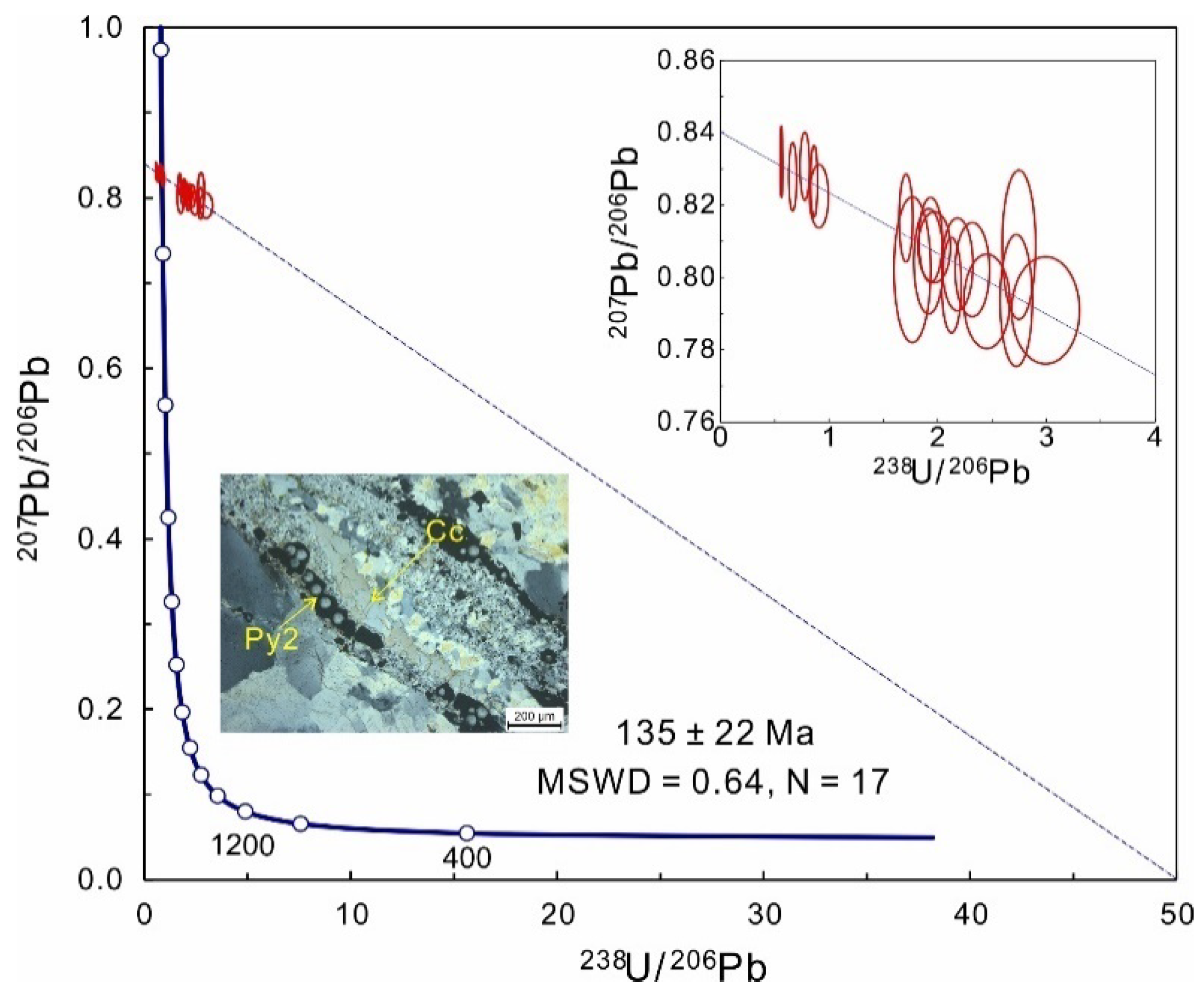
4.4. Calcite U-Pb Age
5. Discussion
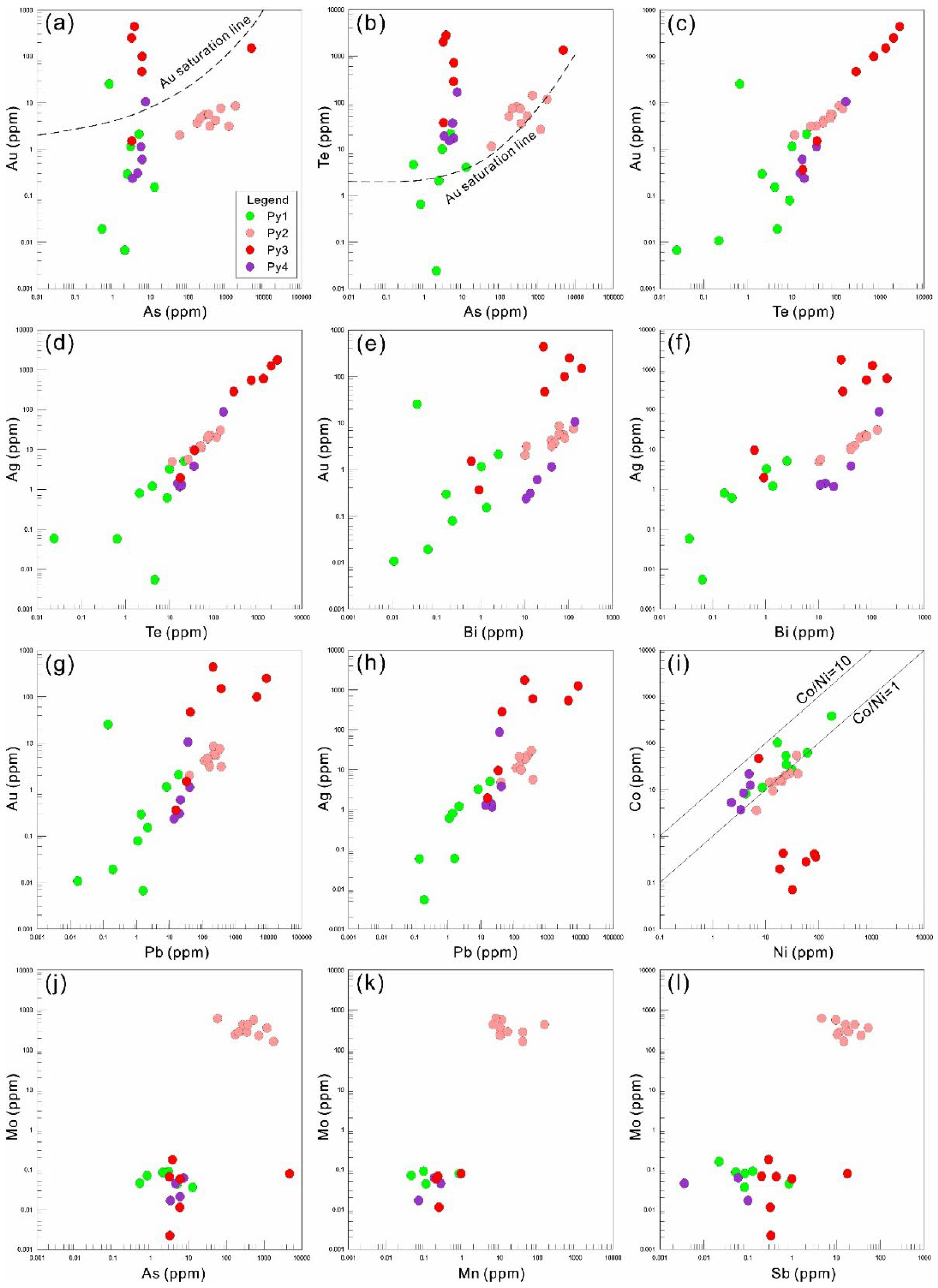
5.1. Trace Element Distribution in Pyrite
5.2. Genesis of Pyrites
5.3. Enrichment Mechanism of Au
5.4. Timing of Te-Au Mineralization
6. Conclusions
- (1)
- Five species of telluride were recognized in the Sanhetun Te-Au deposit, including native Te, tetradymite, tsumoite, hessite and petzite. Tetradymite commonly have close relationship with native gold.
- (2)
- Tellurium occurs as solid solution hosted in the lattice of Py1 and Py2 and Te-Bi-Au submicroscopic inclusions concealing in Py3 and Py4. Gold is mainly present as visible gold and subordinately as invisible gold which occurs as Te-Bi-Au submicroscopic inclusions.
- (3)
- Au enrichment of the Sanhetun Te-Au deposit might be attributed to the existence of Te-Bi-S melts, which can act as an important gold scavenger in the Au-undersaturated ore-forming fluids.
- (4)
- LA-ICP-MS U-Pb dating of calcite from the ore stage revealed the Te-Au mineralization at Sanhetun occurred at 135 Ma during the Early Cretaceous. The S isotopes data suggest a deep source for the metals. Our geochronological and geochemical data suggest that the formation of Sanhetun Te-Au deposit might be related to the subduction of Paleo Pacific plate in Early Cretaceous.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, R.; Xue, C.; Lü, X.; Zhao, X.; Yang, Y.; Li, C. Genesis of the Zhengguang gold deposit in the Duobaoshan ore field, Heilongjiang Province, NE China: Constraints from geology, geochronology and S-Pb isotopic compositions. Ore Geol. Rev. 2017, 84, 202–217. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhou, Z.; OuYang, H. The Ordovician igneous rocks with high Sr/Y at the Tongshan porphyry copper deposit, satellite of the Duobaoshan deposit, and their metallogenic role. Ore Geol. Rev. 2017, 86, 600–614. [Google Scholar] [CrossRef]
- Suo, Q.; Shen, P.; Li, C.; Feng, H.; Chu, X. Mineral chemistry and apatite Sr isotope signatures record overprinting mineralization in the Duobaoshan porphyry Cu deposit, Heilongjiang Province, NE China. Ore Geol. Rev. 2023, 162, 105718. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Cai, W.; Geng, J.; Xue, H.; Wang, X. Fluid evolution and genesis of the Sankuanggou Fe -Cu skarn deposit, Duobaoshan ore field, Northeast China: Evidence from fluid inclusions and H-O-S-Pb isotopes. J. Geochem. Explor. 2023, 252, 107251. [Google Scholar] [CrossRef]
- Yang, H.; Ma, W.L.; Cai, W.Y.; Wang, K.Y.; Sun, F.Y.; Wang, J.H.; Zhou, H.Y.; Kou, B.Y. Metallogenic epoch and tectonic setting of the Xiaoduobaoshan Fe-Cu deposit in Heilongjiang Province, China: Evidence from petrogeochemistry, zircon U-Pb geochronology and Hf isotopic compositions (in Chinese with English abstract). Acta Petrol. Sin. 2020, 36, 856–870. [Google Scholar]
- Zhai, D.G.; Williams-Jones, A.E.; Liu, J.J.; Tombros, S.F.; Cook, N.J. Mineralogical, Fluid Inclusion, and Multiple Isotope (H-O-S-Pb) Constraints on the Genesis of the Sandaowanzi Epithermal Au-Ag-Te Deposit, NE China. Econ. Geol. 2018, 113, 1359–1382. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Santosh, M.; Li, C.; Liu, H.; Yu, H.; Kamoto, M.; Du, B.; Liu, J. Tellurium and Gold enrichment aided by melts and pyrite crystallization kinetics: Insights from the Yongxin gold deposit, Northeast China. Ore Geol. Rev. 2023, 156, 105370. [Google Scholar] [CrossRef]
- Li, C.L.; Li, L.; Yuan, M.W.; Alam, M.; Li, S.R.; Santosh, M.; Deng, C.Z.; Liu, H.; Xu, G.Z. Study on pyrite thermoelectricity, ore-forming fluids and H-O-Rb-Sr isotopes of the Yongxin gold deposit, Central Asian Orogenic Belt: Implications for ore genesis and exploration. Ore Geol. Rev. 2020, 121, 103568. [Google Scholar] [CrossRef]
- Yuan, M.W.; Li, S.R.; Li, C.L.; Santosh, M.; Alam, M.; Zeng, Y.J. Geochemical and isotopic composition of auriferous pyrite from the Yongxin gold deposit, Central Asian Orogenic Belt: Implication for ore genesis. Ore Geol. Rev. 2018, 93, 255–267. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Li, S.; Yuan, M.; Alam, M.; Liu, B.; Zhao, Z.; Li, W.; Yang, Y. Geochronology and genesis of the newly discovered Mengdehe orogenic-type Au deposit in the Xing’an-Mongolia orogenic Belt, NE China. Ore Geol. Rev. 2021, 133, 104083. [Google Scholar] [CrossRef]
- Gao, S.; Xu, H.; Zang, Y.Q.; Wang, T. Mineralogy, ore-forming fluids and geochronology of the Shangmachang and Beidagou gold deposits, Heilongjiang province, NE China. J. Geochem. Explor. 2018, 188, 137–155. [Google Scholar] [CrossRef]
- McNulty, B.A.; Jowitt, S.M. Byproduct critical metal supply and demand and implications for the energy transition: A case study of tellurium supply and CdTe PV demand. Renew. Sust. Energ. Rev. 2022, 168. [Google Scholar] [CrossRef]
- Zweibel, K. The impact of Tellurium supply on Cadmium Telluride photovoltaics. Science 2010, 328, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Fornari, C.I.; Bentmann, H.; Morelhão, S.L.; Peixoto, T.R.F.; Rappl, P.H.O.; Tcakaev, A.; Zabolotnyy, V.; Kamp, M.; Lee, T.; Min, C.; Kagerer, P.; Vidal, R.C.; Isaeva, A.; Ruck, M.; Hinkov, V.; Reinert, F.; Abramof, E. Incorporation of Europium in Bi2Te3 topological insulator epitaxial films. The Journal of Physical Chemistry C 2020, 124, 16048–16057. [Google Scholar] [CrossRef]
- Steven, B.; William, M.; Jinxue, W.; Michael, J.; Farzin, A. Advances in HgCdTe APDs and LADAR receivers. Proc.Spie 2010, 7660. [Google Scholar]
- Gu, H.J.; Li, B.W.; Li, C.L.; Yang, W.P.; Yang, Y.J.; Shi, H.L.; Zhang, M.M. The Discovery and Significance of Tellurideinthe Sanhetun Gold Deposit inthe Northeastern Great Xing’an Range (in Chinese with English abstract). Mineral. Petrol. 2023, 43, 94–104. [Google Scholar]
- Liu, B.S.; Kou, L.L.; Zhang, C.P.; Li, C.L.; Luo, J.; Han, R.P.; Li, B.W. Research on the relationship between mineralization and zircon LA-ICP-MS U-Pb dating of the mylonite from the Sanhetun gold deposit in Nenjiang region, Heilongjiang Province (in Chinese with English abstract). Acta Geologica Sinica 2022, 96, 954–970. [Google Scholar]
- Zhou, J.; Wilde, S.A.; Zhao, G.; Han, J. Nature and assembly of microcontinental blocks within the Paleo-Asian Ocean. Earth-Sci. Rev. 2018, 186, 76–93. [Google Scholar] [CrossRef]
- Miao, L.C.; Fan, W.M.; Zhang, F.Q.; Liu, D.Y.; Jian, P.; Shi, G.H.; Tao, H.; Shi, Y.R. Zircon SHRIMP geochronology of the Xinkailing-Keluo complex in the northwestern Lesser Xing’an Range, and its geological implications. Chinese Science Bulletin 2003, 48, 2315–2323. [Google Scholar]
- Li, B.W.; Gu, H.J.; Li, C.L.; Liu, B.S.; Yang, X.P. Geological Characteristics and Prospecting Potential of Sanhetun Gold Deposit in Heilongjiang Province (in Chinese with English Abstract). Gold Science and Technology 2022, 30, 508–517. [Google Scholar]
- Wang, F.Y.; Ge, C.; Ning, S.Y.; Nie, L.Q.; Zhong, G.X.; White, N.C. A new approach to LA-ICP-MS mapping and application in geology (in Chinese with English abstract). Acta Petrologica Sinica 2017, 33, 3422–3436. [Google Scholar]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Miner. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Gregory, D.D.; Large, R.R.; Halpin, J.A.; Baturina, E.L.; Lyons, T.W.; Wu, S.; Danyushevsky, L.; Sack, P.J.; Chappaz, A.; Maslennikov, V.V.; Bull, S.W. Trace Element Content of Sedimentary Pyrite in Black Shales. Econ. Geol. 2015, 110, 1389–1410. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, J.; Chen, H.; Yang, L.; Cooke, D.; Danyushevsky, L.; Gong, Q. LA-ICP-MS trace element analysis of pyrite from the Chang’an gold deposit, Sanjiang region, China: Implication for ore-forming process. Gondwana Res. 2014, 26, 557–575. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C.; Kesler, S.E. Nanoscale “liquid” inclusions of As-Fe-S in arsenian pyrite. Am. Miner. 2009, 94, 391–394. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Miner. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits; insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Testemale, D.; Skinner, W.; Pring, A. Formation of As(II)-pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochim. Cosmochim. Acta 2013, 100, 1–10. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Hickey, K.A.; Cline, J.S.; Dipple, G.M.; Kilburn, M.R.; Vaughan, J.R.; Longo, A.A. Uncloaking invisible gold: use of nanoSIMS to evaluate gold, trace elements, and sulfur isotopes in pyrite from Carlin-type gold deposits. Econ. Geol. 2009, 104, 897–904. [Google Scholar] [CrossRef]
- Tanner, D.; Henley, R.W.; Mavrogenes, J.A.; Holden, P. Sulfur isotope and trace element systematics of zoned pyrite crystals from the El Indio Au–Cu–Ag deposit, Chile. Contrib. Mineral. Petrol. 2016, 171, 33. [Google Scholar] [CrossRef]
- Du, B.; Shen, J.; Santosh, M.; Liu, H.; Liu, J.; Wang, Y.; Xu, K. Textural, compositional and isotopic characteristics of pyrite from the Zaozigou gold deposit in West Qinling, China: Implications for gold metallogeny. Ore Geol. Rev. 2021, 130, 103917. [Google Scholar] [CrossRef]
- Li, C. Characteristics of Structural Superimposed Halo and Deep Prospecting Prediction of Yongxin Gold Deposit, Duobaoshan Area, Heilongjiang Province (in Chinese with English abstract). Geoscience 2023, 37, 674–689. [Google Scholar]
- Ohmoto, H. Systematics of Sulfur and Carbon Isotopes in Hydrothermal Ore Deposits. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Chaussidon, M.; Lorand, J. Sulphur isotope composition of orogenic spinel lherzolite massifs from Ariege (North-Eastern Pyrenees, France): An ion microprobe study. Geochim. Cosmochim. Acta 1990, 54, 2835–2846. [Google Scholar] [CrossRef]
- Cooke, D.R.; McPhail, D.C. Epithermal Au-Ag-Te Mineralization, Acupan, Baguio District, Philippines: Numerical Simulations of Mineral Deposition. Econ. Geol. 2001, 96, 109–131. [Google Scholar]
- Grundler, P.V.; Brugger, J.; Etschmann, B.E.; Helm, L.; Liu, W.; Spry, P.G.; Tian, Y.; Testemale, D.; Pring, A. Speciation of aqueous tellurium(IV) in hydrothermal solutions and vapors, and the role of oxidized tellurium species in Te transport and gold deposition. Geochim. Cosmochim. Acta 2013, 120, 298–325. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Birch, W.D.; Cook, N.J.; Pring, A.; Grundler, P.V. Petrogenetic significance of Au–Bi–Te–S associations: The example of Maldon, Central Victorian gold province, Australia. Lithos 2010, 116, 1–17. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Wagner, T.; Stanley, C.J. MINERALS OF THE SYSTEM Bi–Te–Se–S RELATED TO THE TETRADYMITE ARCHETYPE: REVIEW OF CLASSIFICATION AND COMPOSITIONAL VARIATION. The Canadian Mineralogist 2007, 45, 665–708. [Google Scholar] [CrossRef]
- Zhen, S.; Wang, D.; Yu, X.; Wang, Q.; Li, Y.; Zha, Z.; Wang, J. Trace Elements and Sulfur Isotopes of Sulfides in the Zhangquanzhuang Gold Deposit, Hebei Province, China: implications for physicochemical conditions and mineral deposition mechanisms. Minerals 2020, 10. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of Trace Element Zonation in Vent Chimneys from the Silurian Yaman-Kasy Volcanic-Hosted Massive Sulfide Deposit (Southern Urals, Russia) Using Laser Ablation-Inductively Coupled Plasma Mass Spectrometry (LA-ICPMS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Rye, R.O. A review of the stable-isotope geochemistry of sulfate minerals in selected igneous environments and related hydrothermal systems. Chem. Geol. 2005, 215, 5–36. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Mao, J.W. Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem. Geol. 2009, 264, 101–121. [Google Scholar] [CrossRef]
- Möller, P.; Kersten, G. Electrochemical accumulation of visible gold on pyrite and arsenopyrite surfaces. Miner. Depos. 1994, 29, 404–413. [Google Scholar] [CrossRef]
- Laird, J.S.; Halfpenny, A.; Ryan, C.G.; Liu, W. Evidence supporting micro-galvanic coupling in sulphides leads to gold deposition. Contrib. Mineral. Petrol. 2021, 176, 1–19. [Google Scholar] [CrossRef]
- Peterson, E.C.; Mavrogenes, J.A. Linking high-grade gold mineralization to earthquake-induced fault-valve processes in the Porgera gold deposit, Papua New Guinea. Geology 2014, 42, 383–386. [Google Scholar] [CrossRef]
- Douglas, N.; Mavrogenes, J.; Hack, A.; England, R. The liquid bismuth collector model: An alternative gold deposition mechanism, in Skilbeck, C.G., and Hubble, T.C.T., eds., Understanding planet Earth; searching for a sustainable future; on the starting blocks of the third millennium: Australian Geological Convention, 15th, Sydney, Abstracts: Sydney, Geological Society of Australia, 135 p. 2000. [Google Scholar]
- Tooth, B.; Brugger, J.; Ciobanu, C.; Liu, W. Modeling of gold scavenging by bismuth melts coexisting with hydrothermal fluids: An experimental study. Geology 2008, 36, 815–818. [Google Scholar] [CrossRef]
- Tooth, B.; Ciobanu, C.L.; Green, L.; O Neill, B.; Brugger, J. Bi-melt formation and gold scavenging from hydrothermal fluids: An experimental study. Geochim. Cosmochim. Acta 2011, 75, 5423–5443. [Google Scholar] [CrossRef]
- Jian, W.; Mao, J.W.; Lehmann, B.; Cook, N.J.; Xie, G.Q.; Liu, P.; Duan, C.; Alles, J.; Niu, Z.J. Au-Ag-Te-rich melt inclusions in hydrothermal gold-quartz veins, Xiaoqinling Lode Gold District, central China. Econ. Geol. 2021, 116, 1239–1248. [Google Scholar] [CrossRef]
- Jian, W.; Mao, J.; Lehmann, B.; Cook, N.J.; Li, J.; Song, S.; Zhu, L. Hyper-enrichment of gold via quartz fracturing and growth of polymetallic melt droplets. Geology 2024. [Google Scholar] [CrossRef]
- Frost, B.R.; Mavrogenes, J.A.; Tomkins, A.G. Partial melting of sulfide ore deposits during medium- and high-grade metamorphism. The Canadian Mineralogist 2002, 40, 1–18. [Google Scholar] [CrossRef]
- Cockerton, A.B.D.; Tomkins, A.G. Insights into the Liquid Bismuth Collector Model Through Analysis of the Bi-Au Stormont Skarn Prospect, Northwest Tasmania. Econ. Geol. 2012, 107, 667–682. [Google Scholar] [CrossRef]
- Oberthur, T.; Weiser, T.W. Gold-bismuth-telluride-sulphide assemblages at the Viceroy Mine, Harare-Bindura-Shamva greenstone belt, Zimbabwe. Mineral. Mag. 2008, 72, 953–970. [Google Scholar] [CrossRef]
- Pals, D.W.; Spry, P.G. Telluride mineralogy of the low-sulfidation epithermal Emperor gold deposit, Vatukoula, Fiji. Mineral. Petrol. 2003, 79, 285–307. [Google Scholar] [CrossRef]
- Bi, S.J.; Li, J.W.; Zhou, M.F.; Li, Z.K. Gold distribution in As-deficient pyrite and telluride mineralogy of the Yangzhaiyu gold deposit, Xiaoqinling district, southern North China craton. Miner. Depos. 2011, 46, 925–941. [Google Scholar] [CrossRef]
- Okamoto, K.; Tanner, L.E. Bi-Te (bismuth-tellurium). In: Massalski, T.B., Ohamoto, K. (Eds.), Binary Alloy Phase Diagrams. Ohio, ASM International, Materials Park, Ohio. 1990, 800-801.
- Deng, J.; Qiu, K.F.; Wang, Q.F.; Goldfarb, R.; Yang, L.Q.; Zi, J.W.; Geng, J.Z.; Ma, Y. In situ dating of hydrothermal monazite and implications for the geodynamic controls on ore formation in the Jiaodong gold province, eastern China. Econ. Geol. 2020, 115, 671–685. [Google Scholar] [CrossRef]
- Zhai, D.G.; Liu, J.J.; Zhang, A.L.; Sun, Y.Q. U-Pb, Re-Os, and 40Ar-39Ar geochronology of porphyry Sn ± Cu ± Mo and polymetallic (Ag-Pb-Zn-Cu) vein mineralization at Bianjiadayuan, Inner Mongolia, Northeast China: Implications for discrete mineralization events. Econ. Geol. 2017, 112, 2041–2059. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Lai, J.; He, H.; Song, X.; Zhai, D.; Li, B.; Wang, Y.; Shi, J.; Zhou, X. In Situ U-Pb Geochronology of Calcite from the World’s Largest Antimony Deposit at Xikuangshan, Southern China. Minerals 2022, 12. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhu, D.C.; Yang, Y.H.; Wang, Q.; Xie, J.C.; Zhao, Z.D. U-Pb Geochronology of Carbonate by Laser Ablation MC-ICP-MS: Method Improvements and Geological Applications. Atom. Spectrosc. 2021, 42, 335–348. [Google Scholar]
- Wu, F.; Lin, J.; Wilde, S.A.; Zhang, X.; Yang, J. Nature and significance of the Early Cretaceous giant igneous event in eastern China. Earth Planet. Sci. Lett. 2005, 233, 103–119. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halliday, A.N. Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of tellurium. Geochim. Cosmochim. Acta 2003, 67, 1117–1127. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Kuhn, T. Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 2020, 1, 158–169. [Google Scholar] [CrossRef]


| Stage | Sample no. | S | Fe | Au | Ag | Te | Se | As | Cu | Pb | Zn | Co | Ni | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Py1 | zk2904g7-1-1 | 52.642 | 46.026 | 0 | 0.011 | 0.043 | 0 | 0.006 | 0 | 0.197 | 0.133 | 0.088 | 0.01 | 99.156 |
| Py1 | zk2904g7-1-2 | 52.869 | 46.347 | 0 | 0.013 | 0 | 0 | 0 | 0 | 0.067 | 0.041 | 0.04 | 0 | 99.377 |
| Py1 | zk2904g7-1-3 | 52.967 | 46.998 | 0 | 0.009 | 0.003 | 0.023 | 0 | 0.017 | 0 | 0.013 | 0.053 | 0 | 100.08 |
| Py1 | zk2904g7-1-4 | 53.01 | 46.579 | 0 | 0.028 | 0 | 0.012 | 0.026 | 0.005 | 0.132 | 0.003 | 0.041 | 0.001 | 99.837 |
| Py1 | zk2904g7-1-5 | 53.032 | 46.623 | 0 | 0.007 | 0.003 | 0.021 | 0.029 | 0 | 0.024 | 0.117 | 0.024 | 0 | 99.88 |
| Py1 | zk2904g7-1-6 | 53.058 | 46.785 | 0.003 | 0.061 | 0 | 0 | 0.022 | 0.005 | 0.014 | 0 | 0.038 | 0 | 99.986 |
| Py1 | zk2904g7-1-7 | 53.146 | 47.022 | 0 | 0 | 0.005 | 0 | 0 | 0 | 0.073 | 0 | 0.059 | 0 | 100.31 |
| Py1 | zk2904g7-1-8 | 53.171 | 46.568 | 0 | 0 | 0 | 0 | 0 | 0.019 | 0.01 | 0.08 | 0.036 | 0 | 99.884 |
| Py1 | zk2904g7-1-9 | 53.215 | 46.814 | 0 | 0 | 0 | 0 | 0 | 0.024 | 0.041 | 0 | 0.01 | 0 | 100.1 |
| Py1 | zk1001g2-1-1 | 53.245 | 46.707 | 0 | 0.004 | 0 | 0 | 0 | 0 | 0.013 | 0.039 | 0 | 0 | 100.01 |
| Py1 | zk1001g2-1-2 | 53.331 | 46.597 | 0 | 0.024 | 0.002 | 0 | 0.01 | 0.041 | 0 | 0 | 0.045 | 0 | 100.05 |
| Py1 | zk1001g2-1-3 | 53.345 | 46.582 | 0.01 | 0.02 | 0.032 | 0.005 | 0 | 0.004 | 0 | 0 | 0.069 | 0 | 100.07 |
| Py1 | zk1001g2-1-4 | 53.35 | 46.893 | 0 | 0.039 | 0 | 0 | 0 | 0.032 | 0.032 | 0 | 0.062 | 0.002 | 100.41 |
| Py1 | zk1001g2-1-5 | 53.357 | 46.86 | 0.019 | 0.022 | 0.067 | 0 | 0 | 0.023 | 0 | 0 | 0.06 | 0 | 100.41 |
| Py1 | zk1001g2-1-6 | 53.392 | 46.481 | 0 | 0.022 | 0 | 0.017 | 0 | 0 | 0.065 | 0.022 | 0.005 | 0 | 100 |
| Py1 | zk1001g2-1-7 | 53.409 | 46.186 | 0 | 0 | 0.099 | 0 | 0 | 0 | 0 | 0.048 | 0.053 | 0 | 99.795 |
| Py1 | zk1001g2-1-8 | 53.419 | 47.138 | 0 | 0 | 0.01 | 0.061 | 0 | 0.017 | 0.07 | 0.039 | 0.021 | 0 | 100.78 |
| Py1 | zk1001g2-1-9 | 53.432 | 46.819 | 0 | 0.041 | 0 | 0.019 | 0.029 | 0.004 | 0.013 | 0.009 | 0.029 | 0 | 100.4 |
| Py1 | zk1001g2-1-10 | 53.446 | 46.346 | 0 | 0 | 0.058 | 0.075 | 0.023 | 0 | 0.03 | 0 | 0.026 | 0.013 | 100.02 |
| Py2 | zk2905g5-1-1 | 53.455 | 46.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.084 | 0.024 | 0 | 99.893 |
| Py2 | zk2905g5-1-2 | 53.468 | 46.577 | 0 | 0 | 0 | 0 | 0.004 | 0.064 | 0 | 0.034 | 0.024 | 0.083 | 100.25 |
| Py2 | zk2905g5-1-3 | 53.476 | 46.996 | 0.013 | 0 | 0 | 0 | 0 | 0.037 | 0.084 | 0 | 0.045 | 0.031 | 100.68 |
| Py2 | zk2905g5-1-4 | 53.484 | 46.682 | 0 | 0.015 | 0.017 | 0 | 0 | 0 | 0.005 | 0.019 | 0.053 | 0 | 100.28 |
| Py2 | zk2905g5-1-5 | 53.484 | 47.057 | 0.003 | 0.065 | 0 | 0 | 0 | 0.021 | 0.024 | 0 | 0.043 | 0 | 100.7 |
| Py2 | zk2905g5-1-6 | 53.492 | 46.626 | 0 | 0.004 | 0 | 0 | 0 | 0 | 0.064 | 0 | 0.01 | 0 | 100.2 |
| Py2 | zk2905g5-1-7 | 53.51 | 46.529 | 0 | 0 | 0.047 | 0.004 | 0 | 0.002 | 0 | 0.026 | 0 | 0 | 100.12 |
| Py2 | zk2905g5-1-8 | 53.564 | 46.848 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0.138 | 0.058 | 0.052 | 0 | 100.71 |
| Py2 | zk2905g5-1-9 | 53.593 | 46.812 | 0 | 0.002 | 0 | 0 | 0 | 0.033 | 0.037 | 0 | 0.014 | 0 | 100.49 |
| Py2 | zk2905g5-1-10 | 53.596 | 46.729 | 0 | 0.002 | 0.035 | 0 | 0 | 0 | 0 | 0.037 | 0.01 | 0 | 100.41 |
| Py2 | zk2905g5-1-11 | 53.647 | 46.742 | 0 | 0 | 0 | 0.004 | 0.019 | 0.026 | 0.035 | 0 | 0.04 | 0 | 100.51 |
| Py2 | zk2905g5-1-12 | 53.651 | 46.756 | 0.019 | 0 | 0 | 0 | 0 | 0 | 0.019 | 0 | 0 | 0.036 | 100.48 |
| Py2 | zk2905g5-1-13 | 53.667 | 46.916 | 0.016 | 0 | 0.015 | 0 | 0 | 0.041 | 0.013 | 0.029 | 0.028 | 0 | 100.73 |
| Py2 | zk2905g5-1-14 | 53.685 | 47.027 | 0 | 0.013 | 0 | 0 | 0.035 | 0 | 0.027 | 0 | 0.083 | 0 | 100.87 |
| Py2 | zk2905g5-1-15 | 53.7 | 47.138 | 0 | 0 | 0 | 0 | 0.003 | 0.038 | 0 | 0 | 0.021 | 0 | 100.9 |
| Py2 | zk2905g5-1-16 | 53.706 | 46.683 | 0.003 | 0 | 0.084 | 0.032 | 0 | 0 | 0.056 | 0 | 0.031 | 0 | 100.6 |
| Py2 | zk2905g5-1-17 | 53.71 | 46.408 | 0 | 0 | 0 | 0.05 | 0.004 | 0 | 0.021 | 0.061 | 0.045 | 0.008 | 100.31 |
| Py2 | zk2905g5-1-18 | 53.724 | 46.985 | 0 | 0.033 | 0.018 | 0 | 0 | 0.013 | 0.072 | 0 | 0 | 0.012 | 100.86 |
| Py2 | zk2905g5-1-19 | 53.742 | 46.905 | 0 | 0.024 | 0 | 0 | 0 | 0 | 0.032 | 0.042 | 0 | 0 | 100.75 |
| Py2 | zk2905g5-1-20 | 53.76 | 46.952 | 0 | 0 | 0.028 | 0 | 0 | 0 | 0.086 | 0.009 | 0 | 0 | 100.84 |
| Py2 | zk2905g5-1-21 | 53.814 | 47.011 | 0 | 0 | 0 | 0 | 0.013 | 0.021 | 0.067 | 0 | 0.055 | 0.01 | 100.99 |
| Py2 | zk2905g5-1-22 | 53.82 | 46.293 | 0 | 0 | 0 | 0 | 0.03 | 0.043 | 0.011 | 0.032 | 0 | 0 | 100.23 |
| Py2 | zk2905g5-1-23 | 53.914 | 46.543 | 0 | 0.024 | 0.012 | 0 | 0.012 | 0.076 | 0 | 0.015 | 0.064 | 0 | 100.66 |
| Py2 | zk2905g5-1-24 | 53.933 | 46.579 | 0 | 0 | 0 | 0 | 0 | 0 | 0.035 | 0 | 0 | 0 | 100.55 |
| Py3 | zk2905g3-1 | 53.947 | 46.519 | 0 | 0.037 | 0 | 0 | 0 | 0 | 0 | 0.076 | 0.105 | 0 | 100.68 |
| Py3 | zk2905g3-2 | 53.982 | 46.644 | 0.016 | 0.022 | 0.033 | 0 | 0 | 0 | 0.064 | 0.006 | 0.026 | 0 | 100.79 |
| Py3 | zk2905g3-3 | 52.543 | 46.366 | 0.022 | 0.074 | 0 | 0 | 0 | 0 | 0 | 0.039 | 0.083 | 0.013 | 99.14 |
| Py3 | zk2905g3-4 | 52.551 | 46.547 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0.038 | 0.034 | 0.035 | 0.007 | 99.292 |
| Py3 | zk2905g3-5 | 53.873 | 46.649 | 0.022 | 0 | 0.007 | 0 | 0.017 | 0.011 | 0.068 | 0.025 | 0.152 | 0.006 | 100.83 |
| Py3 | zk2905g3-6 | 53.879 | 46.842 | 0.022 | 0.007 | 0 | 0.046 | 0.003 | 0 | 0.048 | 0.043 | 0.059 | 0.01 | 100.96 |
| Py3 | zk2905g3-7 | 53.235 | 47.305 | 0.042 | 0 | 0 | 0 | 0 | 0 | 0 | 0.019 | 0.052 | 0 | 100.65 |
| Py3 | zk2905g3-8 | 53.253 | 46.57 | 0.035 | 0.026 | 0.028 | 0.018 | 0.017 | 0 | 0 | 0.054 | 0.024 | 0 | 100.03 |
| Py3 | zk2905g3-9 | 53.335 | 46.853 | 0.029 | 0 | 0 | 0.008 | 0.006 | 0 | 0 | 0 | 0.034 | 0.065 | 100.33 |
| Py3 | zk2905g3-10 | 53.374 | 46.464 | 0.022 | 0 | 0 | 0.014 | 0.004 | 0 | 0 | 0.019 | 0.014 | 0 | 99.911 |
| Py3 | zk2905g3-11 | 53.402 | 46.426 | 0.029 | 0 | 0.048 | 0.026 | 0.044 | 0.021 | 0 | 0.027 | 0.016 | 0 | 100.04 |
| Py3 | zk2905g3-12 | 53.42 | 46.657 | 0.026 | 0 | 0.077 | 0.026 | 0 | 0 | 0.102 | 0 | 0.045 | 0.057 | 100.41 |
| Py3 | zk2905g3-13 | 53.465 | 46.624 | 0.041 | 0 | 0 | 0.015 | 0.001 | 0 | 0.002 | 0 | 0.01 | 0 | 100.16 |
| Py3 | zk2905g3-14 | 53.53 | 46.881 | 0.035 | 0 | 0.022 | 0.006 | 0 | 0.031 | 0.029 | 0.011 | 0.038 | 0.013 | 100.6 |
| Py4 | zk2905g5-2-1 | 53.593 | 47.049 | 0.026 | 0.041 | 0 | 0.012 | 0 | 0.03 | 0.06 | 0 | 0 | 0 | 100.81 |
| Py4 | zk2905g5-2-2 | 53.667 | 46.76 | 0.054 | 0.063 | 0 | 0 | 0.02 | 0.017 | 0.008 | 0 | 0.04 | 0.011 | 100.64 |
| Py4 | zk2905g5-2-3 | 53.677 | 46.804 | 0.035 | 0.052 | 0.082 | 0.006 | 0.01 | 0 | 0 | 0 | 0.053 | 0.002 | 100.72 |
| Py4 | zk2905g5-2-4 | 53.714 | 46.569 | 0.051 | 0.028 | 0 | 0 | 0.006 | 0.028 | 0.029 | 0.075 | 0.038 | 0 | 100.54 |
| Py4 | zk2905g5-2-5 | 53.748 | 46.472 | 0.029 | 0.011 | 0 | 0 | 0.006 | 0.034 | 0 | 0.013 | 0.024 | 0 | 100.34 |
| Py4 | zk2905g5-2-6 | 53.75 | 46.385 | 0.038 | 0.028 | 0.073 | 0 | 0 | 0.007 | 0.109 | 0.008 | 0.017 | 0 | 100.42 |
| Py4 | zk2905g5-2-7 | 53.784 | 46.736 | 0.041 | 0 | 0.003 | 0 | 0 | 0 | 0.033 | 0 | 0.077 | 0 | 100.67 |
| Py4 | zk2905g5-2-8 | 53.812 | 46.257 | 0.061 | 0.004 | 0 | 0.033 | 0 | 0 | 0 | 0.023 | 0.05 | 0 | 100.24 |
| Py4 | zk2905g5-2-9 | 53.846 | 46.81 | 0.083 | 0 | 0 | 0.015 | 0.006 | 0 | 0 | 0.077 | 0.038 | 0.028 | 100.9 |
| Py4 | zk2905g5-2-10 | 53.446 | 46.15 | 0.061 | 0 | 0.04 | 0 | 0 | 0 | 0.142 | 0.074 | 0.06 | 0 | 99.973 |
| Py4 | zk2905g5-2-11 | 53.346 | 46.553 | 0.057 | 0.037 | 0 | 0 | 0.039 | 0 | 0.067 | 0.043 | 0.014 | 0 | 100.16 |
| Py4 | zk2905g5-2-12 | 53.905 | 46.426 | 0.032 | 0.048 | 0 | 0.009 | 0.053 | 0.012 | 0.03 | 0 | 0.022 | 0.006 | 100.54 |
| Py4 | zk2905g5-2-13 | 52.764 | 46.274 | 0.134 | 0.169 | 0.095 | 0.021 | 0 | 0 | 0.04 | 0 | 0.059 | 0 | 99.556 |
| Py4 | zk2905g5-2-14 | 53.156 | 46.488 | 0.061 | 0 | 0.04 | 0 | 0 | 0 | 0.102 | 0.038 | 0.005 | 0 | 99.89 |
| Stage | Sample No. | Ti | Mn | Co | Ni | Cu | Zn | Ge | As | Se | Mo | Ag | Cd | Sb | Te | Au | Tl | Bi | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Py1 | zk2904g7-1 | 1.35 | 0.00 | 34.42 | 24.56 | 0.00 | 0.28 | 4.86 | 0.00 | 0.00 | 0.16 | 0.00 | 0.19 | 0.02 | 0.22 | 0.01 | 0.01 | 0.01 | 0.02 |
| Py1 | zk2904g7-2 | 6.17 | 0.85 | 380.43 | 176.43 | 0.99 | 0.60 | 4.33 | 0.00 | 2.26 | 0.08 | 0.61 | 0.08 | 0.08 | 8.81 | 0.08 | 0.00 | 0.23 | 1.11 |
| Py1 | zk2904g7-3 | 5.59 | 0.11 | 26.51 | 31.80 | 7.12 | 0.18 | 4.79 | 4.97 | 0.00 | 0.04 | 5.08 | 0.06 | 0.85 | 21.36 | 2.11 | 0.05 | 2.53 | 18.90 |
| Py1 | zk2904g7-4 | 1.55 | 0.00 | 0.21 | 0.00 | 0.02 | 0.46 | 4.34 | 2.09 | 1.24 | 0.09 | 0.06 | 0.00 | 0.05 | 0.02 | 0.01 | 0.00 | 0.00 | 1.59 |
| Py1 | zk2904g7-5 | 2.63 | 0.00 | 8.16 | 4.21 | 1.21 | 0.71 | 4.64 | 0.52 | 2.08 | 0.05 | 0.01 | 0.00 | 0.00 | 4.63 | 0.02 | 0.02 | 0.06 | 0.19 |
| Py1 | zk2904g7-6 | 2.14 | 0.10 | 61.71 | 61.14 | 5.16 | 0.76 | 4.87 | 2.98 | 0.67 | 0.09 | 3.22 | 0.00 | 0.13 | 9.99 | 1.15 | 0.00 | 1.04 | 8.18 |
| Py1 | zk1001g2-1 | 0.93 | 0.00 | 102.41 | 16.64 | 0.74 | 0.62 | 5.16 | 12.85 | 2.02 | 0.04 | 1.20 | 0.01 | 0.08 | 4.06 | 0.15 | 0.01 | 1.36 | 2.17 |
| Py1 | zk1001g2-3 | 4.15 | 0.05 | 11.09 | 8.67 | 1.46 | 0.77 | 4.73 | 0.81 | 3.34 | 0.07 | 0.06 | 0.00 | 0.00 | 0.65 | 25.49 | 0.02 | 0.04 | 0.14 |
| Py1 | zk1001g2-4 | 1.21 | 0.00 | 53.04 | 24.08 | 0.78 | 0.14 | 4.23 | 2.44 | 0.69 | 0.08 | 0.79 | 0.00 | 0.00 | 2.08 | 0.29 | 0.00 | 0.16 | 1.38 |
| Py2 | zk2905g5-1-1 | 56.59 | 40.39 | 14.31 | 11.95 | 36.67 | 3.54 | 4.97 | 1784.14 | 2.73 | 163.94 | 20.18 | 0.38 | 14.85 | 117.89 | 8.59 | 0.28 | 61.29 | 215.34 |
| Py2 | zk2905g5-1-2 | 17.05 | 152.54 | 22.61 | 28.74 | 111.99 | 6.12 | 4.41 | 276.23 | 3.63 | 436.09 | 22.97 | 3.33 | 26.11 | 81.84 | 5.60 | 2.15 | 76.29 | 262.73 |
| Py2 | zk2905g5-1-3 | 18.30 | 40.62 | 20.29 | 23.37 | 74.76 | 4.60 | 4.55 | 350.11 | 1.96 | 282.92 | 18.59 | 0.96 | 11.83 | 73.66 | 5.65 | 0.55 | 60.60 | 217.15 |
| Py2 | zk2905g5-1-4 | 87.94 | 11.32 | 9.40 | 13.68 | 64.72 | 3.64 | 4.59 | 535.52 | 4.02 | 572.19 | 10.94 | 0.26 | 9.81 | 51.69 | 4.21 | 0.43 | 40.09 | 115.95 |
| Py2 | zk2905g5-1-5 | 4.66 | 7.87 | 3.55 | 6.65 | 59.57 | 3.45 | 4.33 | 59.24 | 0.84 | 625.39 | 4.87 | 0.66 | 4.65 | 11.43 | 2.03 | 0.67 | 10.15 | 40.36 |
| Py2 | zk2905g5-1-6 | 20.84 | 10.13 | 15.20 | 20.55 | 93.19 | 5.18 | 4.45 | 173.18 | 0.00 | 243.14 | 12.24 | 1.94 | 10.60 | 50.42 | 3.70 | 0.88 | 48.08 | 151.22 |
| Py2 | zk2905g5-1-7 | 60.30 | 6.60 | 15.08 | 15.86 | 57.98 | 3.84 | 4.93 | 372.96 | 0.31 | 436.00 | 9.84 | 1.34 | 16.28 | 35.50 | 3.16 | 0.81 | 40.76 | 163.58 |
| Py2 | zk2905g5-1-8 | 691.12 | 10.16 | 22.02 | 40.89 | 64.56 | 2.92 | 5.00 | 1194.13 | 1.04 | 359.57 | 5.60 | 1.00 | 53.07 | 26.45 | 3.12 | 2.06 | 10.98 | 369.50 |
| Py2 | zk2905g5-1-9 | 222.14 | 10.32 | 54.32 | 38.51 | 98.48 | 2.83 | 4.56 | 731.53 | 2.24 | 231.95 | 30.27 | 1.60 | 36.48 | 142.43 | 7.57 | 3.29 | 128.55 | 335.56 |
| Py2 | zk2905g5-1-10 | 102.91 | 16.09 | 15.77 | 18.57 | 62.97 | 5.81 | 5.17 | 213.34 | 1.06 | 286.36 | 21.23 | 2.66 | 19.07 | 75.39 | 4.73 | 1.99 | 81.51 | 142.46 |
| Py3 | zk1001g2-2 | 5.88 | 0.25 | 46.61 | 7.35 | 25.46 | 1.07 | 5.72 | 5.92 | 0.19 | 0.01 | 282.66 | 0.02 | 0.32 | 284.80 | 47.07 | 0.03 | 28.66 | 43.02 |
| Py3 | zk2905g3-1 | 1.58 | 0.00 | 0.20 | 18.43 | 414.41 | 0.56 | 4.13 | 3.15 | 0.85 | 0.07 | 1253.20 | 4.83 | 0.44 | 2021.51 | 250.14 | 0.03 | 104.29 | 8651.72 |
| Py3 | zk2905g3-2 | 1.50 | 0.21 | 0.36 | 87.78 | 44.87 | 0.44 | 4.60 | 6.01 | 4.21 | 0.06 | 542.55 | 2.44 | 0.98 | 716.70 | 99.76 | 0.05 | 80.14 | 4418.70 |
| Py3 | zk2905g3-3 | 1.51 | 0.00 | 0.41 | 83.01 | 62.20 | 0.54 | 4.66 | 3.78 | 0.75 | 0.18 | 1758.18 | 0.06 | 0.29 | 2801.51 | 440.79 | 0.00 | 26.64 | 209.25 |
| Py3 | zk2905g3-4 | 1.65 | 0.00 | 0.07 | 31.93 | 20.25 | 0.22 | 4.56 | 3.22 | 2.13 | 0.00 | 9.47 | 0.00 | 0.33 | 36.91 | 1.51 | 0.00 | 0.61 | 33.03 |
| Py3 | zk2905g3-5 | 1.06 | 0.23 | 0.43 | 21.37 | 7.54 | 0.49 | 4.89 | 0.00 | 1.77 | 0.07 | 1.93 | 0.49 | 0.20 | 17.60 | 0.36 | 0.01 | 0.92 | 15.79 |
| Py3 | zk2905g3-6 | 1.14 | 0.95 | 0.28 | 57.51 | 66.36 | 0.72 | 4.77 | 4752.33 | 0.77 | 0.08 | 598.01 | 0.36 | 18.00 | 1342.73 | 150.31 | 0.08 | 196.02 | 368.74 |
| Py4 | zk2905g5-1 | 2.25 | 0.00 | 3.67 | 3.36 | 9.63 | 0.43 | 4.17 | 5.99 | 2.00 | 0.02 | 1.16 | 0.02 | 0.00 | 17.07 | 0.60 | 0.00 | 19.27 | 21.63 |
| Py4 | zk2905g5-2 | 1.15 | 0.18 | 8.44 | 3.80 | 22.39 | 0.52 | 4.09 | 7.44 | 4.39 | 0.06 | 87.08 | 0.06 | 0.06 | 166.87 | 10.62 | 0.02 | 139.45 | 36.26 |
| Py4 | zk2905g5-3 | 1.94 | 0.14 | 12.39 | 5.11 | 13.79 | 0.45 | 5.09 | 5.61 | 3.87 | 0.00 | 3.78 | 0.11 | 0.12 | 35.95 | 1.14 | 0.00 | 40.94 | 40.93 |
| Py4 | zk2905g5-4 | 1.88 | 0.28 | 5.26 | 2.25 | 5.98 | 0.63 | 4.97 | 4.59 | 2.92 | 0.04 | 1.40 | 0.00 | 0.00 | 15.23 | 0.31 | 0.01 | 13.41 | 20.18 |
| Py4 | zk2905g5-5 | 2.00 | 0.07 | 21.68 | 4.83 | 6.69 | 0.68 | 4.75 | 3.33 | 2.25 | 0.02 | 1.29 | 0.00 | 0.10 | 19.05 | 0.24 | 0.00 | 10.73 | 13.66 |
| Stage | Sample no. | δ34SV-CDT (‰) |
|---|---|---|
| Py1 | ZK2904g7-1-1 | -0.85 |
| Py1 | ZK2904g7-1-2 | -0.66 |
| Py1 | ZK2904g7-1-3 | -1.20 |
| Py1 | ZK2904g7-1-4 | -0.65 |
| Py1 | ZK2904g7-1-5 | -0.57 |
| Py1 | ZK2904g7-1-6 | -0.69 |
| Py1 | ZK2904g7-1-7 | -0.95 |
| Py2 | ZK2905g5-1-1 | 14.43 |
| Py2 | ZK2905g5-1-2 | 11.09 |
| Py2 | ZK2905g5-1-3 | 5.33 |
| Py2 | ZK2905g5-1-4 | 6.59 |
| Py2 | ZK2905g5-1-5 | 6.88 |
| Py2 | ZK2905g5-1-6 | 11.03 |
| Py2 | ZK2905g5-1-7 | 6.78 |
| Py2 | ZK2905g5-1-8 | 4.75 |
| Py2 | ZK2905g5-1-9 | 4.67 |
| Py2 | ZK2905g5-1-10 | 10.78 |
| Py3 | ZK2905g3-1 | -0.74 |
| Py3 | ZK2905g3-2 | -5.69 |
| Py3 | ZK2905g3-3 | -4.38 |
| Py3 | ZK2905g3-4 | -5.44 |
| Py3 | ZK2905g3-5 | -5.16 |
| Py3 | ZK2905g3-6 | -4.69 |
| Py3 | ZK2905g3-7 | -0.89 |
| Py3 | ZK2905g3-8 | -1.09 |
| Py3 | ZK2905g3-9 | 0.19 |
| Py3 | ZK2905g3-10 | -1.03 |
| Py4 | ZK1001g2-1-1 | 2.90 |
| Py4 | ZK1001g2-1-2 | 2.66 |
| Py4 | ZK1001g2-1-3 | 3.05 |
| Py4 | ZK2905g5-1 | 3.62 |
| Py4 | ZK2905g5-2 | 3.65 |
| Py4 | ZK2905g5-3 | 3.86 |
| Py4 | ZK2905g5-4 | 3.76 |
| Py4 | ZK2905g5-5 | 3.78 |
| Sample no. | Contents (ppm) | 238U/206Pb | 207Pb/206Pb | ||||
|---|---|---|---|---|---|---|---|
| U | Th | Pb | Ratio | 2σ | Ratio | 2σ | |
| Zk2905g5-1 - 1 | 0.43 | 0.43 | 0.82 | 1.7658 | 0.1382 | 0.8021 | 0.0164 |
| Zk2905g5-1 - 2 | 2.18 | 0.52 | 2.13 | 2.9919 | 0.2550 | 0.7909 | 0.0121 |
| Zk2905g5-1 - 7 | 3.31 | 0.49 | 4.29 | 2.7224 | 0.1216 | 0.7936 | 0.0149 |
| Zk2905g5-1 - 8 | 4.48 | 0.60 | 5.60 | 2.4515 | 0.1727 | 0.7934 | 0.0106 |
| Zk2905g5-1 - 9 | 3.39 | 0.68 | 4.55 | 2.3182 | 0.1292 | 0.8022 | 0.0106 |
| Zk2905g5-3 - 1 | 6.04 | 1.42 | 16.58 | 0.9071 | 0.0712 | 0.8225 | 0.0071 |
| Zk2905g5-3 - 2 | 7.99 | 1.62 | 30.65 | 0.8609 | 0.0267 | 0.8266 | 0.0081 |
| Zk2905g5-3 - 3 | 6.26 | 1.72 | 30.37 | 0.6643 | 0.0296 | 0.8278 | 0.0077 |
| Zk2905g5-3 - 4 | 5.15 | 3.84 | 36.04 | 0.5622 | 0.0089 | 0.8297 | 0.0062 |
| Zk2905g5-3 - 5 | 8.16 | 7.34 | 39.36 | 0.7759 | 0.0359 | 0.8308 | 0.0076 |
| Zk2905g5-3 - 6 | 4.05 | 2.64 | 28.05 | 0.5598 | 0.0086 | 0.8322 | 0.0079 |
| Zk2905g5-4 - 1 | 4.67 | 2.14 | 7.06 | 1.9161 | 0.1121 | 0.8045 | 0.0119 |
| Zk2905g5-4 - 2 | 5.43 | 1.63 | 8.95 | 1.9336 | 0.0887 | 0.8104 | 0.0096 |
| Zk2905g5-4 - 3 | 3.68 | 1.19 | 4.73 | 2.7463 | 0.1258 | 0.8090 | 0.0168 |
| Zk2905g5-4 - 4 | 8.73 | 2.85 | 18.42 | 1.7070 | 0.0517 | 0.8164 | 0.0099 |
| Zk2905g5-4 - 5 | 5.25 | 1.36 | 8.83 | 2.1261 | 0.0735 | 0.7978 | 0.0107 |
| Zk2905g5-4 - 6 | 5.58 | 2.27 | 8.25 | 2.1815 | 0.1156 | 0.8036 | 0.0104 |
| Zk2905g5-4 - 7 | 5.71 | 2.03 | 8.45 | 1.9677 | 0.1210 | 0.8083 | 0.0080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).