1. Introduction
The involvement of the Nerve growth factor (NGF) is very well documented in the development of the peripheral nervous system and in determining the phenotype of sensory and sympathetic neurons. In addition, NGF levels increase during various inflammatory conditions and are involved in inducing pain and hyperalgesia [
1,
2,
3]. NGF can bind to its high-affinity receptor, tropomyosin receptor kinase A (TrkA) and its low affinity, p75 neurotrophin receptor (p75NTR). The NGF secreted by resident and inflammatory cells and keratinocytes in injured tissue acts via TrkA receptors present on primary sensory nerve endings and participates in peripheral sensitization [
4]. Hence, NGF/TrkA signaling is considered as a primary target for the development of pain therapeutics [
5] leading to the development of anti-NGF antibodies and TrkA antagonists [
6,
7]. However, for these analgesics to be effective with the least possible side effects, the detailed mechanisms involved in peripheral sensitization need to be evaluated further.
For blocking NGF signaling, several drugs have been developed such as NGF protein inhibitors (Anti-NGF antibodies), NGF/TrkA binding inhibitors and TrkA inhibitors [
8,
9,
10,
11]. TrkA is present on the cell membranes of various cell types, including primary sensory neurons (intraepidermal nerve fibers), immune and skin cells. After the binding of NGF to TrkA, the complex undergoes dimerization and autophosphorylation on the axonal terminals of primary sensory neurons, followed by endocytosis either in a clathrin-dependent or an independent manner. The signaling endosome is retrogradely transported to the cell bodies in dorsal root ganglion (DRG). Before the long-range transport is initiated, the TrkA positive endosomes accumulate the small GTPase from the Rab family, i.e., Rab7 GTPase in the peripheral terminal [
12]. Rab GTPase's are the molecules that determine whether the endosome should travel retrogradely to the cell body for signal transduction or degradation in lysosomes. Once the TrkA signaling endosome associates with Rab7, it moves retrogradely and initiates the downstream signaling for the expression of different inflammatory mediators and neurotransmitters [
13]. Therefore, in this study, we used TrkA and Rab7 pharmacological inhibitors to block the NGF signaling during adjuvant-induced arthritis (AIA) to determine the effect of NGF on glutaminase (GLS) levels in DRG neurons and pain behaviour in AIA animals.
All DRG neurons are glutamatergic as they utilize glutamate to convey information from their peripheral and central terminals. Previous studies linked the peripheral release of glutamate with the process of nociception and sensitization of primary afferent DRG neurons [
14]. Levels of glutamate are elevated in the synovial fluid and epidermis of patients with arthritis and gold-induced skin inflammation, respectively [
15,
16]. The primary afferents convert glutamine to glutamate via the enzyme GLS. This GLS is activated regionally by calcium and phosphate and hence also known as Phosphate-activated Glutaminase (PAG) [
17]. We previously reported that the level of GLS is elevated significantly in DRG cell bodies during chronic inflammation, and peripheral inhibition of GLS reduces sensitization and pain associated with inflammation [
14,
18,
19,
20].
After the initiation of the inflammatory process, the DRG sensory neurons alter the expression of proteins such as Substance P (SP) and Calcitonin gene related peptide (CGRP), mostly attributed to NGF and its high-affinity receptor, TrkA. Although, under normal conditions, the basal expression of GLS is not regulated by NGF [
21], while during the process of inflammation, the role of NGF/TrkA in the regulation of GLS expression needs further evaluation. To identify NGF signaling associated molecules, we evaluated the immunoreactivity of NGF, pTrkA and Rab7 in the ligated sciatic nerve after AIA in Sprague Dawley rats. The goal of this study was to explore the involvement of NGF signaling in the process of neurogenic inflammation by evaluating the levels of GLS in DRG neurons by inhibiting TrkA and Rab7 during peripheral inflammation. The selective TrkA inhibition in the periphery will block the formation of NGF/TrkA complex, while the inhibition of Rab7 will disrupt the NGF/TrkA long-range retrograde signaling, hence affecting the alteration in DRG cell bodies. We used GW441756 (TrkA inhibitor) and CD1067700 (Rab7 inhibitor) to block the NGF signaling in the periphery and evaluated the expression of GLS in DRG cell bodies. We observed that inhibition of both TrkA and Rab7 individually attenuated the levels of GLS, thereby confirming the involvement of NGF retrograde signaling in the glutamate metabolism. We also established the potential of Rab7 as a therapeutic target for treating pain as Rab7 blockade showed reduced pain behaviour in arthritic animals.
2. Results
2.1. Effect of AIA on levels of NGF, pTrkA, and Rab7 in sciatic nerve
For determining the levels of proteins associated with NGF signaling in the sciatic nerve during peripheral inflammation, we ligated the sciatic nerve of adjuvant-induced arthritic animals. The ligation obstructed the axonal transport allowing us to evaluate the levels of NGF, pTrkA, and Rab7. Representative images of the ipsilateral sciatic nerve showed a qualitative increase in the immunoreactivity of NGF, pTrkA, and Rab7 (
Figure 1).
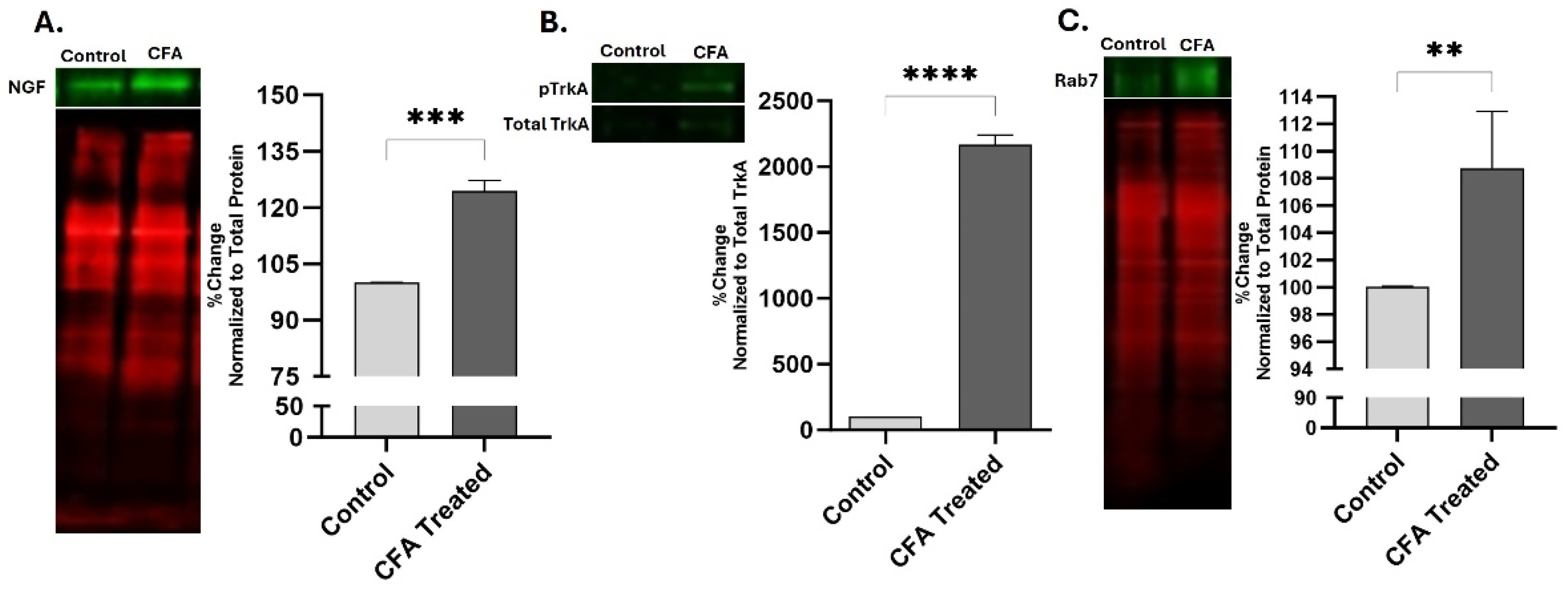
Western blot data detected the band for pro-NGF form at 27 KDa, confirming previous studies [
22]. The NGF-ir was elevated in the sciatic nerve of AIA animals after the initiation of inflammation (
Figure 2A). After 48 hours of AIA, the levels of pTrkA, compared to the total TrkA, were found to be significantly higher (**p = 0.0061) in the sciatic nerve with ligation (
Figure 2B). Quantitative western data indicated a significant increase in the immunoreactivity of Rab7 in the sciatic nerve of CFA treated animals compared to the sham animals (
Figure 2C).
2.2. Effect of TrkA inhibition on GLS expression during AIA
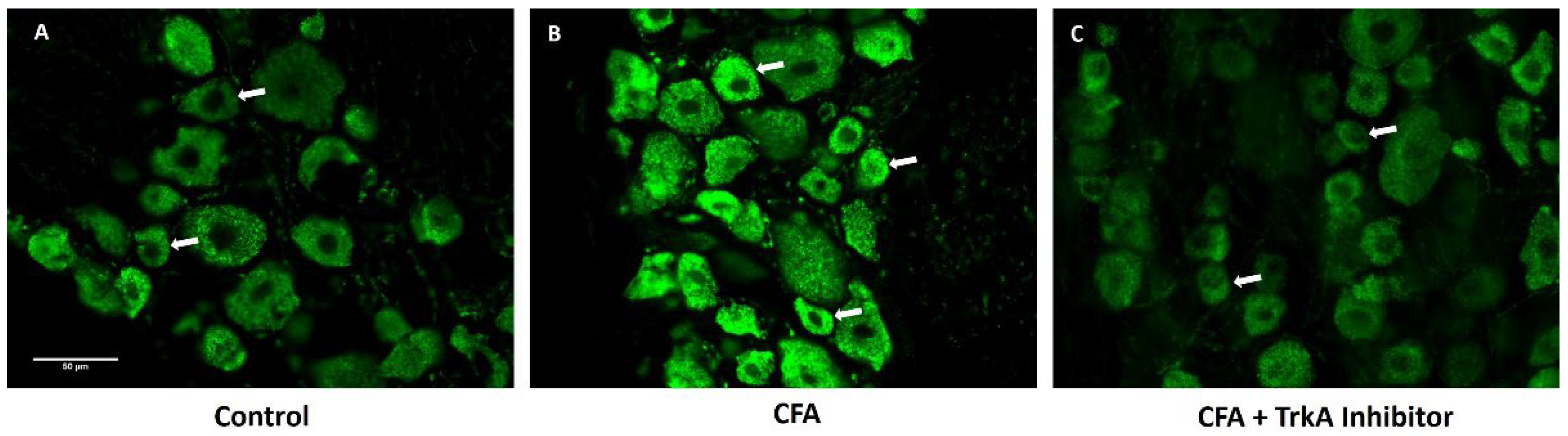
The levels of GLS were evaluated after 48 hours in the L4 and L5 DRG's of rat treated with CFA and selective TrkA inhibitor (GW441756). Almost all the DRG neurons were immunoreactive for GLS, as reported in the previous studies [
14,
20,
23,
24]. The GLS-ir was elevated in the animals treated with CFA as compared to naïve rats. TrkA pharmacological inhibition with selective TrkA inhibitor attenuated the GLS-ir in the DRG neurons compared to the neurons of CFA treated animals (
Figure 3).
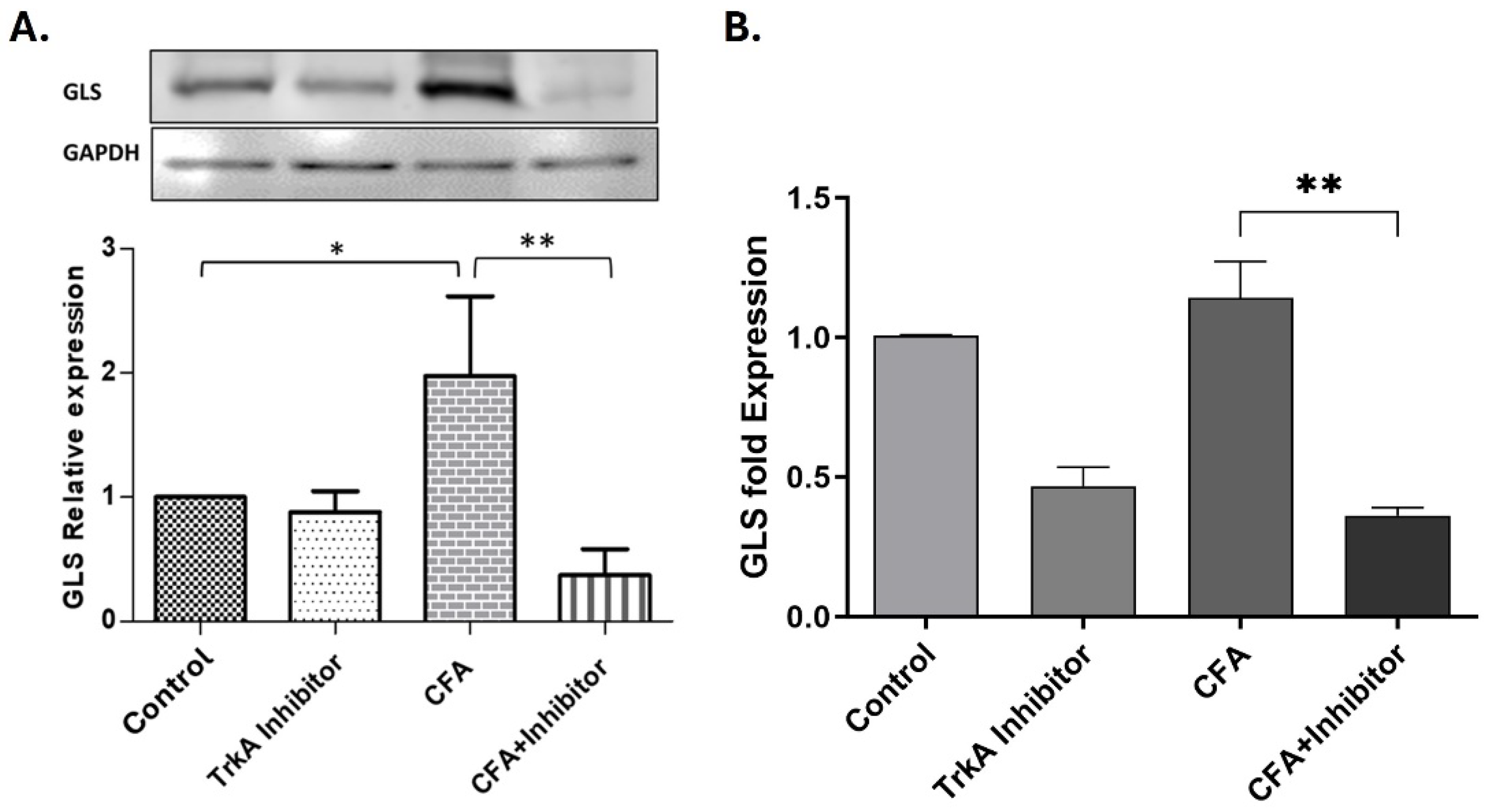
The western blot data indicated a significant reduction in the levels of GLS in the DRG cell bodies of animals treated with the TrkA inhibitor compared to AIA animals (
Figure 4-A). We also evaluated the levels of GLS mRNA in DRG's to determine the transcriptional changes due to NGF signaling blockade via TrkA inhibition. The GLS mRNA levels of TrkA inhibited rats were significantly diminished compared to the CFA–alone group (
Figure 4-B).
2.3. Effect of Rab7 inhibition on GLS expression and pain behavior during AIA.
The peripheral inhibition of Rab7GTPase during CFA-induced inflammation decreased the GLS-ir in the L4 and L5 DRG's. The quantitative western blot data show the elevation of GLS after peripheral inflammation confirming previous findings [
18,
20,
23,
25]. After the Rab7 inhibition, the GLS levels were attenuated significantly in the DRG's of AIA rats (
Figure 5).
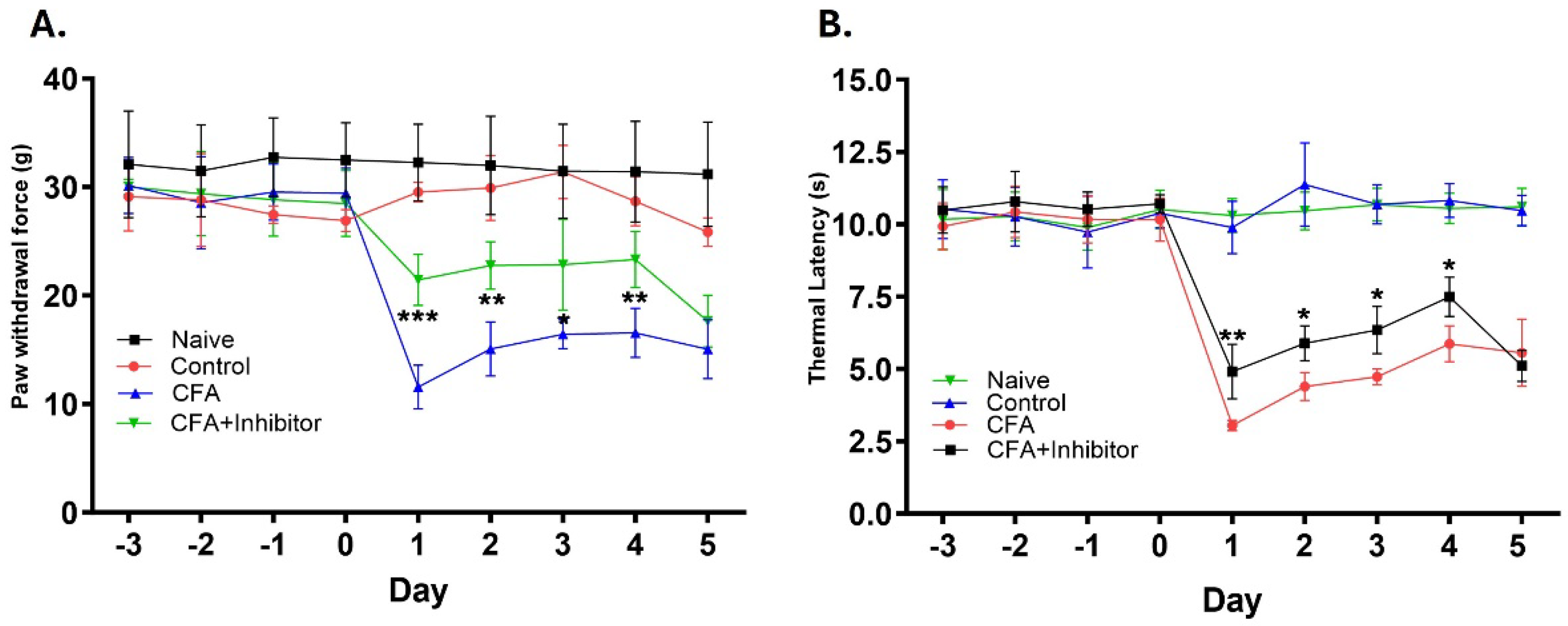
Pain behavior was determined by measuring the mechanical thresholds and thermal latencies in hind paws of animals after injecting and topically applying the CFA and Rab7 receptor antagonist. The reduction in paw withdrawal force and thermal withdrawal latency was interpreted as the presence of mechanical and thermal hyperalgesia, respectively. The mechanical threshold values for animals treated with CFA were significantly reduced compared to control animals starting at 24 hours and remained reduced until day 5 (p = <0.001). The treatment with Rab7 inhibitor along with CFA (Group: CFA + Inhibitor) significantly reduced the mechanical hyperalgesia as compared to CFA only group after Day 1 (p < 0.001), Day 2 (p < 0.01), Day 3 (p < 0.05) and Day 4 (p < 0.01) (
Figure 6-A). Similarly, the thermal withdrawal latencies were increased significantly in animals treated with CFA and Inhibitor as compared to the animals treated with only CFA. The reduced thermal hyperalgesia was observed after Day 1 (p < 0.01), Day 2 (p < 0.05), Day 3 (p < 0.05) and Day 4 (p < 0.05) in rats treated with Rab7 receptor antagonist with CFA (
Figure 6-B).
3. Discussion
Levels of NGF, pTrkA and Rab7 increases during AIA in sciatic nerve.
Our study showed that the unilateral injection of CFA in rat's hind paw elevates the levels of NGF, pTrkA, and Rab7 in the sciatic nerve. These results confirmed the previous findings of the involvement of NGF/TrkA axis in the development of peripheral inflammation [
3,
4,
21,
22,
26,
27]. Levels of NGF are elevated in the cerebrospinal fluid and synovial fluid of patients suffering from inflammatory and autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, respectively [
28,
29]. Different animal models of induced arthritis show increased NGF expression [
2,
30]. Neuropeptides like SP, CGRP, and cytokines like IL-6, IL-β are released after the noxious stimuli and are primary mediators of regulating the inflammatory process. This release of inflammatory mediators might elevate the NGF basal levels as predicted previously [
27,
31].
The NGF antibody we used in this study can bind to mature (13kDa) and pro-NGF (27 and 35kDa) forms [
32] simultaneously, hence we cannot differentiate between the two forms in the immunohistochemistry data. Our western blot analysis also failed to detect the mature NGF (β-NGF) while the pro- NGF form gave a prominent band at 27kDa. The method of tissue processing and western blotting we employed in this study might be the reason of not detecting mature NGF.
TrkA is the high-affinity receptor for NGF and is expressed in various types of cells, including peripheral sensory neurons. After binding to NGF on the cell membrane, TrkA is activated and phosphorylates the tyrosine residues present on the cytoplasmic domain, thus converting from TrkA to pTrkA [
33,
34]. Therefore, for determining the expression of TrkA during the process of inflammation, we used the pTrkA antibody and normalized its expression with total TrkA. After the initiation of inflammation and disrupting the NGF trafficking by sciatic nerve ligation, we found that the levels of pTrkA compared to total TrkA were significantly increased in the sciatic nerve. Previous studies demonstrated that the inflammatory stimulation significantly elevates TrkA expression in the immunologic cells like monocytes, macrophages, B lymphocytes, and T lymphocytes [
35] therefore some of the TrkA inhibition may occur among these cells, also. In addition, the TrkA immunoreactivity and phosphorylation are shown to be strongly upregulated in the lumbosacral DRG's of animals with cyclophosphamide (CYP)-induced cystitis [
36], but we did not attempt to detect these in the DRG in the current study.
We also found the levels of Rab7GTPase increased in the sciatic nerve along with NGF and pTrkA. The Rab family of GTPase is crucial for organelle transport and is responsible for regulating different processes involved in membrane trafficking pathways [
12]. The endosomal GTPase Rab7 is functionally distinct from other members of the Rab superfamily and has been implicated in the transport of late endosomes [
37]. After the endocytosis of NGF/TrkA complex, the signaling endosome travels retrogradely to the cell bodies to initiate signal transduction. Before the initiation of retrograde transport, the TrkA positive endosomes undergo maturation forming Rab5GTPase positive early endosomes to Rab7GTPase positive late endosomes [
38]. Furthermore, a mutation in Rab7 leads to the defective axonal transport and dysregulated NGF/TrkA signaling in DRG neurons, suggesting Rab7's pivotal role in the trafficking of TrkA positive endosomes [
39].
Selective TrkA inhibition attenuates GLS expression during peripheral inflammation.
GLS plays a crucial role in the glutamine-glutamate cycle. Several studies documented the immunoreactivity of GLS in all sizes of DRG neurons [
20,
23]. Our lab previously showed that GLS-immunoreactivity (-ir) and enzyme activity are elevated in DRG neuronal cell bodies during chronic peripheral inflammation [
20], but the mechanism for this GLS elevation has not been fully characterized. In this study, we found that the NGF signaling blockade in the periphery via selective TrkA pharmacological inhibition attenuated the levels of GLS in the DRG during AIA, confirming the interplay between NGF signaling and glutamate metabolism [
21]. During the inflammatory process, elevated amounts of GLS are anterogradely transported to the peripheral nerve terminals causing an increase in glutamate production. The elevated glutamate production and release is responsible for sensitizing the primary sensory afferents, thereby regulating nociceptive transmission [
25]. The data from this study implicate that the NGF signaling inhibition eventually attenuates the glutamate production in the periphery leading to maintenance in nociceptive sensitization.
TrkA inhibition indicates other potential ways of inhibiting NGF signaling apart from anti-NGF antibodies. NGF has a high affinity for TrkA receptors and a low affinity for p75 neurotrophin receptors present at the axonal terminal. Binding of NGF to TrkA promotes endocytosis and the formation of the signaling endosomes. These NGF/TrkA signaling endosomes are structurally and molecularly defined as multivesicular bodies. For eliciting the transcriptional modulation in the cell soma, NGF/TrkA endosomes undergo long-range retrograde travel from the distal axons to the DRG cell bodies for initiating subsequent downstream intracellular signaling [
40,
41,
42]. TrkA activation elevates the expression of SP and CGRP, leading to the sensitization of nociceptors during inflammation [
43,
44]. Therefore, selective TrkA inhibition can decrease inflammation and sensitization, thus reducing the pain behavior in conditions with a prominent inflammatory process like arthritis [
45,
46]. In addition, pharmacological inhibition of TrkA can also be used for studying the mechanisms involved in the trafficking of NGF signaling endosomes during the process of nociception.
Peripheral inhibition of Rab7GTPase decreases the GLS expression and provides analgesia.
Our study shows the pharmacological inhibition of small GTPase Rab7 via receptor antagonist in the periphery attenuates the GLS levels in DRG cell bodies of primary sensory neurons after the initiation of inflammation. Previous studies have linked the Rab7 with the TrkA positive endosomes in neurons and retrograde transport from the axon to the neuronal cell body [
39,
47]. This makes Rab7 as a potential target for disrupting the NGF signaling. The primary function of the Rab7 is to regulate the maturation of early endosomes into late endosomes and are associated with the fusion and clustering [
48]. As Rab7 interacts with TrkA in the multivesicular bodies, Rab7 inhibition leads to an excess of TrkA to be accumulated in the endosomes and hindrance of TrkA endosomal trafficking [
12]. We postulate that the inhibition of NGF/TrkA signaling via Rab7 is responsible for modulating the levels of GLS in DRG, hence suggesting the potential mechanism behind the regulation of glutamate metabolism during peripheral inflammation.
The findings of this study also provide evidence for the analgesic potential of Rab7 inhibition, as the Rab7 receptor antagonist CD1067700 reduced pain behavior associated with the adjuvant-induced inflammation. This offers a novel therapeutic strategy for alleviating arthritic pain. We found the mechanical and thermal hyperalgesia were reduced significantly after Day 1 in groups treated with Rab7 inhibitor. As the long-range NGF/TrkA retrograde signaling takes a considerable amount of time to reach the DRG cell bodies and exert transcriptional changes, the early analgesic effect might be due to the interaction of Rab7 to various other pain-related molecules such as μ-opioid receptors and calcium-/calmodulin-dependent protein kinase 4. These studies suggest Rab7's interaction with different molecules related to pain processing might be necessary for the development of nociceptive processes [
49]. In addition, the involvement of Rab7 in the hereditary sensory neuropathies (HSN) and an autosomal dominant inherited disorder causing peripheral neuropathy, Charcot-Marie-Tooth (CMT) provides a rationale to study this small GTPase closely.
4. Materials and Methods
4.1. Animals
Sprague-Dawley rats (350 – 450g, N = 99) bred and housed in the OSU-CHS animal facility were used in this study. Animals were maintained on a 12 h light: 12 h dark cycle and provided with continuous access to food and water. These studies were performed at Oklahoma State University-Center for Health Sciences (OSU-CHS), and the procedures are approved by the OSU-CHS Institutional Animal Care and Use Committee. All the procedures were performed according to the National Institute of Health and International Association for the Study of Pain guidelines [
50].
4.2. Induction of adjuvant-induced arthritis (AIA) and Sciatic nerve ligation
For inducing unilateral inflammation of the hind paw, Complete Freund's Adjuvant (CFA; Sigma; St. Louis, MO, USA) was used. Rats (n = 12) were anesthetized with isoflurane (initially 5%, then reduced to 2.5%) and 150 µl of a 1:1 emulsion containing CFA and sterile phosphate-buffered saline (PBS) was injected into the plantar surface of the right hind paw. Rats were allowed to recover on a warm towel and then placed back into their cages. After six hours of CFA treatment, animals were anesthetized again, and the right sciatic nerve was exposed and ligated with non-absorbable silk before the trifurcation, 5mm below the sciatic notch [
51]. Sham animals were used as a control group in which the same surgery was performed, but no CFA injection was administered.
4.3. Adjuvant-induced Arthritis (AIA) and Pharmacological Interventions
Rats were anesthetized prior to all injections, and AIA was induced by injecting 150 µl of CFA into the plantar surface of the right hind paw. TrkA inhibitor GW441756 (Tocris Biosciences, Bristol, UK) and Rab7 receptor antagonist CD1067700 (Cayman, Ann Arbor, MI, USA) were dissolved in 5% DMSO in PBS and injected as 20nmol/20µl and 100nmol/20µl respectively in the plantar surface of the right hind-paw. Besides the injection, the inhibitors were also applied topically onto skin with the help of filter paper soaked in DMSO and inhibitor. The inhibitors were injected and topically applied once before 30 mins of CFA treatment and two times: 12 hours and 36 hours after CFA treatment. Rats with respective volume PBS injections as compared to treated were used as controls [
52].
4.4. Immunofluorescence
The animals (n = 9) were perfusion fixed with a fixative containing 0.96% (w/v) picric acid and 0.2% (w/v) formaldehyde in 0.1 M sodium phosphate buffer, pH 7.3 [
21,
53]. The sciatic nerve and the L4 and L5 DRG's were collected from the perfusion fixed rats and immersed in the same fixative for four hours at room temperature. The tissues were transferred to a 10% sucrose solution in PBS (pH 7.3) and incubated at 4°C overnight. The next day, tissues were embedded and frozen in Lipshaw embedding matrix and cut in 14 μm sections on a Leica CM 1850 cryostat (Leica Biosystems; Wetzlar, Germany). The slices were collected on gelatin-coated slides and dried on a slide warmer at 37°C for 1 hour. After rinsing the slides with 1X PBS, the slides were incubated with primary antibodies mentioned in
Table 1 for four days at 4°C [
53]. The slides were washed three times with 1X PBS and incubated with respective fluorescent-labeled secondary antibodies (
Table 1) and incubated for 60 mins at room temperature in the dark. Finally, after incubation in secondary antibodies, the slides were rinsed thrice with 1X PBS and coverslipped with ProLong Gold Mounting Media (Invitrogen; Carlsbad, CA, USA) for image analysis.
4.5. Western Blot analysis
The sciatic nerve and L4, L5 DRG’s were homogenized in lysis buffer (25mM Tris HCl pH-7.4, 150 mM NaCl, 151 1mM EDTA, 5% glycerol and 1% Triton X-100) added with protease inhibitor cocktail (Sigma-Aldrich; St. Louis, MO, USA) for 5 mins with a handheld homogenizer and incubated on ice for 10 mins. The supernatant was collected in a fresh tube after centrifugation of homogenized sample at 14,000 rpm for 15 mins at 4°C. Total protein was estimated by Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Samples (20-50 µg/ml of total protein) were dissolved in loading buffer (10mM Tris Base, 1mM EDTA, 2.5% SDS, 5% β-mercaptoethanol, and 0.01% bromophenol blue) and boiled at 100 °C for 10 min. The sciatic nerve protein samples were separated on 12% Gel, and DRG samples were separated on 7.5% Gel (TGX™ FastCast™ Acrylamide Solutions, Bio-Rad Laboratories, Hercules, CA, USA) along with Spectra™ Multicolor Broad Range Protein Ladder (Thermo Fisher Scientific, Waltham, MA, USA) by SDS -PAGE and blotted on to nitrocellulose membrane in Mini Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA, USA).
TrkA Inhibition study: The membranes were incubated with 5% Carnation milk in Tris-buffered saline Tween (TBST, 20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.5) at room temperature for 1 hour and incubated overnight at 4°C in primary antibody against GLS (
Table 2). After washing with 1XTBST thrice (20 mins each), the membranes were incubated in alkaline phosphatase labeled anti-rabbit IgG (Promega; Madison, WI, USA) secondary antibodies at 1:1000 dilution for 120 min. After washing with 1XTBST three times for 20 mins each, the ECF substrate was used on a Typhoon 9410 Variable Mode Imager for taking the western blot images. The images were analysed by ImageJ (Version – 1.53t, National Institute of Health; Bethesda, MD, USA).
Sciatic nerve ligation and Rab7 inhibition study: After the electrophoretic transfer, the membranes were rinsed with Tris-buffered saline (TBS, 20 mM Tris-HCl, 150 mM NaCl, 0.05%, pH 7.5) and incubated in Revert Total Protein Stain (Li-Cor Biosciences, Lincoln, NE, USA) for 5 mins and rinsed with Revert wash solution and blocked with 5% Carnation milk in TBS at room temperature for 1 hour. All membranes were incubated overnight at 4°C with primary antibodies against NGF, pTrkA, Rab7, and GLS (
Table 2) diluted in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.5). After washing with TBST four times (5 mins each), the membranes were incubated for one hour at room temperature in IRDye 800, and IRDye 680 labeled Donkey anti-rabbit and anti-mouse secondary antibodies (1:20,000). The membranes were rinsed with TBS (no Tween) followed by image acquisition on the Odyssey CLx Infrared Imaging system (Li-Cor Biosciences, Lincoln, NE, USA). The images were normalized against the total protein or total TrkA, and analysis was performed on Image Studio Lite software (Li-Cor Biosciences, Lincoln, NE, USA).
4.6. Quantitative Reverse Transcription PCR
DRG's were collected in liquid nitrogen and stores at -80°C until further use. Total RNA was extracted using Trizol solution (Thermo Fisher Scientific, Waltham, MA, USA). The RNA was reverse transcribed using M-MLV Reverse transcriptase (Promega, Madison, MI, USA), and Quantitative real-time PCR (qRT-PCR) was performed using the ABI StepOneTM system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as an internal reference with primer sequence (
Table 3). The data were expressed as 2-ΔΔCT, which represents the relative amount of target mRNA present in the treated sample to the naïve animal group.
4.7. Pain behavior
The animals (n = 45) were tested for pain behavior by determining the mechanical thresholds and thermal latencies after the initiation of inflammation with CFA. Before the testing started, rats were familiarized in the behavioral room by handling them for at least three days (twice every day for one hour). The baseline values were recorded for three days (-3, -2, -1 day) before the initiation of inflammation. On the day of treatment (day 0), the values were recorded before the CFA injection. All behavioral testing was performed at the same time (late afternoon, around 3 PM) every day to avoid any variation in animal responses. A total of 9 readings were obtained, including four baseline testing (Day -3, -2, -1 and 0) and five after CFA treatment (Day 1, 2, 3, 4, and 5). Mechanical threshold values were obtained using a Dynamic Plantar Aesthesiometer (Ugo Basile, Gemonio, Italy). The maximum force was set to 50 g with the ramp rate of 5 g/sec. Thermal latencies were measured using a Plantar Test apparatus (Ugo Basile, Gemonio, Italy) with the maximum cut off value set at 55°C and the intensity was set at 55 mW/cm2. All the tests were performed by placing the animal in the acrylic apparatus, and readings were obtained once the exploratory behavior ceased. For both mechanical threshold and thermal latency, five readings at intervals of 5 mins were recorded from the ipsilateral hind paw of each rat.
The PCR analysis results were reported as the threshold cycle (Ct), which determined the mRNA of the target gene in relation to the reference gene. The difference between the number of cycles required to detect the PCR products for the target and reference genes was represented by ΔCt. ΔΔCt, was the difference between the naïve animal group and the AIA group. Finally, the relative amount of target mRNA in the CFA-treated sample compared to the control animal group was expressed as 2-ΔΔCt.
4.8. Statistical Analysis
All the data were subjected to Student t-test using GraphPad Prism (version 5.01 for Windows, GraphPad Software, San Diego, CA, USA). p values less than 0.05 were considered significant for all tests. The data presented in the graph are grouped by mean ± SEM (and/or SD).
5. Conclusions
In the adjuvant-induced animal model of persistent inflammation, we have demonstrated that the peripheral inhibition of TrkA and Rab7 can modulate the expression of GLS in DRG, thus affecting the primary afferent's sensitization process. Our data suggest the Rab7GTPase inhibitor CD1067700 provides an analgesic effect in arthritic animals via NGF signaling blockade. Further studies directed towards Rab7 are necessary to understand the trafficking of NGF signaling endosome during the process of inflammation and nociception. We predict that such information on the NGF/TrkA axis can provide potential therapeutic targets for treating chronic inflammation.
Author Contributions
V.G.: Conceptualization, Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization. R.D.P.: Formal analysis, Investigation, Data Curation, Writing - Review & Editing, Visualization. B.M.H.: Writing – Review & Editing. S.D.: Conceptualization, Validation, Supervision, Project administration, Writing - Review & Editing.
Funding
Please add: This research was funded by the National Institute of Health, grant number NIH-AR047410” awarded to Kenneth E. Miller.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of OKLAHOMA STATE UNIVERSITY CENTER FOR HEALTH SCIENCE (74107 - 01/2020) Protocol Number 2020/01.
Data Availability Statement
All relevant data is included in the manuscript.
Acknowledgments
We would like to express our sincere gratitude to the late Kenneth E Miller for his invaluable contributions to this project. K.E.M provided essential funding, guidance, and equipment support, which significantly facilitated our research endeavors. His dedication to advancing scientific knowledge and his unwavering support will always be remembered.
Conflicts of Interest
The authors declare that there is no conflict of interest with regard to this work.
References
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951 Mar;116(2):321–61. [CrossRef]
- Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996/03/29 ed. 1996 Mar 29;351(1338):441–8. [CrossRef]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994 Sep;62(2):327–31. [CrossRef]
- Gujar V, Pande RD, Das S. Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation. International Journal of Molecular Sciences. 2024;25(7). [CrossRef]
- Hirose M, Kuroda Y, Murata E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2016 Feb;16(2):175–82. [CrossRef]
- McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995/08/01 ed. 1995 Aug;1(8):774–80.
- Shelton DL, McMahon SB, Winslow JW, Qao WQ. Neurotrophins and neurotrophin antagonists as potential therapeutics. Restor Neurol Neurosci. 1995/01/01 ed. 1995 Jan 1;8(1):99–100. [CrossRef]
- Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010/12/09 ed. 2010 Dec 7;6:87.
- Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010/10/15 ed. 2010 Oct 14;363(16):1521–31. [CrossRef]
- Ueda K, Hirose M, Murata E, Takatori M, Ueda M, Ikeda H, et al. Local administration of a synthetic cell-penetrating peptide antagonizing TrkA function suppresses inflammatory pain in rats. J Pharmacol Sci. 2010/03/31 ed. 2010;112(4):438–43. [CrossRef]
- Urschel BA, Brown PN, Hulsebosch CE. Differential effects on sensory nerve processes and behavioral alterations in the rat after treatment with antibodies to nerve growth factor. Exp Neurol. 1991/10/01 ed. 1991 Oct;114(1):44–52. [CrossRef]
- Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005/11/25 ed. 2005 Nov 23;25(47):10930–40. [CrossRef]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003/07/10 ed. 2003 Jul 3;39(1):69–84. [CrossRef]
- Hoffman EM, Miller KE. Peripheral inhibition of glutaminase reduces carrageenan-induced Fos expression in the superficial dorsal horn of the rat. Neurosci Lett. 2010 Mar 26;472(3):157–60. [CrossRef]
- McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000/04/01 ed. 2000 Mar;27(3):739–45.
- Nordlind K, Johansson O, Liden S, Hokfelt T. Glutamate- and aspartate-like immunoreactivities in human normal and inflamed skin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993/01/01 ed. 1993;64(2):75–82. [CrossRef]
- Wallace DR, Dawson R Jr. Regional differences in glutaminase activation by phosphate and calcium in rat brain: impairment in aged rats and implications for regional glutaminase isozymes. Neurochem Res. 1993/12/01 ed. 1993 Dec;18(12):1271–9. [CrossRef]
- Hoffman, E.M. The Role of Dorsal Root Ganglion Glutaminase in Nociception—A Novel Therapeutic Target for Inflammatory Pain. Ph.D. Thesis, Oklahoma State University, Ann Arbor, MI, USA, 2009. Volume 3356494.
- Miller KE. Method of alleviating chronic pain via peripheral glutaminase regulation. Patent-US7288246B2, United States; 2010.
- Miller KE, Balbas JC, Benton RL, Lam TS, Edwards KM, Kriebel RM, et al. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following peripheral inflammation. Pain Res Treat. 2012;2012:414697. [CrossRef]
- Hoffman EM, Zhang Z, Anderson MB, Schechter R, Miller KE. Potential mechanisms for hypoalgesia induced by anti-nerve growth factor immunoglobulin are identified using autoimmune nerve growth factor deprivation. Neuroscience. 2011 Oct 13;193:452–65. [CrossRef]
- Gujar V, Anderson MB, Miller KE, Pande RD, Nawani P, Das S. Separation of Rat Epidermis and Dermis with Thermolysin to Detect Site-Specific Inflammatory mRNA and Protein. J Vis Exp [Internet]. 2021/10/19 ed. 2021 Sep 29;(175).
- Miller KE, Douglas VD, Kaneko T. Glutaminase immunoreactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP). Neurosci Lett. 1993 Sep 17;160(1):113–6. [CrossRef]
- Miller KE, Richards BA, Kriebel RM. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002 Aug 2;945(2):202–11. [CrossRef]
- Zhang Z. The role of dorsal root ganglion glutaminase in acute and chronic inflammatory pain [Internet] [Ph.D.]. Vol. 3629945. [Ann Arbor]: Oklahoma State University; 2013. Available from: http://argo.library.okstate.edu/login?url=https://search.proquest.com/docview/1562918358?accountid=4117.
- Mesentier-Louro LA, De Nicolo S, Rosso P, De Vitis LA, Castoldi V, Leocani L, et al. Time-Dependent Nerve Growth Factor Signaling Changes in the Rat Retina During Optic Nerve Crush-Induced Degeneration of Retinal Ganglion Cells. Int J Mol Sci [Internet]. 2017/01/10 ed. 2017 Jan 5;18(1). Available from: https://www.ncbi.nlm.nih.gov/pubmed/28067793. [CrossRef]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997/06/01 ed. 1997 Jun;121(3):417–24.
- Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992/03/01 ed. 1992 Mar;35(3):351–5.
- Falcini F, Matucci Cerinic M, Lombardi A, Generini S, Pignone A, Tirassa P, et al. Increased circulating nerve growth factor is directly correlated with disease activity in juvenile chronic arthritis. Ann Rheum Dis. 1996/10/01 ed. 1996 Oct;55(10):745–8. [CrossRef]
- Manni L, Aloe L. Role of IL-1 beta and TNF-alpha in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol Int. 1998/12/02 ed. 1998;18(3):97–102.
- Steiner P, Pfeilschifter J, Boeckh C, Radeke H, Otten U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor synthesis in rat mesangial cells. Am J Physiol. 1991/11/01 ed. 1991 Nov;261(5 Pt 2):F792-8. [CrossRef]
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988/05/15 ed. 1988 May 15;263(14):6810–5. [CrossRef]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003/04/05 ed. 2003;72:609–42. [CrossRef]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991/03/14 ed. 1991 Mar 14;350(6314):158–60. [CrossRef]
- Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int J Mol Sci [Internet]. 2017 May 11;18(5). Available from: https://www.ncbi.nlm.nih.gov/pubmed/28492466. [CrossRef]
- Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002/11/02 ed. 2002 Dec 9;454(2):200–11. [CrossRef]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995/12/01 ed. 1995 Dec;131(6 Pt 1):1435–52. [CrossRef]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006/10/19 ed. 2006 Oct 19;52(2):293–305. [CrossRef]
- Zhang K, Fishel Ben Kenan R, Osakada Y, Xu W, Sinit RS, Chen L, et al. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J Neurosci. 2013/04/26 ed. 2013 Apr 24;33(17):7451–62. [CrossRef]
- Ascano M, Bodmer D, Kuruvilla R. Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 2012/03/27 ed. 2012 May;22(5):266–73. [CrossRef]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013 Mar;14(3):177–87. [CrossRef]
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007/01/02 ed. 2007 Jan 4;53(1):25–38. [CrossRef]
- Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014/04/11 ed. 2014 Aug;66(8):2188–200. [CrossRef]
- O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004 Nov;201(2):167–80. [CrossRef]
- Ashraf S, Bouhana KS, Pheneger J, Andrews SW, Walsh DA. Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res Ther. 2016/05/06 ed. 2016 May 4;18(1):97. [CrossRef]
- Nwosu LN, Mapp PI, Chapman V, Walsh DA. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2015/08/20 ed. 2016 Jun;75(6):1246–54. [CrossRef]
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002/06/07 ed. 2002 Jun;12(3):268–74. [CrossRef]
- Girard E, Chmiest D, Fournier N, Johannes L, Paul JL, Vedie B, et al. Rab7 is functionally required for selective cargo sorting at the early endosome. Traffic. 2013/12/18 ed. 2014 Mar;15(3):309–26. [CrossRef]
- Kallenborn-Gerhardt W, Moser CV, Lorenz JE, Steger M, Heidler J, Scheving R, et al. Rab7-a novel redox target that modulates inflammatory pain processing. Pain. 2017/04/11 ed. 2017 Jul;158(7):1354–65. [CrossRef]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983 Jun;16(2):109–10. [CrossRef]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, et al. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129(2):473–9.
- Crosby HA, Ihnat M, Miller KE. Evaluating the Toxicity of the Analgesic Glutaminase Inhibitor 6-Diazo-5-Oxo-L-Norleucine in vitro and on Rat Dermal Skin Fibroblasts. MOJ Toxicol [Internet]. 2015/01/01 ed. 2015;1(1). Available from: https://www.ncbi.nlm.nih.gov/pubmed/29750203.
- Hoffman EM, Schechter R, Miller KE. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. 2010 Apr;58(4):329–44. [CrossRef]
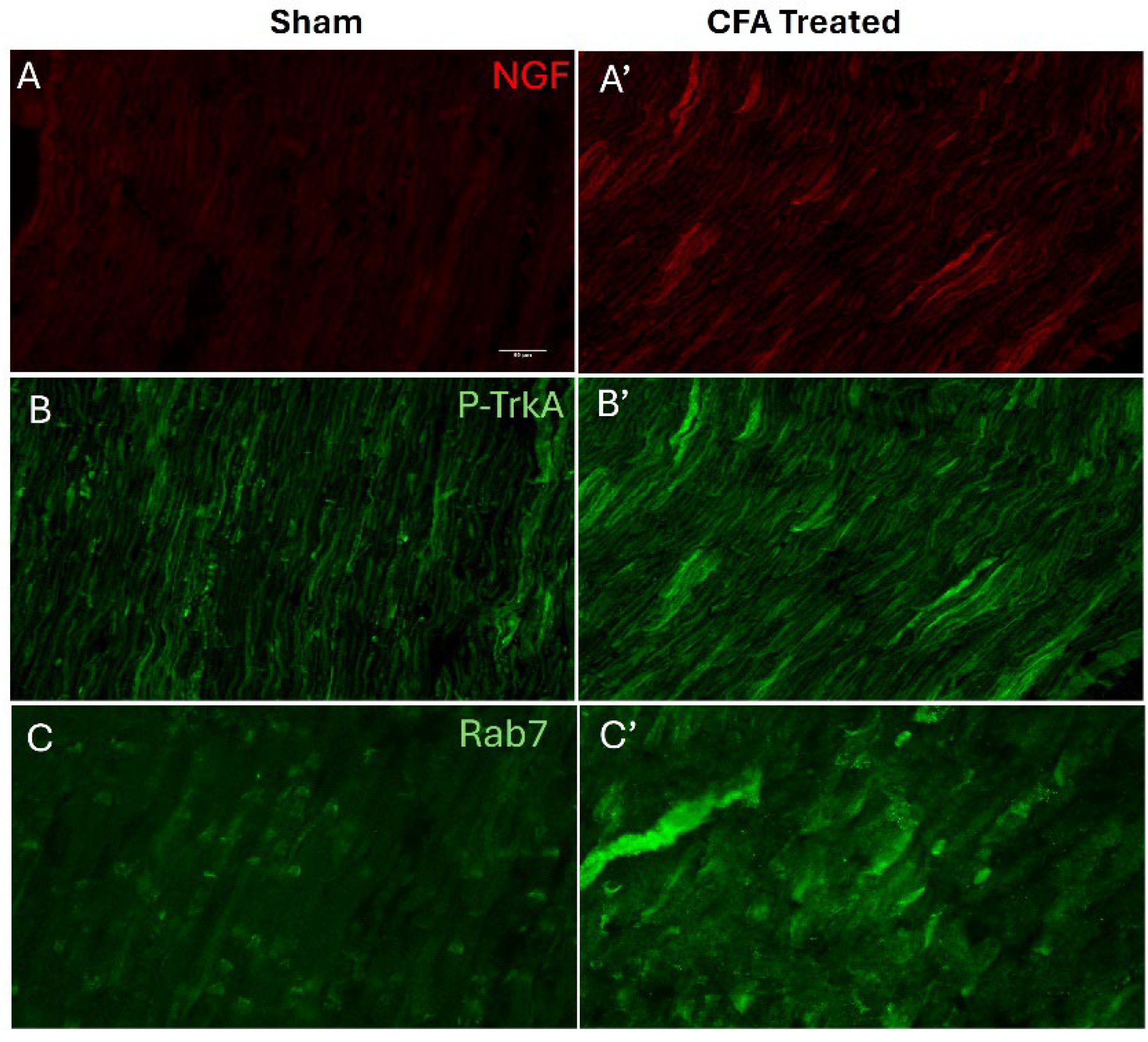
Figure 1.
Peripheral inflammation elevates the levels of NGF, pTrkA, and Rab7 GTPase in ligated sciatic nerve. Increased levels of NGF (A-A'), pTrkA (B-B') and Rab7 (C-C') were detected in sciatic nerve of CFA treated animals as compared to sham animals as determined by qualitative immunohistochemistry. Scale bar = 50 μm, (n=3).
Figure 1.
Peripheral inflammation elevates the levels of NGF, pTrkA, and Rab7 GTPase in ligated sciatic nerve. Increased levels of NGF (A-A'), pTrkA (B-B') and Rab7 (C-C') were detected in sciatic nerve of CFA treated animals as compared to sham animals as determined by qualitative immunohistochemistry. Scale bar = 50 μm, (n=3).
Figure 2.
Peripheral inflammation elevates the levels of NGF, pTrkA and Rab7 GTPase in sciatic nerve after ligation. Increased levels of NGF (A), pTrkA (B) and Rab7 (C) were detected in sciatic nerve of CFA treated animals as compared to Control rats after 48 hrs of inflammation as determined by fluorescence western blot. The NGF and Rab7 data were normalized by total protein while pTrkA data was normalized against total TrkA. **p < 0.01, ***p < 0.001, ****p < 0.0001. (n=4).
Figure 2.
Peripheral inflammation elevates the levels of NGF, pTrkA and Rab7 GTPase in sciatic nerve after ligation. Increased levels of NGF (A), pTrkA (B) and Rab7 (C) were detected in sciatic nerve of CFA treated animals as compared to Control rats after 48 hrs of inflammation as determined by fluorescence western blot. The NGF and Rab7 data were normalized by total protein while pTrkA data was normalized against total TrkA. **p < 0.01, ***p < 0.001, ****p < 0.0001. (n=4).
Figure 3.
Selective TrkA inhibition attenuates GLS levels in DRG during peripheral inflammation: Representative images showing GLS immunoreactivity (green). A: Expression of GLS in DRG of Naïve animal. B: GLS expression in DRG of AIA animal after 48h of inflammation. C: GLS expression in animal treated with CFA and selective TrkA inhibitor (GW441756). Note the expression of GLS in small diameter neurons (white arrows) in animals treated with CFA is higher as compared to the animals treated with TrkA inhibitor along with CFA. Scale bar = 50 μm, was applied to all photomicrographs. (n=3).
Figure 3.
Selective TrkA inhibition attenuates GLS levels in DRG during peripheral inflammation: Representative images showing GLS immunoreactivity (green). A: Expression of GLS in DRG of Naïve animal. B: GLS expression in DRG of AIA animal after 48h of inflammation. C: GLS expression in animal treated with CFA and selective TrkA inhibitor (GW441756). Note the expression of GLS in small diameter neurons (white arrows) in animals treated with CFA is higher as compared to the animals treated with TrkA inhibitor along with CFA. Scale bar = 50 μm, was applied to all photomicrographs. (n=3).
Figure 4.
Selective inhibition of TrkA in the periphery during AIA, changes the expression of GLS expression in rat DRG neurons after 48hrs of inflammation. (A) The levels of GLS protein are upregulated in animals treated with CFA as compared to control but decreased significantly in the group treated with TrkA inhibitor and CFA as determined by quantitative western blot analysis. (B) The GLS mRNA analysis showed that peripheral TrkA inhibition decreased GLS mRNA in both control and CFA treated rats as determined by qRT-PCR. *P < 0.05, **p < 0.01, ***p < 0.001 (Student’s t-test; n=3).
Figure 4.
Selective inhibition of TrkA in the periphery during AIA, changes the expression of GLS expression in rat DRG neurons after 48hrs of inflammation. (A) The levels of GLS protein are upregulated in animals treated with CFA as compared to control but decreased significantly in the group treated with TrkA inhibitor and CFA as determined by quantitative western blot analysis. (B) The GLS mRNA analysis showed that peripheral TrkA inhibition decreased GLS mRNA in both control and CFA treated rats as determined by qRT-PCR. *P < 0.05, **p < 0.01, ***p < 0.001 (Student’s t-test; n=3).
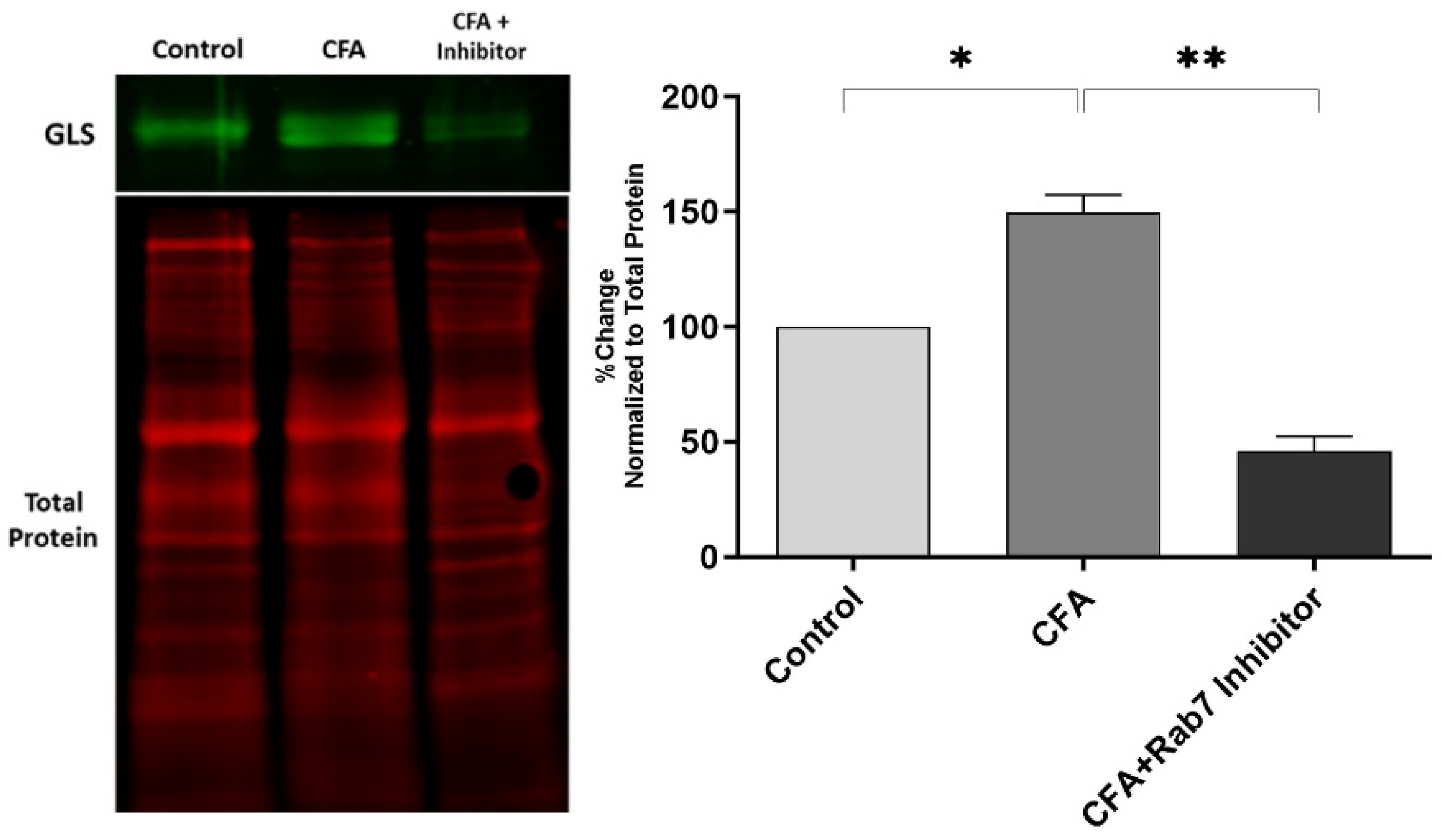
Figure 5.
Inhibition of Rab7 in the periphery during AIA, changes the expression of GLS in rat DRG neurons after 48hrs of inflammation. (A) Image showing GLS bands (66 and 68 kDa) and total proteins after western blotting. (B) The expression of GLS is significantly reduced in the group CFA+Inhibitor as compare to CFA after normalizing with total protein. *P < 0.05, **p < 0.01, ***p < 0.001. (n=4).
Figure 5.
Inhibition of Rab7 in the periphery during AIA, changes the expression of GLS in rat DRG neurons after 48hrs of inflammation. (A) Image showing GLS bands (66 and 68 kDa) and total proteins after western blotting. (B) The expression of GLS is significantly reduced in the group CFA+Inhibitor as compare to CFA after normalizing with total protein. *P < 0.05, **p < 0.01, ***p < 0.001. (n=4).
Figure 6.
Mechanical thresholds and Thermal latencies from rat hind paws after inhibition of Rab7 in the periphery during AIA. (A). Inhibition of Rab7 in the periphery during AIA (CFA+Inhibitor), caused a significant increase in paw withdrawal force from days 1 through 4 as compared to the CFA group. (B). Inhibition of Rab7 in the periphery during AIA (CFA+Inhibitor), caused significant increase in the thermal latencies from days 1 through 4 as compared to the CFA group. Data are graphed as means (±SD). *P < 0.05, **p < 0.01, ***p < 0.001. (Student’s t-test; n=5).
Figure 6.
Mechanical thresholds and Thermal latencies from rat hind paws after inhibition of Rab7 in the periphery during AIA. (A). Inhibition of Rab7 in the periphery during AIA (CFA+Inhibitor), caused a significant increase in paw withdrawal force from days 1 through 4 as compared to the CFA group. (B). Inhibition of Rab7 in the periphery during AIA (CFA+Inhibitor), caused significant increase in the thermal latencies from days 1 through 4 as compared to the CFA group. Data are graphed as means (±SD). *P < 0.05, **p < 0.01, ***p < 0.001. (Student’s t-test; n=5).
Table 1.
Details of Primary and secondary antibodies used in Immunofluorescence studies.
Table 1.
Details of Primary and secondary antibodies used in Immunofluorescence studies.
| Tissue |
Primary Antibodies |
Dilutions |
Secondary Antibodies |
Dilutions |
| Sciatic Nerve |
NGF Anti-mouse
(E-12, Santa Cruz, TX, USA) |
1:1000 |
Donkey anti-mouse Alexa Flour 555
(Invitrogen; Carlsbad, CA, USA) |
1:1000 |
pTrkA Anti-rabbit
(4168S, Cell signaling, MS, USA) |
1:2000 |
Donkey anti-rabbit FITC 488
(Invitrogen; Carlsbad, CA, USA) |
1:1000 |
Rab7 Anti-rabbit
(55469-1-AP, Proteintech, Rosemont, IL, USA) |
1:2000 |
Donkey anti-rabbit FITC 488
(Invitrogen; Carlsbad, CA, USA) |
1:1000 |
| L4 and L5 DRG |
GLS Anti-rabbit
(Norman Curthoys, Colorado State University, Ft. Collins, CO, USA) |
1:1000 |
Donkey anti-rabbit FITC 488
(Invitrogen; Carlsbad, CA, USA) |
1:1000 |
Table 2.
Details of Primary and secondary antibodies used in western blot studies.
Table 2.
Details of Primary and secondary antibodies used in western blot studies.
| Study/Tissue |
Primary Antibodies |
Dilutions |
Secondary Antibodies |
Dilutions |
| Sciatic Nerve Ligation/Sciatic nerve |
NGF Anti-Mouse
(E-12, Santa Cruz, TX, USA) |
1:1000 |
IRDye 800CW Donkey anti-mouse
(Li-Cor, Lincoln, NE, USA) |
1:20,000 |
pTrkA Anti-rabbit
(4168S, Cell signalling, MS, USA) |
1:2000 |
IRDye 800CW Donkey anti-rabbit
(Li-Cor, Lincoln, NE, USA) |
1:20,000 |
Rab7 Anti-rabbit
(55469-1-AP, Proteintech, Rosemont, IL, USA) |
1:2000 |
IRDye 800CW Donkey anti-rabbit
(Li-Cor, Lincoln, NE, USA) |
1:20,000 |
Total TrkA Anti-rabbit
(2505S, Cell signalling, MS, USA) |
1:2000 |
IRDye 800CW Donkey anti-rabbit
(Li-Cor, Lincoln, NE, USA) |
1:20,000 |
| TrkA Inhibition/L4, L5 DRG |
GLS Anti-rabbit
(Norman Curthoys, Colorado State University, Ft. Collins, CO, USA) |
1:1000 |
AP labeled Anti-rabbit IgG (Promega; Madison, WI, USA) |
1:1000 |
| Rab7 Inhibition/L4, L5 DRG |
GLS Anti-rabbit
(Norman Curthoys) |
1:1000 |
IRDye 800CW Donkey anti-rabbit
(Li-Cor, Lincoln, NE, USA) |
1:20,000 |
Table 3.
Primer sequences used for quantitative PCR.
Table 3.
Primer sequences used for quantitative PCR.
| Gene |
Primer Sequence |
| GLS |
GLS-F: 5’- GGGTCTGTTACCTAGCTTGGAAGATTTGC-3’
GLS-R: 5’- GAGTTAATCTTAACATATCCATACACT-3’ |
| GAPDH |
GAPDH-F: 5'- GAACCACGAGAAATATGACAACTCCCTCAAG-3'
GAPDH-R: 5' GCAGTGATGGCATGGACTGTGG-3' |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).