1. Introduction
Ulcerative Colitis (UC) is a chronic inflammatory disorder of the large intestine that is immune-mediated. Characterized primarily by inflammation starting in the rectum and potentially extending proximally to involve sections or the entirety of the colon, UC can manifest at any age. However, it predominantly occurs in individuals, with a peak onset age between 15 and 30 years. This age-related distribution underscores the significant impact UC can have on younger populations, necessitating a focused approach to management and treatment [
1]. Chronic Inflammatory Bowel Disease (IBD) encompasses two principal subtypes of persistent inflammatory conditions of the gastrointestinal tract: Crohn's Disease (CD) and Ulcerative Colitis (UC). Additionally, there exists a category termed IBDU—Inflammatory Bowel Disease Unclassified. Studies estimate that approximately 80% of IBDU cases are reclassified as either CD or UC within an eight-year period. Nevertheless, a distinct subset remains permanently diagnosed as IBDU, constituting a unique clinical entity. Long-term follow-up studies indicate that reclassification rates vary from 5-14%, with a higher incidence of transitioning to Crohn's Disease among adults [
2]. The diagnosis and sub-classification of Inflammatory Bowel Disease (IBD) in clinical practice present significant challenges, often leading to diagnostic inaccuracies. Although ileo-colonoscopy remains the gold standard for diagnosis, a multi-disciplinary approach that integrates clinical evaluation with endoscopic, histological, radiological, and biochemical investigations is essential. IBD affects not only the gastrointestinal tract but also has systemic implications, causing extra-intestinal manifestations that can impact virtually any organ system. These manifestations are a primary source of morbidity and disability. Commonly affected areas include the articular, hepato-biliary, and cutaneous districts. The probability of developing extra-intestinal manifestations escalates with the duration of the intestinal disease and is more prevalent in Crohn’s disease than in Ulcerative Colitis (71% vs 21-22%). While some extra-intestinal manifestations correlate directly with the extent of intestinal inflammation, others, such as those affecting the cardiothoracic district, occur independently of the inflammatory activity within the intestines [
3,
4,
5,
6]. Respiratory complications in Inflammatory Bowel Disease (IBD) are relatively uncommon. The most frequent manifestations involve the larger airways, such as chronic bronchitis and bronchiectasis, although all lung parenchyma can be affected. These pulmonary manifestations typically occur in patients with long-standing IBD and are not directly related to exacerbations of the intestinal condition. Importantly, lung involvement can occur even in the absence of overt clinical symptoms.
Pulmonary manifestations can be categorized based on their underlying mechanisms into several types: drug-induced lung disease (associated with medications such as sulfasalazine, mesalazine, methotrexate, and infliximab), anatomical disease (such as fistulas), overlap syndromes, and immune-mediated processes. The range of pulmonary symptoms in IBD is broad, with patients potentially experiencing progressive respiratory symptoms like breathlessness, chest pain, and cough, or exhibiting changes on radiographic imaging. Interestingly, drugs like sulfasalazine and mesalazine may induce asymptomatic lung lesions more frequently than previously recognized. Typically, symptoms manifest within 2-6 months of initiating therapy; however, in some instances, they can appear within just a few days or, alternatively, after many years [
7,
8].
The medical management of Inflammatory Bowel Disease (IBD) involves pharmacological interventions that either directly or indirectly modulate the inflammatory cascade. Effective drugs for Ulcerative Colitis include mesalazine, corticosteroids, and immunosuppressants such as cyclosporine and azathioprine. Additionally, monoclonal antibodies targeting TNF-alpha (e.g., infliximab) are integral to the therapeutic arsenal. Immunosuppressants represent a relatively recent therapeutic option for treating patients with moderate-to-severe forms of Ulcerative Colitis and Crohn’s Disease. These drugs are particularly valuable for individuals who are refractory or intolerant to conventional treatments, including anti-TNFα agents. This approach underscores the evolving landscape of IBD treatment, which increasingly prioritizes tailored therapies based on individual patient responses and tolerability profiles.
Immunosuppressants, specifically the newer class of integrin antagonist biological agents, have been introduced for treating patients with moderate-to-severe Crohn’s Disease (CD) and Ulcerative Colitis (UC). These agents are particularly useful for patients who have not responded adequately to traditional therapies, including tumor necrosis factor antagonists (TNF-α). These treatments display varying advantages and response rates, highlighting the necessity for personalized medical strategies based on individual patient profiles. On the other hand, surgical therapy presents a distinct approach, often considered when medical management does not achieve sufficient control of symptoms or in cases of complications [
3,
9].
2. Case Presentation
A young man in his third decade of life with a history of ulcerative colitis tragically died suddenly following physical exertion. Despite emergency resuscitation attempts, he was pronounced deceased at the scene. At the time of discovery, his body was surrounded by a substantial pool of blood and foamy, reddish fluid, which had emanated from his oral and/or nasal cavities. Notably, there were no signs of external trauma, though point abrasions from resuscitation efforts were visible on his torso. A blister pack of Mesavanacol was found in his suitcase.
The deceased was subsequently transferred to the Institute of Forensic Medicine in Palermo to further the judicial investigation. An autopsy conducted three days post-mortem revealed no signs of internal bleeding within the peritoneal cavity or gastrointestinal tract, including the absence of erosions or bleeding in the stomach and the large intestine, which also showed no signs of ulcers, perforations, or bleeding.
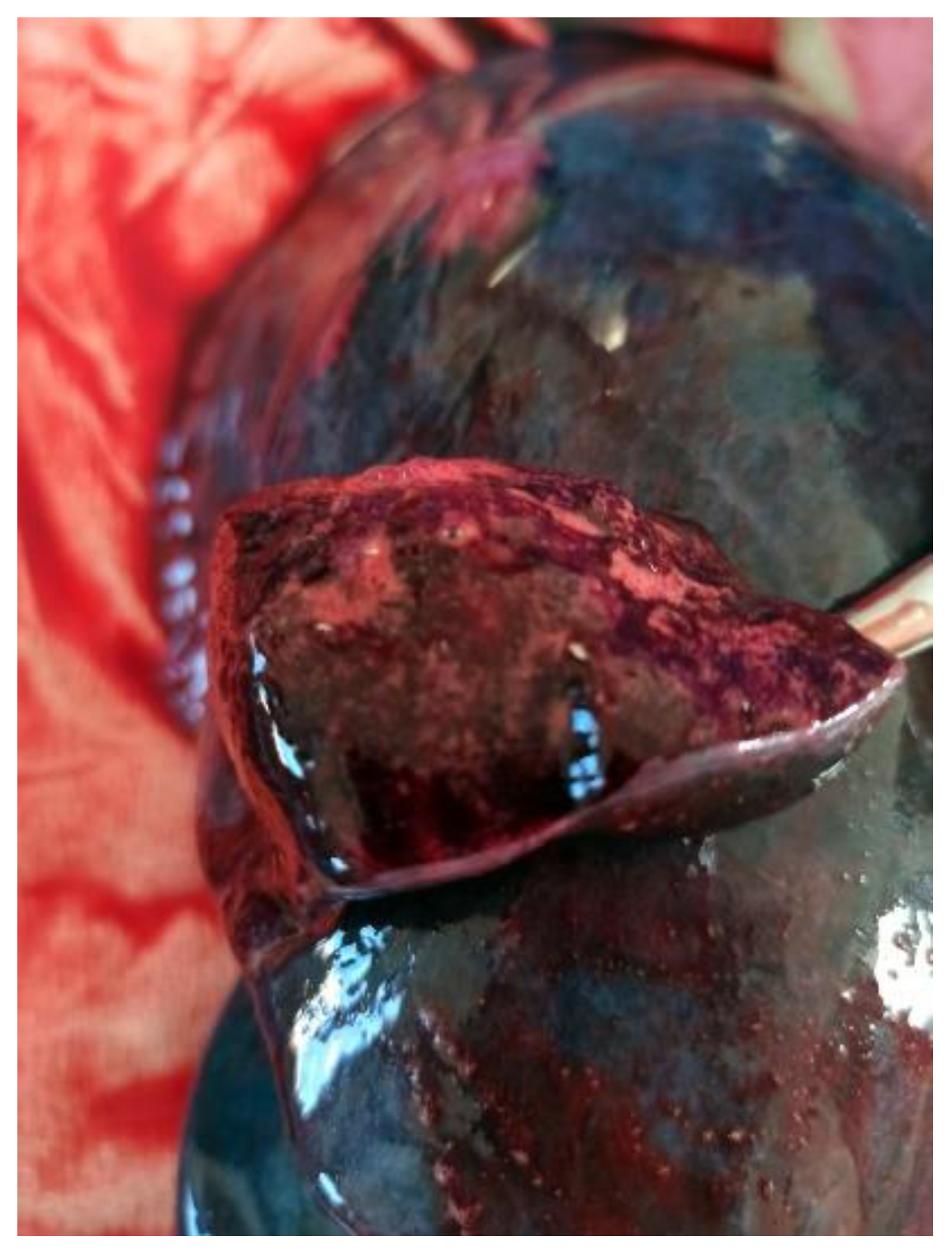
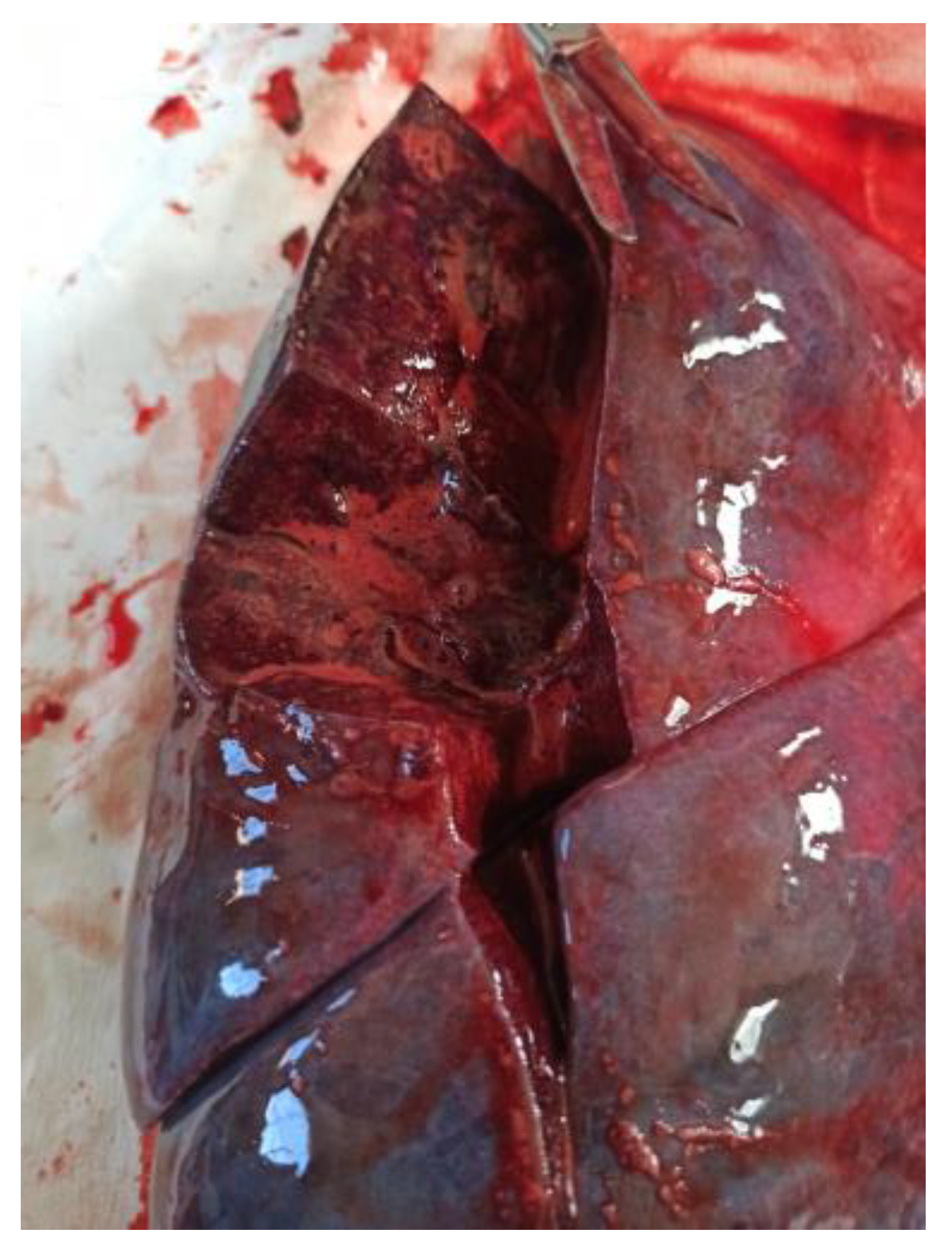
Significant findings included a foamy, blood-stained fluid within the tracheal lumen, glottal edema, and the presence of a similar frothy, reddish liquid upon manipulation of the bronchi. The lungs were congested and markedly hemorrhagic, exuding abundant frothy material upon sectioning. These findings suggest a pulmonary cause of death, potentially linked to an acute exacerbation or complication related to his underlying ulcerative colitis, though further investigation would be necessary to confirm the exact pathophysiological mechanism (
Figure 1 and
Figure 2).
The intestinal mucosa was taken every 5 cm.
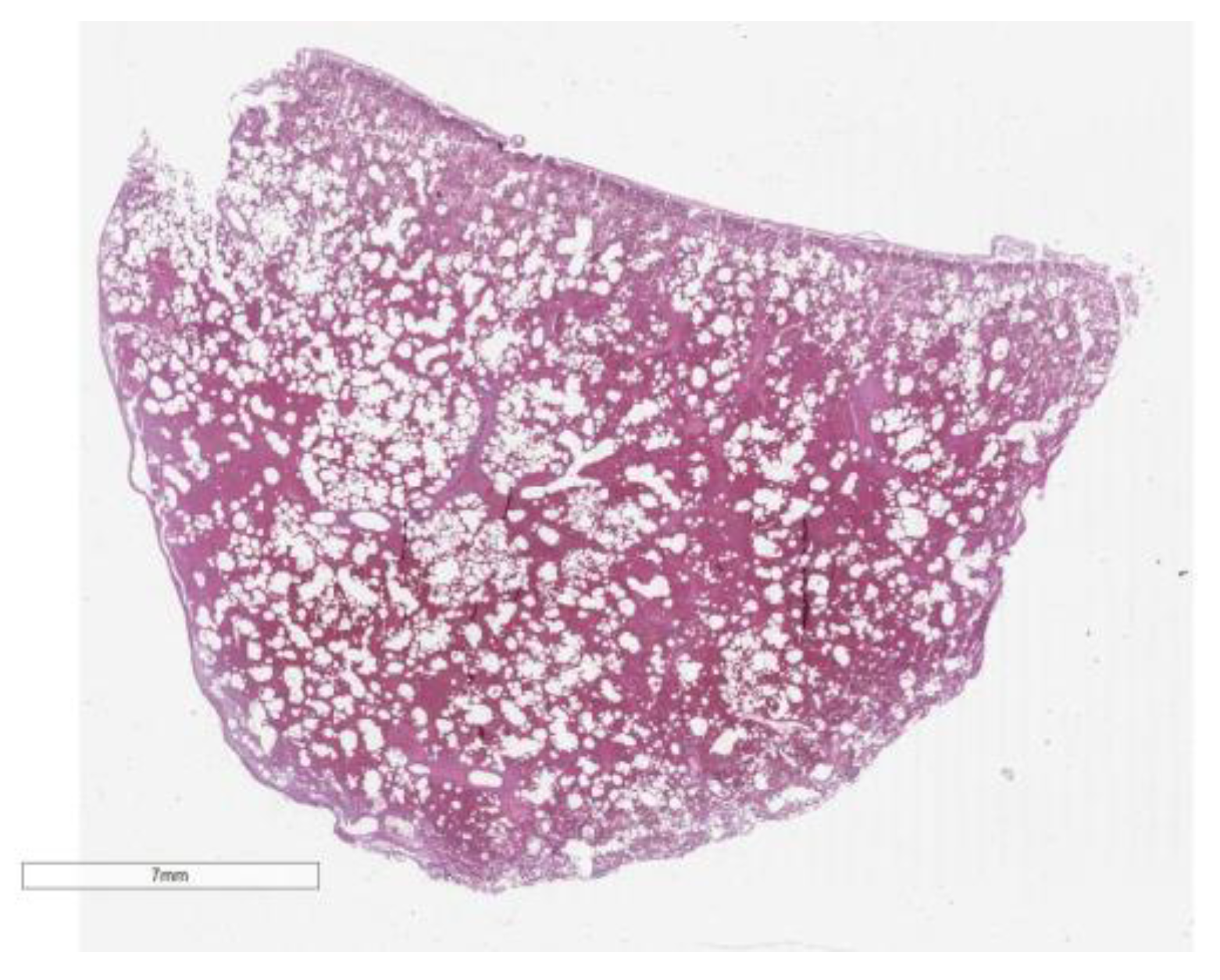
Histological investigations provided significant insights into the pathological conditions affecting various organs. The large intestine exhibited chronic mucosal inflammation with focal inflammation of the submucosa, coupled with ulcerative phenomena. There was also evidence of chronic inflammation within the ileal mucosa. Notably, the heart displayed foci of active lymphocytic myocarditis in the left ventricle and diffuse colliquative myocytolysis, indicating severe cardiac tissue disruption.
Furthermore, the liver was affected by chronic lymphocytic portitis, highlighting an inflammatory response within the portal areas. At the pulmonary level, the findings were particularly severe, with widespread pulmonary hemorrhage and areas of edema. Additionally, emphysematous changes were noted, suggesting alterations in lung tissue structure and function. These comprehensive histological findings underscore the multi-organ impact of the underlying disease processes, contributing to a complex clinical presentation. (
Figure 3 and
Figure 4).
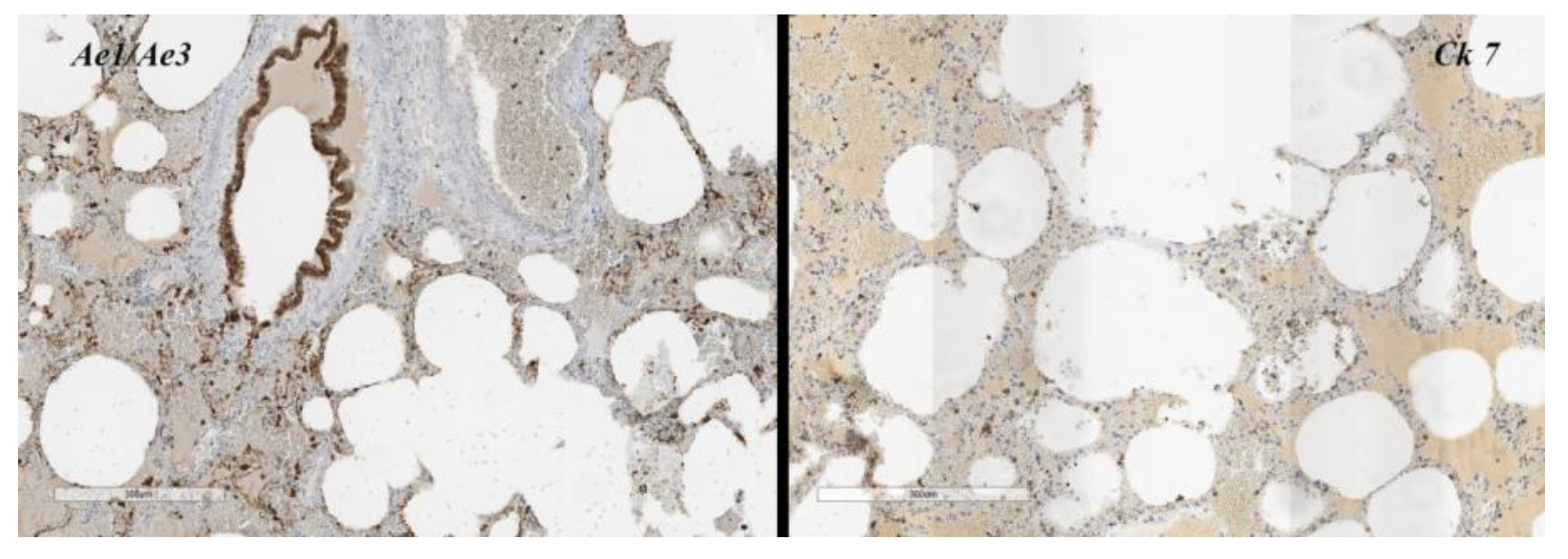
Furthermore, a lymphomonocytic infiltrate (
Figure 5) with a periarteriolar sheath invades the vessel wall with dissociation of the tunica media, and in some tract rupture of alveolar walls. Areas of bronchiolitis, focal pleural fibrosis associated with fibroids of the central lobular and peribronchoacinar interlobar connective tissue.
Toxicological analyses were conducted to screen for a spectrum of substances including opioids, benzodiazepines, cannabinoids, cocaine, and amphetamines, among others. All tests returned negative results, indicating no presence of these drugs in the system at the time of testing.
The clinical history indicated that the patient was diagnosed with ulcerative colitis five years earlier, supported by clinical symptoms, laboratory results, and endoscopic evidence. The clinical presentation included symptoms such as liquid stool discharge, abdominal pain, proctorrhagia, and weight loss. Laboratory analysis confirmed the presence of p-ANCA and PR3-cANCA antibodies, which are indicative of autoimmune activity. An ileo-colonoscopy further substantiated the diagnosis by revealing "backwash ileitis," a condition often associated with this form of inflammatory bowel disease [
2].
Since his diagnosis in 2014, the patient has been under consistent clinical, laboratory, and instrumental surveillance, navigating through periods of remission and exacerbation of ulcerative colitis. Initial treatment involved topical and oral mesalazine, augmented by three cycles of steroids during acute exacerbations. In June 2019, due to insufficient response to these therapies, the patient was switched to monoclonal therapy with infliximab. However, this intervention was halted in October of the same year because of primary failure, evidenced by ongoing active disease both clinically and on ultrasound imaging. Additionally, treatment with azathioprine was terminated prior to this due to pharmacological intolerance.
In December, the patient commenced another monoclonal therapy using a different immunosuppressant, which led to clinical improvement although colonoscopy still showed persistent disease activity. The regimen for this treatment included 300 mg of the immunosuppressant administered intravenously every 8 weeks, and this frequency was increased to every 4 weeks starting in April. Oral mesalazine was continued alongside this therapy.
The patient's last chest X-ray in May 2019 was normal. Subsequent laboratory results from June 2020 indicated moderate inflammation, as evidenced by a C-reactive protein level of 118 mg/L and fecal calprotectin of 275 ng/g. A comprehensive Pancoloscopy conducted on July 27, 2020, revealed colic segments with a tubular appearance. The mucosa lining these segments was hyperemic, fragile, and marked by erosions alongside minor ulcerations. Additionally, several pseudopolyps with an inflammatory appearance and eroded surfaces ranging from 0.1 to 1.5 cm were observed. The rectal ampulla also exhibited hyperemic mucosa and a loss of the vascular pattern. Tragically, on August 23, 2020, only five days following an intravenous infusion of the immunosuppressant, the young man suddenly.
3. Discussion
Immunosuppressants, while pivotal in preventing organ rejection post-transplantation and managing autoimmune diseases, can have detrimental effects on pulmonary health. These drugs can compromise the immune system's ability to combat infections, leading to an increased risk of developing severe respiratory infections such as pneumonia and tuberculosis. Furthermore, certain immunosuppressants are associated with direct toxic effects on lung tissue, which may manifest as drug-induced pneumonitis or pulmonary fibrosis. This condition involves inflammation and scarring of the lung tissue, impairing gas exchange and potentially leading to progressive and irreversible respiratory failure. The balancing act between the benefits of immunosuppression and the risk of pulmonary complications requires vigilant monitoring and management to optimize patient outcomes.
The autopsy confirmed diagnosis of RCU with multiorgan extra-intestinal manifestations (cardiac, hepatic, pulmonary) exceeded the limits of differential diagnostics. Among them was a pulmonary hemorrhage (diffuse endoalveolar).
HDA is well established in scientific literature in ANCA-associated diseases and sometimes associated with monoclonal therapy.
Excluding in the case in question all other causes of HDA (ANCA-associated diseases/vasculitis in relation to the clinical history and autopsy results), despite being present chronic lung damage from drugs, extra-intestinal respiratory manifestations from IBD or overlap exclusion [
2,
7], the acute cause death is due to diffuse alveolar haemorrhage secondary to Immunosuppressant as a diagnosis of exclusion [
10].
Some immunosuppressant is a humanized monoclonal antibody that targets the a4b7-integrin expressed specifically on a subset of gut-homing T lymphocytes and inhibits its binding with mucosal addressing cell adhesion molecule 1 (MAdCAM-1). This binding of a4b7 with MAdCAM-1 is considered gut selective. It is indicated for treatment of adult patients with active ulcerative colitis (moderate to severe) who have shown inadequate response, therapeutic failure or have been found to be intolerant to conventional therapy or administration of an antagonist of tumour necrosis factor alpha (TNFα) [
11].
Recent literature data suggest that although immunosuppressant has a high safety profile, it take action also on the lung. In fact, a4b7 is also moderately expressed on other types of leukocytes and a4b7 is known to have a small role in pulmonary homing through VCAM1 and fibronectin, with the a4b7- fibronectin interaction also being inhibited by immunosuppressant. However, pulmonary homing is more dependent on b1 integrin and it has been suggested that immunosuppressant could induce a shift in integrin expression on lymphocytes with upregulation of b1, leading to an increased migration to extraintestinal sites, thereby facilitating development of extraintestinal inflammation. There are indeed arguments for an interaction between the gastrointestinal and bronchial mucosa mediated by the immune system [
8,
12,
13,
14,
15]. Common respiratory adverse reactions of Immunosuppressant reported include oropharyngeal pain, nasal congestion and coughing. The serum half-life of the drug is about 26 days; the exact route of elimination of Immunosuppressant is not known [
15].
Death was caused by pulmonary hemorrhage (HDA) responsible for the massive loss of blood observed during the judicial inspection and responsible for terminal cardiac arrest (diffuse colliquative myocytosis) compatible with the infusion of Immunosuppressant 5 days before death.
The purpose of our case report is to show the possibility that Vedolizuamb causes pulmonary hemorrhage such as many other monoclonal antibodies to different farmodynamics like Infliximab, Ustekinumab and others [9,18,20]. In fact, rapid onset pulmonary manifestations are known, which if caught in time can be managed safely for the patient [
19,
20].
The classic pulmonary manifestations of pulmonary RU are dictated by the chronic physiological mechanism of the disease.
In fact, upon self-examination, the lungs showed a rapid picture of disease, with rapid onset, and the microscopic examination highlighted a picture of diffuse pulmonary hemorrhage with areas of edema and emphysematous expressions. Furthermore, a lymphomonocytic infiltrate with a periarteriolar sheath invades the vascular wall with dissociation of the tunica media.
In relation to the action that monoclonal therapy has on the migration of the lymphocyte population, and supported by the immunohistochemistry [
Figure 5] which shows a notable lymphocyte infiltrate present in the affected area, we posed the problem in relation to immunosoppresor. However, in cases like ours, in which the rapid onset does not present all the pulmonary clinical manifestations, it leads us to advise specialists to closely follow up the patient who uses this drug, also reporting mini anomalous signs (cough, fatigue, respiratory problems).
4. Conclusions
In conclusion, while immunosuppressants are indispensable for their role in controlling autoimmune disorders and preventing transplant rejection, their use is not without significant pulmonary risks. One of the most severe complications is Diffuse Alveolar Hemorrhage (DAH), a potentially life-threatening condition characterized by bleeding into the alveoli of the lungs. DAH can be precipitated by the immunosuppressive therapy weakening the capillary integrity or through immune-mediated vascular injury. Effective management of patients on immunosuppressant therapy requires a careful consideration of these risks, regular monitoring for early signs of pulmonary distress, and prompt intervention to mitigate adverse outcomes. Balancing these aspects is crucial to harnessing the benefits of immunosuppressants while minimizing their potential harm to lung health.
Author Contributions
Conceptualization, A.A. and E.L.R.; methodology, S.Z. and G.M.; validation, A.A., S.Z. and G.M.; investigation, D.G.; resources, D.G.; data curation, G.D.A.; writing—original draft preparation, A.A. and E.L.R.; writing—review and editing, A.A. and G.M.; visualization, D.G.; supervision, S.Z.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding Grant NextGenerationEU – Grant MUR D.M. 737/2021 – . 522 Research title Supported Grant by University of Palermo, Eurostart 2021–22, cod. n. PRJ-1030. Title: 523 “Tissue markers predictive of damage from substances of abuse and their correlation to preventable 524 adverse cardiovascular events”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Tontini, G.E.; Vecchi, M.; Pastorelli, L.; Neurath, M.F.; Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World J. Gastroenterol. 2015, 21, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Marotto, D.; Atzeni, F.; Ardizzone, S.; Monteleone, G.; Giorgi, V.; Sarzi-Puttini, P. Extraintestinal manifestations of inflammatory bowel diseases. Pharmacological Research 2020, 161, 105206. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; van der Meulen-de Jong, A.E.; Maul, J.; et al. The pathogenesis of extraintestinal manifestations: Implications for IBD research, diagnosis, and therapy. J. Crohns Colitis 2019, 13, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Yangyang, R.Y.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. In Seminars in pediatric surgery; WB Saunder: 2017; Volume 26, pp. 349–355.
- Olpin, J.D.; Sjoberg, B.P.; Stilwill, S.E.; Jensen, L.E.; Rezvani, M.; Shaaban, A.M. Beyond the Bowel: Extraintestinal Manifestations of Inflammatory Bowel Disease. RadioGraphics 2017, 37, 1135–1160. [Google Scholar] [CrossRef] [PubMed]
- Storch, I.; Sachar, D.; Katz, S. Pulmonary Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 10, 239–254. [Google Scholar] [CrossRef]
- Panagi, S.; Palka, W.; Korelitz, B.I.; Taskin, M.; Lessnau, K.D. Diffuse alveolar hemorrhage after infliximab treatment of Crohn's disease. Inflammatory bowel diseases 2004, 10, 274–277. [Google Scholar] [CrossRef]
- Scribano, M.L. Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence. World J. Gastroenterol. 2018, 24, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, O.C.; Stoller, J.K. Diffuse alveolar hemorrhage: Diagnosing it and finding the cause. Clevel. Clin. J. Med. 2008, 75, 258–280. [Google Scholar] [CrossRef]
- De Backer, E.; Bode, H.; Baert, F. New-Onset Diffuse Parenchymal Lung Disease in a 52-Year-Old Woman With Ulcerative Colitis. Gastroenterology 2020, 158, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef]
- Lissner, D.; Glauben, R.; Allers, K.; Sonnenberg, E.; Loddenkemper, C.; Schneider, T.; Siegmund, B. Pulmonary Manifestation of Crohn's Disease Developed Under Treatment With Vedolizumab. Am. J. Gastroenterol. 2018, 113, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Karippot, A.; Oliver, T.M. Vedolizumab-Associated Acute Respiratory Distress Syndrome. Am. J. Ther. 2018, 25, e592–e593. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Shukla, A.; Palanisamy, N.; Rahi, M.S.; Wolff, A. Diffuse alveolar hemorrhage associated with ustekinumab treatment. Am. J. Heal. Pharm. 2021, 78, 1277–1281. [Google Scholar] [CrossRef]
- Myro, A.Z.; Bjerke, G.; Zarnovicky, S.; Holmøy, T. Diffuse alveolar hemorrhage during alemtuzumab infusion in a patient with multiple sclerosis: A case report. BMC Pharmacol. Toxicol. 2018, 19, 1–4. [Google Scholar] [CrossRef]
- Subha Ghosh, MD, MBA; Himanshu Deshwal, MD; Rebecca Haraf, MD; Shine Raju, MD; Mnahi Bin Saeedan, MBBS, MPH.
- Pralay Sarkar, MD, FCCP; Thomas Gildea, MD; Carol F. Farver, MD; and Atul C. Mehta, MD, FCCP. Pulmonary Manifestations of Inflammatory Bowel Disease and Treatment Strategies. Diffuse Lung Disease CHEST Pulmonary Reviews Published: September 04,2023.
- Zhang, J.; Liu, M.-H.; Gao, X.; Dong, C.; Li, Y.-X. Vedolizumab-associated diffuse interstitial lung disease in patients with ulcerative colitis: A case report. World J. Clin. Cases 2022, 10, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).